Abstract
Objective
To investigate the frequency of, risk factors for, and outcomes after elevated levels of vancomycin.
Patients and Methods
We identified hospitalizations among 21,285 individuals in which intravenous vancomycin was given between 08/29/2007 and 10/10/2014. We investigated frequency and risk factors for elevated vancomycin levels (trough levels >30 mg/L) as well as associations with subsequent acute kidney injury (AKI), length of stay, and in-hospital mortality.
Results
Average patient age was 63 years, and 49% were female. Trough levels were checked in 7,422 patients, and 755 elevated levels were detected. Compared to patients with trough levels ≤30 mg/L or no trough levels, patients with elevated levels had longer average duration of vancomycin therapy (6.0 days vs. 3.4 days vs. <1 days, p<.001) and higher maximum dose (1.7 g vs. 1.6 g vs. 1.3 g, p<.001). Patients with higher body-mass index or lower eGFR had more elevated levels. In propensity-matched analyses, patients had higher risk of incident AKI after elevated levels compared to patients without elevated levels (hazard ratio, 1.55, 95% CI: 1.09 to 2.20, p=.02), as well as longer subsequent length of stay (relative risk, 1.14, 95% CI: 1.02 to 1.28, p=.03), but similar in-hospital mortality.
Conclusions
Elevated vancomycin levels were common, particularly in people with higher BMI and lower eGFR, and were associated with greater subsequent AKI and length of stay.
INTRODUCTION
Vancomycin, a glycopeptide antibiotic that inhibits cell wall synthesis in gram positive bacteria, is one of the most commonly used intravenous antibiotics in the world (1). Its use has increased since the epidemic of methicillin-resistant Staphylococcus aureus (MRSA) infections began in the 1990s; currently, MRSA infections affect approximately 80,000 hospitalizations and cause 11,000 related deaths in the United States each year (2, 3). Vancomycin is the guideline-recommended, first-line antibiotic for invasive MRSA infections (4).
Given the widespread use of vancomycin, quantifying the risk of and risk factors for vancomycin-related complications is important. One potential but controversial adverse effect of vancomycin is nephrotoxicity resulting in acute kidney injury (AKI) (5-7). The mechanism of injury may be formation of reactive oxygen species, mitochondrial dysfunction, and apoptosis leading to injury and death of cells of the proximal tubular epithelium, loop of Henle, and collecting duct; kidney biopsies have shown acute tubular necrosis, cast nephropathy, and also acute interstitial nephritis and non-caseating granulomas (8-13). Regardless of cause, patients who sustain AKI face a higher risk of subsequent morbidity, including estimated glomerular filtration rate (eGFR) decline, chronic kidney disease (CKD), cardiovascular events, and mortality (14-16).
Much of the suspected vancomycin nephrotoxicity is thought to be iatrogenic and related to elevated blood levels. Previous studies have found trough levels >15 mg/L to be predictive of nephrotoxicity (17-19). However, many guideline organizations recommend a trough vancomycin concentration between 15 - 20 mg/L, particularly for complicated infections such as bacteremia, endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia caused by methicillin-resistant S. aureus, with a minimum trough level of 10 mg/L in order to avoid development of vancomycin resistance (4, 7). Thus, keeping vancomycin trough levels within a therapeutic range is of high priority both for antimicrobial effectiveness and for avoidance of toxicity.
Using community-based data from a large health care system, we investigated the frequency of, risk factors for, and outcomes after elevated levels of vancomycin in the inpatient setting, testing the hypotheses that 1) elevated trough levels (>30 mg/L) are common; 2) obesity (body-mass index >30 kg/m2) and eGFR <60 ml/min/1.73 m2 are risk factors for elevated vancomycin levels; and 3) elevated trough levels are associated with higher subsequent morbidity, including AKI and longer length of stay.
METHODS
Study Population
This is a retrospective cohort study among primary care patients over 18 years of age in the Geisinger Health System. From this population, we identified 21,285 individuals who received intravenous (IV) vancomycin during a hospitalization in a Geisinger hospital between 08/29/2007 and 10/10/2014. If a person had multiple qualifying hospitalizations, we selected the first hospitalization in which vancomycin was given (Supplemental Figure 1). Geisinger Health System serves more than 3 million residents throughout 45 counties in central and northeastern Pennsylvania, and has 12 hospital campuses with a 551,000-member health plan, all with an integrated electronic medical record. This study was deemed exempt by the Geisinger Medical Center Institutional Review Board as well as the Johns Hopkins University Institutional Review Board.
Vancomycin Dose and Levels
Information on intravenous vancomycin use was abstracted from the electronic medication administration records. Records with incomplete or invalid dose information (e.g., single doses <250 mg or >4 g) were excluded. When multiple dosages were given, the maximum administered dose was recorded. Dosing intervals were determined empirically among people who received at least three doses. Trough levels were defined as a vancomycin check at least eight hours after the last dose. Because guidelines have varied on optimal target trough levels,(20) and the somewhat broad definition of trough used, elevated vancomycin levels were defined conservatively as trough levels >30 mg/L. A sensitivity analysis was conducted using a lower cutoff (>20 mg/L). If multiple levels qualified as elevated, the first was considered the index event.
Covariate Definitions
We abstracted data from the electronic medical record on age, sex, race, body mass index (BMI), and serum creatinine (which was converted to eGFR using the equation developed by the CKD Epidemiology Collaboration) (21). Baseline eGFR was considered the closest antecedent value to vancomycin start date, excluding any value that was measured more than three days prior to the admission date. However, all creatinine values during the hospitalization were evaluated for the purpose of determining the onset of AKI episodes. BMI was defined as the closest outpatient value before admission. History of hypertension, diabetes mellitus (DM), congestive heart failure (CHF), and atherosclerotic cardiovascular disease (ASCVD) (myocardial infarction, stroke, or peripheral vascular disease) were identified from inpatient and outpatient electronic health records using ICD-9-CM diagnostic codes. Information on Intensive Care Unit (ICU) level of care during hospitalization was extracted from hospitalization records. ICD-9-CM diagnosis codes were used to identify sepsis and MRSA infection. Specific coding algorithms are provided in Supplemental Table 1.
Adverse Event Definitions
We defined AKI according to the Kidney Disease Improving Global Outcomes (KDIGO) guideline, which is an increase in serum creatinine of 0.3 mg/dL within 48 hours or 50% within 7 days, and determined if the AKI event preceded or followed the elevated vancomycin level (22). To assess incident AKI after elevated vancomycin levels, we excluded patients with AKI that occurred earlier in the hospitalization (“history of AKI”). In sensitivity analysis, we evaluated KDIGO Stage 2 or worse AKI following the elevated vancomycin level. We also evaluated the length of hospital stay (LOS) after reaching elevated vancomycin level as well as in-hospital mortality.
Statistical Analysis
Baseline characteristics of patients who received IV vancomycin were compared using ANOVA for continuous variables and Chi-squared tests for categorical variables. In analyses by BMI category and eGFR stage, p for trend was estimated using linear, quantile, and logistic regression to evaluate the trend of means, median, and proportion, respectively, within categories. The relationship between vancomycin levels during hospitalization and adverse outcomes after that level was assessed using Cox proportional hazards regression for time to AKI and time to mortality and negative binomial regression for length of stay. This was done using the first elevated level (for patients with an elevated level) or maximum vancomycin trough level (for all others). Vancomycin level was treated as a continuous variable with adjustment for age, sex, race, BMI, eGFR, diabetes, hypertension, CVD, heart failure, sepsis, ICU admission, and length of stay prior to the vancomycin admission. Then, to additionally address confounders of elevated vancomycin level, propensity scores using the same variables were constructed to match patients with elevated levels to those with trough levels <30 mg/L. Cases (elevated vancomycin levels) were matched to controls 1:1 without replacement with calipers set to a quarter of the standard deviation of the calculated propensity scores. Cases and controls were matched exactly on a history of AKI prior to vancomycin level (these patients were excluded from analyses of incident AKI) and vancomycin therapy duration prior to the level. Time at risk began at first elevated trough level among cases and at the matched non-elevated trough level in controls. Analyses were conducted in a propensity score-matched sample and, in a second model, adjusting for covariates that differed between cases and controls. To addressed the missingness of baseline BMI and eGFR (missing in 9.6% and 6.2% of the population, respectively), we used mean-imputation using baseline covariates for adjusted analyses. To increase confidence that the creatinine elevation happened subsequent to the elevated vancomycin level, we performed sensitivity analyses only among patients with serum creatinine checked within 24 hours prior to the elevated trough level. All analyses were conducted using Stata 14.2 (StataCorp, College Station, TX).
RESULTS
Study Population
There were 21,285 patients with hospitalizations in which IV vancomycin was administered. Of these, 8,136 patients had a vancomycin level assessment during hospitalization, and 7,422 had an assessment of a vancomycin trough level (Supplemental Table 2). Average age of the 7,422 patients was 64 years (standard deviation (SD) 16), 44.4% were female, 38.9% had a history of diabetes mellitus, and 49.9% had a history of cardiovascular disease (Table 1). Average pre-vancomycin eGFR was 60 ml/min/1.73 m2 (SD 34), and average body-mass index was 31 (SD 9). Most hospitalizations without any vancomycin monitoring had short duration of vancomycin therapy (95.4% had <1 day). On the other hand, 218 (11.0%) of hospitalizations in which vancomycin was given for more than 7 days had no vancomycin monitoring.
Table 1.
Characteristics of patients and hospitalizations in which intravenous vancomycin therapy was given and trough levels were checked, stratified by vancomycin trough ≤30 mg/L and >30 mg/L
| Overall | Trough Level ≤30 mg/L |
Trough Level >30 mg/L |
P-value | |
|---|---|---|---|---|
| Number of hospitalizations | 7422 | 6667 (89.8%) | 755 (10.2%) | |
| Baseline characteristics | ||||
| Age, mean (SD) | 64.2 (16.0) | 64.6 (16.1) | 60.9 (15.4) | <.001 |
| Female | 3297 (44.4%) | 2899 (43.5%) | 398 (52.7%) | <.001 |
| Black | 129 (1.7%) | 111 (1.7%) | 18 (2.4%) | .15 |
| Diabetes mellitus | 2886 (38.9%) | 2562 (38.4%) | 324 (42.9%) | .02 |
| Hypertension | 5106 (68.8%) | 4592 (68.9%) | 514 (68.1%) | .65 |
| Heart Failure | 2024 (27.3%) | 1789 (26.8%) | 235 (31.1%) | .01 |
| Atherosclerotic Cardiovascular Disease | 3707 (49.9%) | 3367 (50.5%) | 340 (45.0%) | .004 |
| Body mass index, mean (SD) | 31.1 (9.1) | 30.8 (9.0) | 33.0 (9.9) | <.001 |
| eGFR (mL/min/1.73m2), mean (SD) | 59.7 (33.8) | 60.3 (33.6) | 53.6 (34.8) | <.001 |
| Hospitalization Characteristics | ||||
| Intensive care unit stay | 1696 (22.9%) | 1469 (22.0%) | 227 (30.1%) | <.001 |
| Sepsis diagnosis | 2777 (37.4%) | 2430 (36.4%) | 347 (46.0%) | <.001 |
| Methicillin-resistant Staph Aureus infection | 477 (6.4%) | 397 (6.0%) | 80 (10.6%) | <.001 |
Abbreviations: SD, standard deviation; eGFR, estimated glomerular filtration rate
Elevated Vancomycin Trough Levels
Elevated trough levels (vancomycin levels >30 mg/L) occurred in 755 hospitalizations (10.2% of those with trough levels assessed). Nearly half of the elevated trough levels (49%) had a previous trough level checked which was ≤30 mg/L. Elevated trough levels occurred more frequently in younger individuals, women, and those with diabetes or heart failure. Compared to hospitalizations with troughs checked but no elevated levels, hospitalizations with elevated levels had longer duration of vancomycin therapy (median, 6.0 days vs. 3.4 days, p <.001) and slightly higher doses (mean, 1.72 g vs. 1.58 g, p <.001) (Table 2). Among the 1,760 patients who had trough monitoring and more than 7 days of vancomycin therapy, 18.9% had elevated levels.
Table 2.
Vancomycin dosing/monitoring status by vancomycin monitoring type and level
| Characteristic | Total | No Check | No Trough | Trough ≤30 mg/L | Trough >30 mg/L | |
|---|---|---|---|---|---|---|
| N (%) | 21285 | 13149 (61.8) | 714 (3.4) | 6667 (31.3) | 755 (3.5) | |
| Vancomycin Use Durationb (days), median (IQR) | 1.0 (0.0, 2.9) | 0.0 (0.0, 0.9) | 2.5 (1.7, 4.5) | 3.4 (2.0, 6.0) | 6.0 (2.8, 11.9) | |
| Maximum Vancomycin Dose (g), mean (SD) | 1.41 (0.53) | 1.30 (0.48) | 1.61 (0.54) | 1.58 (0.54) | 1.72 (0.60) | |
| Dosing Interval (hours)c,N (%) | Every 6 hrs | 80 (0.8) | 70 (2.6) | 3 (0.4) | 5 (0.1) | 2 (0.3) |
| Every 8 hrs | 1422 (14.2) | 361 (13.7) | 532 (76.2) | 487 (8.1) | 42 (6.0) | |
| Every 12 hrs | 4936 (49.2) | 1423 (53.8) | 108 (15.5) | 3151 (52.7) | 254 (36.3) | |
| Every 24 hrs | 2543 (25.4) | 543 (20.5) | 39 (5.6) | 1735 (29.0) | 226 (32.3) | |
| Every 36 hrs or more | 1042 (10.4) | 246 (9.3) | 16 (2.3) | 604 (10.1) | 176 (25.1) | |
| Maximum Daily Weight-Based Vancomycin dosec(mg/kg/day) | 30.7 (21.3) | 30.5 (28.9) | 51.4 (21.9) | 29.2 (15.7) | 23.9 (16.6) | |
Abbreviations: N, number; IQR, interquartile range; g, grams; SD, standard deviation; hrs, hours; mg/kg/day, milligrams per kiligrams per day
0.0 of vancomycin use duration indicates one-time use.
Restricted to those had 3 or more vancomycin dosing: N = 10023.
Trends in Vancomycin Dosing Patterns and Levels by BMI and eGFR Categories
The duration of vancomycin therapy was similar across BMI categories, but the maximum dose of vancomycin increased with higher BMI, although the opposite was true for weight-based dose (Table 3). There was a trend toward a greater proportion of elevated vancomycin levels in patients with higher BMI (p <.001). For example, among people with BMI <20 kg/m2, 6.9% had elevated levels of vancomycin, compared to 14.0% in people with BMI ≥40 kg/m2.
Table 3.
Vancomycin dosing/monitoring status by BMI level (kg/m2) and eGFR (ml/min/1.73 m2)a
| BMI categoryb |
N (%) | Vancomycin Use Duration (days)d |
Maximum Vancomycin Dose (g) |
Dosing Interval (hours) e, N (%) | Maximum Daily Weight-Based Vancomycin Dosee (mg/kg/day) |
Vancomycin Trough Checked, n (%) |
Elevated Trough Level n (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Every 6 hrs | Every 8 hrs | Every 12 hrs | Every 24 hrs | Every 36 hrs or more | |||||||
| <20 | 1121 (5.8) | 1.0 (0.0, 3.5) | 1.12 (0.26) | 4 (0.7) | 110 (19.0) | 261 (45.2) | 158 (27.3) | 45 (7.8) | 40.1 (21.4) | 418 (37.3) | 29 (6.9) |
| 20-24 | 4056 (21.1) | 1.0 (0.0, 3.0) | 1.23 (0.33) | 10 (0.5) | 288 (15.2) | 880 (46.5) | 533 (28.1) | 183 (9.7) | 34.4 (22.4) | 1359 (33.5) | 103 (7.6) |
| 25-29 | 5373 (27.9) | 0.8 (0.0, 2.6) | 1.33 (0.42) | 20 (0.8) | 320 (13.5) | 1185 (50.1) | 609 (25.7) | 233 (9.8) | 30.4 (16.8) | 1687 (31.4) | 161 (9.5) |
| 30-34 | 3941 (20.5) | 0.8 (0.0, 2.5) | 1.46 (0.53) | 15 (0.9) | 220 (12.5) | 917 (52.1) | 409 (23.2) | 199 (11.3) | 28.9 (23.2) | 1323 (33.6) | 124 (9.4) |
| 35-39 | 2289 (11.9) | 0.8 (0.0,2.8) | 1.60 (0.61) | 13 (1.3) | 152 (14.6) | 562 (54.1) | 202 (19.5) | 109 (10.5) | 29.4 (25.5) | 803 (35.1) | 93 (11.6) |
| ≥40 | 2472 (12.8) | 1.0 (0.0, 3.0) | 1.77 (0.69) | 12 (1.0) | 206 (16.4) | 644 (51.2) | 271 (21.5) | 126 (10.0) | 25.4 (19.6) | 969 (39.2) | 136 (14.0) |
| p-trend | .95 | <.001 | .12 | .85 | <.001 | <.001 | .19 | <.001 | <.001 | <.001 | |
|
eGFR categoryc |
|||||||||||
| 90+ | 4864 (24.4) | 1.0 (0.0, 3.1) | 1.39 (0.50) | 33 (1.2) | 793 (28.7) | 1522 (55.1) | 303 (11.0) | 113 (4.1) | 39.9 (19.4) | 1621 (33.3) | 134 (8.3) |
| 60-89 | 5552 (27.8) | 0.9 (0.0, 2.7) | 1.39 (0.51) | 24 (0.9) | 388 (14.3) | 1664 (61.3) | 499 (18.4) | 138 (5.1) | 33.4 (24.4) | 1825 (32.9) | 151 (8.3) |
| 45-59 | 2921 (14.6) | 1.0 (0.0, 2.9) | 1.43 (0.53) | 12 (0.8) | 103 (7.2) | 830 (57.8) | 406 (28.3) | 85 (5.9) | 29.4 (21.3) | 1001 (34.3) | 114 (11.4) |
| 30-44 | 2721 (13.6) | 1.0 (0.0, 3.1) | 1.44 (0.52) | 7 (0.6) | 44 (3.5) | 496 (39.1) | 566 (44.7) | 154 (12.2) | 23.2 (15.8) | 1013 (37.2) | 117 (11.5) |
| <30 | 3906 (19.6) | 1.0 (0.0, 3.1) | 1.50 (0.60) | 1 (0.1) | 7 (0.5) | 178 (12.5) | 711 (50.1) | 523 (36.8) | 14.6 (9.0) | 1741 (44.6) | 221 (12.7) |
| p-trend | .25 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .02 | <.001 | |
Abbreviations: N, number; IQR, interquartile range; g, grams; SD, standard deviation; hrs, hours; mg/kg/day, milligrams per kiligrams per day
2,033 hospitalizations were excluded because of missing BMI
1,321 hospitalizations were excluded because of missing eGFR.
0.0 of vancomycin use duration indicates one-time use.
Restricted to those had 3 or more vancomycin dosing: N = 8896 for those with BMI measurements and N = 9600 for those with eGFR.
Within categories of eGFR, the duration of vancomycin therapy was slightly longer with lower eGFR. The maximum dose of vancomycin was higher with lower eGFR, but the weight-based daily dose was lower. The proportion with elevated levels was 8.3% among people with eGFR ≥90 ml/min/1.73 m2 and 12.7% among people with eGFR <30 ml/min/1.73 m2. Both low eGFR and higher BMI remained as significant risk factors for elevated levels in adjusted analyses (data not shown).
Association of Vancomycin Levels with Time to Subsequent AKI and Length of Stay
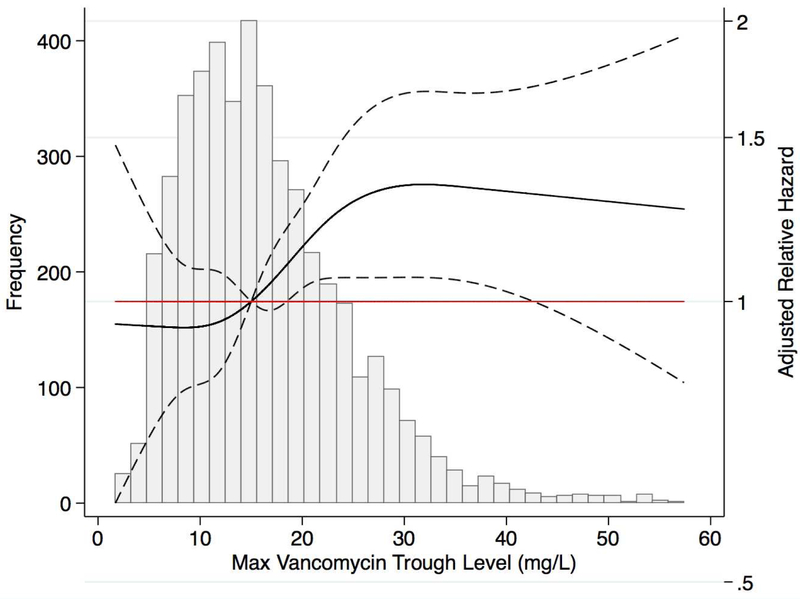
When vancomycin trough level was considered a continuous variable, higher levels were associated with greater risk of adverse outcomes. After excluding the 4,618 people with AKI prior to maximum trough level, there was higher adjusted hazard of incident AKI with higher maximum vancomycin trough levels, with a plateauing of risk after 30 mg/L (Figure 1a). The adjusted hazard ratio of stage 1 AKI was 1.14 (95% CI: 1.03 to 1.27), 1.33 (95% CI: 1.06 to 1.67), 1.31 (95% CI: 1.02 to 1.68), and 1.28 (95% CI: 0.91 to 1.79) at a maximum vancomycin trough level of 20 mg/L, 30 mg/L, 40 mg/L, and 50 mg/L, respectively, compared to the reference of maximum level of 15 mg/L. Risk associations were stronger when AKI was defined as KDIGO Stage 2 or worse (Supplemental Figure 2). A nearly linear association between maximum vancomycin trough levels and longer length of stay was observed, where relative risks were 1.06 (95% CI: 1.02 to 1.11), 1.30 (95% CI: 1.20 to 1.40), 1.44 (95% CI: 1.32 to 1.56), and 1.59 (95% CI: 1.40 to 1.80) at a maximum vancomycin trough level of 20 mg/L, 30 mg/L, 40 mg/L, and 50 mg/L compared to a maximum level of 15 mg/L (Figure 1b).
Figure 1a. Risk of incident Stage1 AKI after maximum vancomycin trough level (N = 2,274) a.
a Excluded 530 hospitalizations with maximum vancomycin level or elevated level achieved on the discharge date and 4,618 with prior AKI.
Figure 1b. Risk of length of stay after maximum vancomycin trough level (N = 6,892) a.
a Excluded 530 hospitalizations with maximum vancomycin level or elevated level achieved on the discharge date
Risks Associated with Elevated Vancomycin Trough Levels in the Propensity-Matched Cohort
There were 679 people with elevated trough levels who were successfully matched to others without elevated trough levels, of whom 397 had AKI prior to the elevated trough level (Supplemental Table 3 and 4). Among those without prior AKI, there were 82 subsequent AKI cases among patients with an elevated trough level and 51 among those without elevated trough levels. The majority of AKI Stage 1 cases (N=79, 96%) occurred within 24 hours of the elevated trough level. A trough level of vancomycin >30 mg/L was associated with a higher hazard of subsequent AKI Stage 1 or worse (hazard ratio, 1.55, 95% CI: 1.09 to 2.20) and Stage 2 or worse (hazard ratio 2.31, 95% CI: 1.06 to 5.03) (Table 4). Sensitivity analyses requiring that a serum creatinine be checked within 24 hours prior to the vancomycin trough level yielded similar results (hazard ratio for subsequent AKI Stage 1 or worse 1.57, 95% CI: 1.10 to 2.25, and Stage 2 or worse, hazard ratio 2.30, 95% CI: 1.01 to 5.26). Among the full matched cohort, a trough level of vancomycin >30 mg/L was associated with longer length of stay (relative risk, 1.14, 95% CI: 1.02 to 1.28), but not higher risk of mortality (HR 1.18, 95% CI:0.88 to 1.58). Risk associations with elevated levels of vancomycin were slightly attenuated when using a lower cut-off point (trough level > 20mg/L) (Supplemental Table 5).
Table 4.
Relative risk for different outcomes associated with elevated trough level of vancomycin (>30 mg/L)
| Outcomes | Propensity Score (PS) Matchinga | PS Matching with Covariate Adjustmentb | |||
|---|---|---|---|---|---|
| Estimated Risk (95%CI) | P value | Estimated Risk (95%CI) | P value | ||
| Mortality | N (cases) | 1,358 (184) | .26 | 1,358 (184) | .16 |
| Hazard Ratio | 1.18 (0.88 to 1.58) | 1.23 (0.92 to 1.64) | |||
| Incident AKI | N (cases) | 564 (133) | .02 | 564 (133) | .01 |
| Hazard Ratio | 1.55 (1.09 to 2.20) | 1.56 (1.10 to 2.21) | |||
| Incident Stage 2 AKI | N (cases) | 564 (32) | .04 | 564 (32) | .03 |
| Hazard Ratio | 2.31 (1.06 to 5.03) | 2.46 (1.13 to 5.35) | |||
| Length of Stay | N (cases) | 1,358 | .03 | 1,358 | .04 |
| Relative Risk | 1.14 (1.02 to 1.28) | 1.12 (1.01 to 1.26) | |||
Abbreviations: AKI, acute kidney injury; PS, propensity score; CI, confidence interval
Used a caliper equals to 1/4 of the standard deviation of propensity scores.
Only covariates that were significantly different after matching were included.
DISCUSSION
Using data from a large healthcare system, we found that supratherapeutic vancomycin levels occurred in 10.2% of hospitalizations with a vancomycin trough level checked, and nearly 20% of those in which vancomycin was given for more than 7 days. Higher BMI and lower eGFR were strong risk factors for supratherapeutic levels, independent of each other and underlying demographic information, suggesting that there may be opportunities to improve vancomycin dosing algorithms in these populations. Finally, there was a strong association between higher maximum vancomycin levels and subsequent AKI (particularly more severe AKI) as well as length of stay. Interventions to improve dosing and monitoring of vancomycin may help improve hospital outcomes.
Our results expand on prior and often smaller studies evaluating risk factors for a heterogeneous group of definitions of vancomycin nephrotoxicity. A retrospective analysis of 188 patients with pneumonia found a 12% increased risk for nephrotoxicity for each additional day on vancomycin (23). A prospective study of 95 patients with MRSA infections found that nephrotoxicity was higher with longer duration of vancomycin therapy: 6% for ≤7 days, 21% for 8–14 days and 30% for >14 days (24). A study of 94 patients demonstrated a 2.6-fold higher odds of nephrotoxicity for ≥2 weeks of therapy compared vancomycin therapy of shorter duration (25). A study of 291 patients found that higher-dose vancomycin regimens (≥4g/day) had higher likelihood of nephrotoxicity compared with lower-dose regimens or Linezolid (34.6%, 10.9%, and 6.7%, respectively) (26). A study of 11,650 patients investigated differences in AKI incidence receiving vancomycin alone, piperacillin-tazobactam alone, or combination therapy, finding that combination therapy resulted in double the odds of AKI compared to either therapy alone.(27) However, most of these studies did not assess trough levels, the definitions of nephrotoxicity varied, and attributing toxicity to vancomycin therapy can be subjective. Our study adds to the existing literature by reporting the occurrence of elevated levels and using propensity matching to ascertain the risk of subsequent AKI, longer hospital stay, and in-hospital mortality.
A priori, we hypothesized that two risk factors – higher BMI and lower eGFR – would be associated with higher occurrence of elevated vancomycin levels. We expected that elevated levels might occur more frequently in those with higher BMI, since vancomycin dosing is partly based on total body weight, and pharmacists often dose by Cockcroft-Gault, which overestimates GFR at higher levels of BMI (7). A previous report found that 64% of vancomycin levels exceeding 20 mg/L occurred in patients weighing more than 100 kg; another suggested that adherence to the Infectious Disease Society of America guideline resulted in more supratherapeutic vancomycin levels in patients with body-mass index >35 kg/m2 (28, 29). Our study found that mean dose was higher but that weight-based daily dose was lower with higher BMI, and that risk of elevated levels more than doubled from BMI <20 kg/m2 to BMI >40 kg/m2, suggesting that dosing in morbid obesity may require titration. Lastly, because of the renal clearance of vancomycin, we expected (and found) higher risk of elevated levels in patients with lower eGFR. Surprisingly, maximum dose of vancomycin was slightly higher with lower eGFR.
A strength of our study is the large study population with extensive clinical data. Most previous studies had far fewer patients; even a recent meta-analysis consisted of only 4033 patients (30). However, our study has certain limitations. The temporal sequence of elevated trough levels and AKI is difficult to characterize perfectly, since we are limited by when creatinine and vancomycin levels were checked. Most of our AKI cases occurred with a day after the vancomycin trough level was obtained. However, most patients had creatinine checked within 24 hours prior to the trough level, and sensitivity analyses including only those patients had similar results. We cannot determine causality. Patients with supratherapeutic levels of vancomycin may be at higher risk of AKI and longer length of stay for reasons unrelated to the vancomycin level. We attempted to address this possible bias by performing propensity matching, but there remains potential for residual confounding. Vancomycin trough timing is notoriously variable,(31) and we chose a broad definition. However, coupled with a relatively conservative definition of elevated vancomycin levels, we believe our specificity is high. Finally, much has changed over the study period, including dosing practices, the definition of AKI, and the rapidity with which antimicrobial sensitivities are reported (7, 22). For these reasons, we chose trough levels of vancomycin as our exposure of interest, and identified AKI by change in creatinine rather than diagnostic code. Still, our pharmacokinetic data is limited to what was checked in routine practice.
Conclusion
We found that elevated vancomycin levels were common, and occurred more frequently when vancomycin was given for more than 7 days, when BMI was high, and when eGFR was low. Further, elevated levels of vancomycin were associated with higher risk of AKI and longer hospital stays. Given the increasing use of IV vancomycin worldwide and potential for nephrotoxicity, careful use and monitoring of vancomycin therapy, particularly in overweight/obese patients or those with chronic kidney disease, may substantially reduce in-hospital morbidity.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Moellering RC. Vancomycin: a 50-year reassessment. Clinical Infectious Diseases. 2006;42(Supplement 1):S3–S4. [DOI] [PubMed] [Google Scholar]
- 2.Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Archives of internal medicine. 2008;168(20):2254–60. [DOI] [PubMed] [Google Scholar]
- 3.Malani PN. National burden of invasive methicillin-resistant Staphylococcus aureus infection. JAMA. 2014;311(14):1438–9. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–92. [DOI] [PubMed] [Google Scholar]
- 5.Han HK, An H, Shin KH, Shin D, Lee SH, Kim JH, et al. Trough concentration over 12.1 mg/L is a major risk factor of vancomycin-related nephrotoxicity in patients with therapeutic drug monitoring. Ther Drug Monit 2014;36(5):606–11. [DOI] [PubMed] [Google Scholar]
- 6.Farber BF, Moellering RC, Jr. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrobial agents and chemotherapy. 1983;23(1):138–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. American Journal of Health-System Pharmacy. 2009;66(1):82–98. [DOI] [PubMed] [Google Scholar]
- 8.Bamgbola O Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab 2016;7(3):136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luque Y, Louis K, Jouanneau C, Placier S, Esteve E, Bazin D, et al. Vancomycin-Associated Cast Nephropathy. J Am Soc Nephrol 2017;28(6):1723–8. Epub 2017/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wicklow BA, Ogborn MR, Gibson IW, Blydt-Hansen TD. Biopsy-proven acute tubular necrosis in a child attributed to vancomycin intoxication. Pediatr Nephrol 2006;21(8):1194–6. Epub 2006/05/25. [DOI] [PubMed] [Google Scholar]
- 11.Wu CY, Wang JS, Chiou YH, Chen CY, Su YT. Biopsy proven acute tubular necrosis associated with vancomycin in a child: case report and literature review. Ren Fail 2007;29(8):1059–61. Epub 2007/12/11. [DOI] [PubMed] [Google Scholar]
- 12.Hong S, Valderrama E, Mattana J, Shah HH, Wagner JD, Esposito M, et al. Vancomycin-induced acute granulomatous interstitial nephritis: therapeutic options. Am J Med Sci 2007;334(4):296–300. Epub 2007/11/22. [DOI] [PubMed] [Google Scholar]
- 13.Plakogiannis R, Nogid A. Acute interstitial nephritis associated with coadministration of vancomycin and ceftriaxone: case series and review of the literature. Pharmacotherapy. 2007;27(10):1456–61. Epub 2007/09/28. [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. [DOI] [PubMed] [Google Scholar]
- 15.Grams ME, Sang Y, Coresh J, Ballew SH, Matsushita K, Levey AS, et al. Candidate surrogate end points for ESRD after AKI. Journal of the American Society of Nephrology. 2016:ASN. 2015070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. American Journal of Kidney Diseases. 2009;53(6):961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. Journal of Antimicrobial Chemotherapy. 1990;25(4):679–87. [DOI] [PubMed] [Google Scholar]
- 18.Han HK, An H, Shin K-H, Shin D, Lee SH, Kim JH, et al. Trough concentration over 12.1 mg/l is a major risk factor of vancomycin-related nephrotoxicity in patients with therapeutic drug monitoring. Therapeutic drug monitoring. 2014;36(5):606–11. [DOI] [PubMed] [Google Scholar]
- 19.Wong-Beringer A, Joo J, Tse E, Beringer P. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. International journal of antimicrobial agents. 2011;37(2):95–101. [DOI] [PubMed] [Google Scholar]
- 20.Martin JH, Norris R, Barras M, Roberts J, Morris R, Doogue M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society Of Infectious Diseases Pharmacists. The Clinical biochemist Reviews. 2010;31(1):21–4. Epub 2010/02/25. [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, et al. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clinical therapeutics. 2012;34(1):149–57. [DOI] [PubMed] [Google Scholar]
- 24.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Archives of internal medicine. 2006;166(19):2138–44. [DOI] [PubMed] [Google Scholar]
- 25.Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clinical therapeutics. 2007;29(6):1107–15. [DOI] [PubMed] [Google Scholar]
- 26.Lodise TP, Lomaestro B, Graves J, Drusano G. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrobial agents and chemotherapy. 2008;52(4):1330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: A retrospective cohort analysis. Journal of hospital medicine. 2017;12(2):77–82. Epub 2017/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosmisky DE, Griffiths CL, Templin MA, Norton J, Martin KE. Evaluation of a new vancomycin dosing protocol in morbidly obese patients. Hospital pharmacy. 2015;50(9):789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto KS TB; Davis J; Dement L; Bello EF Actual body weight dosing of vancomycin in obese patients. Hawaii J Med Public Health. 2013;72(9 Supplement 4). [Google Scholar]
- 30.Ray AS, Haikal A, Hammoud KA, Alan S. Vancomycin and the Risk of AKI: A Systematic Review and Meta-Analysis. Clinical Journal of the American Society of Nephrology. 2016;11(12):2132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy. 2013;33(12):1256–63. Epub 2013/07/31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.