Abstract
Narrow-band imaging is an advanced imaging system that applies optic digital methods to enhance endoscopic images and improves visualization of the mucosal surface architecture and microvascular pattern. Narrow-band imaging use has been suggested to be an important adjunctive tool to white-light endoscopy to improve the detection of lesions in the digestive tract. Importantly, it also allows the distinction between benign and malignant lesions, targeting biopsies, prediction of the risk of invasive cancer, delimitation of resection margins, and identification of residual neoplasia in a scar. Thus, in expert hands it is a useful tool that enables the physician to decide on the best treatment (endoscopic or surgical) and management. Current evidence suggests that it should be used routinely for patients at increased risk for digestive neoplastic lesions and could become the standard of care in the near future, at least in referral centers. However, adequate training programs to promote the implementation of narrow-band imaging in daily clinical practice are needed. In this review, we summarize the current scientific evidence on the clinical usefulness of narrow-band imaging in the diagnosis and characterization of digestive tract lesions/cancers and describe the available classification systems.
Keywords: Early gastric cancer, Colonic polyps, Narrow-band imaging, Chromoendoscopy, Endoscopic classifications, Squamous cell carcinoma, Barrett esophagus, Gastric intestinal metaplasia
Resumo
O sistema de iluminação narrow-band imaging é um sistema de imagem avançada que utiliza ferramentas digitais óticas para realçar imagens endoscópicas e melhorar a observação da superfície e do padrão microvascular da mucosa. O narrow-band imaging tem demonstrado ser um importante adjuvante à endoscopia com luz branca, melhorando a deteção de lesões no tubo digestivo. Tam-bém, possibilita a distinção entre lesões benignas e mali-gnas, guia as biópsias para zonas suspeitas, prediz o risco de cancro invasivo, delimita as margens de ressecção e identifica lesões residuais em cicatrizes. Portanto, em mãos experientes, é uma ferramenta útil que permite ao médico decidir o melhor tratamento (endoscópico ou cirúrgico) e orientação. A evidência atual sugere que esta técnica deve ser utilizada por rotina em doentes com risco aumentado para lesões neoplásicas do tubo digestivo e poderá tornar-se o método de escolha num futuro próxi-mo, pelo menos nos centros de referência. Contudo, são necessários programas de treino adequados para pro-mover a utilização do narrow-band imaging na prática clinica diária. Nesta revisão, resumimos a evidência científi-ca disponível acerca da utilidade do narrow-band imaging no diagnóstico e caracterização das lesões do tubo di-gestivo e descrevem-se os sistemas de classificação dis-poníveis.
Palavras Chave: Narrow-band imaging, Cromoendoscopia, Classificações endoscópicas, Esófago de Barrett, Metaplasia intestinal, Cancro gástrico precoce, Pólipos do cólon
Introduction
Advanced endoscopic imaging techniques (AEITs) are imaging technologies embedded in gastrointestinal scopes that allow changing the white-light (WL) image in order to enhance visualization of the mucosal surface architecture and microvascular pattern, potentially improving endoscopic diagnosis [1, 2].
Indeed, AEITs allow endoscopists to obtain more accurate real-time optical diagnoses and assist clinicians to make better decisions. However, although AEITs are readily available and simpler than dye-based chromoendoscopy, there is a learning curve for their correct use and their advantages are mainly proved in academic centers. On the other hand, in community practice AEITs are less frequently adopted, maybe because they are perceived as cumbersome and time-consuming and requiring special training and also because no AEIT has been shown consistently to be significantly superior to high-definition white light (HDWL) in this setting [1, 2].
The available AEITs are narrowed-spectrum endoscopy, autofluorescence imaging, confocal laser endomicroscopy, and optical coherence tomography [1]. This review focuses on one of the most available and widespread AEITs, i.e., narrow-band imaging (NBI), and aims to explain how it works as well as its utility in gastrointestinal endoscopy, summarizing the available classification systems. It also describes the evidence and the recommendations to use NBI in clinical practice and highlights the importance of specialized training.
Narrow-Band Imaging
NBI was the first narrowed-spectrum technology commercialized [1], and it is based on the penetration properties of light. Shorter wavelengths penetrate only superficially into the mucosa, whereas longer wavelengths can penetrate more deeply. NBI utilizes red-green-and-blue filters to modify WL endoscopy (WLE): the blue light filter (400–430 nm) highlights the capillaries in the superficial mucosa through mean peak absorption of hemoglobin (415 nm), while the green light filter (525–555 nm) penetrates deeper into the mucosa [1]. This results in greater clarity of mucosal surface structures due to the increased contrast between mucosa and superficial vessels, which appear brown/black [3].
Thus, NBI is based on vascular and mucosal patterns that are useful to predict the histological structure of tissues [4]. The normal vascular pattern consists of a regular mucosal capillary network [5] that is altered during the transition of premalignant to malignant lesions, where angiogenesis is critical and a hyperproliferative vascular state is observed [5]. Then, in theory, the vascular pattern allows the early detection and characterization of neoplasms [5, 6]. The mucosal pattern can also be better evaluated with NBI, since it allows better visualization of glands openings (pits) and their specific arrangement (pit pattern), which has a different shape according to different lesions [4].
The first commercially available NBI systems (Lucera Spectrum and Exera II [1, 3]) produced images that were darker than WL (dark NBI), while the second-generation NBI systems released in 2012 (Lucera Elite and Exera III) exhibit an improvement in the light source that allows brighter images (light NBI) [3, 5]. High magnification is defined by the capacity to perform a zoom in an image. Most of the magnification endoscopes available combine optical and digital zoom and permit ×1.5–2 digital magnification and/or an optical magnification up to 150 times. Newer Olympus endoscopes include near-focus imaging, which allows the endoscope to be moved closer (within 2–6 mm) to the area of interest while maintaining the image in focus, theoretically providing similar images to high magnification (> 100 times). NBI with high magnification may increase the accuracy of diagnoses, particularly their specificity [7].
NBI has several advantages over standard dye chromoendoscopy. This technique can be simply activated and deactivated during endoscopy, making it easier to switch between the standard mode and the NBI mode [3]. It reveals both vascular and mucosal patterns without dye application, is easy to perform and user-friendly, is widely available, and allows for inspection of the whole endoscopic field. No reported complications have been attributed to the use of NBI. Disadvantages over WLE alone include a prolonged duration of examination and the need for training to correctly interpret findings and decrease interobserver variability [3, 8].
Esophagus
In the esophagus, WLE provides little detail of the mucosal surface and is not able to accurately differentiate intestinal metaplasia from normal gastric mucosa or dysplastic epithelia. NBI allows a better evaluation of mucosal and vascular patterns that are associated with Barrett esophagus (BE), dysplasia, and esophageal cancer [1, 9].
Magnification endoscopy with NBI (ME-NBI) also allows a better visualization of normal capillary mucosal vessels (intraepithelial papillary capillary loops [IPCLs]) and submucosal vascularity (branching vessels) [1, 10]. Normal IPCLs are observed as brown loops originating from a branching vessel, running perpendicularly in the lamina propria and finally reaching the intraepithelial papillae (Fig. 1a) [11]. On the other hand, in neoplastic lesions the abnormal mucosal and capillary patterns have characteristic features. In squamous neoplastic lesions, IPCLs exhibit characteristic morphological changes, being dilated, tortuous, and irregular in dysplastic lesions, and destructed and replaced by tumor vessels in squamous cell carcinomas (SCCs) [1, 10, 11]. Likewise, in BE a circular and “ridged/villous” pattern with regular vessels is predictive of specialized intestinal metaplasia, and irregular mucosal and vessel patterns are predictive of dysplasia [1, 9].
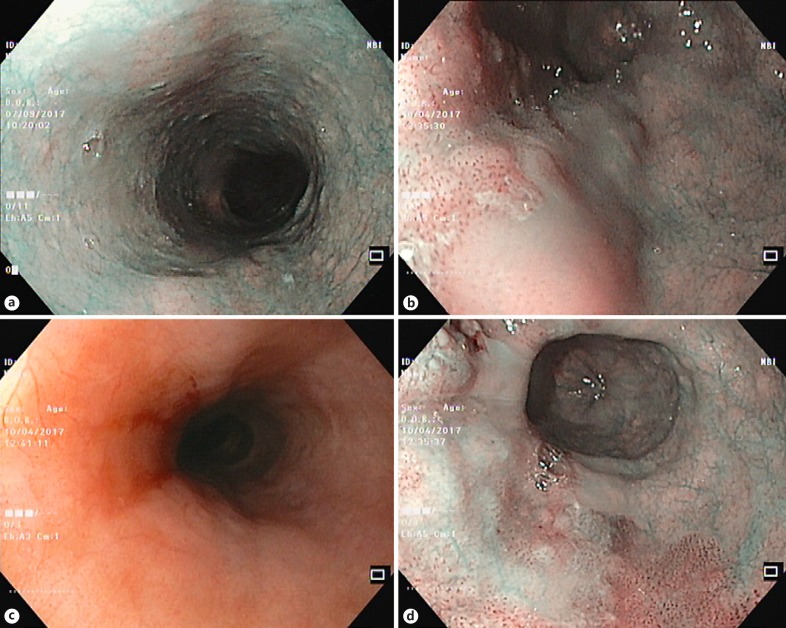
Fig. 1.
Narrow-band imaging features in normal mucosa of the esophagus (a), in squamous cell dysplasia (b), and in cancer (d). c White-light features in cancer.
Squamous Cell Carcinoma
In SCC, NBI seems to be useful in both the detection and the characterization of neoplastic lesions. Indeed, NBI seems to have a better sensitivity for superficial esophageal SCC when compared with WL imaging (97 vs. 55%, p < 0.01) [12, 13, 14]. Regarding the comparison between NBI and Lugol chromoendoscopy, three studies found that NBI and ME-NBI have an increased accuracy and specificity when compared with Lugol, although the sensitivity is similar between the two techniques [15, 16, 17]. These findings were also confirmed in two recent meta-analyses [18].
Two ME-NBI classifications are available to estimate invasion depth in SCC: the IPCL pattern classification (Inoue classification) and a novel classification that is simpler to use in clinical practice [1, 11]. The IPCL classification was described in 2001 by Inoue and describes five different IPCL patterns, allowing the distinction between normal mucosa, atypia, and cancer. Type I corresponds to normal mucosa, type II to inflammation, type III to borderline lesions, i.e., atrophic mucosa or low-grade intraepithelial neoplasia, type IV to high-grade intraepithelial neoplasia, and type V to invasive carcinoma [1, 11]. A novel and easier classification was subsequently proposed, reclassifying the original five categories into three groups: group 1 (nonneoplastic: IPCL types I and II), group 2 (borderline: IPCL types III and IV), and group 3 (cancer: IPCL type V) (Fig. 1) [11]. The advantages of this classification are its easier application and its ability to guide therapy: group 1 lesions require no treatment, group 2 requires careful follow-up or therapy, and group 3 definitely demands therapy. This classification was recently evaluated in a prospective study and an accuracy of 90.5% for the estimation of invasion depth was found [19].
The overall accuracy of pattern IPCL IV or greater was 80.0% (sensitivity 58.5% and specificity 96%) [11]. The sensitivity and specificity of IPCL type V1–2, type V3, and type Vn were 89.5 and 79.6 %, 58.7 and 83.8 %, and 55.8 and 98.6 %, respectively [20]. However, a small study compared diagnoses of the invasion depth of SCC between ME-NBI and WL and showed no additional benefit [21].
Therefore, given the low incidence of SCC in most countries, NBI should only be used routinely in patients at risk of squamous cell cancer (with a history of head and neck cancers, previous biopsies with dysplasia, or caustic esophagitis). In the general population, the value of NBI is still to be determined, but probably it is only justified for improving characterization, guiding biopsies and delimitation if any change in the esophageal mucosa is seen with HDWL.
Barrett Esophagus
Current guidelines recommend endoscopic surveillance in BE, with random 4-quadrant biopsy specimens obtained every 1–2 cm to detect dysplasia (Seattle protocol), in addition to targeted biopsies of suspicious lesions under WLE [22]. Concerning the ability of AEITs to detect dysplasia in BE, two studies showed that NBI with targeted biopsies improves the diagnosis of dysplasia when compared to HDWL examination with the Seattle protocol [9, 23]. Additionally, three recent meta-analyses showed that NBI is an accurate test to diagnose dysplasia in BE, with a sensitivity of 94.2%, a specificity of 94.4%, and a negative predictive value of 97.5% in the most recent meta-analysis [14, 22, 24].
For ME-NBI in BE, four classification systems have been proposed: from Kansas, Amsterdam, Nottingham, and the Barrett's International NBI Group (BING) [1]. The BING system is a simplified NBI classification proposed with the objective of integrating multiple classifications of NBI surface patterns in BE. In this classification, nondysplastic BE has a circular, tubular, or villous mucosal pattern with regular vessels, while dysplasia is characterized by an irregular or absent mucosal pattern and vessels not following the normal glandular architecture (Fig. 2). Validation studies of this classification using ME-NBI showed that the BING classification can predict the presence or absence of dysplasia with a high level of accuracy (> 90%) and very high interobserver agreement [1, 25, 26]. However, without magnification this classification seems less useful, based on a recent study evaluating HD-NBI without magnification in the diagnosis of dysplasia [27]. The specificity and negative predictive value for dysplasia were high (> 85%), although the sensitivity and positive predictive value were suboptimal and inter observer agreement was weak, suggesting that this classification without magnification (or proper training) is not yet ready to replace the Seattle protocol.
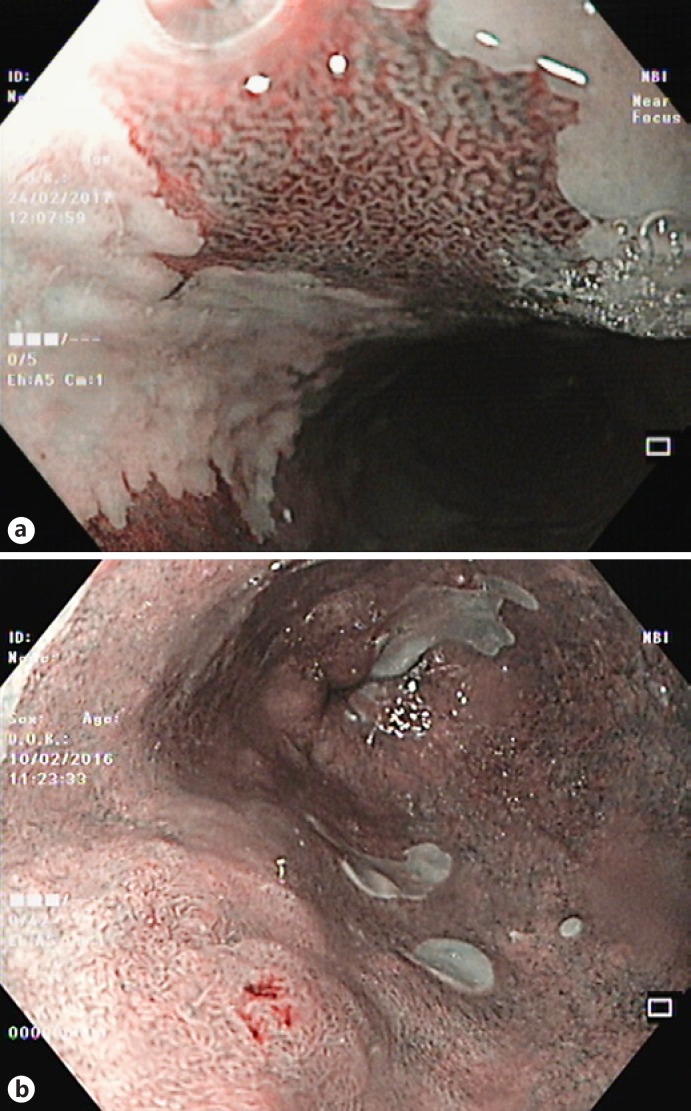
Fig. 2.
Narrow-band imaging features in Barrett esophagus (BE). a Nondysplastic BE. b Dysplastic BE.
Despite some evidence of a benefit from NBI, the British Society of Gastroenterology and the European Society of Gastrointestinal Endoscopy (ESGE) do not recommend the use of AEITs routinely [28, 29], while the American College of Gastroenterology recommends HDWL in conjunction with NBI only after complete elimination of intestinal metaplasia in order to detect mucosal abnormalities that may reflect recurrent intestinal metaplasia and/or dysplasia [30]. However, the Seattle protocol is not widely followed, because it is time-consuming, requires an expensive pathologic analysis, and has the potential for sampling error because of the variable distribution of dysplasia and esophageal adenocarcinoma [22], and thus NBI seems to be an useful tool in the surveillance of patients with BE and may replace the current random biopsy protocols in the future [9, 22]. The role of AEITs is acknowledged in the recently published ESGE guidelines, which recognize that the use of these imaging modalities may be of benefit given their wide availability and the fact that no increased costs are incurred. Therefore, NBI is an important adjunctive tool that can help to target biopsies to suspicious areas and to delineate esophageal lesions for endoscopic resection, and has a promising role in replacing the Seattle protocol in the future at least in reference centers, although more studies are needed before this recommendation can be made.
Stomach
Endoscopic evaluation of the gastric mucosa with WL correlates poorly with histological findings, while NBI can improve the correlation with histology [1, 31]. Several NBI patterns, sometimes different patterns, have been associated with several gastric pathologies, namely, Helicobacter pylori (Hp) gastritis, intestinal metaplasia, dysplasia, intramucosal cancer, and submucosal cancer. It is important to recognize that the normal gastric body and antral mucosa have a slightly different appearance with NBI. The normal gastric body shows a regular arrangement of small round pits, surrounded by a regular capillary network with a honeycomb appearance, while normal antral mucosa has a coil-shaped appearance of a subepithelial capillary network (Fig. 3a) [31, 32, 33, 34]. Even though several authors suggest that Hp gastritis may be confidently diagnosed with NBI, none of them showed the reproducibility of these patterns or tried to validate it [35, 36, 37]. A variable vascular pattern was fairly reproducible and presented an acceptable accuracy. However, in a prospective evaluation it was no better than WLE for the diagnosis of Hp gastritis [31].
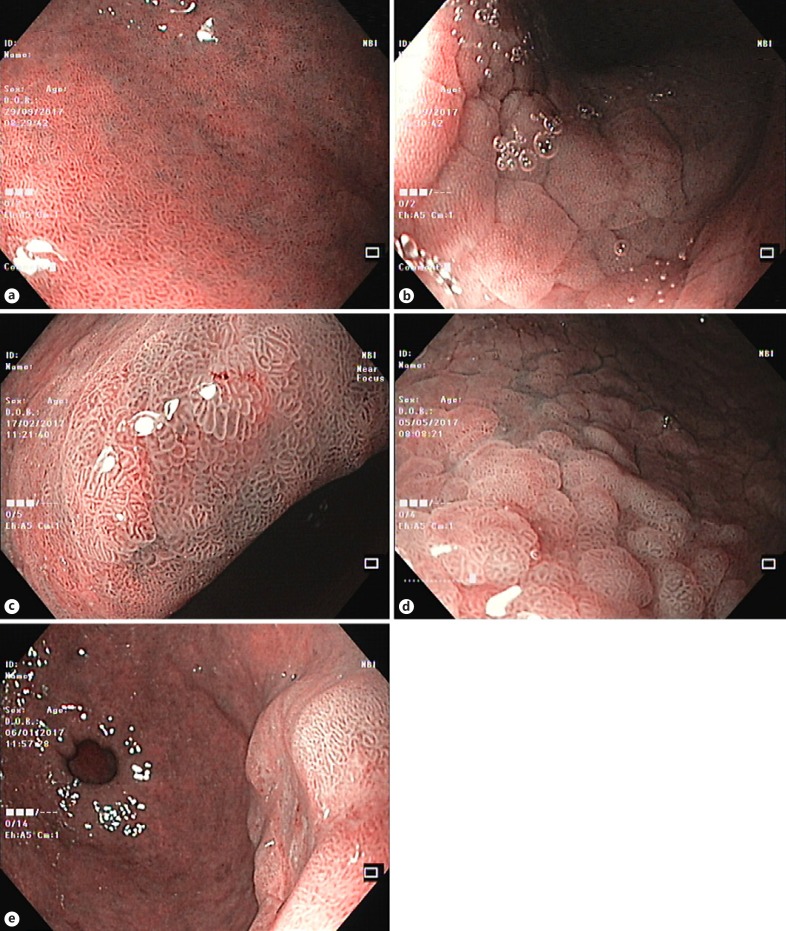
Fig. 3.
Narrow-band imaging simplified classification for gastric lesions. a Pattern Aa (normal antrum, with regular oval/circular mucosa and regular vessels in the center of the gland). b Pattern Ab (normal gastric body, with regular circular mucosa and the gland surrounded by regular vessels). c, d Pattern B corresponds to intestinal metaplasia of the antrum and of the gastric body (regular, ridge, or tubulovillous mucosal patterns with regular vessels; presence of a light-blue crest). e Pattern C is associated with dysplasia/cancer (absent or irregular mucosal patterns with architectural distortion and irregular vascular patterns).
Gastric Intestinal Metaplasia, Dysplasia, and Early Gastric Cancer
With NBI, the presence of regular mucosal and vascular patterns excludes dysplasia, being that ridged or villous patterns are suggestive of intestinal metaplasia [10, 31, 33, 34]. Other features besides pits and vascular patterns were associated with histological findings. For example, areas of intestinal metaplasia can present as a “light-blue crest,” which is defined as a fine, blue line on crests of epithelial surfaces/gyri, being highly specific for the diagnosis of intestinal metaplasia [38]. On the other hand, dysplasia or cancer may present as a “white opaque substance,” which, as the name implies, is characterized by white material above the mucosa [10, 31, 33, 34]. However, a white opaque substance has also been associated with intestinal metaplasia, so it is not a specific marker [39].
For the evaluation of gastric lesions with NBI, three classifications were proposed: a simplified classification system for NBI in the diagnosis of gastric lesions, the Vessels plus Surface Classification, and the classification of gastric lesions proposed by Li [1, 31, 33, 34, 40, 41]. To our knowledge, for the diagnosis of gastric atrophy there is no validated NBI endoscopic pattern or classification.
The simplified NBI classification was proposed for the diagnosis of intestinal metaplasia and dysplasia [1, 33]. This Western classification includes the whole gastric spectrum of carcinogenesis (with the exception of atrophy). It can be applied without magnification and considers three different patterns: pattern A is related to normal mucosa, and is further subdivided into Aa (normal antrum) and Ab (normal gastric body); pattern B corresponds to intestinal metaplasia; and pattern C is associated with dysplasia/cancer (Fig. 3) [33]. An additional pattern of Hp can be included. If it is positive, a plus sign is added to the pattern (e.g., pattern Aa+ for Hp gastritis in normal antral mucosa, pattern B+ for intestinal metaplasia and Hp infection) [33]. This simplified NBI classification demonstrated to be an efficient technique for the diagnosis of gastric intestinal metaplasia and dysplasia (with an accuracy of 83% for normal histology [pattern A], of 84% for intestinal metaplasia [pattern B], and of 95% for dysplasia [pattern C]), with high reproducibility (κ = 0.62) [31]. A different study applying this classification also suggested that more than 90% of individuals with extensive metaplasia could be identified without the need for biopsies [42]. In a multicenter prospective study applying this classification, with some scopes allowing magnification/near focus, the use of NBI after WL significantly increased the sensitivity for the diagnosis of intestinal metaplasia (87 vs. 53%, p < 0.001) and improved the sensitivity for dysplasia (92 vs. 74%) [31]. However, for the detection of Hp gastritis, both WLE and NBI have limitations (74% global accuracy) and low reproducibility; thus, NBI does not replace other diagnostic tests for Hp [31, 33]. These results suggest that, in a real-life scenario, NBI should be used to perform guided instead of random biopsies in a first endoscopic evaluation and, in patients under surveillance, a strategy for NBI-targeted biopsies could potentially remove the need for routine biopsies [31, 42]. In fact, given these results for intestinal metaplasia, an endoscopic grading of gastric intestinal metaplasia with NBI was proposed. This classification considers five different gastric areas: two areas in the antrum, two in the body, and one in the incisura. Each area may have a score of 0 (no intestinal metaplasia), 1 (focal intestinal metaplasia, ≤30% of the area), or 2 points (extensive intestinal metaplasia in that area, > 30% of the area), resulting in a possible total of 10 points. The total score will vary from 0 (normal endoscopy with no areas suggestive of intestinal metaplasia) to 10 (diffuse metaplasia). The letter a or c is added to the score if metaplasia is more evident in the antrum (a) or in the corpus/body (c), suggesting environmental or autoimmune gastritis, respectively [31]. An endoscopic grade of gastric intestinal metaplasia of 5 was identified as the optimal cutoff value to identify patients with extensive intestinal metaplasia deserving surveillance, with a sensitivity of 94.2% and a specificity of 95.2 % [31]. This classification showed a high correlation with histology and is thus a promising tool, although validation studies are still needed.
ME-NBI has also been proven useful in the diagnosis of early gastric cancer, and the Magnifying Endoscopy Simple Diagnostic Algorithm for Early Gastric Cancer (MESDA-G) was recommended for the evaluation of a suspicious gastric lesion [39]. It applies the Vessels plus Surface Classification and suggests evaluation with NBI if a clear border between the suspicious lesion and the background mucosa (demarcation line) exists: if absent, it excludes cancer; if present, microvascular and microsurface patterns should be evaluated. If irregular microvascular and/or microsurface patterns are observed, a diagnosis of gastric cancer can be made [34, 43]. Some studies verified that NBI microvascular and/or microsurface patterns can also predict the histologic type of early gastric cancer (differentiated or undifferentiated) [10, 34, 44, 45].
The classification of gastric lesions proposed by Li describes three distinct patterns associated with different types of gastric lesions and with the depth of cancer invasion: the type A pattern corresponds to noncancerous lesions, the type B pattern corresponds to differentiated adenocarcinoma and intramucosal or superficially invasive cancers, and the type C pattern is indicative of undifferentiated adenocarcinoma or differentiated cancer with deep submucosal invasion [45]. This classification may be a promising tool with good sensitivity, specificity, and accuracy in distinguishing between differentiated and undifferentiated adenocarcinomas (92.3, 89.7, and 90.4%, respectively) and in differentiating between cancerous and noncancerous lesions (97.3, 84.4, and 90.2%, respectively) [45]. However, the validity, reproducibility, and clinical value of this classification are still to be demonstrated.
In conclusion, NBI (with and without magnification) is accurate in the diagnosis of gastric intestinal metaplasia and dysplasia, and is superior to WL [46, 47]. The use of NBI also improves the diagnosis of early gastric cancer [48, 49, 50, 51] and is also helpful in the preoperative demarcation of cancer to prevent positive surgical margins postoperatively [48, 51, 52]. NBI should be seen as a complement to WL, improving the diagnosis and detection of extensive intestinal metaplasia and superficial lesions with dysplasia and cancer [46].
Colon
Normal colonic mucosa presents a circular and regular gland and vessel pattern on NBI. Colon inflammation maintains the same pattern, but with thicker vessels and variable vascular density, which confer a reddish appearance of the mucosa. When this pattern is seen in a polyp or lesion, it suggests a mucosal or inflammatory polyp.
Polyps/Flat Lesions
Most colorectal polyps/superficial lesions are histologically classified into adenomas and serrated polyps (hyperplastic polyps [HPs], sessile serrated adenomas/polyps [SSA/Ps], and traditional serrated adenomas) [53].
NBI provides enhanced vessel and surface patterns of lesions and contributes to the detection and characterization of colorectal polyps. It is helpful for the prediction of histology (real-time optical biopsy) and for estimating the depth of invasion of a colorectal cancer [10, 54].
The use of validated scales allows an improvement of the diagnostic accuracy of in vivo optical diagnosis and decreases interobserver variability [2]. The Kudo classification characterizes the mucosal pit pattern, and the Sano classification assesses the capillary pattern. Both of them were the mainstay of polyp assessment, and the remaining systems were derived from the former ones [5, 55].
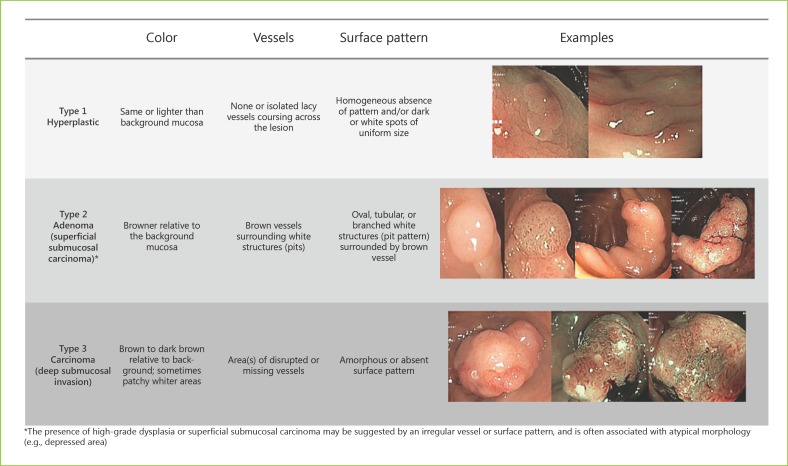
The NBI International Colorectal Endoscopic (NICE) (Fig. 4) and the Japan NBI Expert Team (JNET) classifications simultaneously evaluate surface and capillary patterns [1, 6]. The NICE classification was proposed in 2012 by an international expert group for the diagnosis of colonic lesions [56, 57]. An advantage of this Western validated classification is that it can be applied using NBI with or without optical magnification [1, 56]. It categorizes three types of lesions: type 1 (HP), type 2 (adenoma), and type 3 (deep submucosal invasive colorectal carcinoma) [10, 56, 57].
Fig. 4.
NBI International Colorectal Endoscopic (NICE) classification.
The NICE classification with unmagnified NBI distinguishes neoplastic from nonneoplastic lesions as accurately as does ME-NBI (with a sensitivity, specificity, and negative predictive value of 97.5, 83.3, and 92.6% for unmagnified NBI vs. 97.5, 85.1, and 95.2% for ME-NBI, respectively) [58]. However, with ME-NBI the rate of optical diagnoses of diminutive and small colorectal polyps is significantly improved [59]. The NICE classification is also clinically useful to predict deep submucosal invasive carcinoma (with a sensitivity of 94.9% and a negative predictive value of 95.9%) [57].
The JNET classification was proposed in 2014 [60], aiming to unify previous classifications into one universal ME-NBI classification of colorectal tumors [60]. Lesions are classified into four types [60]. A recent retrospective analysis concluded that types 1, 2A, and 3 of the JNET classification were very reliable indicators of a polyp histology (with a sensitivity, specificity, and accuracy of 87.5, 99.9, and 99.3% for type 1; 74.3, 92.7, and 77.1% for type 2A; and 55.4, 99.8, and 96.6% for type 3, respectively). However, the accuracy for type 2B lesions was lower (with a sensitivity, specificity, and accuracy of 61.9, 82.8, and 78.1%); for this type of lesions, chromoendoscopy with added indigo carmine improves diagnosis [61]. At present, large-scale validation studies of the JNET classification are needed to prove its utility in clinical practice [60].
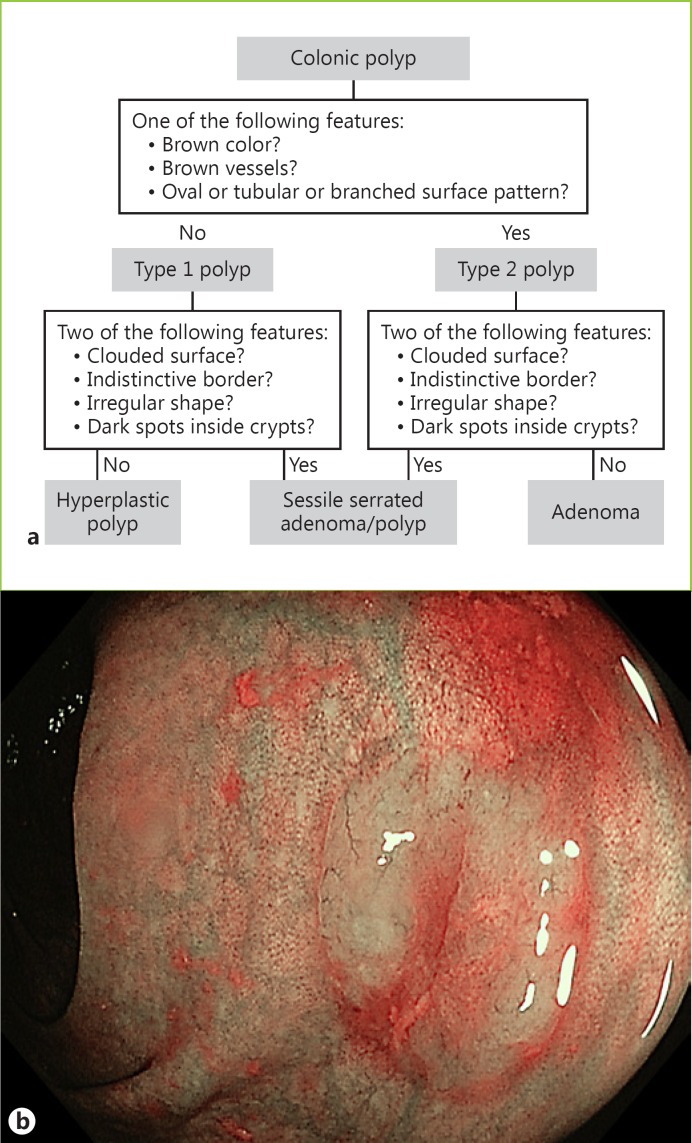
The current classification systems based on NBI do not include serrated adenomas (SSA/Ps and traditional serrated adenomas). These lesions are difficult to differentiate from HPs and sometimes from adenomas [2]. Recently, the Workgroup Serrated Polyps and Polyposis (WASP) classification was developed and validated to allow endoscopic differentiation between adenomas, HPs, and SSA/Ps < 10 mm in a stepwise approach (Fig. 5) [53]. First, colonic polyps are assessed for the presence of adenoma-like features using the NICE criteria. The presence of at least one adenoma-like feature is sufficient to diagnose a type 2 polyp. Subsequently, the diagnostic criteria are used to differentiate between SSA/Ps and HPs for type 1 polyps, and between SSA/Ps and adenomas for type 2 polyps. The presence of at least two SSA/P-like features is considered sufficient for a diagnosis. The introduction of the WASP classification significantly improved the accuracy of the optical diagnosis of serrated lesions, which showed to be sustainable after 6 months [53]. However, more studies are needed before using this classification in clinical practice [1].
Fig. 5.
a Workgroup Serrated Polyps and Polyposis (WASP) classification. b Serrated polyp.
Among patients undergoing screening colonoscopy, previous studies have demonstrated that NBI does not improve the detection of colorectal polyps but seems to be better than standard-definition WL and equal to HDWL [62, 63, 64, 65, 66]. Based on past studies, use of virtual chromoendoscopy is not routinely recommended for improving detection in average-risk populations, only in patients with known or suspected Lynch syndrome/serrated polyposis syndrome [2]. Nevertheless, recent studies suggest that bright NBI can improve adenoma detection [67, 68, 69]. For the detection of colorectal serrated lesions, use of NBI may be promising, but the data are conflicting [70, 71, 72].
Concerning characterization, virtual chromoendoscopy is recommended to predict the risk of invasive cancer in suspected lesions (depressed [Paris 0-IIc] or nongranular/mixed-type laterally spreading tumors), to define the margins of lesions, and to detect residual neoplasia at a scar site. In order to increase the quality of evaluation of colonic lesions, classifications such as the NICE, Kudo, JNET, and WASP systems should be used to describe the surface characteristics of a polyp [1, 73]. A recent meta-analysis showed that the use of AEITs such as NBI was preferable to gross morphological features to differentiate superficial from deeply invasive cancer [74].
In several studies, NBI was demonstrated to allow a reliable optical diagnosis of colonic lesions when used by appropriately trained endoscopists, and to improve diagnostic accuracy in lesion assessment [73, 75, 76, 77]. At present, there is a paradigm shift in the management of diminutive colorectal polyps (≤5 mm), advocating the use of optical biopsy with endoscopic technologies rather than histopathology for polyp characterization and subsequent assignment of surveillance intervals, without affecting its efficacy in reducing the future risk of colorectal cancer [78, 79]. A meta-analysis indicated that NBI allows accurate real-time optical biopsy (the negative predictive value of NBI for adenomatous polyp histology was 91% in general and 93% with expert endoscopists) and supports a “diagnose-and-leave” strategy, in which the endoscopist leaves in situ diminutive rectosigmoid HPs, and a “resect-and-discard” strategy, in which colorectal adenomas ≤5 mm are resected without pathological assessment. This strategy is safe and cost-effective: it reduces the number of resections, associated adverse events, and histological examinations [78, 80].
Community medical centers and nonexpert endoscopists demonstrated an inferior optical biopsy performance, and NBI optical diagnosis cannot be recommended for application in routine clinical practice [76, 77, 78, 81]. Diminutive polyps should be removed and submitted to histopathology to determine the next surveillance colonoscopy interval [78, 82, 83, 84].
Before the widespread implementation of “diagnose-and-leave” and “resect-and-discard” strategies in clinical practice, additional improvements are needed, including developing training and accredited programs, standardization of polyp classification systems based on endoscopic imaging technologies, establishment of standards of practice, and development of quality assurance programs [82, 85].
Thus, in conclusion, NBI may not significantly increase the rate of detection of colorectal neoplasia in average-risk populations, but particularly light NBI could be an option for high-risk patients. Nevertheless, NBI is a useful tool for characterizing lesions (predicting the risk of invasive cancer and defining margins of resection and residual neoplasia in piecemeal polypectomy scars) and in helping to choose the best therapy (endoscopic mucosal resection, endoscopic submucosal dissection, or surgery). In expert centers and under strictly controlled conditions, NBI can also be used for real-time optical diagnosis of diminutive (≤5-mm) colorectal polyps.
Training
Recommendations suggest that training programs can help in achieving a high accuracy and good interobserver agreement in the use of AEITs such as NBI, and that it is a requirement for use in clinical practice [1, 2, 33, 86, 87, 88]. Even for simple NBI patterns in the stomach, a learning curve was observed, with a 10% increase in global accuracy for both trainees and fully trained gastroenterologists [89]. Endoscopists who participated in standardized and continued training using a computer-based module achieved a high performance in the optical diagnosis of colorectal polyps and exceeded thresholds [81]. However, a learning curve exists, and training alone does not guarantee sustainedly high performances in clinical practice [2].
We suggest a staged method of training, beginning with learning the validated endoscopic classifications and recognizing the images and patterns presented by the authors. Then, videos displaying the different pathologies should be watched (at least 20–50 videos). Afterwards, we believe that observation of experts with live explanations may be of great value. At this stage, when confident with NBI diagnosis, we suggest another session of videos, this time without knowing the pathologies, with the goal of more than 90% accuracy. Internet-based e-learning systems are proving their value and should be used whenever possible for this purpose [90]. Finally, we suggest that before using the clinical diagnoses in daily routine, endoscopists should correlate the endoscopic images of their procedures with the correspondent histological diagnoses.
Conclusions
NBI is an advanced endoscopic imaging technique that enhances visualization of the mucosal surface architecture and microvascular details. It is readily available, easy to perform, and safe.
Undoubtedly, NBI is an important adjunctive tool to WLE for improving the diagnosis and characterization of lesions in the digestive tract and assisting the physician to decide on the best treatment (endoscopic mucosal resection, endoscopic submucosal dissection, or surgery). Table 1 summarizes the NBI recommendations in different clinical settings.
Table 1.
NBI recommendations for different clinical settings
| Indication | NBI recommendation |
||
|---|---|---|---|
| detection1 | diagnostic confidence1 | characterization/extension | |
| Squamous cell carcinoma | + + | ++ | ++ |
| Barrett esophagus dysplasia/cancer | + | ++ | ++ |
| Helicobacter pylori gastritis | +/– | + | – |
| Gastric intestinal metaplasia | + | +++ | ++ |
| Dysplasia and early gastric cancer | + | +++ | ++ |
| Polyps/flat lesions | +/– + (high-risk patients) |
+ | +++ |
NBI, narrow-band imaging. +/–, contradictory data; +, little evidence/author's opinion; ++, moderate evidence; +++, strong evidence.
When compared to white-light endoscopy alone or standard biopsy protocols.
Future strategies should focus on adequate training programs to promote the implementation of NBI in daily clinical practice.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, Dekker E, Hassan C, et al. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy. 2016;48:1029–1045. doi: 10.1055/s-0042-118087. [DOI] [PubMed] [Google Scholar]
- 2.Kamiński MF, Hassan C, Bisschops R, Pohl J, Pellisé M, Dekker E, Ignjatovic-Wilson A, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2014;46:435–449. doi: 10.1055/s-0034-1365348. [DOI] [PubMed] [Google Scholar]
- 3.ASGE Technology Committee, Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, et al. Electronic chromoendoscopy. Gastrointest Endosc. 2015;81:249–261. doi: 10.1016/j.gie.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Kudo S, Rubio CA, Teixeira CR, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2001;33:367–373. doi: 10.1055/s-2004-826104. [DOI] [PubMed] [Google Scholar]
- 5.Sano Y, Horimatsu T, Fu KI, Katagiri A, Muto M, Ishikawa H. Magnifying observation of microvascular architecture of colorectal lesions using a narrow-band imaging system. Dig Endosc. 2006;18:S44–S51. [Google Scholar]
- 6.Oba S, Tanaka S, Sano Y, Oka S, Chayama K. Current status of narrow-band imaging magnifying colonoscopy for colorectal neoplasia in Japan. Digestion. 2011;83:167–172. doi: 10.1159/000321807. [DOI] [PubMed] [Google Scholar]
- 7.ASGE Technology Committee High-definition and high-magnification endoscopes. Gastrointest Endosc. 2014;80:919–927. doi: 10.1016/j.gie.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 8.ASGE Technology Committee, Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, et al. Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67:581–589. doi: 10.1016/j.gie.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Hawes RH, Bansal A, Gupta N, Curvers W, Rastogi A, Singh M, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett's oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62:15–21. doi: 10.1136/gutjnl-2011-300962. [DOI] [PubMed] [Google Scholar]
- 10.Boeriu A, Boeriu C, Drasovean S, Pascarenco O, Mocan S, Stoian M, Dobru D. Narrow-band imaging with magnifying endoscopy for the evaluation of gastrointestinal lesions. World J Gastrointest Endosc. 2015;7:110–120. doi: 10.4253/wjge.v7.i2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue H, Kaga M, Ikeda H, Sato C, Sato H, Minami H, Santi EG, et al. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol. 2015;28:41–48. [PMC free article] [PubMed] [Google Scholar]
- 12.Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–1572. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goda K, Dobashi A, Tajiri H. Perspectives on narrow-band imaging endoscopy for superficial squamous neoplasms of the orohypopharynx and esophagus. Dig Endosc. 2014;26((suppl 1)):1–11. doi: 10.1111/den.12220. [DOI] [PubMed] [Google Scholar]
- 14.Chai TH, Jin XF, Li SH, Du RL, Zhang J. A tandem trial of HD-NBI versus HD-WL to compare neoplasia miss rates in esophageal squamous cell carcinoma. Hepatogastroenterology. 2014;61:120–124. [PubMed] [Google Scholar]
- 15.Goda K, Dobashi A, Yoshimura N, Kato M, Aihara H, Sumiyama K, Toyoizumi H, et al. Narrow-band imaging magnifying endoscopy versus Lugol chromoendoscopy with pink-color sign assessment in the diagnosis of superficial esophageal squamous neoplasms: a randomised noninferiority trial. Gastroenterol Res Pract. 2015;2015:639462. doi: 10.1155/2015/639462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CT, Chang CY, Lee YC, Tai CM, Wang WL, Tseng PH, Hwang JC, et al. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy. 2010;42:613–619. doi: 10.1055/s-0030-1255514. [DOI] [PubMed] [Google Scholar]
- 17.Nagami Y, Tominaga K, Machida H, Nakatani M, Kameda N, Sugimori S, Okazaki H, et al. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol. 2014;109:845–854. doi: 10.1038/ajg.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita FH, Bernardo WM, Ide E, Rocha RS, Aquino JC, Minata MK, Yamazaki K, et al. Narrow band imaging versus Lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer. 2017;17:54. doi: 10.1186/s12885-016-3011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, Hirasawa D, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105–112. doi: 10.1007/s10388-016-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato H, Inoue H, Ikeda H, Sato C, Onimaru M, Hayee B, Phlanusi C, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy. 2015;47:122–128. doi: 10.1055/s-0034-1390858. [DOI] [PubMed] [Google Scholar]
- 21.Ebi M, Shimura T, Yamada T, Mizushima T, Itoh K, Tsukamoto H, Tsuchida K, et al. Multicenter, prospective trial of white-light imaging alone versus white-light imaging followed by magnifying endoscopy with narrow-band imaging for the real-time imaging and diagnosis of invasion depth in superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2015;81:1355–1361. doi: 10.1016/j.gie.2014.11.015. e2. [DOI] [PubMed] [Google Scholar]
- 22.ASGE Technology Committee, Thosani N, Abu Dayyeh BK, Sharma P, Aslanian HR, Enestvedt BK, Komanduri S, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett's esophagus. Gastrointest Endosc. 2016;83:684–698. doi: 10.1016/j.gie.2016.01.007. e7. [DOI] [PubMed] [Google Scholar]
- 23.Pascarenco OD, Coroş MF, Pascarenco G, Boeriu AM, Draşovean SC, Onişor DM, Brusnic O, et al. A preliminary feasibility study: narrow-band imaging targeted versus standard white light endoscopy non-targeted biopsies in a surveillance Barrett's population. Dig Liver Dis. 2016;48:1048–1053. doi: 10.1016/j.dld.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Song J, Zhang J, Wang J, Guo X, Yu S, Wang J, Liu Y, et al. Meta-analysis of the effects of endoscopy with narrow band imaging in detecting dysplasia in Barrett's esophagus. Dis Esophagus. 2015;28:560–566. doi: 10.1111/dote.12222. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Bergman JJ, Goda K, Kato M, Messmann H, Alsop BR, Gupta N, et al. Development and validation of a classification system to identify high-grade dysplasia and esophageal adenocarcinoma in Barrett's esophagus using narrow-band imaging. Gastroenterology. 2016;150:591–598. doi: 10.1053/j.gastro.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Kato M, Goda K, Shimizu Y, Dobashi A, Takahashi M, Ikegami M, Shimoda T, et al. Image assessment of Barrett's esophagus using the simplified narrow band imaging classification. J Gastroenterol. 2017;52:466–475. doi: 10.1007/s00535-016-1239-4. [DOI] [PubMed] [Google Scholar]
- 27.Nogales O, Caballero-Marcos A, Clemente-Sánchez A, García-Lledó J, Pérez-Carazo L, Merino B, Carbonell C, et al. Usefulness of non-magnifying narrow band imaging in EVIS EXERA III video systems and high-definition endoscopes to diagnose dysplasia in Barrett's esophagus using the Barrett International NBI Group (BING) classification. Dig Dis Sci. 2017;62:2840–2846. doi: 10.1007/s10620-017-4581-3. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 29.Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, Hassan C, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191–198. doi: 10.1055/s-0042-122140. [DOI] [PubMed] [Google Scholar]
- 30.Shaheen NJ, Falk GW, Iyer PG, Gerson LB, American College of Gastroenterology ACG Clinical Guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol. 2016;111:30–50. doi: 10.1038/ajg.2015.322. quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pimentel-Nunes P, Libânio D, Lage J, Abrantes D, Coimbra M, Esposito G, Hormozdi D, et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy. 2016;48:723–730. doi: 10.1055/s-0042-108435. [DOI] [PubMed] [Google Scholar]
- 32.Aida J, Arima M, Arai T, Sawabe M, Takubo K. Current pathological knowledge of Barrett cancer (in Japanese) Gan To Kagaku Ryoho. 2010;37:1670–1673. [PubMed] [Google Scholar]
- 33.Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, Marcos-Pinto R, Santos C, Rolanda C, Bastos RP, et al. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy. 2012;44:236–246. doi: 10.1055/s-0031-1291537. [DOI] [PubMed] [Google Scholar]
- 34.Muto M, Yao K, Kaise M, Kato M, Uedo N, Yagi K, Tajiri H. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G) Dig Endosc. 2016;28:379–393. doi: 10.1111/den.12638. [DOI] [PubMed] [Google Scholar]
- 35.Alaboudy AA, Elbahrawy A, Matsumoto S, Yoshizawa A. Conventional narrow-band imaging has good correlation with histopathological severity of Helicobacter pylori gastritis. Dig Dis Sci. 2011;56:1127–1130. doi: 10.1007/s10620-010-1414-z. [DOI] [PubMed] [Google Scholar]
- 36.Okubo M, Tahara T, Shibata T, Nakamura M, Kamiya Y, Yoshioka D, Maeda Y, et al. Usefulness of magnifying narrow-band imaging endoscopy in the Helicobacter pylori-related chronic gastritis. Digestion. 2011;83:161–166. doi: 10.1159/000321799. [DOI] [PubMed] [Google Scholar]
- 37.Tahara T, Shibata T, Nakamura M, Yoshioka D, Okubo M, Arisawa T, Hirata I. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246–253. doi: 10.1016/j.gie.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Huang W, Du J, Chen Y, Yang J. Diagnostic yield of the light blue crest sign in gastric intestinal metaplasia: a meta-analysis. PLoS One. 2014;9:e92874. doi: 10.1371/journal.pone.0092874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanemitsu T, Yao K, Nagahama T, Imamura K, Fujiwara S, Ueki T, Chuman K, et al. Extending magnifying NBI diagnosis of intestinal metaplasia in the stomach: the white opaque substance marker. Endoscopy. 2017;49:529–535. doi: 10.1055/s-0043-103409. [DOI] [PubMed] [Google Scholar]
- 40.Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462–467. doi: 10.1055/s-0029-1214594. [DOI] [PubMed] [Google Scholar]
- 41.Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279–284. doi: 10.1016/s0016-5107(02)70194-6. [DOI] [PubMed] [Google Scholar]
- 42.Lage J, Pimentel-Nunes P, Figueiredo PC, Libânio D, Ribeiro I, Jacome M, Afonso L, et al. Light-NBI to identify high-risk phenotypes for gastric adenocarcinoma: do we still need biopsies? Scand J Gastroenterol. 2016;51:501–506. doi: 10.3109/00365521.2015.1101779. [DOI] [PubMed] [Google Scholar]
- 43.Kaise M. Advanced endoscopic imaging for early gastric cancer. Best Pract Res Clin Gastroenterol. 2015;29:575–587. doi: 10.1016/j.bpg.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Ok KS, Kim GH, Park DY, Lee HJ, Jeon HK, Baek DH, Lee BE, et al. Magnifying endoscopy with narrow band imaging of early gastric cancer: correlation with histopathology and mucin phenotype. Gut Liver. 2016;10:532–541. doi: 10.5009/gnl15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li HY, Dai J, Xue HB, Zhao YJ, Chen XY, Gao YJ, Song Y, et al. Application of magnifying endoscopy with narrow-band imaging in diagnosing gastric lesions: a prospective study. Gastrointest Endosc. 2012;76:1124–1132. doi: 10.1016/j.gie.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Kikuste I, Marques-Pereira R, Monteiro-Soares M, Pimentel-Nunes P, Areia M, Leja M, Dinis-Ribeiro M. Systematic review of the diagnosis of gastric premalignant conditions and neoplasia with high-resolution endoscopic technologies. Scand J Gastroenterol. 2013;48:1108–1117. doi: 10.3109/00365521.2013.825315. [DOI] [PubMed] [Google Scholar]
- 47.Song J, Zhang J, Wang J, Guo X, Wang J, Liu Y, Dong W. Meta-analysis: narrow band imaging for diagnosis of gastric intestinal metaplasia. PLoS One. 2014;9:e94869. doi: 10.1371/journal.pone.0094869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q, Wang F, Chen ZY, Wang Z, Zhi FC, Liu SD, Bai Y. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis. Gastric Cancer. 2016;19:543–552. doi: 10.1007/s10120-015-0500-5. [DOI] [PubMed] [Google Scholar]
- 49.Hu YY, Lian QW, Lin ZH, Zhong J, Xue M, Wang LJ. Diagnostic performance of magnifying narrow-band imaging for early gastric cancer: a meta-analysis. World J Gastroenterol. 2015;21:7884–7894. doi: 10.3748/wjg.v21.i25.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv X, Wang C, Xie Y, Yan Z. Diagnostic efficacy of magnifying endoscopy with narrow-band imaging for gastric neoplasms: a meta-analysis. PLoS One. 2015;10:e0123832. doi: 10.1371/journal.pone.0123832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nonaka T, Inamori M, Honda Y, Kanoshima K, Inoh Y, Matsuura M, Uchiyama S, et al. Can magnifying endoscopy with narrow-band imaging discriminate between carcinomas and low grade adenomas in gastric superficial elevated lesions? Endosc Int Open. 2016;4:E1203–E1210. doi: 10.1055/s-0042-117632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horiuchi Y, Fujisaki J, Yamamoto N, Shimizu T, Omae M, Ishiyama A, Yoshio T, et al. Accuracy of diagnostic demarcation of undifferentiated-type early gastric cancer for magnifying endoscopy with narrow-band imaging: surgical cases. Surg Endosc. 2017;31:1906–1913. doi: 10.1007/s00464-016-5192-3. [DOI] [PubMed] [Google Scholar]
- 53.IJspeert JE, Bastiaansen BA, van Leerdam ME, Meijer GA, van Eeden S, Sanduleanu S, Schoon EJ, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut. 2016;65:963–970. doi: 10.1136/gutjnl-2014-308411. [DOI] [PubMed] [Google Scholar]
- 54.Utsumi T, Iwatate M, Sano W, Sunakawa H, Hattori S, Hasuike N, Sano Y. Polyp detection, characterization, and management using narrow-band imaging with/without magnification. Clin Endosc. 2015;48:491–497. doi: 10.5946/ce.2015.48.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, et al. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599–607. doi: 10.1053/j.gastro.2012.05.006. e1. [DOI] [PubMed] [Google Scholar]
- 57.Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the Narrow-Band Imaging International Colorectal Endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625–632. doi: 10.1016/j.gie.2013.04.185. [DOI] [PubMed] [Google Scholar]
- 58.Kim JJ, Hong KS, Kim JS, Jung HC. A randomized controlled clinical study comparing the diagnostic accuracy of the histologic prediction for colorectal polyps depending on the use of either magnified or nonmagnified narrow band imaging. Clin Endosc. 2015;48:528–533. doi: 10.5946/ce.2015.48.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwatate M, Sano Y, Hattori S, Sano W, Hasuike N, Ikumoto T, Kotaka M, et al. The addition of high magnifying endoscopy improves rates of high confidence optical diagnosis of colorectal polyps. Endosc Int Open. 2015;3:E140–E145. doi: 10.1055/s-0034-1391362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team (JNET) Dig Endosc. 2016;28:526–533. doi: 10.1111/den.12644. [DOI] [PubMed] [Google Scholar]
- 61.Sumimoto K, Tanaka S, Shigita K, Hirano D, Tamaru Y, Ninomiya Y, Asayama N, et al. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc. 2017;85:816–821. doi: 10.1016/j.gie.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 62.Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, et al. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363–370. doi: 10.1038/ajg.2011.436. quiz 371. [DOI] [PubMed] [Google Scholar]
- 63.Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012;1:CD008361. doi: 10.1002/14651858.CD008361.pub2. [DOI] [PubMed] [Google Scholar]
- 64.Dinesen L, Chua TJ, Kaffes AJ. Meta-analysis of narrow-band imaging versus conventional colonoscopy for adenoma detection. Gastrointest Endosc. 2012;75:604–611. doi: 10.1016/j.gie.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Jin XF, Chai TH, Shi JW, Yang XC, Sun QY. Meta-analysis for evaluating the accuracy of endoscopy with narrow band imaging in detecting colorectal adenomas. J Gastroenterol Hepatol. 2012;27:882–887. doi: 10.1111/j.1440-1746.2011.06987.x. [DOI] [PubMed] [Google Scholar]
- 66.Omata F, Ohde S, Deshpande GA, Kobayashi D, Masuda K, Fukui T. Image-enhanced, chromo, and cap-assisted colonoscopy for improving adenoma/neoplasia detection rate: a systematic review and meta-analysis. Scand J Gastroenterol. 2014;49:222–237. doi: 10.3109/00365521.2013.863964. [DOI] [PubMed] [Google Scholar]
- 67.Leung WK, Lo OS, Liu KS, Tong T, But DY, Lam FY, Hsu AS, et al. Detection of colorectal adenoma by narrow band imaging (HQ190) vs high-definition white light colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2014;109:855–863. doi: 10.1038/ajg.2014.83. [DOI] [PubMed] [Google Scholar]
- 68.Horimatsu T, Sano Y, Tanaka S, Kawamura T, Saito S, Iwatate M, Oka S, et al. Next-generation narrow band imaging system for colonic polyp detection: a prospective multicenter randomized trial. Int J Colorectal Dis. 2015;30:947–954. doi: 10.1007/s00384-015-2230-x. [DOI] [PubMed] [Google Scholar]
- 69.Ogiso K, Yoshida N, Siah KT, Kitae H, Murakami T, Hirose R, Inada Y, et al. New-generation narrow band imaging improves visibility of polyps: a colonoscopy video evaluation study. J Gastroenterol. 2016;51:883–890. doi: 10.1007/s00535-016-1167-3. [DOI] [PubMed] [Google Scholar]
- 70.Matsuda T, Oka S, Ikematsu H, Matsushita HO, Mori Y, Takeuchi Y, Tamai N, et al. Endoscopic diagnosis of colorectal serrated lesions: current status and future perspectives based on the results of a questionnaire survey. Dig Endosc. 2016;28((suppl 1)):35–42. doi: 10.1111/den.12632. [DOI] [PubMed] [Google Scholar]
- 71.Parikh ND, Chaptini L, Njei B, Laine L. Diagnosis of sessile serrated adenomas/polyps with image-enhanced endoscopy: a systematic review and meta-analysis. Endoscopy. 2016;48:731–739. doi: 10.1055/s-0042-107592. [DOI] [PubMed] [Google Scholar]
- 72.Rex DK, Clodfelter R, Rahmani F, Fatima H, James-Stevenson TN, Tang JC, Kim HN, et al. Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial. Gastrointest Endosc. 2016;83:166–171. doi: 10.1016/j.gie.2015.03.1915. [DOI] [PubMed] [Google Scholar]
- 73.Rutter MD, Chattree A, Barbour JA, Thomas-Gibson S, Bhandari P, Saunders BP, Veitch AM, et al. British Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut. 2015;64:1847–1873. doi: 10.1136/gutjnl-2015-309576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Backes Y, Moss A, Reitsma JB, Siersema PD, Moons LM. Narrow band imaging, magnifying chromoendoscopy, and gross morphological features for the optical diagnosis of T1 colorectal cancer and deep submucosal invasion: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:54–64. doi: 10.1038/ajg.2016.403. [DOI] [PubMed] [Google Scholar]
- 75.Wanders LK, East JE, Uitentuis SE, Leeflang MM, Dekker E. Diagnostic performance of narrowed spectrum endoscopy, autofluorescence imaging, and confocal laser endomicroscopy for optical diagnosis of colonic polyps: a meta-analysis. Lancet Oncol. 2013;14:1337–1347. doi: 10.1016/S1470-2045(13)70509-6. [DOI] [PubMed] [Google Scholar]
- 76.Wu L, Li Y, Li Z, Cao Y, Gao F. Diagnostic accuracy of narrow-band imaging for the differentiation of neoplastic from non-neoplastic colorectal polyps: a meta-analysis. Colorectal Dis. 2013;15:3–11. doi: 10.1111/j.1463-1318.2012.02947.x. [DOI] [PubMed] [Google Scholar]
- 77.Patel SG, Schoenfeld P, Kim HM, Ward EK, Bansal A, Kim Y, Hosford L, et al. Real-time characterization of diminutive colorectal polyp histology using narrow-band imaging: implications for the resect and discard strategy. Gastroenterology. 2016;150:406–418. doi: 10.1053/j.gastro.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.ASGE Technology Committee, Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81:502.e1–502.e16. doi: 10.1016/j.gie.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 79.McGill SK, Evangelou E, Ioannidis JP, Soetikno RM, Kaltenbach T. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut. 2013;62:1704–1713. doi: 10.1136/gutjnl-2012-303965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solon C, Klausnitzer R, Blissett D, Ihara Z. Economic value of narrow band imaging versus white light endoscopy for the characterization of diminutive polyps in the colon: systematic literature review and cost-consequence model. J Med Econ. 2016;19:1040–1048. doi: 10.1080/13696998.2016.1192550. [DOI] [PubMed] [Google Scholar]
- 81.McGill SK, Soetikno R, Rastogi A, Rouse RV, Sato T, Bansal A, McQuaid K, et al. Endoscopists can sustain high performance for the optical diagnosis of colorectal polyps following standardized and continued training. Endoscopy. 2015;47:200–206. doi: 10.1055/s-0034-1378096. [DOI] [PubMed] [Google Scholar]
- 82.Rees CJ, Rajasekhar PT, Wilson A, Close H, Rutter MD, Saunders BP, East JE, et al. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut. 2017;66:887–895. doi: 10.1136/gutjnl-2015-310584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klare P, Haller B, Wormbt S, Nötzel E, Hartmann D, Albert J, Hausmann J, et al. Narrow-band imaging vs high definition white light for optical diagnosis of small colorectal polyps: a randomized multicenter trial. Endoscopy. 2016;48:909–915. doi: 10.1055/s-0042-110650. [DOI] [PubMed] [Google Scholar]
- 84.van der Vlugt M, van Doorn SC, Wang J, Bastiaansen BA, Brosens LA, Fockens P, Dekker E. Optical diagnosis of malignant colorectal polyps: is it feasible? Endosc Int Open. 2016;4:E778–E783. doi: 10.1055/s-0042-107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaltenbach T, Rastogi A, Rouse RV, McQuaid KR, Sato T, Bansal A, Kosek JC, et al. Real-time optical diagnosis for diminutive colorectal polyps using narrow-band imaging: the VALID randomised clinical trial. Gut. 2015;64:1569–1577. doi: 10.1136/gutjnl-2014-307742. [DOI] [PubMed] [Google Scholar]
- 86.Pohl H, Bensen SP, Toor A, Gordon SR, Levy LC, Anderson PB, Anderson JC, et al. Quality of optical diagnosis of diminutive polyps and associated factors. Endoscopy. 2016;48:817–822. doi: 10.1055/s-0042-108432. [DOI] [PubMed] [Google Scholar]
- 87.Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, Imperiale T, et al. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013;144:81–91. doi: 10.1053/j.gastro.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sikong Y, Lin X, Liu K, Wu J, Lin W, Wei N, Jiang G, et al. Effectiveness of systematic training in the application of Narrow-Band Imaging International Colorectal Endoscopic (NICE) classification for optical diagnosis of colorectal polyps: experience from a single center in China. Dig Endosc. 2016;28:583–591. doi: 10.1111/den.12600. [DOI] [PubMed] [Google Scholar]
- 89.Dias-Silva D, Pimentel-Nunes P, Magalhães J, Magalhães R, Veloso N, Ferreira C, Figueiredo P, et al. The learning curve for narrow-band imaging in the diagnosis of precancerous gastric lesions by using Web-based video. Gastrointest Endosc. 2014;79:910–920. doi: 10.1016/j.gie.2013.10.020. quiz 983.e1, 983.e4. [DOI] [PubMed] [Google Scholar]
- 90.Pimentel-Nunes P, Buxbaum J. Internet based e-learning systems: a tool for the future in endoscopy. Endoscopy. 2017;49:936–937. doi: 10.1055/s-0043-117399. [DOI] [PubMed] [Google Scholar]