Summary
Objective:
Cushing disease (CD) is a rare entity caused by ACTH-secreting pituitary tumours, leading to prolonged hypercortisolism. Most cases are sporadic but can rarely occur in the context of familial predisposition, due to germline mutations in genes such as MEN1, leading to multiple endocrine neoplasia type 1, MEN1. We have reported previously that CD can be the first and only presenting manifestation of MEN1. In this report, we describe a cohort of paediatric patients who presented with CD as the first manifestation of MEN1.
Materials and Methods:
A retrospective analysis of paediatric patients admitted to the National Institutes of Health (NIH) Clinical Center for evaluation of hypercortisolism, between 1997 and 2017. MEN1 was diagnosed on a clinical, familial and/or genetic basis.
Results:
Of a total of 238 children with CD, six patients were subsequently diagnosed with MEN1, three males and three females with a mean age at diagnosis of CD at 13.4 ± 2.9 years. Five of the six patients had familial MEN1 and one patient was a sporadic case. Additional manifestations of MEN1 included primary hyperparathyroidism in three patients and hyperprolactinemia in two patients.
Discussion:
This report describes a paediatric patient population with MEN1 in whom CD was the initial manifestation, confirming a previous observation that paediatric patients with MEN1 may present first with an ACTH-producing adenoma. Therefore, germline MEN1 mutations should be sought in paediatric CD and tested for when there is a suggestive family history and/or other manifestations.
Keywords: Cushing disease, MEN1, MEN1 mutations, paediatric patients
1 |. INTRODUCTION
Cushing disease (CD) is a rare disease in paediatrics. It is caused by ACTH overproduction from the pituitary, most often due to an ACTH-secreting pituitary microadenoma. The prolonged hypercortisolism results in the typical Cushing’s syndrome (CS) signs and symptoms: truncal obesity, growth deceleration, skin changes, muscle weakness and hypertension.1,2 Most cases of CD occur sporadically but can rarely occur in the familial setting, most commonly in the context of multiple endocrine neoplasia type 1 (MEN1).1
MEN1 is a familial tumour syndrome that is inherited in an autosomal dominant manner caused by a germline mutation in the MEN1 gene. It includes varying combinations of endocrine and nonendocrine neoplasms. MEN1 is diagnosed on a clinical basis, when a patient is found to have two of the three main MEN1-related endocrine tumours (parathyroid adenomas, entero-pancreatic endocrine tumours, and pituitary tumour); on a familial basis, when a patient has one MEN1-associated tumour and an affected first-degree relative; and finally on a genetic basis, when an individual has an MEN1 pathogenic mutation.3,4 Other neoplasms associated with MEN1 include neuroendocrine and adrenocortical tumours, facial angiofibromas, collagenomas, lipomas, meningiomas, ependymomas and leiomyomas.3,5
The prevalence of pituitary tumours in MEN1 varies widely from 10% to 60%, with pituitary tumours presenting as the first clinical manifestation of MEN1 in 25% of sporadic and 10% of familial cases.6,7 ACTH-secreting tumours are generally rare in MEN1, representing only approximately 2% of cases.4 The literature on patients with MEN1 over the age of 18 years includes a limited number of cohorts with CS.8-10 In this report, we describe a cohort of six paediatric patients who presented with CD as their first manifestation of MEN1. Three patients from our cohort had been previously included in papers published from our institution by Simmonds et al8 and Stratakis et al.11 The present report aims to expand the cohort of MEN1 patients presenting with CD to include more recently discovered cases as well as patients found to have MEN1 through novel methods of testing, including whole exome sequencing.
2 |. MATERIALS AND METHODS
2.1 |. Clinical studies on CS and CD
We performed a retrospective chart review of paediatric patients (<21 years at diagnosis) admitted to our institution for evaluation of hypercortisolism between 1997 and 2017 and recruited under the research protocol 97-CH-0076 of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study was approved by the Investigational Review Board of NICHD and all patients and/or their parents gave written assent/consent. The NIH Clinical Research Information System was used to obtain medical records and clinical data.
All patients admitted to NICHD for evaluation of possible CS underwent laboratory and imaging testing to provide accurate diagnosis and classification of the disease, as per published diagnostic guidelines. This testing included including serum midnight and morning cortisol and ACTH levels, 24-hr urinary free cortisol (UFC) and 17-hydroxycorticosteroid (17OHCS) collections, high dose dexamethasone suppression test, ovine CRH stimulation test, inferior petrosal sinus sampling and pituitary magnetic resonance imaging (MRI) using thin sections and high resolution with contrast (gadolinium).1,12
2.2. Diagnostic evaluation for MEN1
Biochemical testing for MEN1 was performed only when there was suggestive family history and/or other manifestations on presentation and included measurement of plasma levels of parathyroid hormone, calcium, prolactin and gastrointestinal tract hormone profile (gastrin, glucagon, vasointestinal polypeptide, pancreatic polypeptide, chromogranin A and insulin with an associated fasting glucose level). Additional imaging was performed as indicated in individual cases and included thyroid sonography (US) and sestamibi parathyroid scan, renal US and DEXA scans to evaluate for hyperparathyroid-associated complications, including renal calculi and osteoporosis. A dermatology consult was obtained to evaluate for skin manifestations of MEN1 (angiofibromas, lipomas, collagenomas, hypomelanotic macules). Genetic testing of the MEN1 gene was performed when DNA was available.
3 |. RESULTS
3.1 |. Patients
Of a total of 238 patients with CD evaluated at our institution since 1997, we identified 6 patients with an additional diagnosis of MEN1 at the time of their discharge after the initial evaluation of hypercortisolism. Our cohort included three males and three females with a mean age at diagnosis of 13.4 ± 2.9 years old (Table 1). The most common presenting symptoms included weight gain and poor growth, similar to that of all our other patients with CD occurring sporadically (Table 1). Follow-up evaluation was carried out at 6 months and 1 year after the surgery. One patient was lost to follow-up (patient 3). For two cases (patients 4 and 5), longer follow-up evaluations were available at 6-7 years after their initial inpatient admission.
TABLE 1.
Biometrics and presenting signs and symptoms for MEN-1 patients with Cushing’s disease
| Patient ID | Age (Gender) |
Race | Height (cm) (Z-score) |
Weight (kg) (Z-score) |
BMI (kg/m2) (Z-score) |
SBP (mm Hg) (Z-score) |
DBP (mm Hg) (Z-score) |
Presenting signs and symptoms |
|---|---|---|---|---|---|---|---|---|
| 1 | 11.0 (M) | Black/AA | 122.1 (−3.20) | 49.6 (1.43) | 33.3 (2.49)a | 102 (0.56) | 49 (−0.79) | Wt gain, ht arrest, irritability |
| 2 | 15.1 (M) | White | 171.3 (0.10) | 92.3 (2.28)a | 31.5 (2.17)b | 131 (1.53)b | 71 (0.54) | Wt gain, headaches, bone aches, stretch marks |
| 3 | 9.2 (M) | White | 135.3 (0.11) | 65.5 (2.94)a | 35.8 (2.71)a | 129 (2.60)a | 89 (2.45)a | Wt gain, ht arrest, headaches, facial plethora |
| 4 | 17.2 (F) | White | 174 (1.80)a | 105.5 (2.47)a | 34.8 (2.17)a | 133 (1.74)b | 61 (−0.66) | Wt gain, facial plethora, facial hirsutism, menstrual irregularities |
| 5 | 11.4 (F) | White | 151.5 (0.83) | 75.5 (2.64)a | 32.9 (2.46)a | 114 (0.65) | 59 (−0.34) | Wt gain, headaches, |
| 6 | 16.7 (F) | White | 151.9 (−1.69) | 107.7 (2.39)a | 46.7 (2.56)a | 120 (1.10) | 88 (2.11)b | Wt gain, ht arrest, headaches, muscle weakness, hirsutism at neck and chest |
AA, African American; Ht, height; Wt, weight.
>99th percentile.
>95th percentile.
All the six patients had elevated UFC in at least three urine samples prior to their evaluation at the NIH. Four of six patients (patients 1-3,6) had documented elevated midnight serum cortisol levels (>4.4 μg/dL) and a positive response to HDDST (Table 2). The two patients to whom diurnal serum cortisol measurements were not indicative of CS (patient 4 and 5, Table 2) had either positive diagnostic tests (patient 4) or exhibited spontaneous resolution of CS stigmata after pituitary apoplexy (patient 5).
TABLE 2.
Biochemical, imaging and pathology evaluation of Cushing’s disease in patients with MEN-1
| Patient ID |
Diurnal cortisol (μg/dL) pm/am |
UFCa | ACTH (am) |
HDD | CRH stim test |
Pit MRI | IPSS | CT adrenals |
Surgical intervention and operative findings |
Pathology | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.5/7.1 | 143.3 (ref range < 91) | 23.9 | Pos | N/A | 1 cm pit adenoma | Pos | Normal | TSS-two adenomas at L anterior pit and R posterior pit | L adenoma (0.5 × 0.3 × 0.2 cm) pos for prl and R adenoma (0.2 × 0.2 × 0.1 cm) pos for ACTH | In remission | 3 y |
| 2 | 11.1/10.6 | 124.0 (ref range 4-56) | 40.4 | Pos | Pos | Normal | Pos | Small L adrenal nodule | TSS-two adenomas at L superior pit and midline inferior pit | L superior adenoma (0.2 × 0.3 × 0.3 cm) pos for prl, neg for ACTH. Midline inferior adenoma (0.2 × 0.2 × 0.2 cm) pos for prl, neg for ACTH. | In remission | 1 y |
| 3 | 17/20.25 | 110.0 (ref range 2.6-37) | 49.1 | Pos | Pos | Normal | Pos | Normal | TSS-one adenoma at R lateral pit | R adenoma (0.4 × 0.4 × 0.2 cm) pos for ACTH | In remission | N/A |
| 4 | 3.0/13.9 | 10.2 (ref range < 91) | 43.2 | N/A | Posb | Normal | Pos | Normal | TSS ×2 within 2 weeks due to persistent hypercortisolism | No adenoma identified | In remission-panhypopit | 6 y |
| 5 | 2.3/15 | 39.2 (ref range 8-77) | 78.6 | N/A | Negb | 5 mm pit adenoma | Not indicated | Normal | None | None | Spontaneous resolution of symptoms s/p likely pituitary apoplexy | 7 y |
| 6 | 20/19.1 | 151.8 (ref range 4-56) | 23.4 | Pos | Pos | 8 mm pit adenoma | Not indicated | L adrenal adenoma | TSS-R sided pit adenoma | R adenoma (0.9 × 0.6 × 0.6 cm) pos for ACTH | In remission-panhypopit | 8 m0 |
HDD, high dose dexamethasone suppression test-positive response is indicative of Cushing’s disease; Positive CRH stimulation test is indicative of Cushing’s disease; IPSS, inferior petrosal sinus sampling- positive response is indicative of Cushing’s disease; Pos, positive; TSS, Transsphenoidal sinus surgery; UFC, 24 h urinary free cortisol μg/24 h ref (urine cortisol assays used at our institution have changed over the years thus reference ranges are different based on the assay that was used at the time the patient was evaluated and provided in the table.
Average of 3 different 24 h-urine samples obtained during the same hospitalisation as the diurnal serum cortisol and ACTH levels.
Dex-CRH stimulation test was performed.
Patient 4 was a challenging case to diagnose as she turned out to have cyclical CD. She presented to our institution for evaluation of excessive weight gain, development of thick abdominal striae and irregular menses at the age of 13 years. Prior evaluation was equivocal for CD, including only one abnormal 24 hours-urinary free cortisol collection. During her initial hospitalisation at our institution, midnight cortisol level was 4.3 μg/dL (normal < 4.4) with normal diurnal variation. Additional UFC samples obtained during her hospitalisation at the NIH were all within the normal range. After discharge, she was asked to collect several 24 hours-urine samples over the next 2 months and bring these to our laboratory for cortisol analysis. Thirteen samples were evaluated in our laboratory and all but 3, were above the upper normal limit of 91 μg/24 h (ranging from 110 to 153.8). Her clinical symptoms were worsening and she underwent IPSS, which showed a baseline central-to-peripheral ACTH ratio at 6.57 increased to 33.44 at 10 minutes post-CRH administration. TSS was eventually performed but she stayed hypercortisolemic; she was eventually cured from her CD after a second TSS and remains eucortisolemic to date.
Patient 5 was initially found to have a 2 cm pituitary tumour in a brain MRI performed as part of an extensive work-up for persistent chronic headaches, visual disturbances, marked weight gain and development of violaceous abdominal striae. Serial imaging of the pituitary over the next 2 years showed collapse and near complete resolution of the cystic structure along with marked decrease in the pituitary lesion. Correlation with clinical information led us to the assumption that she likely underwent pituitary apoplexy; at that time, she suffered an episode of an intensely severe headache, described as the worst headache of her life and shortly after, her headaches and prior visual complains had all resolved and her weight gain stabilised. Pituitary MRI imaging was performed after this episode and revealed essentially complete involution of the sellar cystic mass with normal-sized pituitary gland.
Pituitary MRI was performed in all the six patients; only three of them showed evidence of pituitary adenoma. IPSS was performed when pituitary MRI failed to identify an adenoma or if it was otherwise clinically indicated. Adrenal CT showed an incidental adrenal adenoma and/or nodule in two patients (patient 2 and 6); however, patient 2 had a positive IPSS result indicating CD and patient 6 had pituitary MRI and biochemical testing highly suggestive of CD; transsphenoidal surgery (TSS) was performed in both these cases. In total, five of six patients underwent TSS; four of these patients had one or two pituitary microadenomas identified in surgery and confirmed by pathology (patients 1, 2, 3, 6). Patient 4, in whom no adenoma was identified, underwent TSS twice within a 2-weeks period due to persistent hypercortisolism. Patient 5 never had a surgical intervention as her CS signs and symptoms spontaneously resolved after what appeared to be a pituitary apoplexy.
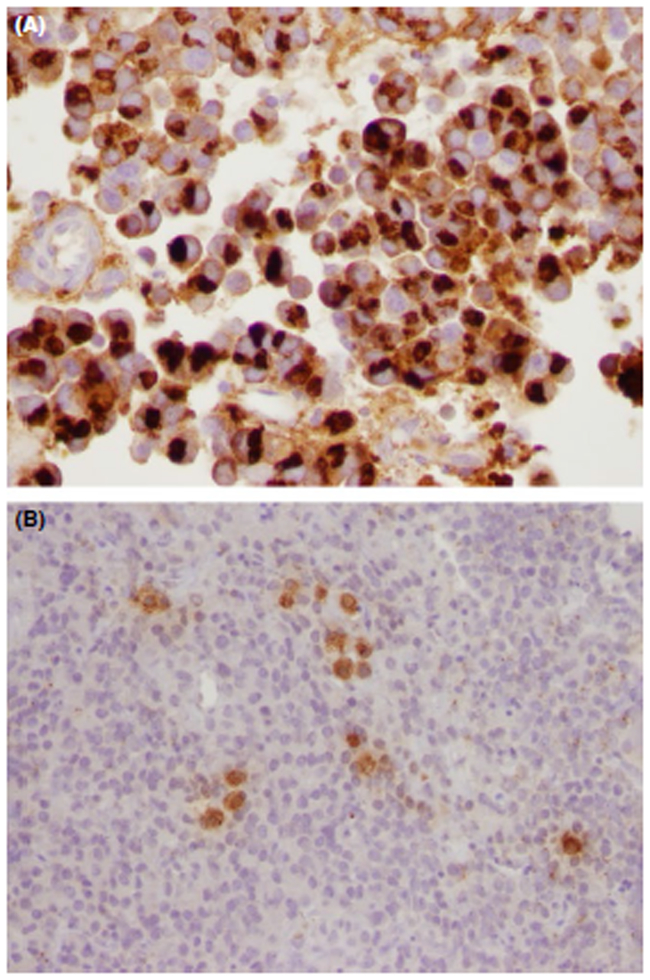
Immunohistochemical staining was performed for all the surgically excised specimens. Two patients (patients 1 and 6) had adenomas that stained positive both for ACTH and prolactin (Figure 1), indicative of the variability of pituitary tumours in MEN1. Two patients had more than one pituitary adenomas identified (patient 1 and 2); patient 1 had an ACTH-immunostaining positive adenoma and a second distinct prolactin-positive adenoma and patient 2 had two distinct prolactin-staining adenomas. All the pituitary adenomas identified in our patient cohort were microadenomas. A total of four patients had pituitary adenomas identified (patients 1, 2, 3 and 6). Patient 2 was the only case that the pituitary adenomas stained negative for ACTH; however, CD was biochemically confirmed pre-op followed by clinical and biochemical resolution post-TSS.
FIGURE 1.
Pathology Specimen from Patient 6, Staining Positive for Both Prolactin (A) and ACTH (B) [Colour figure can be viewed at wileyonlinelibrary.com]
3.2 |. Evaluation for MEN1
Five of six patients included in our cohort had a strong family history of MEN1 or MEN1-related tumours in close relatives that were identified either at the time of the first interview or after genetic testing became available. Three patients (patients 2,5,6) had elevated calcium and PTH levels on presentation. Thyroid US and/or sestamibi parathyroid scan were performed to evaluate the parathyroid glands. Parathyroid hyperplasia was identified in three patients (patients 2, 5, 6) who underwent 2.5, 3.5 and 1 gland parathyroidectomies, respectively. Serum calcium levels normalised in all patients with the exception of patient 5 who eventually developed recurrent asymptomatic hypercalcemia and therefore no additional intervention is planned. Screening prolactin and gastrin levels were obtained to evaluate for common MEN1 related tumours, including prolactinomas and pancreatic neuroendocrine tumours. Prolactin was elevated at >80 μg/L in two patients (patients 1,6), who also had a pituitary microadenoma, that was stained positively to prolactin on pathology. Patient 6 reported menstrual irregularities, but no galactorrhea was present in either patient. Both patients were successfully treated with cabergoline to address their hyperprolactinemia status and repeat prolactin levels were within the normal range. Of note, patient 2 had normal serum prolactin levels, despite having two distinct small prolactin-staining tumour on immunohistochemistry measuring 3 mm at the biggest diameter. Serum prolactin levels were confirmed with serial measurements at 20 minutes intervals ranging from 10.5 to 13.7 μg/mL. While it is established that even minimal prolactin elevations can be associated with prolactinomas, the only explanation we have for this discrepancy is the relatively small size of the tumour. Screening gastrin levels were obtained for five patients and were within the normal range for all of them.
A dermatology evaluation showed that one patient (patient 1) had nasal angiofibromas consistent with MEN1, whereas another (patient 5) had possible face angiofibromas in the setting of acne vulgaris, that were unable to differentiate from acneiform lesions. Additional screening imaging for MEN1 related disease, had revealed a stable asymptomatic pancreatic mass in patient 5, which is followed regularly by abdominal scans.
3.3 |. Genetic and DNA studies
Germline MEN1 mutational testing results were available for all the patients included in our cohort (Table 3). Five of six patients had familial MEN1 that was identified either at the time of the first interview or after genetic testing became available. One patient was a de novo MEN1 case (patient 6). The MEN1 gene was sequenced; if there were no mutations, deletion testing was performed. MEN1 mutational testing was only performed in patients when there was suggestive family history and/or other manifestations on presentation (eg hypercalcemia or hyperprolactinemia found on routine testing). The remaining patients from our cohort of 238 patients did not have MEN1 DNA testing thus our cohort may include a few more undiagnosed cases of MEN1 that were not sequenced for.
TABLE 3.
Evaluation of MEN-1 on presentation
| Pt ID | Family history | iCal | PTH | Prl | Gastrin | MEN1 mutation (NM_130799.2) |
Skin | Surgical intervention for MEN1-related disease |
|---|---|---|---|---|---|---|---|---|
| 1 | +MEN1 in father, PGF, and P-uncles | 1.33 | 33.4 | 188.7 | 26 | Deletion exons1-2 | Nose angiofibromas | None |
| 2 | +MEN1 in PGF and P-cousin | 1.52 | 75.7 | 13.7 | 15 | c.1243C>T, p.R415a | Neg | 2.5 gland parathyroidectomy |
| 3 | +MEN1 in PGGM. Mother and multiple maternal relatives with parathyroidectomy and nephrocalcinosis | 1.26 | 31.3 | 7.6 | 31 | c.1192C>T, p.Q398a | n/a | None |
| 4 | M-aunt with prolactinoma | 2.26a | n/a | n/a | n/a | c.55G>C, p.V19L | n/a | None |
| 5 | +MEN1 at father, 4 P-uncles and 2 P-aunts | 1.54 | 150.2 | 12 | 12 | c.307delC frameshift, p.L103Cfs*16 | Questionable face angiofibromas | 3.5 glands parathyroidectomy |
| 6 | None | 3.2a | 139.5 | 262 | 70 | c.251-252delCT, S84Yfs*32 | Neg | Parathyroid adenoma excision |
iCal, ionised calcium normal range 1.17-1.31 mmol/L; M-uncle, maternal uncle; P-cousin, paternal cousin; PGF, paternal grandfather; PGGM, paternal great grand-mother; Pt ID, patient identification; PTH, parathyroid hormone normal range 6-40 pg/mL; P-uncles, paternal uncles; Prolactin normal range 1.0-25.0 μg/L; Gastrin normal range 0-99 pg/mL; Skin findings after dermatology evaluation, negative for angiofibromas, lipomas or collagenomas.
Total calcium available: normal range 2.05-2.5 mmol/L.
3.4 |. Clinical outcome and follow-up
All the patients included in our cohort were cured with resolution of their CS-associated symptoms, including one patient with spontaneous resolution of symptoms s/p likely pituitary apoplexy. Serum cortisol levels were normalised in all patients, both in the immediate post-op period as well as their follow-up visit (apart from patient 3 who never returned for follow-up). Biochemical remission was defined as midnight cortisol <4.4 μg/dL or urinary free cortisol levels within the normal range at the first follow-up visit at 6-12 months post-TSS.
Two patients developed post-op panhypopituitarism (patients 4 and 6). The remaining patients for whom follow-up data were available (patients 1, 2 and 5) had documented recovery of their HPA axis (hypothalamus-pituitary-adrenal axis) as evident by peak cortisol levels >18 μg/dL during an ACTH stimulation test at 12-18 months post-TSS or after what appeared to be pituitary apoplexy for patient 5 Patient 4 underwent partial hypophysectomy as a distinct pituitary adenoma was not identified intra-operatively or in pathology analysis and eventually achieved clinical and biochemical resolution of her hypercortisolemia. However, she went on to develop central diabetes insipidus, GH deficiency, hypogonadotropic hypogonadism, central hypothyroidism and central adrenal insufficiency, for which she is now on hormone replacements as clinically indicated. Patient 6 was only recently evaluated at our institution and at her 8-month follow-up visit after surgery, the midnight cortisol levels were <1 μg/dL and her hyperprolactinemia had also resolved. However, she was found to have low plasma IGF-1 levels, low free thyroxine with inappropriately normal TSH, and low gonadotropins, suggesting surgical panhypopituitarism. She remains on hydrocortisone replacement therapy as her stimulated cortisol level at 60 minutes post-ACTH was <18 μg/dL.
All the three patients who underwent parathyroidectomy (patients 2, 5 and 6), had initial resolution of their hypercalcemia; patient 5 had recurrent hypercalcemia at 3 years follow-up but remained as-ymptomatic without end-organ damage. Patient 1 had normalisation of both cortisol and prolactin levels at 3 years follow-up.
4 |. DISCUSSION
This report describes a cohort of paediatric patients with MEN1 in whom CD was the initial clue exhibiting the presence of the syndrome. MEN1 is an autosomal dominant familial cancer syndrome with high penetrance. Manifestations can initiate as early as 5 years of age and it is estimated that 95% of the patients will develop tumours by the fifth decade of life.3 The most common tumours found in MEN1 patients are tumours of the parathyroid glands (85%). For patients who do not present with primary hyperparathyroidism, the most common initial manifestation is a gastrinoma or prolactinoma.7
Among the anterior pituitary tumours that have been reported in MEN1 patients, most cases involve a microadenoma (defined as diameter < 1 cm).4 Almost every type of anterior pituitary adenoma has been reported in the MEN1 literature4,13: prolactinoma is the most common type representing 20% of cases, followed by GH/PRL co-secreting adenomas, GH-secreting adenomas and nonfunctioning adenomas, each representing 5% of cases. ACTH-secreting pituitary adenomas, that lead to CD, account only for 2% of the cases.4 Endocrine tumours that occur in the context of MEN1 are thought to be more aggressive and resistant to treatment as compared with the respective tumours occurring in non-MEN1 patients, likely due to the tumour multiplicity found in MEN1 patients.3,8
In our cohort, manifestations of MEN1 included primary hyperparathyroidism in three patients and hyperprolactinemia in two patients. All patients in our cohort presented with the typical stigmata of CS, including excessive weight gain, arrest in linear growth, facial plethora, proximal muscle weakness, and in females menstrual irregularities and hirsutism. All the three patients with hyperparathyroidism were incidentally found to have elevated calcium levels during work-up for CS. Two of these patients had a positive family history of MEN1 that was previously not recognised. Hypercalcemia resolved in all the three patients after parathyroid surgery. Both patients with hyperprolactinemia were started on cabergoline therapy prior to surgical excision of the pituitary tumour and both exhibited resolution of hyperprolactinemia after surgery. Two patients in our cohort have not yet exhibited any MEN1-related manifestations (patients 3, 4); however, a genetic diagnosis was made after they presented with CS and family history suggestive of MEN1.
With regards to the aetiology of CS in paediatric patients with MEN1, all the six patients included in the current paper, had CD. Our group had previously published one patient with ectopic CS, who was found to have a germline de novo MEN1 mutation as part of genetic MEN1 screening (patient 4 in the article published by Karageorgiadis et al.14 Simonds et al8 recently described 19 adult MEN1 patients with CS; 79% had CD and 21% had ACTH-independent CS.
In our case series, none of the patients was previously diagnosed with MEN1 despite the fact that in most cases there was a strong family history suggestive of MEN1-related tumours. While published guidelines recommend screening first degree relatives of MEN1 patients, this was not the case in the patients included in this cohort. What is unique in these patients is that CD was the initial manifestation of MEN1. As Stratakis et al11 previously suggested the present study confirms that in a small number of paediatric patients with CD, an ACTH-producing adenoma may be the first manifestation of MEN1. Although a rare finding in patients with CS, the diagnosis of an underlying familial cancer syndrome is of paramount importance for the prognosis and long-term surveillance of these patients, and it behoves clinicians that diagnose children with CD to inquire carefully on family history suggestive of MEN1 and if suspected, MEN1 gene and deletion testing should be pursued.
Acknowledgments
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interests in this work.
REFERENCES
- 1.Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am. 2012;41(4):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holst JM, Horváth-Puhó E, Jensen RB, et al. Cushing’s syndrome in children and adolescents: a Danish nationwide population-based cohort study. Eur J Endocrinol. 2017;176(5):567–574. [DOI] [PubMed] [Google Scholar]
- 3.Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990–3011. [DOI] [PubMed] [Google Scholar]
- 4.Brandi ML. CONSENSUS: guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. [DOI] [PubMed] [Google Scholar]
- 5.Giusti F, Cianferotti L, Boaretto F, et al. Multiple endocrine neoplasia syndrome type 1: institution, management, and data analysis of a nationwide multicenter patient database. Endocrine. 2017;58:349–359. [DOI] [PubMed] [Google Scholar]
- 6.Syro LV, Scheithauer BW, Kovacs K, et al. Pituitary tumors in patients with MEN1 syndrome. Clinics. 2012;67(S1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falchetti A, Marini F, Luzi E, et al. Multiple endocrine neoplasia type 1 (MEN1): not only inherited endocrine tumors. Genet Med. 2009;11(12):825–835. [DOI] [PubMed] [Google Scholar]
- 8.Simonds WF, Varghese S, Marx SJ, Nieman LK. Cushing’s syndrome in multiple endocrine neoplasia type 1. Clin Endocrinol. 2012;76(3):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skogseid B, Larsson C, Lindgren PG, et al. Clinical and genetic features of adrenocortical lesions in multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 1992;75(1):76–81. [DOI] [PubMed] [Google Scholar]
- 10.Waldmann J, Bartsch DK, Kann PH, Fendrich V, Rothmund M, Langer P. Adrenal involvement in multiple endocrine neoplasia type 1: results of 7 years prospective screening. Langenbecks Arch Surg. 2007;392(4):437–43. [DOI] [PubMed] [Google Scholar]
- 11.Stratakis CA, Tichomirowa MA, Boikos S, et al. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78(5):457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120(3):e575–86. [DOI] [PubMed] [Google Scholar]
- 13.Corbetta S, Pizzocaro A, Peracchi M, Beck-Peccoz P, Faglia G, Spada A. Multiple endocrine neoplasia type 1 in patients with recognized pituitary tumours of different types. Clin Endocrinol (Oxf). 1997;47(5):507–12. [DOI] [PubMed] [Google Scholar]
- 14.Karageorgiadis AS, Papadakis GZ, Biro J, et al. Ectopic adrenocorticotropic hormone and corticotropin-releasing hormone co-secreting tumors in children and adolescents causing cushing syndrome: a diagnostic dilemma and how to solve it. J Clin Endocrinol Metab. 2015;100(1):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]