Abstract
Regadenoson is an FDA approved adenosine receptor agonist which increases blood-brain barrier (BBB) permeability in rodents. Regadenoson is used clinically for pharmacologic cardiac stress testing using SPECT or CT imaging agents that do not cross an intact BBB. This study was conducted to determine if standard doses of regadenoson transiently disrupt the human BBB allowing higher concentrations of systemically administered imaging agents to enter the brain. Patients without known intracranial disease undergoing clinically indicated pharmacologic cardiac stress tests were eligible for this study. They received regadenoson (0.4 mg) followed by brain imaging with either 99mTc-sestamibi for SPECT or visipaque for CT imaging. Pre-and post-regadenoson penetration of imaging agents into brain were quantified [SPECT: radioactive counts, CT: Hounsfield units (HU)] and compared using a matched-pairs t-test. Twelve patients (33% male, median 60 yo) were accrued: 7 SPECT and 5 CT. No significant differences were noted in pre-and post-regadenoson values using mean radionuclide counts (726 vs. 757) or HU (29 vs. 30). While animal studies have demonstrated that regadenoson transiently increases the permeability of the BBB to dextran and temozolomide, we were unable to document changes in the penetration of contrast agents in humans with intact BBB using the FDA approved doses of regadenoson for cardiac evaluation. Further studies are needed exploring alternate regadenoson dosing, schedules, and studies in patients with brain tumors; as transiently disrupting the BBB to improve drug entry into the brain is critical to improving the care of patients with CNS malignancies.
Keywords: Blood-brain barrier permeability, Regadenoson, Visipaque, 99mTc-sestamibi, Drug entry
Introduction
The blood-brain barrier (BBB) remains a major obstacle limiting the delivery of most systemically administered pharmacologic agents to the central nervous system. Even in diseases such as glioblastoma, where the BBB is usually considered to be severely disrupted, drug distribution at the growing edges of the tumor is known to be severely restricted [1]. The BBB provides an anatomic and physiologic barrier comprised of astrocytes, and pericytes, tight junctional proteins, and multidrug transporter proteins. These work together to allow lipophilic and small molecules to enter the brain while preventing the entry of larger, hydrophilic substances. As a result, many patients with systemic cancers treated with efficacious agents which do not reach therapeutic concentrations have excellent control of their systemic disease but progressive metastases in the central nervous system [2–4].
Several methods have been studied in an effort to increase the penetration of chemotherapy into the CNS. Examples include transiently opening the BBB with intra-arterial mannitol and chemotherapy, bypassing the BBB with chemotherapy containing polymers placed in the brain at the time of surgery, or the insertion of intracranial catheters for direct injections or infusions into brain parenchyma. Additional studies have explored the use of bradykinin and adenosine agonists to increase BBB permeability [5–11]. Cereport, a bradykinin analog, has been extensively evaluated in preclinical and clinical studies with the goal to transiently open the BBB to carboplatin. While preclinical studies demonstrated increased permeability and an antitumor effect, clinical studies failed to show a survival benefit of adding cereport to carboplatin in patients with recurrent malignant glioma [12, 13]. Adenosine can result in vasodilation and in rodents causes transient disruption of the BBB, by decreasing expression of tight junction proteins [14, 15]. Short term exposure with regadenoson, an FDA approved adenosine receptor A2A agonist, resulted in both small and large Dalton (D) molecule passages to the brain as evidenced by fluorescent dextran (10–70 kD) uptake measurements [15]. Further studies in rodents have shown that when temozolomide is administered systemically after intravenous administration of regadenoson, there is a 60% increase in the concentration of temozolomide in the brain without changes in the systemic pharmacology this agent [16]. Additionally, preclinical studies of regadenoson with Gemcitabine (263 D), Epirubicin (543 D) and Evans blue dye which is bound to albumin (60,000 D) have demonstrated the ability of adenosine A2A receptor agonism to cause transient increased drug entry in mice with normal brains [17–19].
Clinical studies have demonstrated the initial half-life phase of regadenoson is 2–4 min; While the intermediate half-life of 30 min correlates with resolution of its pharmacodynamics effect. By 2 h, the terminal phase half-life results in a decline of systemic plasma concentrations. No clinical studies have been performed to evaluate the steady state drug level effects on CNS drug entry. Preclinical rat studies demonstrated an elimination half-life of 18–24 min with 90% eliminated in 24 h after a single dose of regadenoson. Accordingly, past rodent studies evaluating CNS impact revealed the effect of regadenoson to transiently increase drug delivery to the CNS within 30 min-2 h after administration [14–19]. These provocative findings in rodents led us to conduct the studies reported in this manuscript to determine if FDA approved doses of regadenoson transiently disrupt an intact BBB in humans.
The integrity of the of BBB has been assessed radio-graphically for decades initially using radionuclide brain scans and more recently using computed tomography (CT) and magnetic resonance (MR) imaging [20–22]. The basic principle underlying these studies are that water soluble agents with a molecular weight greater than ≥160 D do not pass through an intact BBB but do enter regions of the brain where the BBB is disrupted [23, 24]. As a result, these contrast agents extravasate into areas of the brain with leaky blood vessels in patients with tumors, strokes, and infections where they are easily visualized using these imaging techniques. Quantification of isotope entry with SPECT imaging is done voxel by voxel to assess radioactivity concentration (absolute or relative) within varied brain regions. This technique is known to be less sensitive for smaller lesions or less disrupted areas [25–27]. CT has been shown to be more sensitive than SPECT in detecting smaller areas of BBB disruption because SPECT results in low spatial resolution [22].
SPECT and computed tomography (CT) are routine non-invasive imaging techniques used to diagnose coronary artery disease [28]. At our institution the imaging agents used for cardiac imaging are 99mTc-sestamibi (Tc-99m) radionuclide (778 D) for SPECT imaging and Iodixanol (Visipaque) (1550 D) for CT imaging. Tc-99m has both a hydrophilic cation portion and an isonitrile hydrophobic section that allows cell membrane uptake but has previously been noted as a substrate for the ATP binding cassette transporter, P-glycoprotein [29–32]. Its cellular uptake is strongly dependent on mitochondrial activity and the integrity of the cell membrane but its distribution, retention and clearance within viable tissue is very slow [33]. In contrast, Iodixanol is a nonionic hydrophilic compound without evidence that it is a substrate for any multi-drug resistance proteins/ABC transporters [34]. Both of these are considered water soluble imaging agents which have been shown to enter the brain only in regions where the blood-brain barrier is disrupted using CT and SPECT imaging [35–37].
Imaging of the heart and lungs is routinely done with two separate injections of the imaging agent, one before and one after regadenoson administration. In normal coronary arteries, regadenoson induces a profound increase in cardiac blood flow. Both of these imaging agents have been used in patients with primary brain tumors and thus it is known that they enter the brain only in regions where the BBB is disrupted [35, 36]. As a result, we asked patients undergoing clinically indicated pharmacologic cardiac stress tests if we could add brain imaging to their routine cardiac imaging, as part of a study, to determine if this would affect the brain penetration, distribution, and retention of Tc-99m and visipaque in this patient population. Additionally, we evaluated enhancement changes at varied times after regadenoson administration, in an attempt to discover the optimal time course of BBB disruption following adenosine receptor activation.
Materials/methods
Study subjects
Patients were eligible for this study if they were ≥18 years old, had been scheduled for a regadenoson pharmacologic stress test with SPECT and/or CT imaging, were not pregnant, and were able to provide written informed consent.
Imaging agents
The reagents, Regadenoson (Astellas Pharma, Northbrook, IL), Tc-99m Sestamibi (Mallinckrodt, St. Louis, MO) and Visipaque (GE Healthcare, Princeton, NJ) were all supplied by the Johns Hopkins pharmacy or a regional radiopharmacy. Patients were enrolled on one of two protocols which evaluate brain uptake of tracer agent by SPECT or CT. Each patient enrolled on these Johns Hopkins IRB approved trials received the standard doses of the regadenoson and the cardiac imaging agent for cardiac imaging. Regadenoson was given only once to each enrolled participant. For SPECT imaging, Tc-99m was given once for rest cardiac imaging and then again after regadenoson administration for stress cardiac imaging. For CT imaging, Visipaque was given once for rest cardiac imaging and then again after regadenoson administration for stress cardiac imaging. All participating patients gave written informed consent to participate on trial and both the clinical protocol and informed consent documents were approved by the Johns Hopkins Institutional Review Board.
SPECT studies
Patients were enrolled at the time of a clinically indicated cardiac stress test. After enrollment, patients were injected with 8–12 mCi of a 99mTc-labeled imaging radiotracer at rest according to clinical protocol. Sixty minutes later, rest gated myocardial SPECT images were acquired for 13–18 min using a dual-head gamma camera per the clinical standard. Brain imaging was added to the clinical protocol at varied time points from 0 to 60 min post radiotracer injection. Patients then underwent their clinically indicated pharmacologic stress testing using Regadenoson 400 mcg. Approximately 1–2 min following Regadenoson injection, the time of maximal cardiac blood flow increase, patients were injected with a second dose of 24–36 mCi (based on patient weight) of 99mTc-sestamibi imaging radiotracer. Approximately 60 min after radiotracer injection testing, stress gated myocardial SPECT images were acquired for 13–18 min using a dual-head gamma camera per the clinical standard. Again, brain SPECT images were acquired for 12–15 min using the same gamma camera as for the clinically indicated cardiac imaging. Brain imaging was repeated at 10–25, 26–41 or 42–57 min, depending on the imaging group, after radiotracer injection.
Brain SPECT images were reconstructed, stripped of any patient identifiers, coded using study specific numbers, and analyzed by Nuclear Medicine study personnel for quantitative evaluation. Brain SPECT images were reviewed closely for any abnormal radiotracer uptake. Brain SPECT images were reconstructed using the following parameters: OSEM, 2 iterations, 10 subsets, Butterworth filter with 0.55 critical frequency, power 10, no attenuation correction. Rest and stress images for each patient were registered using a rigid body algorithm (Mirada Medical). An isocontour region-of-interest (ROI) was automatically defined (PMOD) so as to encompass the whole head. This ROI was subsequently shrunk so as to produce a new irregularly shaped ROI that included only the brain. The brain ROI was then applied to both the registered rest and stress SPECT images and the total counts were recorded for each image. The counts at stress were scaled to account for the higher injected activities at stress compared to rest (i.e. the number or radioactive molecules accumulated in the brain).
CT studies
Patients undergoing standard cardiac CT had an additional non-contrast brain CT. This was followed by 40–75 mL of intravenous iodinated contrast (Visipaque®−320) was given as an infusion at a rate of 3–6 mL/sec. Approximately 20 min later, a repeat CT of the brain was performed. After approximately 21 min from the first Visipaque injection, 40–75 mL (based on patient weight) of Visipaque was injected, and within 2 min 0.4 mL of Regadenoson was administered. Repeat CT brain imaging was last obtained 10 min and then again 10 min later.
Four brain CT images were taken. The CT imaging obtained 2 mm slices with 4 detector rows at 120 kV, 200 mA at a 1.0 s rotation speed. Depending on patient size, we estimate the total dose was 0.052 rem per person. The 2 mm slices were obtained at the level of the basal ganglia with the goal of evaluating Visipaque uptake within the cortex, deep gray matter and white matter.
All CT imaging was performed on the Aquilion ONE, ViSION Edition scanner. This system is a 2nd generation 320 detector CT scanner with a 0.5 mm quantum detector, 320 rows with 640-slice reconstruction with 0.275 s rotation time. Several radiation dose reduction methods were implemented including: reduced scan length, low tube voltage, and iterative reconstruction methods. For brain CT imaging, a 2 mm slice reconstruction was performed.
Results
A total of twelve patients were accrued to this study and analyzed. Demographic and imaging information are provided in Table 1. The average age of the patients studied was 60 years old and 8 of the 12 patients were female. Seven patients received brain SPECT imaging and 99mTc-sestamibi with and without regadenoson. Five patients received brain CT imaging and visipaque with and without regadenoson. All patients tolerated the studies without unexpected adverse events. There were no incidental findings noted with either imaging techniques.
Table 1.
Patient demographics and imaging modalities of cardiac stress patients
| Patient | Gender | Age | Imaging modality |
|---|---|---|---|
| 1 | F | 66 | SPECT |
| 2 | M | 48 | SPECT |
| 3 | F | 57 | SPECT |
| 4 | F | 62 | SPECT |
| 5 | F | 74 | SPECT |
| 6 | F | 60 | SPECT |
| 7 | M | 50 | SPECT |
| 8 | F | 66 | CT |
| 9 | M | 53 | CT |
| 10 | M | 67 | CT |
| 11 | F | 62 | CT |
| 12 | F | 55 | CT |
In the patients undergoing SPECT imaging, the mean 99mTc-sestamibi uptake pre-regadenoson and post-regadenoson were 725.9 ± 230 and 757.0 ± 236 counts respectively (P = 0.69). As seen in Fig. 1, there was no evident differences in mean isotope uptake within the brain, when imaging was performed an average of 26 min (21–38 min) after regadenoson administration. In the patients undergoing brain CT with and without regadenoson there were no differences in the mean HU recorded: 29.3 ± 2.4 versus 30.2 ± 1.8 respectively (P=0.13). Figure 2, depicts the lack of statistically significant difference between contrast enhancement obtained 20 min after visipaque injection, with or without regadenoson administration.
Fig. 1.
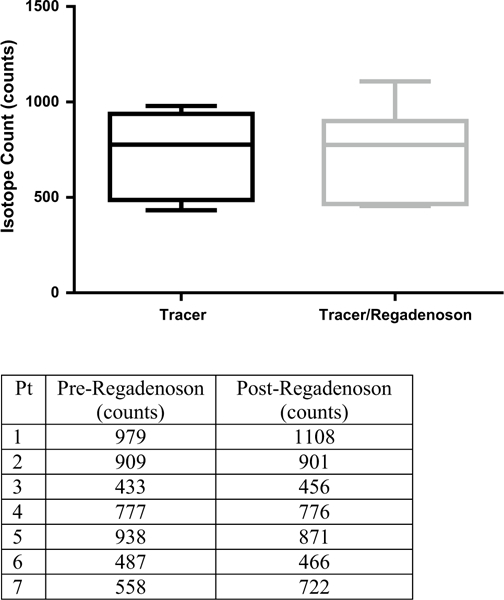
Tc99-Sestamibi mean brain uptake with varied conditions. There was no significant difference seen after regadenoson administration (P = 0.69)
Fig. 2.
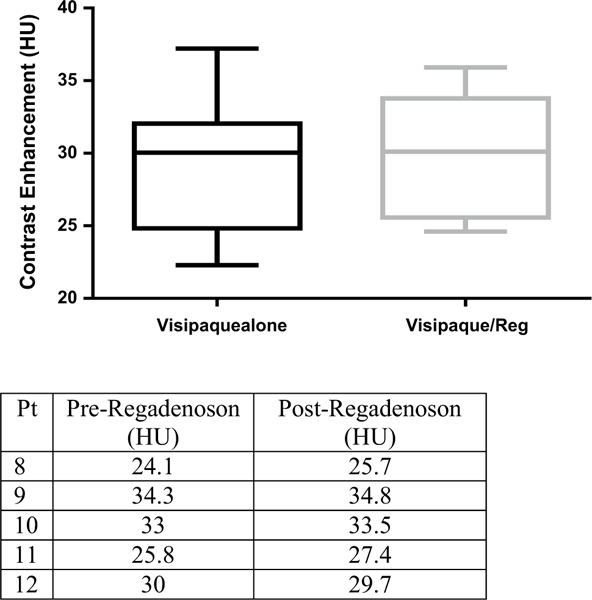
Mean brain visipaque contrast uptake with varied conditions. Regadenoson poses little effect on contrast enhancement within the brain 20 min after administration (P = 0.13)
Discussion
The blood-brain barrier affords maximal protection against natural infections and toxins. It also presents a formidable obstacle for the delivery of administered agents to the brain in therapeutic concentrations. This likely plays a major role in the failure to make the progress in the treatment of CNS malignancies that has been observed in other systemic, cancers. The study described in this manuscript represents an effort to repurpose an FDA approved drug routinely used for chemical cardiac stress tests to one that could transiently disrupt the BBB allowing higher concentrations of oral or intravenously administered agents to penetrate the brain. Although this agent appears to do this effectively in rodents, the results presented in this manuscript fail to demonstrate that regadenoson allows even small contrast agents to penetrate an intact human BBB.
The data using an adenosine A2A receptor agonist for this purpose in rodents is quite convincing. Bynoe et al. demonstrated that regadenoson increases delivery of high molecular weight dextran (MW 70,000 D) and a hydrophilic chemotherapeutic agent (gemcitabine, MW 263 D) by decreasing the expression of multidrug resistance and tight junction proteins [14, 15, 18]. In these studies, the best results were found when regadenoson was administered 30 min after administration of the dextran or gemcitabine as demonstrated using fluorescence and drug quantitative assays. In addition, rodent studies evaluating the penetration of temozolomide (MW 194 D) are also intriguing. This agent is the only one that produces a clear survival benefit in patients with malignant brain tumors and yet brain levels are only about 20% of those found in blood [38, 39]. Regadenoson resulted in a 60% increase in the concentrations of temozolomide in the brains of rodents with an intact BBB without affecting its systemic pharmacology [16].
These observations in rodents provided the basis for the imaging study reported in this manuscript. By design, diagnostic agents used for brain imaging allow changes in the integrity of the BBB to be readily visualized using modern imaging techniques. As a result, we expected to see some changes after the administration of regadenoson in humans with normal blood brain barriers using 99mTc-sestamibi or visipaque as imaging agents. There are several potential reasons why this was not observed: (1) regadenoson does not have the same effect in humans because the human and rodent BBB are different, (2) the administered dose of regadenoson was not effective, (3) the schedule of regaden-oson administration was ineffective, (4) the wrong imaging time was used or (5) the signal relative to noise with the imaging agents was insufficient to detect small changes in radiotracer uptake. Each of these issues is addressed below.
This study was conducted in patients with suspected cardiac disease who had a clinical indication for the administration of regadenoson. As per our IRB approval, we asked patients permission to merely add brain imaging to their pre-planned cardiac studies. As a result, we were restricted to the standard dose of 0.4 mg of regadenoson and to the imaging times other than when the heart was being imaged. It is quite possible that the optimal regadenoson dose and schedule for cardiac stress testing is different than the ideal dose or schedule needed to transiently disrupt the blood-brain barrier. Other doses and schedules have been explored. Fields et al. demonstrated the efficacy of a continuous low-dose regadenoson infusion over 12 h in patients with sickle cell disease [40]. Additionally, they found that steady state regadenoson plasma concentrations occurred 6 h from the start of infusion when given at a dose of 1.44 μg/kg/h. In preclinical studies, Kochanek et al. used MRI to assess cerebral blood flow after continuous A2A receptor stimulation. They reported increased cerebral blood flow after intrahippocampal administration in uninjured rat brains [41]. The receptor activation increased ipsilateral hippocampal and cortex blood flow by four to five times compared to the normal contralateral brain. These studies suggest that a lower continuous dose of A2A receptor stimulation (systemic or local administration) may have a more robust effect on cerebral vasculature aiding in transient BBB disruption. It is also possible that repeated dosing of regadenoson could provide additional benefit in disrupting the blood-brain barrier. Previous studies using three doses of regadenoson in rodents demonstrated a clear increase in 10 kD dextran within the brain [15]. Moreover, human studies using three doses of regadenoson have found this to be safe and feasible with resultant higher serum concentrations for up to 4 h [42].
In addition, the best time to evaluate the effect of regadenoson on human blood-brain barrier has not yet been explored. Our study was restricted by the clinically indicated and standard cardiac stress test procedures. As a result, both CT and SPECT imaging were performed approximately 0–60 min after the regadenoson administration. Yet, the regadenoson rodent studies demonstrated a therapeutic effect that lasted from 30 to 120 min [15, 16, 18]. As suggested by the data presented above, it is possible that regadenoson will work in humans as it does in rodents with different dosing and administration schedules. As a result, future studies of regadenoson’s ability to transiently disrupt the BBB should explore multiple drug doses, variations in schedule, and imaging times.
It is also possible that co-administration of regadenoson with other vasoactive agents that could result in a more pronounced therapeutic effect. For example, cereport is a bradykinin B2 receptor agonist that preclinically demonstrated increased drug entry to high molecular weight dextran (70 kD), Evans blue (60 kD when bound to albumin) and carboplatin following intracarotid or intravenous administration [43, 44]. Additionally, its effect on concentrations of chemotherapy in the brain were maintained beyond the short half-life of the drug (2–5 min) [45]. However, when cereport was administered with carboplatin in adults and children with high grade gliomas there was no obvious clinical benefit [8, 13]. The apparent mismatch between findings in the rodents and humans is strikingly similar to our findings with regadenoson and deserves further study. However, combining these two vasoactive receptor agonists may provide a measurable effect in humans.
Finally, a small regadenoson effect on the blood-brain barrier may have gone undetected by our imaging methods. Modest uptake of both 99mTc-sestamibi and the background noise inherent in SPECT and/or CT studies may yield results that are below the level of detection with these methods. For example, a doubling of uptake of very low uptake tracer may remain difficult to detect if the method is operating near its sensitivity floor. In addition, with the IV contrast, some of the signal may be intravascular, and this could mask some of the brain parenchymal activity. Also possible is that both entry and exit of the imaging agents were equally affected by the regadenoson, potentially meaning more tracer entered but more also leaked out resulting in a net “no change” in tracer accumulation to the brain. We believe these scenarios are unlikely, but may complicate the assessment of minor alternations to the blood brain barrier. Unfortunately, direct pharmacologic measurements of administered agents require invasive placements of microdialysis catheters or brain biopsy.
In summary, while animal studies have demonstrated that regadenoson transiently increases the permeability of the BBB to dextran and temozolomide, we were unable, to document changes in the penetration of contrast agents in humans with two different non-invasive imaging methods using the dose of regadenoson which is FDA approved for cardiac evaluation. Pursuing this line of investigation is critical to improving the delivery of therapeutic agents in patients with malignancies of the central nervous system. As noted above, additional research is required to explore alternate regadenoson doses, schedules, and combination regimens designed to transiently disrupt the blood-brain barrier and allow higher concentrations of systemically administered chemotherapy to reach the central nervous system.
Acknowledgements
Thank you to the JH Nuclear Medicine and Cardiology departments for assistance with patient recruitment, and image acquisition.
Funding This work was supported in part by fellowship support T32GM066691 in Johns Hopkins Clinical pharmacology training program (Sadhana Jackson).
Footnotes
Compliance with ethical standards
Conflict of interest None.
References
- 1.Pokorny JL, et al. (2015) The efficacy of the Weel inhibitor MK-1775 combined with temozolomide is limited by heterogeneous distribution across the blood-brain barrier in glioblastoma. Clin Cancer Res 21(8):1916–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders NR, et al. (2016) The biological significance of brain barrier mechanisms: help or hindrance in drug delivery to the central nervous system?. F1000Res. doi: 10.12688/f1000research.7378.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brastianos HC, Cahill DP, Brastianos PK (2015) Systemic therapy of brain metastases. Curr Neurol Neurosci Rep 15(2):518. [DOI] [PubMed] [Google Scholar]
- 4.Lockman PR et al. (2010) Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16(23):5664–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carman AJ et al. (2015) Expert consensus document: mind the gaps-advancing research into short-term and long-term neuropsychological outcomes of youth sports-related concussions. Nat Rev Neurol 11(4):230–244 [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ et al. (2005) A Phase I study of concurrent RMP-7 and carboplatin with radiation therapy for children with newly diagnosed brainstem gliomas. Cancer 104(9):1968–1974 [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ et al. (2005) Phase 1 study of concurrent RMP-7 and carboplatin with radiotherapy for children with newly diagnosed brainstem gliomas. Cancer 104(6):1281–1287 [DOI] [PubMed] [Google Scholar]
- 8.Warren K et al. (2006) Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children’s Oncology Group. Cancer Chemother Pharmacol 58(3):343–347 [DOI] [PubMed] [Google Scholar]
- 9.Doolittle ND et al. (2014) Delivery of chemotherapeutics across the blood-brain barrier: challenges and advances. Adv Pharmacol 71:203–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlongan CV, Emerich DF (2003) Facilitation of drug entry into the CNS via transient permeation of blood brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, Cereport. Brain Res Bull 60(3):297–306 [DOI] [PubMed] [Google Scholar]
- 11.Zhang X et al. (2004) The effect of RMP-7 and its derivative on transporting Evans blue liposomes into the brain. Drug Deliv 11(5):301–309 [DOI] [PubMed] [Google Scholar]
- 12.Bartus RT et al. (2000) Evidence that Cereport’s ability to increase permeability of rat gliomas is dependent upon extent of tumor growth: implications for treating newly emerging tumor colonies. Exp Neurol 161(1):234–244 [DOI] [PubMed] [Google Scholar]
- 13.Prados MD et al. (2003) A randomized, double-blind, placebo¬controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol 5(2):96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bynoe MS et al. (2015) Adenosine receptor signaling: a key to opening the blood-brain door. Fluids Barriers CNS 12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carman AJ et al. (2011) Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci 31(37):13272–13280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson S et al. (2016) The effect of regadenoson-induced transient disruption of the blood-brain barrier on temozolomide delivery to normal rat brain. J Neurooncol 126(3):433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DG, Bynoe MS (2015) A2A Adenosine receptor regulates the human blood-brain barrier permeability. Mol Neurobiol 52(1):664–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DG, Bynoe MS (2016) A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J Clin Invest 126(5):1717–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan Y et al. (2016) Preliminary study on assessment of lexis-can-induced blood-brain barrier opening and its level by CT perfusion imaging. Zhonghua Yi Xue Za Zhi 96(35):2825–2829 [DOI] [PubMed] [Google Scholar]
- 20.Walovitch RC, Williams SJ, Lafrance ND (1990) Radiolabeled agents for SPECT imaging of brain perfusion. Int J Rad Appl Instrum B 17(1):77–83 [DOI] [PubMed] [Google Scholar]
- 21.Leggett DA, Miles KA, Kelley BB (1999) Blood-brain barrier and blood volume imaging of cerebral glioma using functional CT: a pictorial review. Eur J Radiol 30(3):185–190 [DOI] [PubMed] [Google Scholar]
- 22.Abraham T, Feng J (2011) Evolution of brain imaging instru¬mentation. Semin Nucl Med 41(3):202–219 [DOI] [PubMed] [Google Scholar]
- 23.Levin VA et al. (1984) Relationship of octanol/water partition coefficient and molecular weight to cellular permeability and partitioning in s49 lymphoma cells. Pharm Res 1(6):259–266 [DOI] [PubMed] [Google Scholar]
- 24.Levin VA, Patlak CS, Landahl HD (1980) Heuristic modeling of drug delivery to malignant brain tumors. J Pharmacokinet Biop-harm 8(3):257–296 [DOI] [PubMed] [Google Scholar]
- 25.Garrigue P et al. (2016) Single photon emission computed tomography imaging of cerebral blood flow, blood-brain barrier disruption, and apoptosis time course after focal cerebral ischemia in rats. Int J Stroke 11(1):117–126 [DOI] [PubMed] [Google Scholar]
- 26.Gilad R et al. (2012) SPECT-DTPA as a tool for evaluating the blood-brain barrier in post-stroke seizures. J Neurol 259(10):2041–2044 [DOI] [PubMed] [Google Scholar]
- 27.Veksler R, Shelef I, Friedman A (2014) Blood-brain barrier imaging in human neuropathologies. Arch Med Res 45(8):646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patchett ND, Pawar S, Miller EJ (2016) Visual identification of coronary calcifications on attenuation correction CT improves diagnostic accuracy of SPECT/CT myocardial perfusion imaging. J Nucl Cardiol 1–10 [DOI] [PubMed] [Google Scholar]
- 29.Rao VV et al. (1994) Expression of recombinant human multidrug resistance P-glycoprotein in insect cells confers decreased accumulation of technetium-99m-sestamibi. J Nucl Med 35(3):510–515 [PubMed] [Google Scholar]
- 30.Levine GN, (2014) Cardiology secrets Secrets series 4th ed., Philadelphia, PA: Elsevier/Saunders. p [Google Scholar]
- 31.Gupta Y et al. (2007) P-glycoprotein expression is associated with sestamibi washout in primary hyperparathyroidism. Br J Surg 94(12):1491–1495 [DOI] [PubMed] [Google Scholar]
- 32.Dyszlewski M et al. (2002) Characterization of a novel 99mTccarbonyl complex as a functional probe of MDR1 P-glycoprotein transport activity. Mol Imaging 1(1):24—35 [DOI] [PubMed] [Google Scholar]
- 33.Travin MI, Bergmann SR (2005) Assessment of myocardial viability. Semin Nucl Med 35(1):2–16 [DOI] [PubMed] [Google Scholar]
- 34.Spencer CM, Goa KL (1996) Iodixanol. A review of its pharma¬codynamic and pharmacokinetic properties and diagnostic use as an X-ray contrast medium. Drugs 52(6):899–927 [DOI] [PubMed] [Google Scholar]
- 35.Cecchin D et al. (2009) Presurgical (99m)Tc-sestamibi brain SPET/CT versus SPET: a comparison with MRI and histological data in 33 patients with brain tumours. Nucl Med Commun 30(9):660–668 [DOI] [PubMed] [Google Scholar]
- 36.Ak I et al. (2003) Tc-99m MIBI uptake and its relation to the proliferative potential of brain tumors. Clin Nucl Med 28(1):29–33 [DOI] [PubMed] [Google Scholar]
- 37.Horsch AD et al. (2016) Relation between stroke severity, patient characteristics and CT-perfusion derived blood-brain barrier per¬meability measurements in acute ischemic stroke. Clin Neuroradiol 26(4):415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portnow J et al. (2009) The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res 15(22):7092–7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostermann S et al. (2004) Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res 10(11):3728–3736 [DOI] [PubMed] [Google Scholar]
- 40.Field JJ et al. (2013) Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood 121(17):3329–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochanek PM et al. (2005) Characterization of the effects of adenosine receptor agonists on cerebral blood flow in uninjured and traumatically injured rat brain using continuous arterial spinlabeled magnetic resonance imaging. J Cereb Blood Flow Metab 25(12):1596–1612 [DOI] [PubMed] [Google Scholar]
- 42.Townsend R et al. (2015) Safety and tolerability of intravenous regadenoson in healthy subjects: a randomized, repeat-dose, placebo-controlled study. J Nucl Cardiol 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inamura T, Black KL (1994) Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J Cereb Blood Flow Metab 14(5):862–870 [DOI] [PubMed] [Google Scholar]
- 44.Emerich DF et al. (1999) Enhanced delivery of carboplatin into brain tumours with intravenous Cereport (RMP-7): dramatic differences and insight gained from dosing parameters. Br J Cancer 80(7):964–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emerich DF et al. (2001) The development of the bradykinin agonist labradimil as a means to increase the permeability of the blood-brain barrier: from concept to clinical evaluation. Clin Pharmacokinet 40(2):105–123 [DOI] [PubMed] [Google Scholar]


