Abstract
Background
HeartFailure (HF) is a progressive clinical and pathophysiological syndrome caused by cardiovascular and noncardiovascular abnormalities. Childhood HF has not been well studied in Sub-Sharan Africa, particularly in Ethiopia. Hence, this study aimed at describing the pattern and outcome of pediatrics HF at a referral-teaching hospital.
Methods
Medical records of 216 HF children aged 2 months to 14 years, and admitted between January 2014 and January 2016 were reviewed. Clinical information was collected, analyzed and presented in tables and pie charts.
Results
A total of 2000 children were admitted to Hawassa University Hospital during the study period. HF accounted for 10.8% (216) of pediatrics admissions, 51.9% males. The median age of the study subjects was 6 years. Functionally, NYHA/Ross class III and IV consisted 65(30.1%) and 139(64.4%) of HF. Structural heart disease was the commonest cause of HF, 144(66.7%): Rheumatic heart disease (RHD),75(52%), and congenital heart disease (CHD),64(44.5%). Anemia and renal cases contributed to 50(23.1) and 12(5.6%) of HF.CHD was predominantly documented in <5 years. Pneumonia 66(42.9%), and infective endocarditis 29(18.8%) were the common precipitating/comorbid conditions with HF. The case fatality rate of HF was 13.9 %(30).
Conclusion
In this study, HF accounted for a tenth of pediatrics admissions. Structural heart disease was the commonest cause of heart failure. CHD and RHD affected predominantly children of <5 years of age and >5 years of age. Echocardiographic screening of HF cases for structural heart disease and optimal care for patients with underlying structural heart disease are recommended.
Keywords: Heart failure, children, pattern, outcome, Ethiopia
Introduction
Heart Failure(HF) is a progressive clinical and pathophysiological syndrome caused by cardiovascular and noncardiovascular abnormalities (1). Childhood cardiovascular disorders including HF are associated with high morbidity and mortality especially in sub-Saharan Africa where palliative and definitive treatments seem far from reach (2–5).
Causes of HF are significantly different in children, and many cases are due to congenital malformations such as left-to-right shunts (6). Cardiomyopathies are common causes of HF in developed nations, though not all present with HF (7–8). The developing countries have a double burden of HF causes: congenital heart disease, myocarditis and cardiomyopathies with additional burden of rheumatic heart disease, nutritional problems and tropical diseases. Although, the exact prevalence of HF in children is not known in developing countries due to limited data (9–10). Pediatrics HF incurs a considerable treatment cost than adult population because of a longer hospital stay and increased procedure requirement. This in turn affects the structure and the economic productivity of families (1,11).
Clinical features suggestive of HF in infants include tachypnea, feeding difficulty, diaphoresis, irritability with poor feeding and even refusal of feeds. Edema of face and limbs is uncommon in infants and young children. Tachycardia, tachypnea, gallop rhythm and hepatomegaly are features of HF in infants. Older children and adolescents present with fatigue, effort intolerance, dyspnea, orthopnea, abdominal pain, dependent edema and ascites. The New York Heart Association (NYHA) HF classification isnot readily applicable to younger children (12). The modified Ross classification is used for HF severity classification in children (13). Transthoracic echocardiography is indicated in all cases of pediatric HF to exclude possible structural disease. Chest X-ray, electrocardiography, tests for infectious or precipitating factors are also indicated (10,12).
Pediatrics HF management principles are developed from a combination of clinical experience, small scale studies and extrapolation of adult studies (14). Treatment principles include treatment of the cause, correction of any precipitating event and alleviation of systemic or pulmonary congestion. Precipitating factors include infections, anemia, drug discontinuation, infective endocarditis and rheumatic recurrence (12).
The status of HF in children is not well studied in Sub-Saharan Africa with very limited reports and only a single study from Ethiopia (10, 15–16). We aimed at describing the clinical characteristics of pediatrics HF at a tertiary hospital in Hawassa, Southern Ethiopia. The study will provide additional knowledge to the underdocumented pediatrics HF and also serves as a resource information for future intervention.
Methods
Study area: The study was conducted in the Department of Pediatrics and Child Health, Hawassa University Comprehensive Specialized hospital. The hospital is located in Hawassa City, 275 km south of Ethiopia's capital city-Addis Ababa. The hospital is a referral and tertiary teaching center. The department has a cardiac unit staffed with trained Pediatric echocardiographer with Echocardiography machines (2-D, M-mode and color Doppler imaging facilities), electrocardiography and radiology imaging services.
Study Design: Medical records of study subjects were retrieved from the central medical records archive. Demographic, clinical and echocardiographic data were collected from medical records of study subjects using questionnaires developed.
Study population: All pediatric HF cases who were admitted to Hawassa University Comprehensive Specialized Hospital (HUCSH) between January 2014 and January 2016 were included in the study.
Inclusion criteria: All admitted pediatrics HF cases aged 2 months to 14 years were included.
Exclusion criteria: Patients with incomplete medical records and patients with suspected structural heart disease with no echocardiography report were excluded from the study.
A sample size of 214 was calculated based on the assumptions of 16.7% HF admissions (15), 95% confidence interval with 5% margin of error. However all 216 HF study subjects fulling the predefined criteria during the study period were included in the study.
Operational definitions: Heart failure was defined by simultaneous presence of 3 or all of the criteria:
tender hepatomegaly- with liver edge palpable 3cm below the right costal margin along the midclavicular line.
Significant tachycardia for age (heart rate >160 bpm in infancy, >140 bpm at the age of 2 years, >120 bpm the age of 4 years, and >100 bpm above 6 years of age).
significant tachypnea for age (respiratory rate >60/min in newborn, 2months up to 12 months >50/min, 1 year and above 40/min).
cardiomegaly (cardiothoracic ratio >60% in <5 year of age and >50% in >5 years of age) (17).
Severity of HF was assessed by New York Heart Association (NYHA) or modified Ross classification for younger children (13,18). Structural heart disease was defined as a disease of the heart that is either congenital (present from birth) or acquired (19). Diagnosis of pneumonia in HF patients was considered if fever, elevated white blood count for age, and chest x-ray evidence of infiltrates were documented (20). Severe acute malnutrition was defined by a very low weight for height (below -3z scores of the median WHO growth standards), by visible severe wasting, or by the presence of nutritional edema (21). Anemia was defined when hemoglobin concentration was <11g/dl, <11.5g/dl, and <12g/dl in under five years, 5–11 years and 12–14 years of agerespectively (22). Outcome refers to the hospital discharge outcome of patients. World heart federation and other standard criteria were used for diagnosis and classification of rheumatic heart disease and congenital heart disease (23,24).
Statistical methods: Data were double entered into excel spread sheet and transferred into SPSS software version 20 for analysis. Descriptive statistics were used to analyze variables.
Ethics: Ethical clearance was obtained from institutional review board of College of Medicine and Health Sciences, Hawassa University. Written permission was obtained from Hawassa University comprehensive specialized hospital administration for retrieving medical records of study subjects.
Results
Clinical characteristics: A total of 2000 children were admitted between 2 months and 14 years of age to HUCSH in the two years period (January 2014–January 2016). HF accounted for 10.8%(216) of pediatric inpatient admissions. The gender composition was comparable, 112 (51.9%) were males with male to female ratio of 1.1:1. The median age of the study subjects was 6 years. Children above the age of 5 years dominated the study population, 124 (57.4%).
The most common presenting symptoms were cough, feeding interruption, body swelling and fast breathing in 60(27.8%), 55(25.5%), 34(15.7%) and 29(13.4%) of the cases. The majority of the patients had moderate to severe symptoms with NYHA/Ross class III and IV comprising 65(30.1%) and 139(64.4%) of HF cases (Table 1). Pneumonia and infective endocarditis were the common precipitating factors for HF in 66(42.9%) and 29(18.8%) of the study subjects.
Table 1.
Clinical characteristics of Pediatric heart failure at Hawassa University Comprehensive Specialized Hospital, January 2014– January 2016
| Variable | Frequency(n) | Percentage (%) |
| Age in years (n=216) | ||
| <2 | 54 | 25 |
| 2–5 | 38 | 17.6 |
| 5–10 | 56 | 25.9 |
| ≥10 | 68 | 31.5 |
| Sex (n=216) | ||
| Male | 112 | 51.9 |
| Female | 104 | 48.1 |
| Presenting Symptom (n=216) | ||
| Cough | 60 | 27.8 |
| Fast breathing | 29 | 13.4 |
| Feeding interruption | 55 | 25.5 |
| Body swelling | 34 | 15.7 |
| Easy fatigability | 22 | 10.2 |
| Fever | 4 | 1.9 |
| Others* | 12 | 5.5 |
| Heart failure cause (n=216) | ||
| Structural heart disease | 144 | 66.7 |
| Anemia | 50 | 23.1 |
| Renal cause(hypertension) | 12 | 5.6 |
| Corpulmonale | 5 | 2.3 |
| Others+ | 5 | 2.3 |
| Functional Classification | ||
| (NYHA/Modified Ross) (n=216) | ||
| Class IV | 65 | 30.1 |
| Class III | 139 | 64.3 |
| Class II | 12 | 5.6 |
| Precipitating factor/comorbidity(n=154) | ||
| Pneumonia | 66 | 42.9 |
| Infective endocarditis | 29 | 18.8 |
| Severe acute malnutrition | 10 | 6.5 |
| Down syndrome | 19 | 12.3 |
| Others++ | 30 | 19.4 |
| Outcome (n=216) | ||
| Improved | 178 | 82.4 |
| Self-discharged against medical advice |
8 | 3.7 |
| Died | 30 | 13.9 |
include orthopnea, palpitation, and paroxysmal nocturnal dyspnea;
include tuberculous pericarditis, malnutrition;
Arrhythmias, rheumatic recurrence
Causes of heart failure: Structural heart disease was the commonest cause of heart failure, 144(66.7%) of HF cases. Anemia and renal causes contributed for 50(23.1) and 12(5.6%) of HF cases. Hypertension related to acute post streptococcal glomerulonephritis was the cause for renal cases.
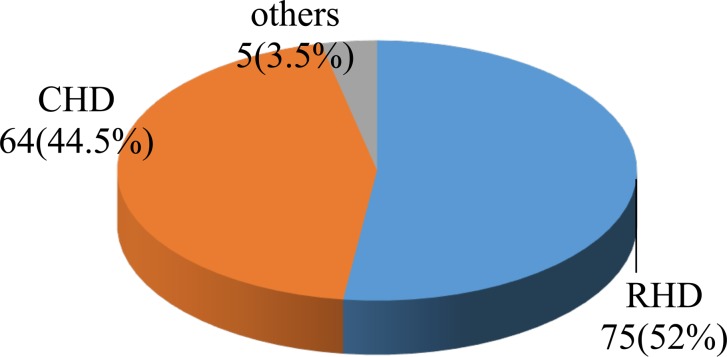
Rheumatic valvular heart disease (RHD) and congenital heart disease contributed to 75(52%) and 64(44.5%) of children with structural heart disease. Other causes like cardiomyopathies constituted only 5(3.5%) of HF cases (Figure 1). Mitral and aortic valves were severely affected by rheumatic heart disease and almost all (96.2%) of RHD cases had moderate to severe valvular insufficiency.
Figure 1.
Pattern of structural heart disease in childhood heart failure at Hawassa University Comprehensive specialized hospital, January 2014– Janurary 2016.
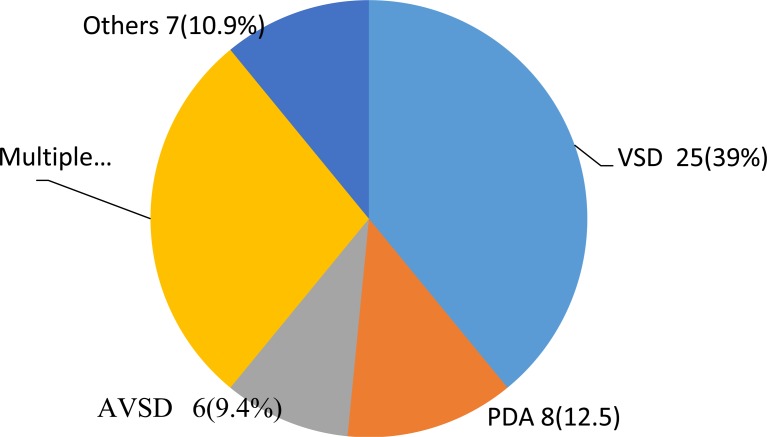
Eighty three (57.6%) of children with structural heart disease were aged 5 years and above. All RHD cases were documented in children above 5 years of age. CHD was documented in 41(64%), 14(21.9%) and 9(14%) of HF cases among children aged between 2 months and 24 months, 24 months to five years, and above 5 years of age, respectively. Ventricular septal defect was the commonest CHD, 25(39%). Multiple CHDs comprising ASD with VSD, VSD with PDA, VSD and PS with D-TGA etc contributed to 18(28%) of CHDs. Others including tricuspid atresia accounted for 7(10.9%) of CHD (Figure 2). Anemia was the second most common cause of heart failure, 50(24.3%). Hematologic malignancy, iron deficiency anemia and megaloblastic anemia contributed to 18(36%), 15(30%) and 13(26%) of anemia cases.
Figure 2.
Pattern of congenital heart disease among children with heart failure at Hawassa University Comprehensive Specialized Hospital, January 2014– January 2016
Treatment and Outcome: Various combinations of diuretics, angiotensin converting enzyme inhibitors, β-blockers and digoxin were used to treat HF. Outof 216 HF cases, 30 died making a case fatality rate of 13.9%. One hundred seventy eight (82.4%) were discharged improved, and 8(3.7%) left the hospital against medical advice. Twenty eight (93.3%) of deaths were documented among patients with structural heart disease. CHD and RHD accounted for 14 deaths each.
Discussion
Heart failure accounted for 10.8 % (95% CI 9.5, 12.2) of pediatric admissions in this study. This is higher than reports from the United Kingdom and Ireland, Nigeria, Kenya and Ethiopia (7,10,17). However, it is lower than a study conducted in Nigeria (15). It is comparable to a Belgian study (10.4%) (25). Differences could be attributed to the difference in the age of inclusion and the capacity of the hospitals. A report from the United Kingdom and Ireland was collected from multiple cardiac centers on a prospective basis and that might have contributed to the noted difference.
In this study, the majority of HF patients are aged 5 years and above with comparable gender composition. Age distribution is in agreement with Ethiopian study but our study subjects are older than Kenyan and Nigerian report (10,15,16). The reason for older age could be the high occurrence of rheumatic heart disease above 5 years of age in our study and the high RHD burden in Ethiopia (26). Additionally, diagnosis of HF in young children could be difficult owing to the similarity of HF symptoms to pneumonia, reactive airway disease and viral respiratory infections and these resulting in misclassification of final patients' diagnosis. In underprivileged setting like ours young children with severe congenital heart disease could also die of the underlying disease early (12,13).
In our study, the majority of patients were in NYHA/Ross class III and IV. This is similar to studies conducted in Ethiopia, Kenya and Belgium. In a Nigerian study, class I and II NYHA dominate (10,15,16,25). The difference could be attributed to the high occurrence of structural heart disease in our case which depletes the reserve of the heart for acute crisis state and HF in Nigerian report followed infectious diseases in normally structured heart (27,28).
Pneumonia was the commonest comorbid or precipitating condition for HF in our study.
This is in agreement with other studies in children with structural heart disease (16,17,29,30). The increased pulmonary circulation in HF was ascribed as a cause for pneumonia (29,31). IE was also a common precipitator for HF in our study. This is similar to reports from Kenya and Ethiopia (32,33). Infective endocarditis complicates patients with structural heart disease and commonly results in heart failure.
Regarding causes of HF, structural heart disease was documented as the commonest cause in our study. This is in agreement with another study from Ethiopia (16). This is opposed to the other Sub-Saharan studies where infectious causes dominated (10,17,29,34). Studies from developed nations have documented congenital heart disease and cardiomyopathies as common cause for HF (7, 25). Significant reduction of infectious diseases, community addressed primary health education, early diagnosis and treatment of common childhood infectious diseases at primary health care unit by health extension workers in Ethiopia could explain the absence of infectious etiologies as a cause of HF in our study (35–38). The difference could also be attributed to differences in level of diagnosis, care and treatment for congenital heart disease where CHD cases are underestimated in Africa owing to poor outcome (39). Childhood HF could also mirror the high burden of rheumatic heart disease in Ethiopia(26). The estimated CHD or cardiovascular congenital defects prevalence in Ethiopia is 7.9/1000 live births (40). Our finding calls for more robust community based studies to document the true burden of CHD and HF. The types of CHD in our study are similar to reports from other studies (16,17,29).
Anemia was the second common cause of HF in our study, and this is in agreement with findings from Nigeria and Kenya (10,15,17). This goes in line with the high prevalence of childhood anemia in Ethiopia, and also with the reported nutritional anemias occurrence mong patients with structural heart disease (41,42). Our finding of renal causes is comparable to report from Nigeria (17). Untreated hypertension following acute glomerulonephritis is reported to result in HF.
In this study, the case fatality rate was 13.9%. It is lower than reports from Nigeria and Ethiopia (15,16), but higher than a report from Kenya (10). Higher fatality rates from Nigeria might be related to infectious diseases and its prognosis. The high occurrence of severe acute malnutrition in a study conducted at Addis Ababa could also explain the noted high mortality. A lower case fatality identified in Kenya could be attributed to the availability of better care and surgical intervention for children with structural heart disease in their setting.
Our study has several limitations. It is a single center and hospital based study. It is likely that only patients with moderate and severe symptoms presented to our hospital, and this might not reflect the full context of the disease in the community/nation. Moreover, our study is retrospective, and many important variables were missing thus making the detailed analysis of determinant variables difficult. Nevertheless, this study provides the deviating pattern of pediatrics heart failure in Ethiopia from that of sub-Saharan African countries with structural heart disease being the commonest cause. It also calls for more large scale multicenter studies to document the real burden of pediatrics heart failure and gives insight into the needed future intervention. In this study, heart failure accounted for a tenth of pediatrics admissions. Structural heart disease was the commonest cause of heart failure. CHD and RHD affected predominantly children of less than 5 years of age and those above 5 years of age. Echocardiographic based screening of HF patients for structural heart disease and targeted treatment of HF cases with underlying structural heart disease are recommended.
Acknowledgements
We would like to thank the school of graduate studies of Hawassa University for financial support. We would also like to thank the College of Medicine and Health sciences administration for facilitation of the study.
References
- 1.Hsu DT, Pearson GD. Heart failure in children part I: history, etiology, and pathophysiology. Circ Heart Fail. 2009;2(1):63–70. doi: 10.1161/CIRCHEARTFAILURE.108.820217. [DOI] [PubMed] [Google Scholar]
- 2.Kassebaum N, Kyu HH, Zoeckler L, Olsen HE, Katie Thomas K, Pinho C, et al. Child and Adolescent Health From 1990 to 2015 Findings From the Global Burden of Diseases, Injuries, and Risk Factors 2015 Study The Global Burden of Disease Child and Adolescent Health Collaboration. JAMA Pediatr. 2017;171(6):573–592. doi: 10.1001/jamapediatrics.2017.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and, author
- 4.national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global, author. [DOI] [PMC free article] [PubMed]
- 5.Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.JIeH, author. The global burden of congenital heart disease. Cardiovasc J Afr. 2013;24(4):141–145. doi: 10.5830/CVJA-2013-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewitson J, Zill P. Children's heart disease in sub-Saharan Africa: Challenging the burden of disease. SA Heart Journal. 2010;7:18–29. [Google Scholar]
- 8.World health organization, author. The selection and use of essential medicines: report of the WHO Expert Committee, March 2009 (including the 16th WHO model list of essential medicines and the 2nd WHO model list of essential medicines for children) Switzerland: 2009. WHO technical report series. [Google Scholar]
- 9.Andrews RE, Fenton MJ, Ridout DA, Burch M. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United kingdom and Ireland. Circulation. 2008;117(1):79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348(17):1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 11.Ramakrishnan S. Pediatric Heart Failure in the Developing World. Rev Recent Clin Trials. 2014;9(2):86–90. doi: 10.2174/1574887109666140908125841. [DOI] [PubMed] [Google Scholar]
- 12.Ogeng'o J, Gatonga PM, Olabu BO, Nyamweya DK, Ong'era D. Pattern of congestive heartfailure in a kenyan paediatric population. Cardiovasc J Afr. 2013;24(4):117–120. doi: 10.5830/CVJA-2013-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster G, Zhang J, Rosenthal D. Comparison of the epidemiology and co-morbidities of heart failure in the pediatric and adult populations: a retrospective, cross-sectional study. BMC Cardiovasc Disord. 2006;6:23. doi: 10.1186/1471-2261-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayaprasad N. Heart Failure in Children. Heart Views. 2016;17(3):92–99. doi: 10.4103/1995-705X.192556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cradiol. 1992;13(2):72–75. doi: 10.1007/BF00798207. [DOI] [PubMed] [Google Scholar]
- 16.Kay JD, Colan SD, Graham TP., Jr Congestive heart failure in pediatric patients. Am Heart J. 2001;142(5):923–928. doi: 10.1067/mhj.2001.119423. [DOI] [PubMed] [Google Scholar]
- 17.Duru CO, Mesiobi-Anene N, Akinbami FO. Pediatric heart failure among emergency room admissions in a Tertiary Health Centre in Southern Nigeria. Nig J Cardiol. 2016;13:62–66. [Google Scholar]
- 18.Gebremariam S, Moges T. Pediatric Heart Failure, Lagging, and Sagging of Care in Low Income Settings: A Hospital Based Review of Cases in Ethiopia. Cardiol Res Pract. 2016;2016:7147234. doi: 10.1155/2016/7147234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagunju IA, Omokhodion SI. Childhood heart failure in Ibadan. West Afr J Med. 2003;22(1):42–45. doi: 10.4314/wajm.v22i1.27978. [DOI] [PubMed] [Google Scholar]
- 20.Dolgin Martin, New York Association . Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston: Little, Brown; 1994. [Google Scholar]
- 21.Steinberg Daniel H, Staubach Stephan, Franke Jennifer, Sievert Horst. Defining structural heart disease in the adult patient: current scope, inherent challenges and future directions. European Heart Journal Supplements. 2010:12. [Google Scholar]
- 22.Hu D, Liu Y, Tao H, Gao J. Clinical value of plasma B-type natriuretic peptide assay in pediatric pneumonia accompanied by heart failure. Exp Ther Med. 2015;10(6):2175–2179. doi: 10.3892/etm.2015.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World health organization, United Nations Children's Fund, author. WHO child growth standards and the identification of severe acute malnutrition in infants and children. switzerland: A Joint Statement by the World Health Organization and the United Nations Children's Fund; 2009. [PubMed] [Google Scholar]
- 24.World Health Organization, author. Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World. [Google Scholar]
- 25.Health Organization, 2011 (WHO/NMH/NHD/MNM/11.1) ( http://www.who.int/vmnis/indicators/haemoglobin.pdf, accessed on July 31, 2018), author
- 26.Reményi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease--an evidence-based guideline. Nat Rev Cardiol. 2012;9(5):297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai WW, Mertens LL, Cohen MS, Geva T. Echocardiography Pediatric and congenital heart disease: from fetus to adult. 2nd ed. Wiley-Blackwell; 2016. [Google Scholar]
- 28.Massin MM, Astadicko I, Dessy H. Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol. 2008;31(8):388–391. doi: 10.1002/clc.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dejuma Y, Abraha H, Abraham H, et al. Prevalence of rheumatic heart disease among school children in Ethiopia: A multisite echocardiography -based screening. Int J Cardiol. 2016;221:260–263. doi: 10.1016/j.ijcard.2016.06.232. [DOI] [PubMed] [Google Scholar]
- 30.Vanderlaan RD, Caldarone CA, Backx PH. Heart failure in congenital heart disease: the role of genes and hemodynamics. Pflugers Arch. 2014;466(6):1025–1035. doi: 10.1007/s00424-014-1447-9. [DOI] [PubMed] [Google Scholar]
- 31.Knudson JD, Cabrera AG. The Pathophysiology of Heart Failure in Children: The Basics. Curr Cardiol Rev. 2016;12(2):99–103. doi: 10.2174/1573403X12666151119164525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadoh WE, Osarogiagbon WO. Underlying congenital heart disease in Nigerian children with pneumonia. Afr Health Sci. 2013;13(3):607–612. doi: 10.4314/ahs.v13i3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andres S, Bauer G, Rodríguez S, Novali L, Micheli D, Fariña D. Hospitalization due to respiratory syncytial virus infection in patients under 2 years of age with hemodynamically significant congenital heart disease. J Pediatr (Rio J) 2012;88(3):246–252. doi: 10.2223/jped.2202. [DOI] [PubMed] [Google Scholar]
- 34.Owayed AF, Campbell DM, Wang EE. Underlying causes of recurrent Pneumonia in children. Arch Pediatr Adolesc Med. 2000;154(2):190–194. doi: 10.1001/archpedi.154.2.190. [DOI] [PubMed] [Google Scholar]
- 35.Moges T, Gedlu E, Isaakidis P, Kumar A, et al. Infective endocarditis in Ethiopian children: a hospital based review of cases in Addis Ababa. Pan Afr Med J. 2015;20:75. doi: 10.11604/pamj.2015.20.75.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyungu E, Nabakwe E, Esamai F. Factors that precipitate heart failure among children with rheumatic heart disease. East Afr Med J. 2011;88(11):384–387. [Google Scholar]
- 37.Omokhodion SI, Lagunju IA. Prognostic indices in childhood heart failure. West Afr J Med. 2005;24(4):325–328. doi: 10.4314/wajm.v24i4.28226. [DOI] [PubMed] [Google Scholar]
- 38.The Federal Democratic Republic of Ethiopia Ministry of Health, author. HSTP: Health Sector Transformation Plan: 2015/16 – 2019/20 (2008–2012 EFY) Addis Ababa: Federal Democratic Republic of Ethiopia Ministry of Health; 2015. [Google Scholar]
- 39.Abrahim O, Linnander E, Mohammed H, Fetene N, Bradley E. A Patient-Centered Understanding of the Referral System in Ethiopian Primary Health Care Units. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0139024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fetene N, Linnander E, Fekadu B, Alemu H, Omer H, Canavan M, et al. The Ethiopian Health Extension Program and Variation in Health Systems Performance: What Matters? PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0156438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misganaw A, Haregu TN, Deribe K, Tessema GA, Deribew A, Melaku YA, et al. National mortality burden due to communicable, non-communicable, and other diseases in Ethiopia, 1990–2015: findings from the Global Burden of Disease Study 2015. Population Health Metrics. 2017;15:29. doi: 10.1186/s12963-017-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zühlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart. 2013;99(21):1554–1561. doi: 10.1136/heartjnl-2013-303896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christianson A, Howson CP, Modell B. March of Dimes. Global report on birth defects. The hidden toll of dying and disabled children. New York: March of dimes birth defects foundation; 2006. [Google Scholar]
- 44.Shah R, Agarwal AK. Anemia associated with chronic heart failure: current concepts. Clin Interv Aging. 2013;8:111–122. doi: 10.2147/CIA.S27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melku M, Takele WW, Anlay DZ, Ekubagewargies DT, Getaneh Z, Abebe M, Abebe Z. Male and undernourished children were at high risk of anemia in Ethiopia: a systematic review and meta-analysis. Ital J Pediatr. 2018;44(1):79. doi: 10.1186/s13052-018-0513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]