Abstract
Objectives
Activation of β3-adrenoceptor (ADRB3) is essential in the process of human adipose tissue browning, but obese subjects suffered from reduced ability of brown adipose tissue activation. The present study aims to detect the adipocyte ADRB3 expression in overweight individuals and the relationship between adipocyte ADRB3 expression and adiposity in adults.
Methods
Visceral adipose tissue samples were obtained from 85 subjects who underwent abdominal surgery. ADRB3 mRNA and protein expression levels in mature adipocytes and adipose tissue stromal vascular cells were examined by quantitative real-time PCR and Western blot assay, respectively. UCP-1mRNA expression levels in mature adipocytes were examined by quantitative real-time PCR.
Results
The data revealed that ADRB3 mRNA (p = 0.021) and protein (p = 0.025) expression levels in mature adipocytes were significantly higher in the normal-weight than in the overweight group. Similar results were also found for ADRB3 mRNA (p = 0.041) and protein (p = 0.025) expressions of stromal vascular cells. An inverse correlation was verified between mature adipocyte ADRB3 mRNA expression and BMI (r = −0.362, p = 0.012). UCP-1 mRNA expression levels in mature adipocytes were higher in the normal-weight group compared with the overweight group (p = 0.045).
Conclusion
Adipocyte ADRB3 expression levels were down-regulated before the onset of obesity, which indicated that the reduction of ADRB3 expression might be the cause of compromised adipose tissue browning and obesity rather than the result. Thus, the interference of the ADRB3 pathway in adipocytes may provide a potential treatment target for obesity.
Keywords: β3-adrenoceptor, Overweight, Adipocyte, Human
Introduction
Obesity is an important global public health problem, which is an independent risk factor of diabetes, hypertension, hyperlipidemia, ischemic heart disease and some cancers, and there are still no effective therapeutic strategies yet [1]. Brown adipose tissue (BAT) can dissipate chemical energy in the form of heat through the activation of uncoupling protein 1 (UCP1), thereby preventing the development of obesity. Classical brown adipocytes mainly reside in infants and young children, and rarely in human adults. But when exposed to cold stimuli, some adipocytes in white adipose tissue can express high levels of UCP1 and are termed as beige or brite adipocytes [2, 3]. Adipose tissues with UCP1-positive adipocytes in human are known as human brown adipose tissue (hBAT) [4]. β3-adrenoceptor (ADRB3) is a dominant signaling pathway in the process of adipose tissue browning, and some researchers have focused on adipose tissue browning in mice [5] and humans [6, 7] by activating ADRB3 in order to treat obesity. But the increase of hBAT activity during the process of sympathetic nerve stimulation is lower in obese patients compared with non-obese individuals [8]. This is due to the reduced ADRB3 function as shown in a few animal [9, 10] and one human [11] study; however, these studies recruited humans and animals with severe obesity and thus cannot explain the causal relationship between ADRB3 disfunction / adipose tissue browning disability and obesity. The present study proposed that compromised adipocyte ADRB3 function may appear before the onset of obesity, which would reduce adipose tissue response to browning stimuli, which result in decreased hBAT activation and eventually contribute to the development of obesity. To verify the above-mentioned hypothesis, the present study is designed to investigate adipocyte ADRB3 expression in overweight individuals, and its relationship to adipose tissue browning and adiposity.
Material and Methods
Participants
Omental adipose tissue samples (2–6 g) were collected from 85 patients who underwent abdominal surgery. Entry criteria were age between 20 and 75 years and BMI between 18 and 29.9 kg/m2. Patients who have suffered from cancer, thyroid diseases, diabetes, and serious infectious diseases as well as those who have been taking thyroxine, insulin, thiazolidinedione, lipid-lowering agents and GLP-1 analogues in last 3 months were excluded. Height, weight, systolic blood pressure and diastolic blood pressure were measured before surgery. Fasting (8–10 h) blood samples were collected from all participants. Low-density lipoprotein cholesterol (LDLC) was calculated with the Friedewald formula, and triglycerides (TG) were determined by conventional enzymatic assays. BMI was calculated as weight (kilograms) divided by height (meters) squared. Participants were divided into two subgroups according to BMI: normal-weight group (NW group; BMI < 25 kg/m2) and overweight group (OW group; (25 ≤ BMI < 30 kg/m2) [12]. This study was conducted in terms of Declaration of Helsinki and approved by the local Ethical and Research Committee of Jinan Central Hospital (Shandong, China). All participants signed informed consent.
Isolation of Human Mature Adipocytes and Stromal Vascular Cells
Adipose tissues were minced and digested with 1 mg/mL collagenase in 37°C shaking H2O bath for 15–30 min. Then, the digested tissues were filtered through 250 micron mesh and left to settle for 5 min. The floating mature adipocytes were collected from the top layer. The remaining cell suspension was spun down by centrifugation at 2,000 rpm (10 min), and stromal vascular (SV) cells were obtained in the pellet. Cells were washed twice in PBS and stored at −80°C for RNA and protein extraction.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was extracted from the mature adipocytes and SV cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's recommendations. RNA was dissolved in nuclease-free water and quantified using spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA (1 μg) was reverse-transcribed using the Reverse Transcription Reaction Kit (TaKaRa Bio Inc., Kusatzu, Japan) and quantitative real-time PCR (qRT-PCR) was performed on the ABI 7,500 Sequence Detection system (Applied Biosystems, Foster City, CA, USA). The relative mRNA expression levels were calculated using the 2–ΔΔCt method after standardization to18S mRNA levels. The qRT-PCR reactions were performed using ADRB3 primers: 5′-TACTCTGCGCTGGCTTTTGA-3′ (forward), and 5′-AAGGCTCAAGCTCACTCCC-3′ (reverse); UCP-1 primers: 5′-AACGAAGGACCAACGGCTTTC-3′ (forward), and 5′-CACAGTCCATAGTCTGCCTTG-3′ (reverse); and 18S [13] primers: 5′-GCAATTATTCCCCATGAACG-3′ (forward) and 5′-GGCCTCACTAAACCATCCAA-3′ (reverse).
Western Blot Analysis
Mature adipocytes and SV cells were homogenized in RIPA lysis buffer and protease inhibitor. Protein concentrations were determined by BCA Protein Assay kit (Thermo Fisher Scientific). The total 50 μg protein of each sample was separated by 10% SDS-PAGE and transferred onto PVDF membranes (0.45 μm pore size, Merck Millipore, Burlington, MA, USA). The membranes were blocked in non-fat milk for 1 h at room temperature and then incubated overnight at 4°C with specific primary antibodies consisting of anti-ADRB3 (1: 500, Abcam, Cambridge, UK) and anti-GAPDH (1: 4000, Proteintech Group, Rosemont, IL, USA), which was followed by incubation with appropriate secondary anti-rabbit/mouse IgG conjugated with HRP(Beijing Zhongshan Gold Bridge Biotechnology, Beijing, China) for 1 h at room temperature. The protein bands were detected using the Chemiluminescent HRP Substrate present in FluorChemE system (Cell Biosciences, Santa Clara, CA, USA) and analyzed with Image J software.
Statistical Analysis
SPSS version 22.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Normal distribution data were expressed as mean ± SD and analyzed statistically using the nonpaired Student's t test. The non-normal distribution variables were presented as median (interquartile range) and log-transformed before analysis. ADRB3 and UCP-1 expression levels of the NW and OW groups were compared using the nonpaired Student's t test. Correlations of ADRB3 mRNA with age and BMI were tested by univariate correlation analyses. Stepwise multiple linear regression analysis was performed to find correlation between independent variables (gender, age and ADRB3 mRNA) and BMI. p < 0.05 was considered statistically significant.
Results
Subject Characterization
85 subjects with an average age of 55 years (43 females, 42 males) were included in this study. Because of the limited amount of adipose tissue samples provided by surgery, we cannot get all ADRB3 mRNA and protein expression data of mature adipocytes and SV cells from each sample. ADRB3 mRNA and protein expression levels in mature adipocytes were examined from 47 and 25 specimens, respectively (Table 1). ADRB3 mRNA and protein levels of SV cells were analyzed from 40 and 46 specimens, respectively (Table 2). UCP-1 mRNA expression levels in mature adipocytes were examined from 35 specimens (Table 3). The NW group and OW group were well-matched by age and gender.
Table 1.
Clinical and laboratory parameters analysis of ADRB3 in mature adipocyte group
| ADRB3 mRNA | ADRB3 protein | |||
|---|---|---|---|---|
| N | NW group 22 | OW group 25 | NW group 11 | OW group 14 |
| N | 22 | 25 | 11 | 14 |
| Gender, m/f | 10/12 | 14/11 | 8/3 | 9/5 |
| Age, years | 58.59±12.11 | 58.12±10.31 | 58.09±15.64 | 58.43±11.61 |
| BMI, kg/m2 | 22.88±1.49 | 27.53±1.48# | 22.51±2.19 | 27.18±1.64# |
| DBP, mm Hg | 78.73±11.11 | 84.64±8.87 | 78.73±11.56 | 85.07±9.73 |
| SBP, mm Hg | 126.23±16.14 | 142.28±21.44* | 125.45±14.35 | 137.93±20.43 |
| LDLC, mmol/L | 2.66±0.95 | 3.13±0.60 | 2.61±1.09 | 2.78±0.75 |
| TG, mmol/L | 1.10±0.37 | 1.39±0.70 | 1.12±0.34 | 1.42±0.84 |
Data show as mean ± SD for continuous variables.
p < 0.001,
p < 0.05 compared with OW group.
Table 2.
Clinical and laboratory parameters analysis of ADRB3 in SV cells group
| ADRB3 mRNA |
ADRB3 protein |
|||
|---|---|---|---|---|
| NW group | OW group | NW group | OW group | |
| N | 20 | 20 | 23 | 23 |
| Gender, m/f | 7/13 | 9/11 | 12/11 | 14/9 |
| Age, years | 57.40±13.59 | 58.95±10.06 | 54.22±15.68 | 55.83±13.87 |
| BMI, kg/m2 | 22.84±1.83 | 27.69±1.57# | 22.77±1.78 | 27.73±1.61# |
| DBP, mm Hg | 80.80±12.42 | 86.60±7.37 | 77.70±9.95 | 84.39±6.97# |
| SBP, mm Hg | 129.40±19.26 | 141.60±19.94 | 124.61±13.51 | 136.39±19.33# |
| LDLC, mmol/L | 2.39±0.71 | 3.30±0.47* | 2.48±0.84 | 2.78±0.77 |
| TG, mmol/L | 1.02±0.38 | 1.37±0.58# | 1.19±0.45 | 1.39±0.73 |
Data show as mean ± SD for continuous variables.
p < 0.001,
p < 0.05 compared with OW group.
Table 3.
Clinical and laboratory parameters analysis of UCP-1 in mature adipocyte group
| NW group | OW group | |
|---|---|---|
| N | 17 | 18 |
| Gender, m/f | 9/8 | 11/7 |
| Age, years | 57.18±11.93 | 58.28±11.62 |
| BMI, kg/m2 | 22.86±1.33 | 27.61±1.47# |
| DBP, mm Hg | 77.82±12.14 | 84.11±9.30 |
| SBP, mm Hg | 124.53±16.89 | 143.67±23.63* |
| LDLc, mmol/L | 2.72±1.02 | 3.06±0.63 |
| TG, mmol/L | 1.10±0.36 | 1.33±0.73 |
Data show as mean ± SD for continuous variables.
p < 0.001,
p < 0.05 compared with OW group.
ADRB3 mRNA and Protein Expression Levels in Mature Adipocytes
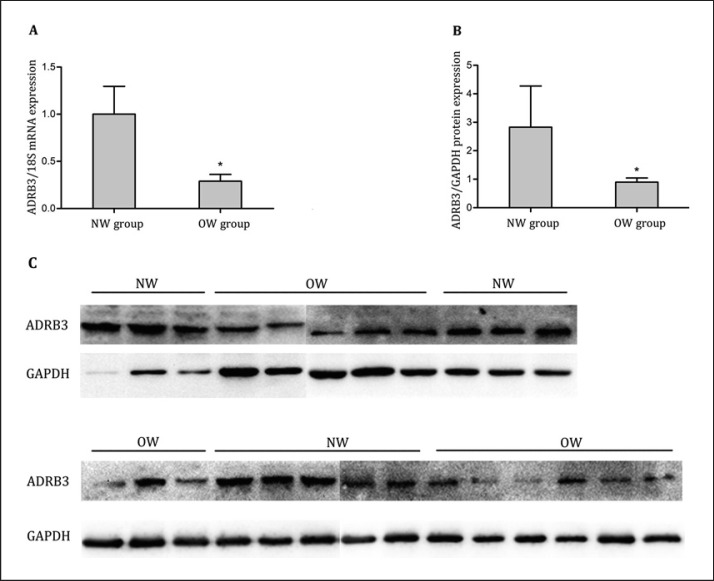
ADRB3 mRNA and protein expression levels in mature adipocytes were detected by qRT-PCR and Western blot. The data showed that ADRB3 mRNA expression levels of mature adipocytes in the NW group were significantly higher compared with the OW group (p = 0.021) (Table 4; Fig. 1A); similar results were achieved for ADRB3 protein expression levels in mature adipocytes (p = 0.025) (Table 4, Fig. 1B, C).
Table 4.
ADRB3 and UCP-1 expression analysis in adipocytes
| NW group | OW group | p value | |
|---|---|---|---|
| ADRB3 mRNA expression in mature adipocyte | 1.33 (0.31, 2.60) (n = 22) |
0.41 (0.16, 0.71) (n = 25) |
0.021 |
| ADRB3 protein expression in mature adipocyte | 1.32 (1.14, 2.01) (n = 11) | 0.72 (0.56, 1.12) (n = 14) | 0.025 |
| ADRB3 mRNA expression in SV cells | 1.00 (0.38, 2.54) (n = 20) | 0.47 (0.11, 1.25) (n = 20) | 0.041 |
| ADRB3 protein expression in SV cells | 0.79 (0.66, 1.24) (n = 23) | 0.72 (0.43, 0.76) (n = 23) | 0.025 |
| UCP-1 mRNA expression in mature adipocyte | 0.82 (0.36, 2.71) (n = 17) | 0.56 (0.19, 1.12) (n = 18) | 0.045 |
The variables were analyzed using Student's t test, after logarithm transformation. Data were showed as median (interquartile range) for non-normal distribution variables.
Fig. 1.
ADRB3 mRNA and protein expression levels in mature adipocytes. A ADRB3 mRNA expression levels were examined from 22 normal-weight and 25 overweight individuals by qRT-PCR. B, C ADRB3 protein expression levels were detected in 11 normal-weight and 14 overweight individuals by Western blot. Bands of ∼45 kDa (ADRB3) and ∼36 kDa (GAPDH) were detected in all samples using specific antibodies. NW means normal-weight group; OW means overweight group. * p < 0.05.
ADRB3 mRNA and Protein Expression Levels in SV Cells
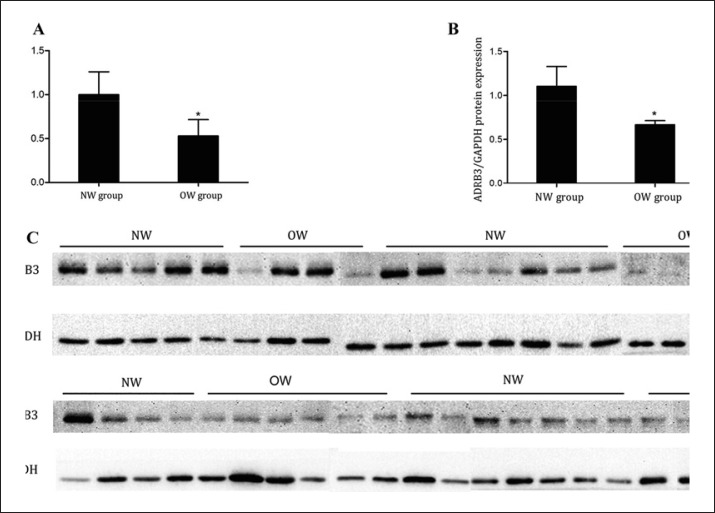
ADRB3 mRNA and protein expression levels in SV cells were examined by qRT-PCR and Western blot. The data showed that the ADRB3 mRNA expression levels in SV cells were higher in the NW group than in the OW group (p = 0.041) (Table 4; Fig. 2A), similar results were achieved for ADRB3 protein expression levels in SV cells (p = 0.025) (Table 4; Fig. 2B, C).
Fig. 2.
ADRB3 mRNA and protein expression levels in SV cells. A ADRB3 mRNA expression levels were examined from 20 normal-weight and 20 overweight individuals by qRT-PCR. B, C ADRB3 protein expression levels were detected in 23 normal-weight and 23 overweight individuals by western blot. Bands of ∼45 kDa (ADRB3) and ∼36 kDa (GAPDH) were detected in all samples using specific antibodies. NW means normal-weight group; OW means overweight group. * p < 0.05.
The Relationship between ADRB3 mRNA Expression in Mature Adipocytes and BMI
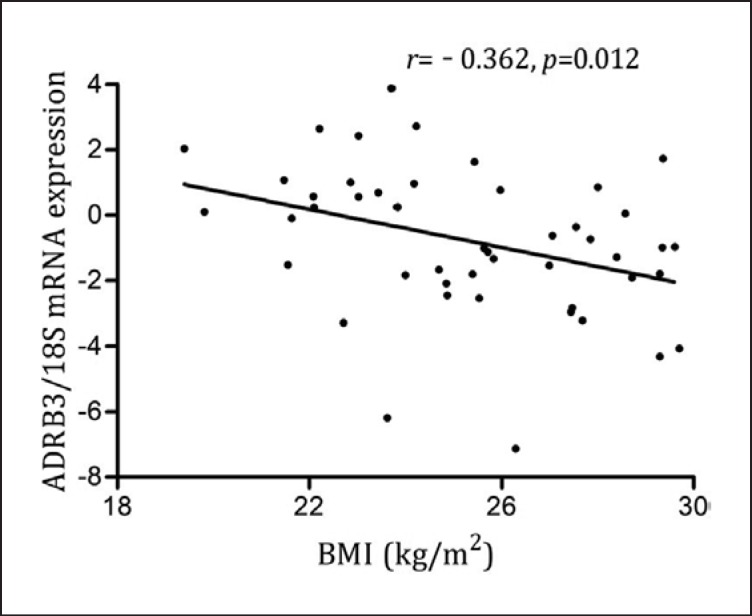
Correlation analysis showed that ADRB3 mRNA levels in mature adipocytes were negatively correlated with age (r = −0.307, p = 0.036) and BMI (r = −0.362, p = 0.012) (Fig. 3). Stepwise multiple linear regression analysis on BMI (dependent variable) that entered gender, age, and mature adipocytes ADRB3 mRNA levels (logarithmic transformation data) as independent variables revealed that the ADRB3 mRNA expression level was still negatively correlated with BMI even after excluding the effect of age (Table 5). We did not find any statistically significant relationships between ADRB3 (both mRNA and protein) expression levels and BMI in SV cells.
Fig. 3.
Relationship between ADRB3 mRNA expression levels in mature adipocytes and BMI. ADRB3 mRNA expression levels were examined in 47 individuals, ADRB3 mRNA expression levels in mature adipocytes were negatively associated with BMI (r = −0.362, p = 0.012).
Table 5.
Stepwise multiple linear regression analysis results showed ADRB3 mRNA expression levels in mature adipocytes were independent correlates to BMI
| BMI | B | SE | β | p value |
|---|---|---|---|---|
| Constant | 24.99 | 0.405 | − | <0.001 |
| ADRB3 mRNA | −0.448 | 0.172 | −0.362 | 0.012 |
Variables excluded in the multiple regression analysis were gender and age. B, unstandardized coefficients; β, standardized coefficients; SE, standardized error. F = 6.774, p = 0.012.
UCP-1 mRNA Expression Levels in Mature Adipocytes
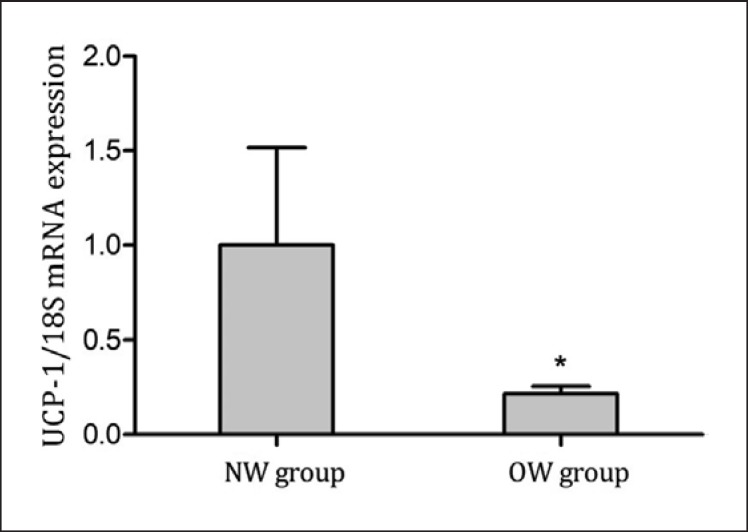
UCP-1 mRNA expression levels in mature adipocytes were detected by qRT-PCR. The results showed that the expression levels of UCP-1 mRNA in mature adipocytes were higher in the NW than in the OW group (p = 0.045) (Table 4; Fig. 4).
Fig. 4.
UCP-1 mRNA expression levels in mature adipocytes. UCP-1 mRNA expression levels were examined in 17 normal-weight and 18 overweight individuals by qRT-PCR. NW means normal-weight group; OW means overweight group. * p < 0.05.
Discussion
The hBAT activity is increasing through transdifferentiation from white adipocytes to beige adipocytes or de novo differentiation of precursor cells to beige adipocytes when exposing to the cold or other stimuli [14, 15]. Exposure to the cold [16, 17, 18] or selective ADRB3 agonists [7] resulted in higher resting metabolic rate and hBAT activity in healthy individuals as measured via 18F-FDG/PET-CT, in whom the ADRB3 pathway was considered as a dominant signaling pathway to promote the activation or differentiation of beige adipocytes [17, 19, 20]. ADRB3 belong to G protein-coupled receptors and are abundant in adipose tissue, especially in the BAT [17, 21]. Trp64Arg polymorphisms of ADRB3 genes are associated with a variety of body weight [22, 23]. A prospective study [24] showed that ADRB3 function is an important predictor of long-term changes in body weight.
hBAT activation capacity was reduced in obese subjects [12, 16]. Furthermore, it was demonstrated that the reduced ADRB3 expression in adipose tissue of obese rats [9, 10] increased after weight loss following Roux-en-Y gastric bypass surgery [25]. Moreover, treatment with ADRB3 agonists caused a noticeable increase of adipose tissue browning and UCP-1 protein level in lean rather than in obese mice [26, 27]. Taking both results together, one may presume that the decreased adipose tissue browning in obese rats was associated with a down-regulated expression of ADRB3 in adipose tissue. There was also study in humans showing that ADRB3 expression levels in adipose tissue were significantly reduced in severely obese subjects (BMI > 40 kg/m2) in a Caucasian population [11]. However, the results mentioned above mainly came from data of obese / severely obese animals or humans, which cannot indicate a causal relationship between adipose tissue ADRB3 expression and obesity. The present study therefore focused on a pre-obesity population and investigated the adipose tissue ADRB3 expression levels in overweight individuals.
We were able to show that overweight individuals have reduced ADRB3 and UCP-1 expression levels in mature adipocytes compared with normal-weight persons, and ADRB3 mRNA expression levels in mature adipocytes were inversely correlated with BMI. The data indicated that mature adipocyte ADRB3 expression was down-regulated before the onset of obesity (BMI 25–30 kg/m2), which may lead to weak beige adipocytes activation and adipose tissue browning ability induced by the cold or ADRB3 agonists. Moreover, UCP-1 mRNA expression was also lowered, potentially influencing body weight in the future.
Differentiation of pre-adipocytes into beige adipocytes is an important way to increase hBAT activity [28, 29], and the ADRB3 signaling pathway also plays a role in this process. The present study also examined SV cell ADRB3 mRNA and protein expression in overweight individuals to explore whether a disorder of beige adipocyte differentiation was involved. The results showed that overweight individuals had obviously lower ADRB3 mRNA and protein expression levels in SV cells compared with normal-weight persons, which indicated that ADRB3 expression levels were decreased in some adipose-derived stem cells, resulting in a decline of differentiation into beige adipocytes, which can be induced by certain stimuli, and thus in a decreased hBAT activity.
The present study demonstrated that ADRB3 expression in mature adipocytes and SV cells were down-regulated at an overweight stage, before the onset of obesity, which reduced the activation of beige adipocyte and differentiation of pre-adipocytes to beige adipocytes induced by low temperature or other inducers, resulting in decreased activity of hBAT and, finally, in weight gain and overt obesity. Thus, we hypothesize that the down-regulation of ADRB3 expression in mature and SV cells may be the cause rather than the result of obesity and that adipocyte ADRB3 expression may be a potential interference target for obesity treatment.
One of strengths of the present study was that human adipose tissues were clinically collected from overweight adults. Thus, we were able to detect the molecular mechanisms before the onset of obesity. Secondly, ADRB3 and UCP-1 expression in mature adipocytes and SV cells in adipose tissue of overweight subjects were analyzed separately for the first time, providing us with information of both beige adipocyte activation and differentiation. However, only abdominal visceral adipose tissue was included in present study, and it is known that human adipose tissue browning was closely associated with the anatomical site [4]. Further studies are needed to elucidate the specific molecular mechanism of ADRB3 signaling pathway and how it affects human adipose tissue browning and adipose tissue depot-specific characteristics of ADRB3 expression.
Financial Support
This work was financially supported by National Natural Science Foundation of China (No. 81300686).
Disclosure Statement
The authors have nothing to disclose.
Acknowledgments
We are thankful to Li Fang and Qin Dandan for technical assistance with data analysis and to Prof. Cheng for kindly providing adipose tissue. We also acknowledge volunteers who participated in the study.
References
- 1.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013 Oct;1((2)):152–62. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012 Jul;150((2)):366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012 Jan;302((1)):E19–31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 4.Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, et al. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab. 2013 Sep;98((9)):E1448–55. doi: 10.1210/jc.2013-1265. [DOI] [PubMed] [Google Scholar]
- 5.Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012 Apr;15((4)):480–91. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordicchia M, Pocognoli A, D'Anzeo M, Siquini W, Minardi D, Muzzonigro G, et al. Nebivolol induces, via β3 adrenergic receptor, lipolysis, uncoupling protein 1, and reduction of lipid droplet size in human adipocytes. J Hypertens. 2014 Feb;32((2)):389–96. doi: 10.1097/HJH.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 7.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015 Jan;21((1)):33–8. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, et al. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013 Jan;56((1)):147–55. doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- 9.Komai AM, Musovic S, Peris E, Alrifaiy A, El Hachmane MF, Johansson M, et al. White adipocyte adiponectin exocytosis is stimulated via beta3-adrenergic signaling and activation of epac1: catecholamine resistance in obesity and type 2 diabetes. Diabetes. 2016 Nov;65((11)):3301–13. doi: 10.2337/db15-1597. [DOI] [PubMed] [Google Scholar]
- 10.Jiao J, Han SF, Zhang W, Xu JY, Tong X, Yin XB, et al. Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food Nutr Res. 2016 Sep;60((1)):31304. doi: 10.3402/fnr.v60.31304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurylowicz A, Jonas M, Lisik W, Jonas M, Wicik ZA, Wierzbicki Z, et al. Obesity is associated with a decrease in expression but not with the hypermethylation of thermogenesis-related genes in adipose tissues. J Transl Med. 2015 Jan;13((1)):31. doi: 10.1186/s12967-015-0395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisonkova S, Muraca GM, Potts J, Liauw J, Chan WS, Skoll A, et al. Association between prepregnancy body mass index and severe maternal morbidity. JAMA. 2017 Nov;318((18)):1777–86. doi: 10.1001/jama.2017.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homøe P, Loft A, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013 May;17((5)):798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015 Oct;22((4)):546–59. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013 Jun;15((6)):659–67. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 16.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009 Apr;360((15)):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 17.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009 Apr;360((15)):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 18.van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013 Aug;123((8)):3395–403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki T, Hosaka K, Lim S, Fischer C, Honek J, Yang Y, et al. Endothelial PDGF-CC regulates angiogenesis-dependent thermogenesis in beige fat. Nat Commun. 2016 Aug;7:12152. doi: 10.1038/ncomms12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Berry DC, Graff JM. Distinct cellular and molecular mechanisms for β3 adrenergic receptor-induced beige adipocyte formation. eLife. 2017 Oct;6:e30329. doi: 10.7554/eLife.30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari A, Longo R, Fiorino E, Silva R, Mitro N, Cermenati G, et al. HDAC3 is a molecular brake of the metabolic switch supporting white adipose tissue browning. Nat Commun. 2017 Jul;8((1)):93. doi: 10.1038/s41467-017-00182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brondani LA, Duarte GC, Canani LH, Crispim D. The presence of at least three alleles of the ADRB3 Trp64Arg (C/T) and UCP1 -3826A/G polymorphisms is associated with protection to overweight/obesity and with higher high-density lipoprotein cholesterol levels in Caucasian-Brazilian patients with type 2 diabetes. Metab Syndr Relat Disord. 2014 Feb;12((1)):16–24. doi: 10.1089/met.2013.0077. [DOI] [PubMed] [Google Scholar]
- 23.Yamakita M, Ando D, Tang S, Yamagata Z. The Trp64Arg polymorphism of the β3-adrenergic receptor gene is associated with weight changes in obese Japanese men: a 4-year follow-up study. J Physiol Anthropol. 2010;29((4)):133–9. doi: 10.2114/jpa2.29.133. [DOI] [PubMed] [Google Scholar]
- 24.Andersson D, Wahrenberg H, Löfgren P. Beta3-adrenoceptor function and long-term changes in body weight. Int J Obes. 2009 Jun;33((6)):662–8. doi: 10.1038/ijo.2009.54. [DOI] [PubMed] [Google Scholar]
- 25.Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF, et al. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab. 2015 Mar;4((5)):427–36. doi: 10.1016/j.molmet.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghorbani M, Teimourian S, Farzad R, Asl NN. Apparent histological changes of adipocytes after treatment with CL 316,243, a β-3-adrenergic receptor agonist. Drug Des Devel Ther. 2015 Feb;9:669–76. doi: 10.2147/DDDT.S73891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung YW, Ahmad F, Tang Y, Hockman SC, Kee HJ, Berger K, et al. White to beige conversion in PDE3B KO adipose tissue through activation of AMPK signaling and mitochondrial function. Sci Rep. 2017 Jan;7((1)):40445. doi: 10.1038/srep40445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013 Oct;19((10)):1338–44. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YH, Thacker RI, Hall BE, Kong R, Granneman JG. Exploring the activated adipogenic niche: interactions of macrophages and adipocyte progenitors. Cell Cycle. 2014;13((2)):184–90. doi: 10.4161/cc.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]