Abstract
Impulsivity is an important personality trait associated with several clinical syndromes including drug abuse. While repeated drug exposure is known to increase certain behavioral responses, such as locomotion, to subsequent drug exposure, few studies have examined whether such sensitization develops for impulsive behavior. In the current study we tested the effects of methamphetamine acutely, during the course of, and upon discontinuation of chronic methamphetamine treatment on impulsive behavior in two models, the 5-choice serial reaction time task (5-CSRTT) and the delay-discounting task which measure impulsive action and impulsive choice, respectively. We also examined whether the trace amine-associated receptor 1 (TAAR1) agonist RO5263397 attenuated methamphetamine-induced effects in parallel tests. Acute methamphetamine dose-dependently increased premature responses in the 5-CSRTT and shifted the delay function upward in delay discounting. Up to 40 days of methamphetamine treatment did not significantly alter the dose-effect curve of methamphetamine-induced premature responses, but produced a significant effect in the delay-discounting task. RO5263397 attenuated acute methamphetamine-induced premature responses, but this effect became non-significant over the course of chronic treatment. RO5263397 did not significantly alter the delay-discounting performance. Discontinuation of methamphetamine treatment increased premature responses, which was attenuated by RO5263397, but did not significantly alter the delay discounting function. These results suggest that acute discontinuation from prolonged methamphetamine treatment increases impulsivity, which can be reduced by a TAAR1 agonist.
Keywords: Methamphetamine, TAAR1, impulsivity, 5-CSRTT, delay discounting, rat
1. Introduction
Behavioral control and decision-making are important personality characteristics closely related to impulsivity, a complex trait in its own right determined by several neural circuits and neurochemical processes (Evenden, 1999; Robbins, 2002). Impulsivity is a multifactorial construct typically segregated into impulsive action – related to the ability to withhold a response until the appropriate time – and impulsive choice – associated with the control of reward-based responding – and is associated with clinical syndromes including addiction and gambling (see Dalley and Robbins, 2017 for review). In the case of substance use disorders, it is uncertain to what degree impulsivity is a predisposing trait to drug abuse as opposed to how much it is a consequence of chronic drug exposure. For instance, both stimulant abusers and their non-user siblings displayed comparable impairments in clinical tests of impulsivity, but only the stimulant abusers exhibited hypoactivity in the ventrolateral prefrontal cortex and anterior cingulate cortex during the task relative to healthy controls (Morein-Zamir et al., 2013). Moreover, a high-impulsive phenotype in rats predicts high rates of cocaine self-administration and reinstatement to cocaine-seeking after abstinence (Dalley et al., 2007; Economidou et al., 2009), while withdrawal from cocaine or methamphetamine self-administration produces long-term behavioral disturbances including increased impulsivity (Dalley et al., 2006; Mendez et al., 2010).
The most popular behavioral tasks used to assess impulsive action and impulsive choice in rats are the 5-choice serial reaction time task (5-CSRTT) and the delay-discounting task, respectively. In the 5-CSRTT, rats are trained to nosepoke in response to a brief stimulus light pseudo-randomly presented in one of five holes in order to receive a reinforcer; premature nosepokes prior to stimulus presentation are reflective of impulsive action. In delay-discounting task, rats are trained to associate responses on one lever with small but immediate reinforcers, and on another lever with larger but sometimes delayed reinforcers; the rate at which a rat discounts the larger reinforcers on the basis of the delay until delivery is reflective of impulsive choice. The acute administration of psychostimulants produces well-documented increases in premature responding (van Gaalen et al., 2006) and, enigmatically, increases in responding for the larger reinforcer in delay discounting (Winstanley et al., 2003) although opposing results have also been reported (Evenden and Ryan, 1996; Maguire et al., 2014). While the ability of repeated drug administration is well-documented to increase sensitivity to later drug exposure in certain behavioral measures such as locomotion (Pascoli et al., 2011; Thorn et al., 2014b), few studies have systematically examined how drug-induced impulsivity is affected during chronic psychostimulant exposure. Among the studies that have, results have been inconsistent (Paine et al., 2003; Richards et al., 1999; Stanis et al., 2008; Winstanley et al., 2009). To gain a better understanding of this, we performed an investigation using methamphetamine, a widely-abused drug which carries a $23.4 billion burden of care (Courtney and Ray, 2014).
The trace amine-associated receptor 1 (TAAR1) is a G protein-coupled receptor recently identified as an important modulator of the dopaminergic system and, as such, has relevance to a range of dopamine-related neuropsychiatric diseases including drug abuse (Berry, 2007; Pei et al., 2016). TAAR1 agonists such as RO5263397 and RO5166017 are capable of attenuating several psychostimulant abuse-related behavioral indices in rats including locomotor sensitization, conditioned place preference, and intravenous self-administration (Jing et al., 2014; Liu et al., 2016; Thorn et al., 2014a; Thorn et al., 2014b). Interestingly, the literature also suggests that TAAR1 interacts with D2 and/or D3 receptors (Espinoza et al., 2011; Siemian et al., 2017b), which were shown by Dalley and colleagues to be important determinants of trait impulsivity in rats (2007). However, the effects of TAAR1 agonists on psychostimulant-induced impulsivity have not been previously investigated. Thus, the purpose of this study was two-fold: first, to investigate how acute and chronic treatment with methamphetamine influenced baseline and methamphetamine-induced impulsivity and second, to determine whether the TAAR1 agonist RO5263397 attenuated these potential methamphetamine-induced effects on impulsivity.
2. Methods
2.1. Animals
A total of 64 adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250–350 g at experiment onset were individually housed on a 12/12-hour light/dark cycle with behavioral experiments conducted during the light period. The 48 rats used in 5-CSRTT and delay-discounting tasks had free access to water except during test sessions, but restricted access to standard rodent chow, such that their bodyweights were maintained at approximately 90% of their free-feeding counterparts. The 16 additional rats used in the locomotion experiment had free access to both water and standard rodent chow. Rats were maintained and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington, DC, 2011), and were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York (Buffalo, NY).
2.2. Five-Choice Serial Reaction Time Task
Twenty-four rats were trained under a 5-CSRTT procedure according to published protocols (Bari et al., 2008) using parameters and equipment as previously described (Siemian et al., 2017a). Briefly, sessions were conducted in standard chambers designed for the 5-CSRTT (Med Associates, Inc., Georgia, VT) measuring within sound-attenuating, ventilated enclosures. Session programs were controlled and data collected through a PC-interface setup and Med-PC IV software (Med Associates, Inc.). Briefly, sessions began with illumination of the house light and food magazine and delivery of one food pellet (45 mg dustless precision pellets; Bio Serv Inc., Frenchtown, NJ). Once the food pellet was collected, the inter-trial interval (ITI) began and only the house light was illuminated. At the end of the ITI, one of the five response holes on the chamber wall opposing the food magazine was illuminated for a brief amount of time, the stimulus duration (SD). A correct response into this hole within the limited hold (LH) period turned off the target stimulus, turned on the food magazine light, and delivered one food pellet. Once the food pellet was collected, the next ITI began. The target stimulus varied pseudorandomly between trials. A response into the non-target hole was considered an incorrect response, and a failure to respond was considered an omission; each caused a 5 s timeout period in which all chamber lights were extinguished, followed by an initiation of the next ITI. Responses during the ITI prior to target stimulus presentation also caused a timeout and were considered premature responses. Reponses during the timeout period reset the timeout period and were considered timeout responses. Responses occurring after a correct response but before food pellet collection were considered perseverative responses.
Sessions lasted for 100 total trials or 60 min, whichever occurred first, and were conducted 7 days per week. At the onset of training, the SD, LH, and ITI were 30, 30, and 5 s, respectively. Over the course of training, the SD and LH decreased according to each rat’s performance (Bari et al., 2008). Once the SD and LH reached 2.5 and 5 s, respectively, the LH remained constant for the rest of the experiment. The SD was further adjusted by 0.25 s increments until performance was maintained at a stable level of >70% accuracy and <30 omissions for three consecutive sessions, between 1.0 and 1.5 s across the rats.
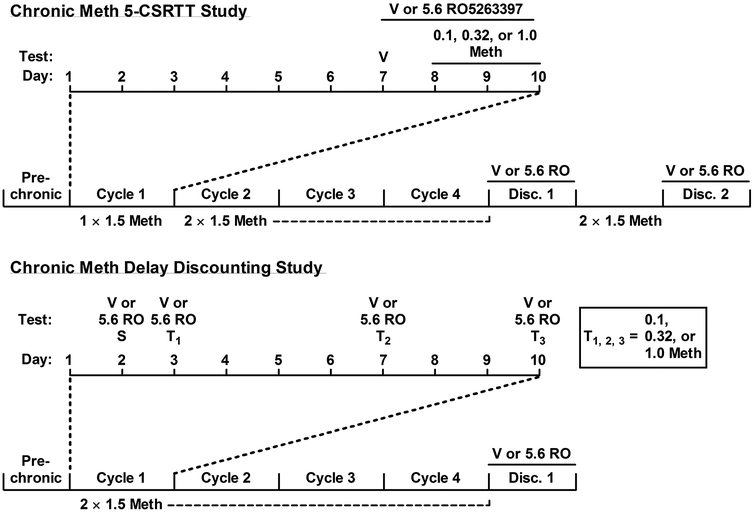
A schematic diagram of the experimental timeline was shown in Fig 1. For acute drug testing, an initial group of 8 naïve rats received injections of vehicle, methamphetamine (0.1 – 1.0 mg/kg, 20 min prior to session start), RO5263397 (3.2 – 10 mg/kg, 30 min prior to session start), or a combination of methamphetamine and RO5263397 according to a Latin-square design. Rats were always placed in the testing chambers and allowed to habituate for 5 min prior to the start of the session, and at least two days of stable performance (>70% accuracy and <30 omissions) were interspersed between drug tests.
Fig. 1.
Experimental timeline of the chronic methamphetamine-treatment 5-CSRTT and delay discounting studies. Top: following acute testing with methamphetamine, two groups of rats were treated with 1.5 mg/kg methamphetamine per day for 6 days. On days 7 – 10, performance in the 5-CSRTT was examined following administration of vehicle or 5.6 mg/kg RO52633397, according to group assignment, in combination with saline or 0.1 – 1.0 mg/kg methamphetamine, according to Latin-square design. The remainder of methamphetamine to reach 1.5 mg/kg was administered following the tests. This 10-day ‘cycle’ was repeated another three times, except that methamphetamine was increased to 2 daily 1.5 mg/kg injections. Following cycle 4, daily methamphetamine was discontinued and 5-CSRTT performance was examined twice per day for 3 days; during selected tests 5.6 mg/kg RO5263397 was administered (see Section 2.2). Following 7 days of abstinence, twice-daily methamphetamine was resumed before a second discontinuation occurred, with treatment groups reversed according to a crossover design. Bottom: the delay discounting study followed the same design as above with the following exceptions: 1) Methamphetamine tests were interspersed over the course of the 10 days, occurring on days 3, 7, and 10 with doses administered according to a Latin-square design; 2) 2 daily 1.5 mg/kg methamphetamine injections were given during cycle 1; and 3) only the first round of methamphetamine discontinuation was performed.
For chronic drug testing, during the first series of tests (‘pre-chronic’), 16 naïve rats received a single injection of vehicle or methamphetamine (0.1, 0.32, or 1.0 mg/kg), according to a Latin square design. Rats were then evenly matched into two groups of 8 rats each based on their premature responses after methamphetamine treatment, and assigned to either vehicle or RO5263397 treatment. During the first chronic treatment phase (‘cycle 1’), rats received an injection of 1.5 mg/kg methamphetamine immediately after daily training sessions for 7 days. On day 7, rats received an injection of either vehicle and saline or 5.6 mg/kg RO5263397 and saline prior to the session, depending on group assignment. On days 8 through 10, tests were conducted in which the rats received injections of either vehicle or 5.6 mg/kg RO5263397 based on their group assignment 10 min before injections of methamphetamine (0.1, 0.32, or 1.0 mg/kg, according to a Latin square design). One test was conducted for each rat per day. After methamphetamine tests, the remainder of the methamphetamine dose was administered after the session to reach the total daily dose of 1.5 mg/kg. For example, if 0.1 mg/kg methamphetamine was administered before the test, then 1.4 mg/kg methamphetamine was administered afterward. This 10-day cycle (7 days of treatment, 3 days of methamphetamine testing) was identically repeated another 3 times (‘cycles 2 – 4’), with the exception that for cycles 2 – 4 the rats received 2 injections of 1.5 mg/kg methamphetamine per day, one immediately after the training session and the other at least 6 hours later. Group assignments on test days (vehicle vs. RO5263397) remained constant throughout this phase of the experiment.
Following the fourth cycle (equal to 40 days of methamphetamine treatment), daily administration of methamphetamine was discontinued for 7 days. Due to the short duration of methamphetamine discontinuation-induced effects (McGregor et al., 2005), two daily training sessions were administered during the first three days of methamphetamine discontinuation, one in the morning and one in the late afternoon, to closely monitor the temporal changes of methamphetamine discontinuation-induced changes in attention or impulsivity. The vehicle group was always trained first, and 5.6 mg/kg RO5263397 was only administered to the other group when the vehicle group showed increased levels of premature responding relative to pre-discontinuation; this occurred most prominently during the afternoon training session of the first day (data not shown after the first day). Following these 7 days of forced abstinence, twice-daily methamphetamine treatment was resumed for an additional 7 days before another treatment discontinuation, in which a crossover design was implemented such that the previously-RO5263397-treatment group was trained first and received vehicle, and the previously-vehicle group now received 5.6 mg/kg RO5263397 before the afternoon session on the first day of discontinuation.
2.3. Locomotion
Locomotor activity was monitored by an infrared motor-sensor system (AccuScan Instruments, Columbus, OH) fitted outside clear acrylic chambers (40 × 40 × 30 cm). Prior to the test, all rats were handled for at least 3 days by the experimenter. On the test day, rats received an injection of either saline (n = 8) or 1.5 mg/kg methamphetamine (n = 24) immediately before being placed into the chamber for 2.5 hours. These 16 rats were those used in the 5-CSRTT, which had been receiving chronic exposure to methamphetamine. The locomotion test occurred on day 31 of chronic methamphetamine treatment.
2.4. Delay Discounting
A total of 24 rats were trained on the delay-discounting task using the same parameters and equipment as previously described (Siemian et al., 2017a). Briefly, rats were first trained to press either of two levers for food reinforcement. At the beginning of the session, the houselight and both lever lights were illuminated. A response on either lever turned off both lever lights and delivered one food pellet immediately followed by a re-illumination of the lever lights and another opportunity to respond. After 3 sessions in which 50 pellets were earned, the number of food pellets delivered for responding on the non-preferred lever was increased to three. The delay for large reinforcer delivery after responding was gradually increased according to each rat’s performance in training sessions in which the rat was trained to alternate its responding for the small and large reinforcer on each trial. Timeout periods, in which all lights were extinguished and lever presses had no programmed consequence, were also introduced during this training period after each reinforcer delivery.
Training sessions were conducted 7 days per week. For each rat, three days of stable responding in which the delay function did not vary by more than 20% was required before drug testing began.
For acute drug testing, an initial group of 8 naïve rats received injections of vehicle or RO5263397 (3.2 – 10 mg/kg, 20 min prior to session start) according to a Latin-square design (Fig. 1). Rats were always placed in the testing chambers and allowed to habituate for 5 min prior to the start of the session, and at least two days of stable performance were interspersed between drug tests.
The chronic treatment testing design was similar to that used for the 5-CSRTT. Throughout the experiment, the following 10-day repeated testing schedule was used: D, S, T1, D, ND, D, T2, D, D, T3, where “D” was a normal delay training session, “ND” was a no-delay training session, in which no delay was applied to the large reinforcer across blocks, “S” was a delay session with a saline pretreatment, and “T1,” “T2,” and “T3” were delay sessions with either 0.1, 0.32, or 1.0 mg/kg methamphetamine pretreatment, which were administered according to a Latin square design. During the first series of tests (‘pre-chronic’), 16 naïve rats were administered vehicle or methamphetamine 10 min prior to being placed in the chamber, after which the program was started 5 min later. Rats were then evenly matched into two groups of 8 rats each based on the delay functions produced by saline and methamphetamine, and assigned to either vehicle or RO5263397 treatment.
During the chronic treatment phase (‘cycles 1 – 4’), 4 cycles of the above 10-day schedule were performed, during which rats received two daily injections of 1.5 mg/kg methamphetamine, one immediately after the training session and one at least 6 hours later. Rats received an injection of vehicle or 5.6 mg/kg RO5263397 10 min prior to the methamphetamine injection, according to their group assignment. After methamphetamine tests, the remainder of the methamphetamine was administered after the testing session to the final total dose of 1.5 mg/kg.
Following the fourth cycle (equal to 40 days of methamphetamine treatment), daily administration of methamphetamine was discontinued for 7 days. In the same manner as the 5-CSRTT experiment, two daily training sessions were administered for the first day of methamphetamine discontinuation, one in the morning and one in the late afternoon, to closely monitor the potential temporal changes of methamphetamine discontinuation-induced changes in delay-discounting performance. The vehicle group was trained first, and 5.6 mg/kg RO5263397 was administered to the other group during the afternoon session on the first day of methamphetamine discontinuation.
2.5. Data Analysis
All graphs and statistical analyses were performed using Prism 7.0 (GraphPad Software, Inc., Dan Diego, CA). P < 0.05 was considered significant for all statistical analyses. For the 5-CSRTT, the primary measures were response accuracy (correct responses divided by total responses multiplied by 100%), omissions, premature responses, timeout responses, and perseverative responses which were plotted as a function of drug dose. Effects of acute RO5263397 treatment were analyzed using one-way repeated-measures ANOVA. Effects of RO5263397 treatment on methamphetamine-induced changes on 5-CSRTT performance were analyzed using two-way repeated-measures ANOVA with both methamphetamine dose and RO5263397 dose entered as within-subject factors. Effects of chronic methamphetamine treatment were analyzed using two-way repeated measures ANOVA with both methamphetamine dose and treatment duration (cycle) entered as within-subject factors. Effects of RO5263397 treatment were analyzed using two-way mixed-model ANOVA with methamphetamine dose as the within-subject factor and RO5263397 treatment as the between-group factor. Discontinuation data were analyzed using paired one-tailed t-tests to compare effects within-group, and unpaired one-tailed t-tests to compare effects between treatment groups.
Locomotion data were plotted as distance traveled (in cm) during each 5 min bin. These data were analyzed using two-way mixed-model ANOVA with time as the within-subject factor and either acute methamphetamine treatment or chronic methamphetamine treatment as the between-group factor.
For delay discounting, the percent choice of the larger reinforcer was calculated by dividing the number of large rewards chosen by the total number of responses made in each block and multiplying by 100%, and plotted as a function of delay. Area under the curve with base-10 logarithmic transformation (AUClog d) was calculated using GraphPad software from individual subject delay functions, after converting percent choice to a value between 0 and 1, and transforming the delay value to a base-10 logarithmic unit. This AUC calculation method was used to avoid the disproportionate contribution of indifference points at long delays to the total AUC with minimal contribution from indifference points at short delays (Borges et al., 2016). Effects of acute RO5263397 treatment were analyzed by two-way repeated-measures ANOVA on the delay function and by one-way repeated-measures ANOVA on AUC. Effects of chronic methamphetamine treatment on AUC were analyzed using two-way repeated measures ANOVA with both methamphetamine dose and treatment duration entered as within-subject factors. Effects of RO5263397 treatment on methamphetamine-induced changes in AUC of the delay functions were analyzed using two-way mixed-model ANOVA with methamphetamine dose as the within-subject factor and RO5263397 treatment as the between-group factor. Discontinuation data were analyzed using two-way repeated measures ANOVA with both delay and day entered as within-subject factors.
2.6. Drugs
d-Methamphetamine hydrochloride (Sigma-Aldrich, St. Louis, Missouri, USA) was dissolved in normal saline. RO5263397 (provided by Dr. Yanan Zhang, Research Triangle Institute, with chemical structure detailed by Revel et al., 2013) was dissolved in a vehicle of 1 part 190 proof ethanol, 1 part Alkamuls EL-620 (Rhodia, Cranbury, NJ) and 18 parts normal saline. Both drugs were administered intraperitoneally in an injection volume of 1 ml/kg.
3. Results
3.1. 5-CSRTT
In the 5-CSRTT, acute RO5263397 treatment (3.2 – 10 mg/kg) did not significantly affect any behavioral measure (one-way repeated-measures ANOVA; Fig. 2, left column). When administered in combination, for accuracy two-way repeated-measures ANOVA revealed a significant RO5263397 × methamphetamine interaction [F(6, 42) = 2.90, p < 0.05] with a significant main effect of RO5263397 [F(2, 14) = 8.71, p < 0.01] but not methamphetamine. For omissions, there was a significant RO5263397 × methamphetamine interaction [F(6, 42) = 12.18, p < 0.0001] with significant main effects of RO5263397 [F(2, 14) = 22.96, p < 0.0001] and methamphetamine [F(3, 21) = 12.05, p < 0.0001]. For premature responses, there was a significant RO5263397 × methamphetamine interaction [F(6, 42) = 4.18, p < 0.01] with significant main effects of RO5263397 [F(2, 14) = 15.74, p < 0.001] and methamphetamine [F(3, 21) = 7.46, p < 0.01]. For timeout responses, only RO5263397 produced a significant main effect [F(2, 14) = 11.64, p < 0.01]. Neither drug produced significant effects on perseverative responses (Fig. 2, right column).
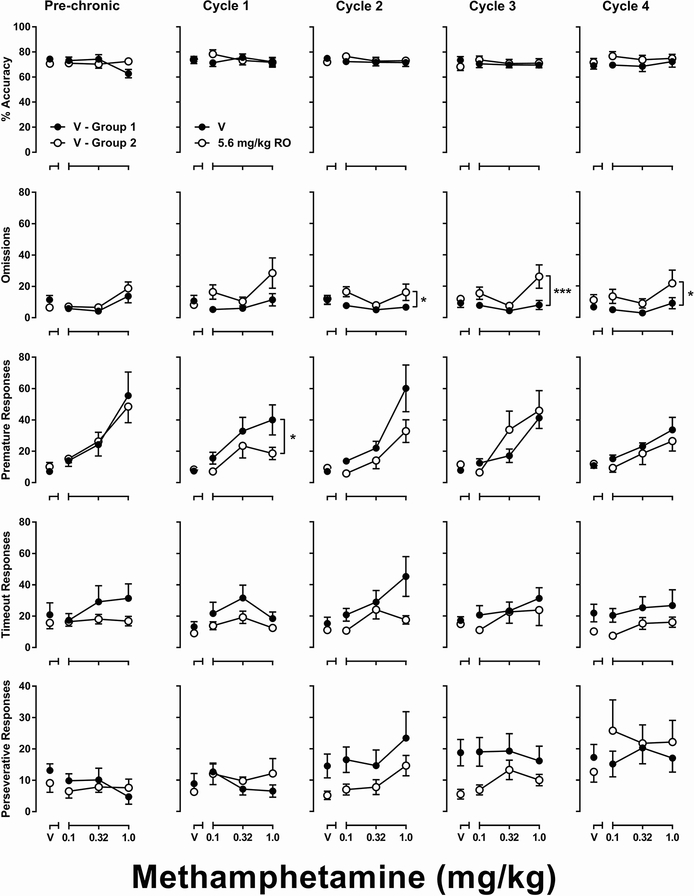
Fig. 2.
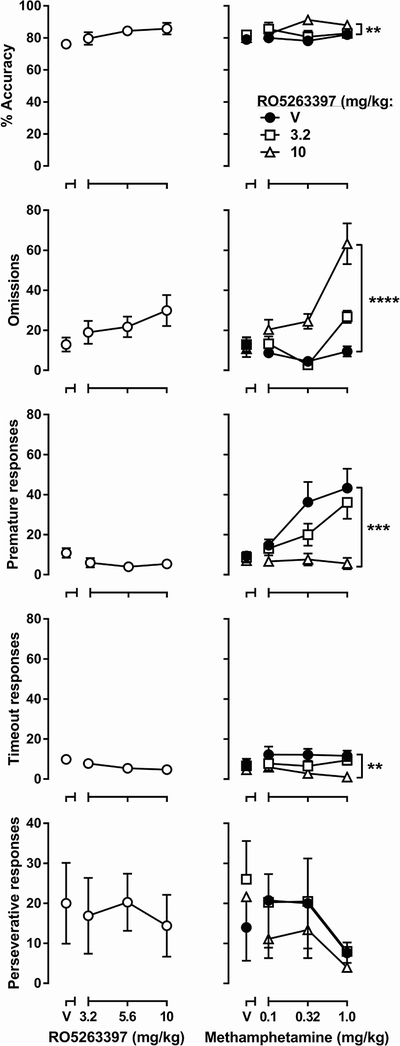
Effects of RO5263397 (left column) and methamphetamine alone or in combination with RO5263397 (right column) on 5-CSRTT performance. Ordinates are percent accuracy (first row), omissions (second row), premature responses (third row), timeout responses (fourth row), and perseverative responses (fifth row). Abscissas are the dose of RO5263397 (left) or methamphetamine (right) in mg/kg. **P < 0.01, ***P < 0.001, ****P < 0.0001 main effect of RO5263397 treatment according to two-way repeated-measures ANOVA.
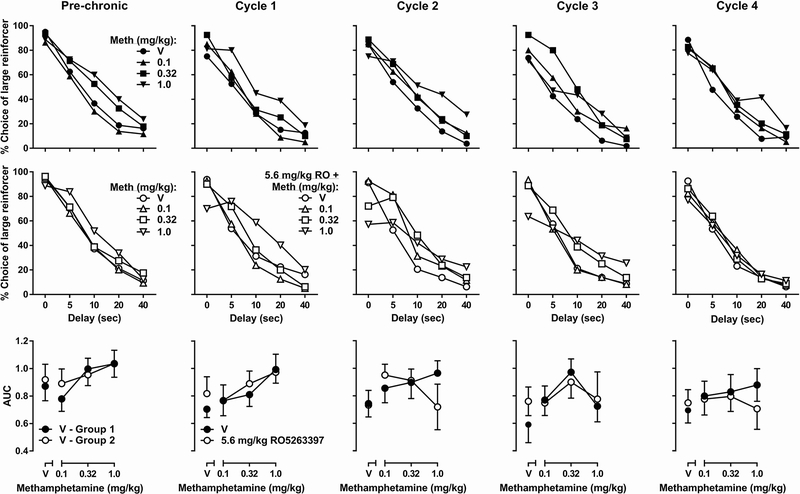
For the chronic treatment experiment, rats were assigned to vehicle- or 5.6 mg/kg RO5263397-treatment groups after acute methamphetamine testing (Fig. 3, left column labeled ‘pre-chronic’). Methamphetamine produced significant main effects on omissions [F(3, 42) = 8.35, p < 0.001] and premature responses [two-way mixed-model ANOVA: F(3, 42) = 15.80, p < 0.0001] but not accuracy, timeout responses, or perseverative responses, whereas group assignment did not produce significant main effects in any of these measures. During chronic methamphetamine treatment (Fig. 3, columns labeled ‘cycle 1’ through ‘cycle 4’) in the vehicle-treatment group, the methamphetamine dose but not treatment duration produced a significant main effect on omissions [F(3, 21) = 3.45, p < 0.05], premature responses [F(3, 21) = 15.19, p < 0.0001], and timeout responses [F(3, 21) = 5.61, p < 0.01]. In contrast, treatment duration but not methamphetamine dose produced a significant main effect on perseverative responses [F(4, 28) = 3.34, p < 0.05]. Accuracy was unaffected by either methamphetamine dose or treatment duration. Pretreatments with 5.6 mg/kg RO5263397 produced significant main effects on omissions during cycles 2 [two-way mixed-model ANOVA: F(1, 14) = 6.76, p < 0.05], 3 [F(1, 14) = 17.88, p < 0.0001], and 4 [F(1, 14) = 4.97, p < 0.05], as well as on premature responses during cycle 1 [F(1, 14) = 5.84, p < 0.05] as compared to the vehicle-treatment group.
Fig. 3.
Effects of methamphetamine alone or in combination with RO5263397 on 5-CSRTT performance during chronic methamphetamine treatment. ‘Pre-chronic’ (column 1) represent data collected prior to initiation of chronic methamphetamine, no RO5263397 was administered in either group. ‘Cycle 1’ (column 2) represent data collected on days 7 – 10 of daily methamphetamine treatment. ‘Cycle 2,’ ‘cycle 3,’ and ‘cycle 4’ (columns 3 – 5) represent data collected on days 17 – 20, 27 – 30, and 37 – 40 of chronic methamphetamine treatment, respectively. In cycles 1 – 4, 5.6 mg/kg RO5263397 was administered to the group labeled ‘5.6 RO’ prior to each test. See Figure 1 for ordinates. Abscissas are the dose of methamphetamine in mg/kg. *P < 0.05, ***P < 0.001 main effect of RO5263397 treatment according to two-way mixed-model ANOVA.
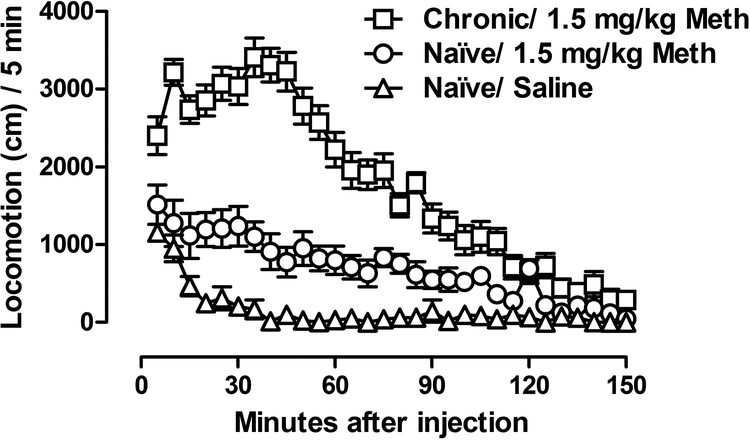
At the beginning of cycle 4 on day 31 of chronic methamphetamine treatment, the rats were tested for their locomotor response to methamphetamine as compared to naïve rats (Fig. 4). Among the naïve rats, between rats injected with saline and 1.5 mg/kg methamphetamine, two-way mixed model ANOVA revealed a significant methamphetamine × time interaction [F(29, 290) = 4.12, p < 0.0001] with significant main effects of both 1.5 mg/kg methamphetamine [F(1, 290) = 21.32, p < 0.001] and time [F(29, 290) = 16.75, p < 0.0001]. Among the rats treated with 1.5 mg/kg methamphetamine, two-way mixed model ANOVA revealed a significant chronic methamphetamine treatment × time interaction [F(29, 580) = 5.71, p < 0.0001] with significant main effects of both chronic methamphetamine treatment [F(1, 580) = 67.30, p < 0.0001] and time [F(29, 580) = 21.33, p < 0.0001].
Fig. 4.
Effects of saline or methamphetamine on locomotor activity in naïve rats or rats on day 31 of chronic methamphetamine treatment. Ordinates are locomotion counts per 5 min, abscissas are time (min).
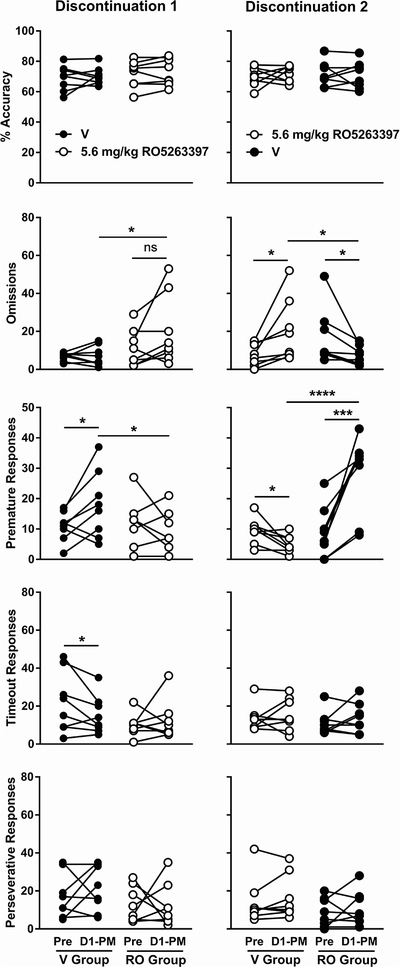
Discontinuation of twice-daily methamphetamine administration produced a significant increase in premature responses during the afternoon session on abstinence day 1 relative to the previous day in vehicle-treated rats (Fig. 5, left column) [paired one-tailed t-test: t(7) = 2.29, p < 0.05]. This within-group effect was not observed when RO5263397 was administered prior to the session [t(7) = 1.15, p > 0.05], and the RO5263397-treated rats exhibited significantly less premature responses than the vehicle-treated rats [unpaired one-tailed t-test: t(14) = 1.98, p < 0.05]. A within-group decrease in timeout responses was observed in the vehicle-treatment group [t(7) = 2.04, p < 0.05] but the other behavioral measures were unaffected by the treatment discontinuation. While there was no significant within-group effect of RO5263397 on omissions [t(7) = 1.81, p > 0.05], the RO5263397-treated rats displayed statistically more omissions than the vehicle-treated group [t(14) = 1.91, p < 0.05]. A second discontinuation was performed according to a crossover design (Fig. 5, right column; see section 2.2). Rats treated with vehicle before the afternoon session on abstinence day 1 exhibited a significant increase in premature responses relative to pre-discontinuation [t(7) = 4.93, p < 0.001] whereas rats treated with RO5263397 displayed a significant decrease in premature responses [t(7) = 2.83, p < 0.05]. The RO5263397-treated rats displayed statistically fewer premature responses than the vehicle-treated group [t(14) = 5.08, p < 0.0001]. Inversely, rats treated with vehicle before the afternoon session on abstinence day 1 exhibited a significant decrease in omissions relative to pre-discontinuation [t(7) = 2.43, p < 0.05] whereas rats treated with RO5263397 displayed a significant increase in omissions [t(7) = 2.63, p < 0.05]. The RO5263397-treated rats displayed statistically more omissions than the vehicle-treated group [t(14) = 2.09, p < 0.05].
Fig. 5.
Effects of acute discontinuation of twice-daily methamphetamine on 5-CSRTT performance. For each group, the data labeled ‘Pre’ were collected in a normal daily session, after which methamphetamine was not administered. Data labeled ‘D1-PM’ were collected in the afternoon session the following day; 5.6 mg/kg RO5263397 was administered prior to this second session in groups represented by open circles. ‘Discontinuation 1’(left column) represents data in which group assignments were the same as previous in the experiment, ‘discontinuation 2’(right column) represents data in which treatment assignments were reversed according to a crossover design. See Figure 1 for ordinates. *P < 0.05, ***P < 0.001, ****P < 0.0001 according to paired one-tailed t-test.
3.2. Delay Discounting
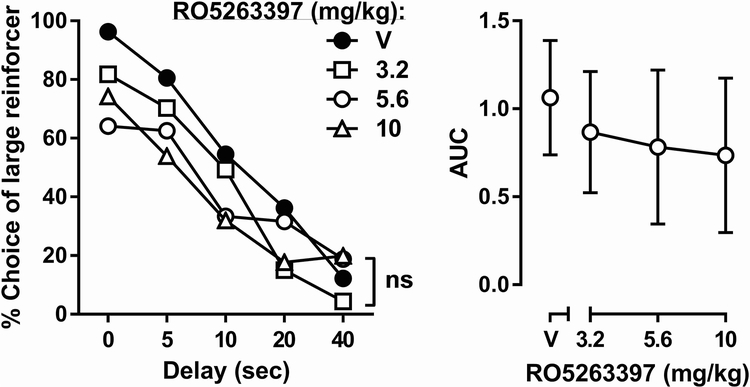
In the delay-discounting study, acute RO5263397 (3.2 – 10 mg/kg) treatment produced no significant delay × RO5263397 interaction nor a main effect of RO5263397 treatment, though a significant main effect of delay [F(4, 28) = 26.28, p < 0.0001] was found (two-way repeated-measures ANOVA; Fig. 6, left). Likewise, acute RO5263397 did not significantly affect AUC according to one-way repeated-measures ANOVA (Fig. 6, right).
Fig. 6.
Effects of RO5263397 on delay discounting. Ordinates are percent choice of the larger reinforcer (left) or area under the curve (right). Abscissas are the delay of large reinforcer delivery (left) or dose of RO5263397 in mg/kg (right). Error bars were omitted from the left panel for clarity.
For the chronic methamphetamine treatment experiment, rats were assigned to vehicle- or RO5263397-treatment groups after acute methamphetamine testing (Fig. 7, left column labeled ‘pre-chronic’). Methamphetamine produced significant main effects on the AUC of the delay functions [two-way mixed-model ANOVA: F(3, 42) = 9.28, p < 0.0001]. During chronic methamphetamine treatment (Fig. 7, columns labeled ‘cycle 1’ through ‘cycle 4’) in the vehicle-pretreatment group, two-way repeated-measures ANOVA revealed a significant methamphetamine × treatment duration interaction [F(12, 84) = 2.14, p < 0.05] and significant main effects of both methamphetamine [F(3, 21) = 7.91, p < 0.01] and treatment duration [F(4, 28) = 3.36, p < 0.05] on AUC. In the RO5263397 treatment group, two-way repeated-measures ANOVA revealed a significant main effect of treatment duration [F(4, 28) = 3.49, p < 0.05] but not methamphetamine or methamphetamine × treatment duration interaction. Within each cycle, two-way mixed-model ANOVAs revealed significant main effects of methamphetamine in treatment cycles 1 [F(3, 42) = 6.95, p < 0.001] and 3 [F(3, 42) = 4.15, p < 0.05], but RO5263397 treatment or RO5263397 × methamphetamine interactions were not significant during any treatment cycle.
Fig. 7.
Effects of methamphetamine alone or in combination with RO5263397 on delay discounting during chronic methamphetamine treatment. ‘Pre-chronic’ (column 1) represent data collected prior to initiation of chronic methamphetamine, no RO5263397 was administered in either group. ‘Cycle 1,’ ‘cycle 2,’ ‘cycle 3,’ and ‘cycle 4’ (columns 2 – 5) represent data collected between days 3 – 10, 13 – 20, 23 – 30, and 33 – 40 of twice-daily methamphetamine treatment, respectively; 5.6 mg/kg RO5263397 was administered to the group labeled ‘5.6 RO’ prior to each test. For the top 2 rows, see Figure 5, left for ordinates and abscissas. For the bottom row, see Figure 5, right for ordinates and abscissas
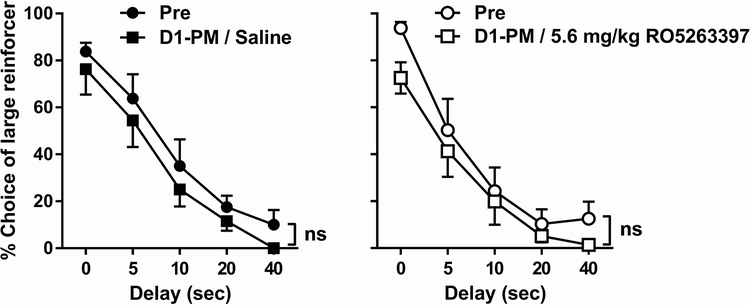
Discontinuation of twice-daily methamphetamine administration did not cause a significant effect on the delay-discounting performance (Fig. 7). Two-way repeated-measures ANOVA revealed a significant main effect of delay [F(4, 28) = 37.57, p < 0.0001] but not day, although there was a non-significant trend [F(1, 7) = 5.48, p = 0.0517] in the saline-treated group. In the RO5263397-treated group, similar results were found with a significant main effect of delay [F(4, 28 = 41.63, p < 0.0001] but not day [F(1, 7) = 3.04, p = 0.125].
4. Discussion
Impulsivity is an important and complex trait that is a determinant of other characteristics such as behavioral control and decision-making. While the ability to act and make decisions quickly can be advantageous in some settings, these impulsive decisions and actions become risky and maladaptive when employed in excess. Such high impulsivity is associated with several problematic syndromes such as drug addiction, gambling, and attention-deficit hyperactivity disorder. Progress has been made in elucidating neural networks underlying forms of impulsivity, but one remaining issue in the context of drug addiction is how much impulsivity is a result of the neuroadaptations produced by drug exposure versus how much impulsivity puts drug use at risk in the first place. Numerous studies support either scenario, which may suggest that a combined model is most accurate. In the animal literature, rats prescreened for high rates of premature responding self-administered cocaine more rapidly than less impulsive counterparts (Dalley et al., 2007) while, in the opposite direction, chronic cocaine exposure in rats increased premature responding and steepened delay discounting (Mendez et al., 2010; Winstanley et al., 2009). Interestingly, in the latter scenario these effects appear to be intake-dependent. That is, rats self-administering larger amounts of drug displayed greater impulsive choice after three weeks of abstinence (Mitchell et al., 2014), but in contrast to the results of Dalley and colleagues (2007), the high- and low-intake groups did not differ from each other prior to drug experience. Thus, further research is required to better understand the determinant factors in this relationship. These and other studies have examined impulsive behavior during drug abstinence, often testing several weeks after drug discontinuation. However, fewer studies have examined how acute drug-induced impulsivity is altered during the course of ongoing drug exposure.
In the current study, acute methamphetamine dose-dependently increased premature responses and shifted the delay function upward in naïve rats, similar to data previously reported for d-amphetamine (van Gaalen et al., 2006; Winstanley et al., 2003). Interestingly, a significant effect of chronic methamphetamine treatment on methamphetamine-influenced performance was observed in the delay-discounting task but not the 5-CSRTT. However, while the chronic treatment produced statistically significant effects on AUC in delay discounting, the effect size was small and visual inspection appears to indicate the possible development of tolerance as opposed to sensitization. Indeed, main effects of methamphetamine on AUC were no longer significant by treatment cycle 4. In the 5-CSRTT, only a small increase in methamphetamine-induced perseverative responses was found over the course of the chronic treatment. Perseverative responses are generally interpreted as compulsive behavior, but this interpretation has not been well-validated and thus this finding is difficult to interpret. In contrast, acute methamphetamine treatment in the 5-CSRTT rats after 30 days of methamphetamine treatment induced a robust hyperactivity, which was significantly greater than that produced by the same acute treatment in naïve rats, evidence that the treatment protocol did induce sensitization as measured by a different behavioral endpoint.
These findings are in general agreement with a previous report, in which repeatedly-administered d-amphetamine induced behavioral sensitization (locomotion and stereotypy) to d-amphetamine, but did not alter the effects of d-amphetamine on delay discounting performance (Stanis et al., 2008). Thus, a typical “sensitizing” regimen of an amphetamine or amphetamine derivatives appears to be insufficient to change drug-induced impulsivity. Furthermore, the neuroadaptations associated with sensitization are likely distinct from those involved in impulsivity. Indeed, potentiated excitatory transmission in D1-receptor-expressing medium-sized spiny neurons in NAc induced by repeated drug exposure may account for locomotor sensitization (Pascoli et al., 2011); if D2/D3 receptors determine impulsivity more than D1 receptors do, then the relative stability of D2/D3 receptor sensitivity during drug treatment may explain this dissociation (Collins et al., 2011). On the one hand, such stability of drug effect confers validity to previous pharmacological studies using these impulsivity models, which require repeated drug testing due to their incompatibility with cumulative-dosing procedures that would limit the occasions of drug exposure. On the other hand, the apparent dissociative nature of drug exposure on different behavioral endpoints may necessitate future studies to employ intravenous self-administration procedures, as opposed to experimenter-administered designs, to uncover the potential influence of ongoing drug experience on drug-induced impulsivity.
In contrast to the results found during the period of continuous methamphetamine administration, rats exhibited significantly greater numbers of premature responses at an early time point following methamphetamine discontinuation. This effect and temporal changes appears to correlate well with previous studies in rats and with human-reported amphetamine-craving data (McGregor et al., 2005; Winstanley et al., 2009). However, no acute effect of methamphetamine discontinuation was apparent in the delay-discounting task. It is becoming clear that these two forms of impulsivity are associated with different neural circuits – impulsive action in the 5-CSRTT governed by NAc shell and its afferents such as the infralimbic cortex, insula, and ventral hippocampus as well as the cingulate cortex and dorsal striatum (Abela et al., 2013; Chudasama et al., 2003; Dalley et al., 2002; Muir et al., 1996), and impulsive choice in the delay-discounting task governed by NAc core, hippocampus, basolateral amygdala, and lateral and medial orbitofrontal cortex (Abela and Chudasama, 2013; Winstanley et al., 2004; Zeeb et al., 2010). As such, the results of the current study suggest that acute methamphetamine cessation may more robustly affect those areas involved in impulsive action than impulsive choice. While some studies have shown that circadian rhythm plays a significant role in determining behaviors, particularly those which involve dopaminergic components (Abarca et al., 2002; McClung, 2007), it is unlikely that the abstinence-associated effect on 5-CSRTT performance is due to the time of test since afternoon sessions on subsequent days showed comparable performance as compared to normal daily sessions. The current study did not explore the potential emergence of impulsive behaviors occurring at longer time points (e.g., > 3 weeks) following drug exposure that other investigations have reported because the behavioral changes recovered only one day after methamphetamine discontinuation.
The effects of the TAAR1 agonist RO5263397 in the present study were in general agreement with the previous literature regarding TAAR1 and psychostimulant abuse-related behavioral indices. TAAR1 has been established as an important modulator of the dopaminergic system (Borowsky et al., 2001; Bunzow et al., 2001), and research has shown RO5263397 to attenuate cocaine- or methamphetamine-induced locomotor sensitization, conditioned place preference, drug intake via self-administration, and reinstatement (Jing et al., 2014; Thorn et al., 2014a). Interestingly, TAAR1 is expressed in both NAc core and shell and microinjections of another TAAR1 agonist, RO5166017, into these regions attenuated cue- or drug-induced reinstatement of cocaine self-administration, respectively (Liu et al., 2017). Additionally, TAAR1 signaling appears to modulate D2 and/or D3 receptor functions (Harmeier et al., 2015; Siemian et al., 2017b; Xie et al., 2007). Given the apparent correlation between D2/D3 receptor levels in NAc and trait impulsivity, it was conceivable for TAAR1 activation to modulate methamphetamine-induced impulsivity. While RO5263397 attenuated methamphetamine-induced premature responses acutely and at early time points in the chronic treatment experiment, this effect disappeared at later stage of chronic treatment. This, however, may simply be a result of a flattening of the methamphetamine dose-effect curve in the vehicle-pretreatment group over the course of methamphetamine treatment. In the delay-discounting study, RO5263397 did not produce a significant main effect on the AUC. Despite this, within-group analysis showed that acute methamphetamine did not produce a significant main effect in the RO5263397 group over the course of the chronic treatment. While this result is difficult to interpret due to the lack of significance in between-group comparisons in any cycle, it nonetheless may suggest a small impact of TAAR1 activation on methamphetamine-induced temporal discounting.
Importantly, the increased premature responding observed during the afternoon of the first day of methamphetamine discontinuation (approximately 46 hours after the last methamphetamine injection) was significantly attenuated by RO5263397. This appeared to be a stable effect as it was also observed when the treatment assignments were reversed during a second discontinuation according to a crossover design, which argues against the concern that this effect resulted from repeated exposure to RO5263397 over the duration of the experiment. This was further supported by findings in a different group of methamphetamine-naïve rats, in which 7 days of daily 5.6 mg/kg RO5263397 administration before 5-CSRTT training did not significantly alter any performance measure including omissions (data not shown). Thus, this finding is different from several previously observed effects of TAAR1 agonists on abuse-related behaviors, which were described in procedures generally involving re-exposure to a drug (e.g., acute treatment to stimulate locomotion, drug-primed reinstatement) or contextual cue (e.g., unrestricted chamber access in CPP, cue-primed reinstatement) after one or more conditioning periods (e.g., acquisition/extinction, saline-versus drug-pairing). In this study, a cessation of the daily methamphetamine injections was sufficient to induce premature responses in an otherwise constant, ongoing daily procedure. The attenuation of this effect by TAAR1 activation suggests that TAAR1 ligands have potential utility to directly treat abstinence-associated performance impairment, and may have implications distinct from findings in which TAAR1 activation attenuates an elicited behavioral response by a stimulus re-exposure during the abstinence period.
4.1. Conclusion
Chronic methamphetamine did not significantly alter methamphetamine-induced impulsive action, and produced small but significant effects on methamphetamine-influenced temporal discounting while inducing robust locomotor sensitization. Discontinuation of methamphetamine injections significantly increased premature responses, which was attenuated by RO5263397 treatment, but did not significantly affect delay discounting. This study is the first to report an attenuation of a TAAR1 agonist treatment on forced abstinence-induced impulsivity. These results, when considered with findings from previous studies, suggest that TAAR1 agonists may be promising therapeutic agents for reducing relapse susceptibility during abstinence.
Fig. 8.
Effects of acute discontinuation of twice-daily methamphetamine on 5-CSRTT performance. For each group, the data labeled ‘Pre’ were collected in a normal daily session, after which methamphetamine was not administered. Data labeled ‘D1-PM’ were collected in the afternoon session the following day; 5.6 mg/kg RO5263397 was administered prior to this session in the right panel. See Figure 5, left for ordinates and abscissas.
Acknowledgements
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (award no. R01DA034806 and R21DA040777).
Footnotes
Competing Interests
None declared.
References
- 2011. Guide for the Care and Use of Laboratory Animals. National Academy of Sciences, Washington DC. [Google Scholar]
- Abarca C, Albrecht U, Spanagel R, 2002. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A 99, 9026–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abela AR, Chudasama Y, 2013. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. Eur J Neurosci 37, 640–647. [DOI] [PubMed] [Google Scholar]
- Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y, 2013. Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb Cortex 23, 1396–1409. [DOI] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW, 2008. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3, 759–767. [DOI] [PubMed] [Google Scholar]
- Berry MD, 2007. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials 2, 3–19. [DOI] [PubMed] [Google Scholar]
- Borges AM, Kuang J, Milhorn H, Yi R, 2016. An alternative approach to calculating Area-Under-the-Curve (AUC) in delay discounting research. Journal of the Experimental Analysis of Behavior 106, 145–155. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C, 2001. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A 98, 8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK, 2001. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 60, 1181–1188. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW, 2003. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146, 105–119. [DOI] [PubMed] [Google Scholar]
- Collins GT, Truong YN, Levant B, Chen J, Wang S, Woods JH, 2011. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity. Psychopharmacology (Berl) 215, 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA, 2014. Methamphetamine: An update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend 143, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW, 2007. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315, 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DEH, Pena Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW, 2006. Enduring Deficits in Sustained Visual Attention during Withdrawal of Intravenous Methylenedioxymethamphetamine Self-Administration in Rats: Results from a Comparative Study with d-Amphetamine and Methamphetamine. Neuropsychopharmacology 32, 1195–1206. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Robbins TW, 2017. Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci 18, 158–171. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW, 2002. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology 26, 716–728. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ, 2009. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry 65, 851–856. [DOI] [PubMed] [Google Scholar]
- Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, Caron MG, Gainetdinov RR, 2011. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol 80, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, 1999. Varieties of impulsivity. Psychopharmacology (Berl) 146, 348–361. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN, 1996. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 128, 161–170. [DOI] [PubMed] [Google Scholar]
- Harmeier A, Obermueller S, Meyer CA, Revel FG, Buchy D, Chaboz S, Dernick G, Wettstein JG, Iglesias A, Rolink A, Bettler B, Hoener MC, 2015. Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur Neuropsychopharmacol 25, 2049–2061. [DOI] [PubMed] [Google Scholar]
- Jing L, Zhang Y, Li JX, 2014. Effects of the trace amine associated receptor 1 agonist RO5263397 on abuse-related behavioral indices of methamphetamine in rats. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Siemian JN, Seaman R Jr., Zhang Y, Li JX, 2017. Role of TAAR1 within the Subregions of the Mesocorticolimbic Dopaminergic System in Cocaine-Seeking Behavior. J Neurosci 37, 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Thorn DA, Zhang Y, Li JX, 2016. Effects of Trace Amine-associated Receptor 1 Agonists on the Expression, Reconsolidation, and Extinction of Cocaine Reward Memory. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Henson C, France CP, 2014. Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology 87, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, 2007. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. ScientificWorldJournal 7, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM, 2005. The nature, time course and severity of methamphetamine withdrawal. Addiction 100, 1320–1329. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, Setlow B, 2010. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci 124, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Ouimet DJ, Fuchs RA, Morgan D, Setlow B, 2014. Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behav Neurosci 128, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein-Zamir S, Simon Jones P, Bullmore ET, Robbins TW, Ersche KD, 2013. Prefrontal hypoactivity associated with impaired inhibition in stimulant-dependent individuals but evidence for hyperactivation in their unaffected siblings. Neuropsychopharmacology 38, 1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW, 1996. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6, 470–481. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC, 2003. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res 147, 135–147. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Luscher C, 2011. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature 481, 71–75. [DOI] [PubMed] [Google Scholar]
- Pei Y, Asif-Malik A, Canales JJ, 2016. Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications. Frontiers in Neuroscience 10, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, Metzler V, Chaboz S, Groebke Zbinden K, Galley G, Norcross RD, Tuerck D, Bruns A, Morairty SR, Kilduff TS, Wallace TL, Risterucci C, Wettstein JG, Hoener MC, 2013. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 18, 543–556. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H, 1999. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 146, 432–439. [DOI] [PubMed] [Google Scholar]
- Robbins T, 2002. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163, 362–380. [DOI] [PubMed] [Google Scholar]
- Siemian JN, Xue Z, Blough BE, Li JX, 2017a. Comparison of some behavioral effects of d- and l-methamphetamine in adult male rats. Psychopharmacology (Berl) 234, 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemian JN, Zhang Y, Li JX, 2017b. Trace amine-associated receptor 1 agonists RO5263397 and RO5166017 attenuate quinpirole-induced yawning but not hypothermia in rats. Behav Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanis JJ, Marquez Avila H, White MD, Gulley JM, 2008. Dissociation between long-lasting behavioral sensitization to amphetamine and impulsive choice in rats performing a delay-discounting task. Psychopharmacology (Berl) 199, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM, Zhang Y, Li JX, 2014a. Effects of the Trace Amine-Associated Receptor 1 Agonist RO5263397 on Abuse-Related Effects of Cocaine in Rats. Neuropsychopharmacology 39, 2309–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang C, Zhang Y, Li JX, 2014b. The trace amine associated receptor 1 agonist RO5263397 attenuates the induction of cocaine behavioral sensitization in rats. Neurosci Lett 566, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ, 2006. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 187, 73–85. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Bachtell RK, Theobald DE, Laali S, Green TA, Kumar A, Chakravarty S, Self DW, Nestler EJ, 2009. Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontal cortex. Cereb Cortex 19, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW, 2003. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 170, 320–331. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW, 2004. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci 24, 4718–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, Miller GM, 2007. Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther 321, 116–127. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA, 2010. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 211, 87–98. [DOI] [PubMed] [Google Scholar]