Abstract
The reaction between Mo(O)(CHAro)(ORF6)2(PMe3) (Aro = ortho-methoxyphenyl, ORF6 = OCMe(CF3)2) and two equivalents of LiOHMT (OHMT = O-2,6-(2,4,6-Me3C6H2)2C6H3) leads to Mo(O)(CHAro)(OHMT)2, an X-ray structure of which shows it to be a trigonal bipyramidal anti benzylidene complex in which the o-methoxy oxygen is coordinated to the metal trans to the apical oxo ligand. Addition of one equivalent of water (in THF) to the benzylidyne complex, Mo(CArp)(OR)3(THF)2 (Arp = para-methoxyphenyl, OR = ORF6 or OC(CF3)3 (ORF9)) leads to formation of {Mo(CArp)(OR)2(μ-OH)(THF)}2(μ-THF) complexes. Addition of one equivalent of a phosphine (L) to Mo(CArp)(ORF9)3(THF)2 in THF, followed by addition of one equivalent of water, all at room temperature, yields Mo(O)(CHArp)(ORF9)2(L) complexes in good yields for several phosphines (e.g., PMe2Ph (69% by NMR), PMePh2 (59%), PEt3 (69%), or P(i-Pr)3 (65%)). The reaction between Mo(O)(CHArp)(ORF9)2(PEt3) and two equivalents of LiOHMT proceeds smoothly at 90 °C in toluene to give Mo(O)(CHArp)(OHMT)2, a four-coordinate syn alkylidene complex. Mo(O)(CHArp)(OHMT)2 reacts with ethylene (1 atm in C6D6) to give (in solution) a mixture of Mo(O)(CHArp)(OHMT)2, Mo(O)(CH2)(OHMT)2, and an unsubstituted square pyramidal metallacyclobutane complex, Mo(O)(CH2CH2CH2)(OHMT)2, along with ethylene and ArpCH=CH2. Mo(O)(CHArp)(OHMT)2 also reacts with 2,3-dicarbomethoxynorbornadiene to yield syn and anti isomers of the “first-insertion” products that contain a cis C=C bond.
The neopentylidyne ligand in (t-BuCH2)3M≡C-t-Bu (M = W1 or Mo2) complexes is formed through what amounts to an intramolecular deprotonation of an α carbon atom in one alkyl ligand by another in some multialkyl intermediate to give an alkylidene first,3 followed by a second (more facile) α deprotonation of that alkylidene by an alkyl to give the alkylidyne.4 Synthesis of M(C-t-Bu)(1,2-dimethoxyethane)Cl3 and M(C-t-Bu)(OR)3 complexes (OR is a sterically demanding alkoxide or aryloxide) and the demonstration that the latter are initiators for catalytic alkyne metathesis5 led to the development of four-coordinate alkene metathesis initiators of the type M(NR’)(CHR”)(OR)2 (M = Mo or W).6,7 Synthetic routes to imido alkylidene complexes of Mo and W are based on a single α hydrogen abstraction/deprotonation reaction in a dineopentyl or dineophyl complex.8 Syntheses of oxo alkylidenes, which have been proposed to be the type of alkene metathesis catalysts present in “classical” heterogeneous catalyst systems,9 by analogous methods have been more challenging. Tungsten oxo alkylidene complexes were prepared through α hydrogen abstraction in tungsten dialkyls in 2012,10 but syntheses of molybdenum oxo alkylidene complexes that are active for metathesis of olefins have remained elusive.11. Recently we began to explore the synthesis of Mo oxo alkylidene complexes through addition of water to alkylidyne complexes.7,12
Addition of one equiv of water to Mo(CAro)(ORF6)3(dme) (Aro = ortho-methoxyphenyl) gives the dimeric benzylidyne hydroxo complex, {Mo(CAro)(ORF6)2(μ-OH)}2(μ-dme) in which each bridging hydroxo proton is hydrogen-bonded to the ortho methoxy oxygen in the o-methoxybenzylidyne ligand. (This is a rare example of a controlled hydrolysis of a high oxidation state alkylidene or alkylidyne.13,14) Addition of PMe3 to {Mo(CAro)(ORF6)2(μ-OH)}2(μ-dme) led to formation of Mo(O)(CHAro)(ORF6)2(PMe3), from which Mo(O)(CHAro)Cl2(PMe3) and Mo(O)(CHAro)(OHIPT)Cl(PMe3) (OHIPT = O-2,6-(2,4,6-i-Pr3C6H2)2C6H315) were prepared. In the presence of B(C6F5)3 Mo(O)(CHAro)(OHIPT)Cl(PMe3) was shown to be active for the stereoselective ring-opening metathesis polymerization of 2,3-dicarbomethoxynorbornadiene (DCMNBD) and rac-2,3-dicarbomethoxy-5-norbornene (DCMNBE), and the homocoupling of 1-decene to 9-octadecene, consistent with analogous reactions that use tungsten-based initiators16 and with removal of PMe3 to form active metathesis initiators. Important questions are whether an ortho-methoxy group in the benzylidyne and benzylidene ligands is required for a controlled reaction involving water and an alkylidyne complex and whether phosphine-free complexes that are active for olefin metathesis can be prepared and isolated. Some answers to these questions are provided in this communication.
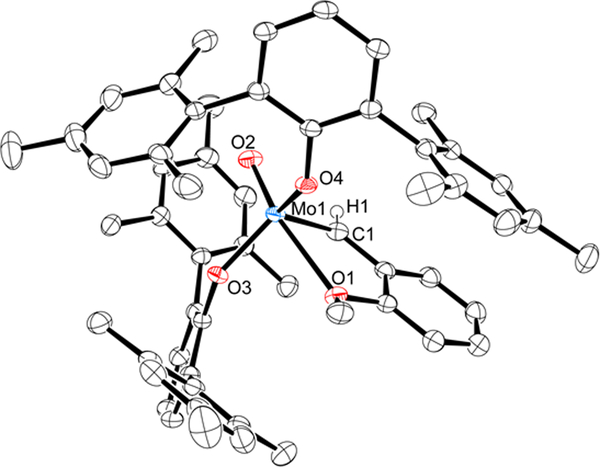
The synthesis and stability of W(O)(CH-t-Bu)(OHMT)2 (OHMT = O-2,6-(2,4,6-Me3C6H2)2C6H3 or hexamethylterphenoxide17)10a suggested that the phosphine-free target should be Mo(O)(CHAro)(OHMT)2 (1-Aro); it was prepared from Mo(O)(CHAro)(ORF6)2(PMe3) as shown in eq 1. Trimethylphosphine is lost in the process as a consequence of sterichindrance overall in combination with binding of the methoxide oxygen in the Aro group in the anti alkylidene to the metal trans to the oxo ligand, as shown through an X-ray study (Fig 1; see SI). The τ value18 (0.78) suggests that the configuration at the metal is closest to a trigonal bipyramid (O2-Mo1-O1 = 166.27(7)°). Alkylidene proton resonances were observed in the initial proton NMR spectrum of 1-Aro at 11.92 ppm for the anti isomer (97%, JCH = 156 Hz), the isomer found in the solid state (Fig 1), and at 12.02 ppm for the syn isomer (JCH = 134 Hz), in which the alkylidene has rotated19 by 180° and the methoxide is not bound to the metal. After 1 h at 50 °C an 85:15 equilibrium ratio of anti to syn isomers was observed.
Figure 1.
A drawing of the structure of 1-Aro; Mo1-C1 = 1.933(2) Å, Mo1-O2 = 1.6698(14) Å, Mo1-O1 = 2.4865(17) Å, Mo1-O3 = 1.915(2) Å, Mo1-O4 = 1.9295(15) Å.
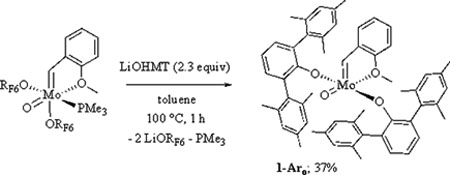 |
(1) |
Preliminary studies showed that the rate of reaction of 1-Aro with ethylene is slow, perhaps because the lower energy “methoxy-bound” anti form only slowly converts to the more reactive syn form. We therefore turned to a synthesis of the p-methoxybenzylidene analog where intramolecular binding of the methoxy oxygen is not possible.
The para-methoxybenzylidyne complex, Mo(CArp)(dme)Br3 was prepared by the “low oxidation state” procedure.20 From it Mo(CArp)(ORF6)3(dme)21 and Mo(CArp)(ORF6)3(THF)222 were prepared without complications. Addition of one equivalent of water (in THF, diethyl ether, or dichloromethane) to Mo(CArp)(ORF6)3(THF)2 led to the formation of {Mo(CArp)(ORF6)2(μ-OH)}2(THF)3 (2-ArpF6) in good yield (eq 2). It was crystallized from a mixture of pentane and CH2Cl2 at – 40 °C in the presence of several equivalents of THF and isolated in 70% yield as pale red crystals. Similar reactions starting with Mo(CArp)(ORF6)3(dme) and one equivalent of water (in THF) also led to 2-ArpF6, but in poor yield (~10%), while addition of water in dme to Mo(CArp)(ORF6)3(dme) did not lead to an isolable benzylidyne hydroxo complex. Dme appears to be detrimental and THF beneficial for formation of benzylidyne hydroxo complexes in these circumstances.
 |
(2) |
An X-ray study of 2-ArpF6 (Fig 2) shows its structure to be analogous to that of previously reported12 {Mo(CAro)(ORF6)2(μ-OH)}2(μ-dme) with one of the THFs bridging the two Mo centers and one THF hydrogen-bonded to each of the two bridging hydroxos. The OH...O angles and O...O distances are consistent with a “classical” hydrogen bond between the bridging hydroxo protons and THF oxygens.23 The hydroxo proton resonance in 2-ArpF6 is found at 6.60 ppm in the proton NMR spectrum in CD2Cl2 (cf. 9.30 ppm for the hydroxo protons in {Mo(CAro)(ORF6)2(μ-OH)}2(μ-dme)). The Mo-O distance to the bridging THF is 2.485(2) Å. The Mo-C-Cipso angles are 179.4(2)°, in contrast to 168.57(7)° and 167.33(7)° in {Mo(CAro)(ORF6)2(μ-OH)}2(μ-dme), which result from the hydroxo protons being hydrogen bonded to the o-methoxy oxygens in the Aro group instead of to THF, as found in 2-ArpF6. Other distances and angles are not unusual (see SI). In solution, proton NMR resonances for the bridging THF are distinct from those for the hydrogen-bonded THFs or THF in solution. The hydrogen-bonded THFs are readily lost from solid samples, especially in vacuo, to give {Mo(CArp)(ORF6)2(μ-OH)}2(μ-THF), and exchange rapidly on the NMR scale with free THF in solution, as shown through proton NMR studies. Addition of dioxane to an NMR sample of {Mo(CArp)(ORF6)2(μ-OH)}2(μ-THF) led to formation of a mixture of the μ-THF (δOH at 6.63 ppm) and μ-dioxane (δOH at 6.51 ppm) complexes.
Figure 2.
End view of the structure of 2-ArpF6.
Addition of one equivalent of water (in THF) to Mo(CArp)(ORF9)3(THF)2 in CH2Cl2 led to formation of {Mo(CArp)(ORF9)2(μ-OH)}2(μ-THF), which could be isolated in 43 % yield as lime-green crystals; in the presence of additional THF it crystallizes as {Mo(CArp)(ORF9)2(μ-OH)(THF)}2(μ-THF) (2-ArpF9). An X-ray structural study showed its structure to be entirely analogous to that for 2-ArpF6 (see SI for details). The two molecules of hydrogen-bonded THF in 2-ArpF9 again are lost readily from solid samples, especially in vacuo. The μ-OH resonance is found at 8.21 ppm in 2-ArpF9 in CD2Cl2 (cf. 6.60 ppm in 2-ArpF6).
Reactions between 2-ArpF6 or 2-ArpF9 and PMe3 or PEt3 (L) do not yield Mo(O)(CHArp)(OR)2(L) complexes readily and in high yield. However, addition of one equivalent of L to Mo(CArp)(ORF9)3(THF)2, followed by addition of one equivalent of water, all at room temperature in THF, yields Mo(O)(CHArp)(OR)2(L) complexes (3) in good yields for several phosphines L (e.g., PMe2Ph (69% by NMR), PMePh2 (59%), PEt3 (69%), or P(i-Pr)3 (65%)). For example, Mo(O)(CHArp)(ORF9)2(PEt3) (3-PEt3) can be prepared in this manner (eq 3). These results suggest that in most cases it is best to avoid formation of bis(μ-OH) dimers. We propose that THF discourages formation of a hydroxo benzylidyne dimer from monomeric Mo(CArp)(ORF9)2(OH)(THF)x (x unknown). We also propose that the hydroxo proton migrates to the benzylidyne α carbon intramolecularly in intermediate Mo(CArp)(ORF9)2(OH)(THF)x to give Mo(O)(CHArp)(ORF9)2(THF)x, which is then trapped by L to give Mo(O)(CHArp)(ORF9)2(L) complexes. Phosphines can also bind to Mo in Mo(CArp)(ORF9)2(OH)(THF)x and help prevent formation of μ-OH products and promote (perhaps along with THF itself) migration of the proton from O to C. Formation of ORF6 analogs of 3 at room temperature in THF does not appear to be as successful, most likely because the migrating proton is less acidic in ORF6 hydroxo benzylidyne complexes. Studies that address multiple mechanistic issues are ongoing.
 |
(3) |
Mo(O)(CHArp)(ORF9)2(PEt3) (3-PEt3) was chosen to proceed further toward the goal of preparing a four-coordinate complex. The reaction between 3-PEt3 and two equivalents of LiOHMT proceeds smoothly at 90 °C in toluene over a period of 1.5 h to give Mo(O)(CHArp)(OHMT)2 (1-Arp) which could be isolated as plum-purple needles (eq 4). The alkylidene proton resonance is found at 11.20 ppm (in C6D6) with JCH = 130 Hz, consistent with the alkylidene being in the syn conformation. The corresponding anti isomer was not found in solution even upon heating a sample of 1-Arp at 100 °C, which suggests that coordination of an ortho methoxide in 1-Aro (Fig 1) stabilizes the anti form. The OHMT ligands in the proton NMR spectrum at 22 °C are equivalent on the NMR time scale through mirror symmetry. The purple color of 1-Arp (λmax = 532 nm with ε532 ~ 400) is unusual for a “d0” complex of this general type. We propose that the purple color arises from a weak LMCT transition that involves the p-methoxybenzylidene ligand. Compound 1-Arp also was prepared from 3-PMePh2 in 66% yield on a 500 mg scale at 70 °C in 3.5 h.
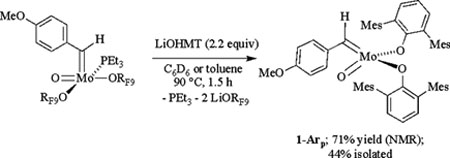 |
(4) |
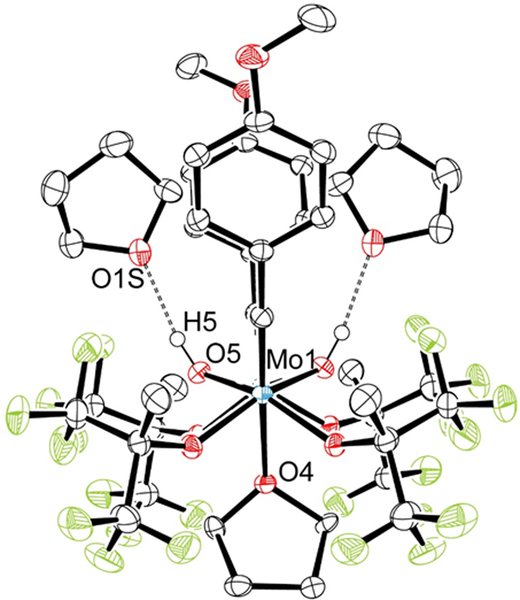
An X-ray structural study of 1-Arp (Fig 3) reveals that the OHMT terphenyl groups are roughly “interlocked,” as they are in W(O)(CH2)(OHMT)2,10b although they rotate readily around the Mo-O bonds on the NMR time scale in solution at 22 °C. The relevant distances and angles are similar to those in 1-Aro (see Fig 1 and SI). Together the two OHMT ligands prevent PEt3 or PMePh2 from remaining bound to the metal in the final product and also provide a significant degree of steric protection against bimolecular decomposition reactions.
Figure 3.
A drawing of the structure of 1-Arp;
Mo1-C1 = 1.9004(13) Å, Mo1-O1 = 1.6803(10) Å, Mo1-O3 = 1.9064(9) Å, Mo1-O4 = 1.9113(9) Å, Mo1-C1-Cipso = 137.42(10)°, Mo1-O3-Cipso = 143.11(8)°, Mo1-O4-Cipso = 127.58(8)°.
Compound 1-Arp reacts with ethylene (1 atm in C6D6 at 22 °C) to give an orange solution that contains a mixture of 1-Arp, Mo(O)(CH2)(OHMT)2, and an unsubstituted metallacyclobutane complex, Mo(O)(CH2CH2CH2)(OHMT)2, along with ArpCH=CH2, and ethylene, according to proton NMR studies. At a molybdenum concentration of 17 mM in C6D6, the ratio of Mo=CHArp : Mo=CH2 : Mo(C3H6) : ArpCH=CH2 is 57:5:38:43 (43% conversion) after 1.5 h. The 1:1:2:2 pattern of the four metallacycle proton resonances in Mo(O)(CH2CH2CH2)(OHMT)2 at 2.92, 2.39, 1.68, and 0.45 ppm suggest that it has a square pyramidal structure.24 So far, efforts to isolate Mo(O)(CH2CH2CH2)(OHMT)2, an analog of isolable W(O)(CH2CH2CH2)(OHMT)2,10b have not been successful.
Compound 1-Arp does not react readily with several equivalents of Z-5-decene at 22°C. It does react with 2,3-dicarbomethoxynorbornadiene (4 equiv) to yield a 17:1 mixture of syn (δ Hα at 11.63 ppm; eq 5) and anti isomers (not shown in eq 5) of the first insertion product, each of which contains a cis C=C bond.25 (The yield (by NMR) is ~90%; see SI for details.) Both results are consistent with a relatively sterically demanding coordination sphere that limits access to an alkylidene, in contrast to Mo(O)(CHAro)(OHIPT)Cl formed through removal of PMe3 from Mo(O)(CHAro)(OHIPT)(PMe3)Cl, which forms polymers from 2,3-dicarbomethoxynorbornadiene readily.12
 |
(5) |
We conclude that the o-methoxy group is not required, either for forming a benzylidene ligand from a benzylidyne ligand or for stabilizing a bis(OHMT) oxo benzylidene complex. The most successful syntheses of oxo alkylidene complexes involve intramolecular α proton migrations from O to C, where C is the benzylidyne α carbon, and are most successful when OR is ORF9, the solvent is THF, and a phosphine is available to trap the oxo benzylidene product and possibly help form it. Several phosphine adducts of molybdenum oxo benzylidene complexes are accessible via reactions between Mo(CArp)(OR)3(THF)2 complexes and water. We can now predict that other metathesis-active molybdenum oxo alkylidene complexes will be observed or isolated under the right circumstances, and look forward to comparing their metathesis reactions with those catalyzed by tungsten analogs.10,16
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for financial support from the National Institutes of Health (GM-59426). We also thank the NSF for support of X-ray diffraction instrumentation (CHE-0946721).
Footnotes
Supporting Information
Experimental details of the syntheses of all compounds, NMR and spectral data, details of the metathesis experiments, and X-ray crystallographic files. This material is available free of charge via the Internet at http://pubs.acs.org.
Accession Codes
CCDC 1865865−1865868 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interests.
REFERENCES
- (1).Clark DN; Schrock RR Multiple Metal–Carbon Bonds. 12. Tungsten and Molybdenum Neopentylidyne and Some Tungsten Neopentylidene Complexes. J. Am. Chem. Soc 1978, 100, 6774–6776. [Google Scholar]
- (2).(a) McCullough LG; Schrock RR Multiple Metal–Carbon Bonds. 34. Metathesis of Acetylenes by Molybdenum(VI) Alkylidyne Complexes. J. Am. Chem. Soc 1984, 106, 4067–4068. [Google Scholar]; (b) McCullough LG; Schrock RR; Dewan JC; Murdzek JS Multiple Metal-Carbon Bonds. 38. Preparation of Trialkoxymolybdenum(VI) Alkylidyne Complexes, Their Reactions with Acetylenes, and the X-ray Structure of Mo[C3(CMe3)2][OCH(CF3)2]2(C5H5N)2. J. Am. Chem. Soc 1985, 107, 5987–5998. [Google Scholar]
- (3).Schrock RR Alkylcarbene Complex of Tantalum by Intramolecular α-Hydrogen Abstraction. J. Am. Chem. Soc 1974, 96, 6796–6797. [Google Scholar]
- (4).Guggenberger LJ; Schrock RR A Tantalum Carbyne Complex. J. Am. Chem. Soc 1975, 97, 2935. [Google Scholar]
- (5).Schrock RR in Handbook of Metathesis, Grubbs RH, Ed., Wiley-VCH, Weinheim, 2003, p. 173–189. [Google Scholar]
- (6).(a) Schrock RR in Handbook of Metathesis, Grubbs RH, Ed., Wiley-VCH, Weinheim, 2003, p. 8–46. [Google Scholar]; (b) Schrock RR High Oxidation State Multiple Metal–Carbon Bonds. Chem. Rev. 2002, 102, 145–180. [DOI] [PubMed] [Google Scholar]; (c) Schrock RR Recent Advances in High Oxidation State Mo and W Imido Alkylidene Chemistry. Chem. Rev 2009, 109, 3211–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Wengrovius JH, Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, Massachusetts, 1982, pages 283 and 290. [Google Scholar]; (b) Rocklage SM; Schrock RR; Churchill MR; Wasserman HJ Multiple Metal Carbon Bonds. Part 29. Facile Conversion of Tungsten(VI) Neopentylidyne Complexes into Oxo and Imido Neopentylidene Complexes and the Crystal Structure of W(CCMe3)(PHPh)(PEt3)2Cl2. Organometallics 1982, 1, 1332–1338. [Google Scholar]
- (8).Schrock RR; Czekelius CC Recent Advances in the Syntheses and Applications of Molybdenum and Tungsten Alkylidene and Alkylidyne Catalysts for the Metathesis of Alkenes and Alkynes. Adv. Synth. Catal 2007,349, 55–77. [Google Scholar]
- (9).(a) Copéret C; Chabanas M; Saint-Arroman RP; and Basset J-M Bridging the Gap between Homogeneous and Heterogeneous Catalysis through Surface Organometallic Chemistry. Angew. Chem. Int. Ed 2003, 42, 156–181. [DOI] [PubMed] [Google Scholar]; (b) Copéret C; Comas-Vives A; Conley MP; Estes DP; Fedorov A; Mougel V; Nagae H; Núñez-Zarur F; Zhizhko PV Surface Organometallic and Coordination Chemistry toward Single-Site Heterogeneous Catalysts: Strategies, Methods, Structures, and Activities. Chem. Rev 2016, 116, 323–421. [DOI] [PubMed] [Google Scholar]; (c) Solans-Monfort X; Copéret C; Eisenstein O Oxo vs Imido Alkylidene d0-Metal Species: How and Why Do They Differ in Structure, Activity, and Efficiency in Alkene Metathesis? Organometallics 2012,. 31, 6812–6822. [Google Scholar]
- (10).(a) Peryshkov DV; Schrock RR; Takase MK; Müller P; Hoveyda AH Z-Selective Olefin Metathesis Reactions Promoted by Tungsten Oxo Alkylidene Complexes. J. Am. Chem. Soc 2011, 133, 20754–20757. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Peryshkov DV; Schrock RR Synthesis of Tungsten Oxo Alkylidene Complexes. Organometallics 2012, 31, 7278–7286. [Google Scholar]; (c) Peryshkov DV; Forrest WP Jr.; Schrock RR; Smith SJ; Müller PB (C6F5)3 Activation of Oxo Tungsten Complexes That Are Relevant to Olefin Metathesis. Organometallics 2013, 32, 5256–5259. [Google Scholar]
- (11).(a) Fairhurst SA; Hughes DL; Marjani K; Richards RL Insertion of Alkynes into Molybdenum–Phosphine and –Carbon bonds. Crystal Structures of the Alkyne–Ylide Complex [MoO(SC6H2Pri3-2,4,6)2{η2-CHC(tol)}{C(tol)CHPMePh2}] (tol = C6H4Me-4) and the Phosphonium–Alkylidene complex [MoO(SC6H2Pri3-2,4,6)3{=C(Ph)CH=C(Ph)CH2PMe2Ph}]. J. Chem. Soc., Dalton Trans 1998, 1899–1904. [Google Scholar]; (b) Hughes DL; Marjani K; Richards RL Insertion of Alkynes into Molybdenum–Phosphine and Molybdenum–Carbon Bonds. X-ray Structure of the Phosphonium–Alkylidene Complex [MoO{=C(Ph)CH=C(Ph)CH2PMe2Ph}(SC6H2iPr3-2,4,6)3]. J. Organomet. Chem 1995, 505, 127–129. [Google Scholar]; (c) Varjas CJ; Powell DR; Thomson RK Rapid Access to an Oxido-Alkylidene Complex of Mo(VI). Organometallics 2015, 34, 4806–4809. [Google Scholar]
- (12).Bukhryakov KV; Schrock RR; Hoveyda AH; Tsay C; Müller P Syntheses of Molybdenum Oxo Alkylidene Complexes through Addition of Water to an Alkylidyne Complex. J. Am. Chem. Soc 2018, 140, 2797–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Garden JA; Pike SD Hydrolysis of Organometallic and Metal–Amide Precursors: Synthesis Routes to Oxo-Bridged Heterometallic Complexes, Metal-Oxo Clusters and Metal Oxide Nanoparticles. Dalton Trans 2018, 47, 3638–3662. [DOI] [PubMed] [Google Scholar]
- (14).(a) Feinstein-Jaffe I; Dewan JC; Schrock RR Preparation of Anionic Tungsten(VI) Alkyl Complexes Containing Oxo or Sulfido Ligands and the X-ray Structure of [N(C2H5)4]{WO2[OC(CH3)2C(CH3)2O][CH2C(CH3)3]}. Organometallics 1985, 4, 1189–1193. [Google Scholar]; (b) Feinstein-Jaffe I; Pedersen SF; Schrock RR Aqueous Tungsten(VI) Alkyl Chemistry. J. Am. Chem. Soc 1983, 105, 7176–7177. [Google Scholar]; (c) Feinstein-Jaffe I; Gibson D; Lippard SJ; Schrock RR; Spool A A Molecule Containing the OWOWO Unit. Synthesis, Structure, and Spectroscopy of W2O3(CH2CMe3)6. J. Am. Chem. Soc 1984, 106, 6305–6310. [Google Scholar]; (d) Schoettel G; Kress J; Fischer J; Osborn JA Controlled Hydrolysis of Molybdenum(VI) Alkyls and Alkylidenes: X-ray Structure of the Molybdate-Bridged Trimetallic Complex [{Mo(NBut)(CH2But)3}2(MoO4)]. J. Chem. Soc., Chem. Commun 1988, 914–915. [Google Scholar]; (e) Morton LA; Miao M; Callaway TM; Chen T; Chen S-J; Tuinman AA; Yu X; Lu Z; Xue Z-L Reactions of d0 Tungsten Alkylidyne Complexes with O2 or H2O. Formation of an Oxo Siloxy Complex through Unusual Silyl Migrations. Chem. Commun 2013, 49, 9555–9557. [DOI] [PubMed] [Google Scholar]; (f) O’Reilly ME; Ghiviriga I; Abboud KA; Veige AS A New ONO3- Trianionic Pincer-Type Ligand for Generating Highly Nucleophilic Metal–Carbon Multiple Bonds. J. Am. Chem. Soc 2012, 134, 11185–11195. [DOI] [PubMed] [Google Scholar]; (g) Chen P; Zhang L; Xue Z-L; Wu Y-D; Zhang X Density Functional Theory Study of the Reaction between d0 Tungsten Alkylidyne Complexes and H2O: Addition versus Hydrolysis. Inorg. Chem 2017, 56, 7111–7119. [DOI] [PubMed] [Google Scholar]
- (15).Stanciu C; Richards AF; Stender M; Olmstead MM; Power PP New Terphenylphenoxides of Group 13 and 14 Elements. Polyhedron 2006, 25, 477–483. [Google Scholar]
- (16).(a) Forrest WP; Axtell JC; Schrock RR Tungsten Oxo Alkylidene Complexes as Initiators for the Stereoregular Polymerization of 2,3-Dicarbomethoxynorbornadiene. Organometallics 2014, 33, 2313–2325. [Google Scholar]; (b) Forrest WP; Weis JG; John JM; Axtell JC; Simpson JH; Swager TM; Schrock RR Stereospecific Ring-Opening Metathesis Polymerization of Norbornadienes Employing Tungsten Oxo Alkylidene Initiators. J. Am. Chem. Soc 2014, 136, 10910–10913. [DOI] [PubMed] [Google Scholar]; (c) Autenrieth B; Jeong H; Forrest WP; Axtell JC; Ota A; Lehr T; Buchmeiser MR; Schrock RR Stereospecific Ring-Opening Metathesis Polymerization (ROMP) of endo-Dicyclopentadiene by Molybdenum and Tungsten Catalysts. Macromolecules 2015, 48, 2480–2492. [Google Scholar]
- (17).Dickie DA; MacIntosh IS; Ino DD; He Q; Labeodan OA; Jennings MC; Schatte G; Walsby CJ; Clyburne JAC Synthesis of the Bulky m-Terphenyl Phenol Ar*OH (Ar* = C6H3-2,6-Mes2, Mes = 2,4,6-Trimethylphenyl) and the Preparation and Structural Characterization of Several of Its Metal Complexes. Can. J. Chem 2008, 86, 20–31. [Google Scholar]
- (18).Addison AW; Rao TN; Van Rijn J; Veschoor GC; Reedijk J Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) Perchlorate. J. Chem. Soc., Dalton Trans 1984, 1349–1356. [Google Scholar]
- (19).Oskam JH; Schrock RR Rotational Isomers of Mo(VI) Alkylidene Complexes and Cis/Trans Polymer Structure: Investigations in Ring-Opening Metathesis Polymerization. J. Am. Chem. Soc 1993, 115, 11831–11845. [Google Scholar]
- (20).(a) von Kugelgen S; Bellone DE; Cloke RR; Perkins WS; Fischer FR Initiator Control of Conjugated Polymer Topology in Ring-Opening Alkyne Metathesis Polymerization. J. Am. Chem. Soc 2016, 138, 6234–6239. [DOI] [PubMed] [Google Scholar]; (b) Bittner C; Ehrhorn H; Bockfeld D; Brandhorst K; Tamm M Tuning the Catalytic Alkyne Metathesis Activity of Molybdenum and Tungsten 2,4,6-Trimethylbenzylidyne Complexes with Fluoroalkoxide Ligands OC(CF3)nMe3–n (n = 0–3). Organometallics 2017, 36, 3398–3406, . [Google Scholar]; (c) Heppekausen J; Stade R; Goddard R; Fürstner A Practical New Silyloxy-Based Alkyne Metathesis Catalysts with Optimized Activity and Selectivity Profiles. J. Am. Chem. Soc 2010, 132, 11045–11057. [DOI] [PubMed] [Google Scholar]
- (21).(a) Haberlag B; Wu X; Brandhorst K; Grunenberg J; Daniliuc CG; Jones PG; Tamm M Preparation of Imidazolin‐2‐iminato Molybdenum and Tungsten Benzylidyne Complexes: A New Pathway to Highly Active Alkyne Metathesis Catalysts. Chem. Eur. J 2010, 16, 8868–8877. [DOI] [PubMed] [Google Scholar]; (b) von Kugelgen S; Sifri R; Bellone D; Fischer FR Regioselective Carbyne Transfer to Ring-Opening Alkyne Metathesis Initiators Gives Access to Telechelic Polymers. J. Am. Chem. Soc 2017, 139, 7577–7585. [DOI] [PubMed] [Google Scholar]
- (22).An analogous THF monoadduct was reported byHaberlag B; Freytag M; Jones PG; Tamm M Tungsten and Molybdenum 2,4,6‐Trimethylbenzylidyne Complexes as Robust Pre‐Catalysts for Alkyne Metathesis. Adv. Synth. Catal 2014, 356, 1255–1265.
- (23).Steiner T The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed 2002, 41, 48–76. [DOI] [PubMed] [Google Scholar]
- (24).For a recent discussion of metallacyclobutane complexesGordon CP; Yamamoto K; Liao W-C; Allouche F; Andersen RA; Copeŕet C; Raynaud C; Eisenstein O Metathesis Activity Encoded in the Metallacyclobutane Carbon-13 NMR Chemical Shift Tensors. ACS Cent. Sci 2017, 3, 759–768.
- (25).For a related circumstance that leads only to a monoinsertion productGerber LCH; Schrock RR Synthesis of Methylidene Complexes that Contain a 2,6-Dimesitylphenylimido Ligand and Ethenolysis of 2,3-Dicarbomethoxynorbornadiene. Organometallics 2013, 32, 5573–5580.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.