Abstract
Background:
Hemodialysis (HD) patients frequently experience pain. Previous studies of HD patients suggest increased opioid prescribing through 2010. It remains unclear if this trend continued after 2010 or declined with national trends.
Methods:
Longitudinal cohort study of 484,745 HD patients in the United States Renal Data System/Medicare data. We used Poisson/negative binomial regression to estimate annual incidence rates of opioid prescribing between 2007–2014. We compared prescribing rates with the general U.S. population using IQVIA’s National Prescription Audit data. Outcomes included: percent of HD patients receiving an opioid prescription, rate of opioid prescriptions, quantity, days supply, morphine milligram equivalents (MME) dispensed per 100 person-days, and prescriptions per person.
Results:
In 2007, 62.4% of HD patients received an opioid prescription. This increased to 63.2% in 2010 then declined to 53.7% by 2014. Opioid quantity peaked in 2011 at 73.5 pills per 100 person-days and declined to 62.6 pills per 100 person-days in 2014. MME peaked between 2010 and 2012 then declined through 2014. In 2014, MME rates were 1.8-fold higher among non- Hispanic patients and 1.6-fold higher among low-income patients. HD patients received 3.2-fold more opioid prescriptions per person compared to the general U.S. population and were primarily prescribed oxycodone and hydrocodone. Between 2012 and 2014, HD patients experienced greater declines in opioid prescriptions per person (18.2%) compared to the general U.S. population (7.1%).
Conclusion:
Opioid prescribing among HD patients declined between 2012 and 2014. However, HD patients continue receiving substantially more opioids than the general U.S. population.
Keywords: Trends, Opioid prescribing, Hemodialysis, United States
INTRODUCTION
Initiatives designed to improve the identification and management of pain in the United States1,2,3,4 coincided with a four-fold increase in the sale of prescription opioids and overdose deaths among Americans.5,6,7,8,9,10 Approximately 50% of patients undergoing hemodialysis (HD) report chronic pain11,12 and achieving adequate levels of analgesia in these patients can be challenging.13 Non-opioid analgesics such as non-steroidal anti-inflammatory drugs can negatively affect renal function and many commonly prescribed opioids are contraindicated among those on HD.14 Given the prevalence of chronic pain is higher in patients with end-stage renal disease (ESRD) compared to those in the general U.S. population,15 it is important to understand whether and how broader secular trends in opioid prescribing observed in the general U.S. population have influenced changes in prescribing among patients undergoing HD.
Prior studies examining the prevalence of opioid prescribing among HD patients do not span beyond 2010.11,15,16,17,18 Altogether, these studies suggest the proportion of HD patients receiving opioids increased from 5% in 1996 to 63% in 2010. Since 2010, a number of stakeholders, including the Centers for Medicare and Medicaid Services, Food and Drug Administration, and Drug Enforcement Administration, have broadly implemented policies and programs to curb excess opioid prescribing in the general U.S. population.19,20, 21,22,23,24 These efforts have likely contributed to recent reductions in opioid use in the general U.S. population;25,26 however, it remains unclear if similar declines in opioid prescribing occurred among patients undergoing HD.
Prior work examining the trends and prevalence of opioid prescribing among HD patients has primarily focused on the percentage of patients filling at least one opioid prescription.11,15,16,17,18 Unfortunately, this crude measure of prescribing overlooks other important underlying drivers of the total amount of opioids dispensed. Quantifying changes in the rates of prescriptions, quantity, days supply, and total Morphine Milligram Equivalents (MME), as well as stratifying these rates by sociodemographic characteristics may assist stakeholders in identifying sub-groups of patients commonly receiving large quantities of opioids for extended periods of time.
The goals of this study were to: 1) comprehensively examine temporal changes in the rates of opioid prescribing among HD patients, 2) identify sub-groups of patients commonly prescribed opioids, and 3) compare trends in opioid prescribing between HD patients and the general U.S. population and by opioid molecule.
MATERIALS AND METHODS
Study population and data for HD patients
In this retrospective cohort study, we selected prevalent in-center maintenance HD patients in the United States Renal Data System (USRDS) who were over 18 years of age and had no prior kidney transplants between January 1, 2007 and December 31, 2014. To ensure we captured the entirety of each patients’ prescription fills, we limited our population to patients consistently receiving HD and enrolled in Medicare Parts A, B (MPAB), and D.
We ascertained relevant sociodemographic data from the USRDS which compiles detailed demographic, diagnostic, enrollment, and treatment history information from a variety of data sources for all Medicare beneficiaries with ESRD. The CMS ESRD Medical Evidence Report form (CMS 2728) is used to register patients at the onset of ESRD, establish Medicare eligibility, and captures patients’ sociodemographic information, such as age, sex, race, ethnicity, employment, cause of ESRD, and geographic location, as well as diagnostic values and comorbidities.
The Prescription Drug Event Standard Analytics Files (SAFs) in the USRDS provide detailed information on Part D enrollment, dual-eligibility for Medicare and Medicaid, prescription drug claims, including brand name, generic name, National Drug Code (NDC), quantity dispensed, strength, days supply, and date of service as described below.
Opioid prescribing measures
To calculate annual measures of opioid prescribing, we identified a single observation period for each patient during which all eligibility criteria were met. Patients contributed observation time from the start of HD, MPAB, or Part D coverage until death, transplantation, or the end of HD, MPAB, or Part D coverage. For patients who started HD, MPAB, or Part D coverage on different days we selected the most recent date. We then identified opioid claims for selected molecules (Appendix Table 1) filled during each patient’s observation period.
We examined seven measures of opioid prescribing. First, we calculated the 1) annual percentage of HD patients who filled at least one opioid prescription between 2007 and 2014. We then calculated incidence rates of 2) prescriptions, 3) quantity dispensed (pills, patches, sublingual films), 4) days supply, and 5) MME during each calendar year. Finally, we calculated the 6) annual number of opioid prescriptions per person and 7) the annual MME dispensed per 100 person-days by opioid molecule.
We calculated the percentage of HD patients receiving an opioid prescription by dividing the number of HD patients who received opioids in a given year by the total number HD patients observed in that same year. To account for differences in observation time between patients, we restricted this measure to HD patients that were observed for the entire year. We calculated annual rates of opioid prescribing, by first obtaining annual totals of each measure (prescriptions, quantity, days supply and MME), by summing the patient-specific annual totals of each measure among patients who contributed at least one day of person-time in a given year. We then divided the annual totals for each measure by the total number of person-days contributed by all HD patients observed in that year regardless of opioid receipt. Thus, these rates represent the incidence of opioid prescribing within the entire HD population and are not restricted to HD patients who received opioids. To calculate the number of opioid prescriptions dispensed per patient, we divided the total number of annual opioid prescriptions dispensed to observed HD patients, by the total number of HD patients observed in each year. In the general U.S. population, we divided the projected total number of annual opioid prescriptions filled in the United States by the total U.S. population over 18 years of age in the given year.27 Finally, we calculated annual MME dispensed per 100 person-days observed for each opioid molecule by dividing the total MME for each opioid molecule in a given year, by the total number of person- days observed in that year.
In order to calculate the annual incidence rate of MME, we used the NDC to link the opioid claims data in the Part D SAFs to their respective oral MME conversion factors in the 2017 Centers for Disease Control (CDC) master opioid file.28 These conversion factors were derived from prior work characterizing long-term opioid use for noncancer pain.29 To obtain the MME for each opioid prescription, we multiplied the quantity dispensed, strength in milligrams and the equianalagesic morphine conversion factor. Converting each prescription to MME allowed us to account for differences in molecule type, formulation and bioavailability.
Statistical analyses of HD patients
We examined the characteristics of the HD population overall and then in 2007 and 2014. Sociodemographic characteristics of interest included: age category (18–35, 36–50, 51–65, ≥66), sex, race (Black, White, Native American/Asian, Other/Unknown), ethnicity (Hispanic, Non- Hispanic, Other/Unknown), dual-eligibility for Medicare and Medicaid, employment status (Unemployed, Full or Part-time, Retired, Retired Disabled, Other), diabetes status, cause of ESRD (Diabetes, Hypertension, Other), and region. To compare opioid prescribing between states with similar economic and social factors30, we categorized patients based on their state of residence into Bureau of Economic Analysis regions (New England, Mideast, Great Lakes, Plains, Southeast, Southwest, Rocky Mountain, Far West)31. We excluded <1% of our sample for missing sociodemographic information or residing outside of the continental United States and Hawaii.
To quantify opioid use in each calendar year, we first calculated the proportion of HD patients filling at least one opioid prescription. We then calculated incidence rates using Poisson regression for each measure of opioid prescribing. For each model, we included the annual total of each outcome measure as the dependent variable and the calendar year as the sole independent variable. To obtain incidence rates, annual totals were offset by the log of total person-days observed in each year. We then calculated incidence rates stratified by the previously mentioned sociodemographic characteristics of interest. We ran separate models for each characteristic. Each model included two independent variables: the calendar year and the sociodemographic characteristic of interest. The stratified Poisson models showed evidence of overdispersion; therefore, we reran these models using a negative binomial distribution. We evaluated the assumption that each predictor had a constant multiplicative effect across years by examining plots of the crude incidence rates for each outcome and predictor combination and adding interaction terms by calendar year to our models. Based on these assessments we concluded the effect of the selected predictors did not vary over time.
We based our inferences regarding trends in prescribing on the 95% confidence intervals for each estimate. We compared the 95% confidence intervals for the incidence rates between years and considered non-overlapping intervals a statistically significant difference.32 Finally, we plotted the 1) annual number of opioid prescriptions dispensed per patient in our study sample and the general U.S. population and the 2) annual rate of MME dispensed per 100 person-days by opioid molecule.
In some cases, private insurers cover patients’ first 30 months of dialysis. We excluded this period of time in our main analysis; however patients may have still initiated HD and obtained MPAB coverage on different dates. As a sensitivity analysis, we restricted our sample to 270,210 patients who started HD and MPAB coverage on the same day. All analyses were conducted using SAS version 9.3.
Comparison of HD patients with general U.S. population
To compare the number of opioid prescriptions dispensed per patient between HD patients in our study and the general U.S. population, we used data from the IQVIA National Prescription Audit (NPA). The NPA provides annual estimates of opioid prescriptions dispended from retail pharmacies to the general U.S. population. These estimates are national projections based on retail outlets included in the IQVIA sample. IQVIA estimates the number of prescriptions dispensed by non-sample pharmacies using projection factors based on the distance between sample and non-sample stores. IQVIA then combines the totals for each product to estimate dispensing at the national level.
RESULTS
Characteristics of HD population
Among the 484,745 patients undergoing HD, 47.1% were female, 40.3% were aged ≥66 years, and 60.1% were white (Table 1). The annual total of patients observed in our study sample increased from 163,558 in 2007 to 208,807 in 2014. Overall, the sociodemographic characteristics of the study population were similar in 2007 and 2014. However, the proportion of HD patients aged ≥66 years decreased from 46.8% in 2007 to 29.3% in 2014. During this time period, the proportion of patients with a diagnosis of diabetes increased from 39.1% to 55.8%.
Table 1.
Sociodemographic characteristics of hemodialysis patients
| 2007–2014 (N=484,745) | 2007 (N=163,558) | 2014 (N=208,807) | |
|---|---|---|---|
| % | % | % | |
| Age (years) | |||
| 18–35 | 5.7 | 4.3 | 7.4* |
| 36–50 | 18.1 | 16.1 | 23.2* |
| 51–65 | 36.0 | 32.8 | 40.1* |
| ≥66 | 40.3 | 46.8 | 29.3* |
| Female | 47.1 | 49.2 | 46.3* |
| Race | |||
| Black | 34.4 | 40.3 | 36.8* |
| White | 60.1 | 54.0 | 57.4* |
| Native American/Asian | 5.2 | 5.4 | 5.6 |
| Other/Unknown | 0.2 | 0.3 | 0.2* |
| Ethnicity | |||
| Hispanic | 14.5 | 15.0 | 15.9* |
| Non-Hispanic | 85.0 | 83.4 | 83.8* |
| Other/Unknown | 0.6 | 1.6 | 0.3* |
| Dual-Eligible | 50.6 | 58.4 | 51.3* |
| Employment | |||
| Unemployed | 25.3 | 26.5 | 28.4* |
| Full or Part-time | 6.5 | 6.6 | 7.4* |
| Retired | 35.4 | 30.6 | 31.9* |
| Retired Disabled | 25.3 | 25.2 | 25.7* |
| Other | 7.5 | 11.1 | 6.6* |
| Diabetes | 51.6 | 39.1 | 55.8* |
| Cause of ESRD | |||
| Diabetes | 49.4 | 49.4 | 49.5 |
| Hypertension | 29.7 | 28.8 | 30.9* |
| Other | 20.9 | 21.8 | 19.6* |
| U.S. Geographic Region | |||
| New England | 3.4 | 3.2 | 3.3 |
| Mideast | 16.6 | 16.1 | 16.7* |
| Great Lakes | 15.4 | 14.7 | 15.0 |
| Plains | 5.4 | 5.4 | 4.9* |
| Southeast | 30.6 | 31.8 | 30.2* |
| Southwest | 13.6 | 14.1 | 14.1 |
| Rocky Mountain | 1.5 | 1.5 | 1.5 |
| Far West | 13.6 | 13.1 | 14.4* |
statistically significant difference based on 95% CI 2014 vs. 2007
Proportion of HD patients receiving opioids
The percentage of patients undergoing HD who received an opioid prescription slightly increased from 62.4% (95% CI: 62.1%, 62.7%) in 2007 to 63.2% (95% CI: 62.9%, 63.5%) in 2010, then declined to 60.2% (95% CI: 59.9%, 60.5%) until 2013. By 2014, the proportion of patients undergoing HD that received an opioid declined to 53.7% (95% CI: 53.4%, 54.0%) (Appendix Table 2). Since their peak, the total number of opioid prescriptions, quantity, days supply, and MME also declined until 2014.
Overall rates of opioid prescriptions, quantity, days supply and MME among HD patients
The rate of prescriptions, quantity, days supply, and MME peaked between 2010 and 2012 then declined until 2014 (Appendix Table 3). Despite an increase in the incidence rate of opioid prescriptions between 2007 and 2010; the rate of prescriptions remained approximately 1.2 opioid prescriptions per 100 person-days from 2007 to 2012. By 2014, the rate of opioid prescriptions declined to 1.0 prescription per 100 person-days. The incidence rate of opioid quantity increased from 61.5 in 2007 to 73.5 per 100 person-days in 2011, then declined to 62.6 per 100 person-days in 2014. Thus, the quantity of opioids dispensed in 2014 was sufficient to provide all HD patients observed in our sample (opioid recipients and non-recipients) with approximately 63 opioid pills, patches, or sublingual films per 100 person-days. Since their respective peaks in 2009, 2011, and 2012, the rate of prescriptions, quantity and days supply declined by 21%, 15%, and 12% by 2014.
Opioid prescription rates by sociodemographic factors
Annual incidence rates of opioid prescribing decreased across all sociodemographic factors in 2014 (Appendix Table 4). Between 2007 and 2014, the rate of opioid prescribing was consistently 1.5-fold higher among dual-eligible HD patients compared to those without dual- eligibility. For example, in 2010, dual-eligible patients received 1.5 opioid prescriptions per 100 person-days, whereas their non-eligible counterparts received 0.9 opioid prescriptions per 100 person-days. In 2014, non-Hispanic HD patients also experienced a 1.5-fold higher rate of opioid prescribing when compared to Hispanics. Overall, incidence rates of opioid prescriptions were highest among HD patients who were 36–50 years old, female, black, non-Hispanic, dual- eligible, retired and disabled, diabetic, had ESRD from causes other than diabetes or hypertension, or were living in the Rocky Mountain region of the United States.
Annual rates of opioid quantity, days supply and MME
Rates of opioid quantity, days supply, and MME followed similar trends as opioid prescriptions (Table 2). In 2011, near the peak of opioid prescribing, HD patients 36–50 years old received 99 opioid pills, patches, or sublingual films per 100 person-days. By 2014, HD patients in this age category received 83 opioid pills, patches or sublingual films per 100 person-days. Across the study period, black and white HD patients received a similar quantity and days supply of opioids. However, white HD patients received a total MME at a higher rate than black patients in 2014 (1030mg vs. 961mg).
Table 2.
| Quantity | Days Supply | MME | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2011 | 2014 | 2007 | 2011 | 2014 | 2007 | 2011 | 2014 | ||
| Age (years) | ||||||||||
| 18–35 | 67.0 | 79.7 | 66.8 | 17.8 | 21.4 | 18.6 | 1082.1 | 1168.6 | 1020.0 | |
| 36–50 | 83.3 | 99.1 | 83.0 | 22.5 | 27.0 | 23.5 | 1310.5 | 1415.3 | 1235.3 | |
| 51–65 | 66.8 | 79.5 | 66.5 | 18.2 | 21.8 | 19.0 | 933.8 | 1008.5 | 880.2 | |
| ≥66 | 43.0 | 51.2 | 42.9 | 12.4 | 14.8 | 12.9 | 529.0 | 571.3 | 498.7 | |
| Sex | ||||||||||
| Female | 65.2 | 78.1 | 66.6 | 18.5 | 22.0 | 19.5 | 896.5 | 973.2 | 871.4 | |
| Male | 57.8 | 69.3 | 59.1 | 15.8 | 18.8 | 16.6 | 851.2 | 924.1 | 827.4 | |
| Race | ||||||||||
| Black | 64.6 | 74.1 | 65.5 | 18.5 | 20.9 | 19.0 | 908.6 | 889.5 | 960.6 | |
| White | 65.6 | 75.3 | 66.6 | 18.0 | 20.4 | 18.5 | 973.7 | 953.3 | 1029.5 | |
| Native American/Asian | 33.5 | 38.4 | 34.0 | 9.6 | 10.9 | 9.9 | 394.0 | 385.7 | 416.6 | |
| Other/Unknown | 51.3 | 58.9 | 52.1 | 14.0 | 15.9 | 14.4 | 821.1 | 803.9 | 868.2 | |
| Ethnicity | ||||||||||
| Hispanic | 42.5 | 53.1 | 45.1 | 11.7 | 14.4 | 12.6 | 494.3 | 565.2 | 485.7 | |
| Non-Hispanic | 62.3 | 77.9 | 66.1 | 17.5 | 21.6 | 18.9 | 903.8 | 1033.4 | 888.1 | |
| Other/Unknown | 79.4 | 99.3 | 84.2 | 22.4 | 27.7 | 24.3 | 1252.9 | 1432.5 | 1231.0 | |
| Dual Eligible | ||||||||||
| Non-Eligible | 46.4 | 56.2 | 48.4 | 12.7 | 15.4 | 13.7 | 637.4 | 695.6 | 631.4 | |
| Dual-Eligible | 72.1 | 87.3 | 75.1 | 20.1 | 24.3 | 21.7 | 1046.8 | 1142.4 | 1037.0 | |
| Employment | ||||||||||
| Unemployed | 68.8 | 80.2 | 67.5 | 19.0 | 22.2 | 19.4 | 1012.5 | 1059.2 | 933.4 | |
| Full or Part-time | 47.9 | 55.8 | 47.0 | 13.0 | 15.2 | 13.3 | 649.0 | 678.9 | 598.3 | |
| Retired | 44.6 | 52.0 | 43.8 | 12.7 | 14.8 | 12.9 | 558.8 | 584.5 | 515.1 | |
| Retired Disabled | 81.9 | 95.5 | 80.4 | 22.3 | 26.1 | 22.8 | 1238.6 | 1295.7 | 1141.8 | |
| Other | 58.3 | 68.0 | 57.3 | 16.3 | 19.0 | 16.6 | 809.5 | 846.8 | 746.2 | |
| Diabetes | ||||||||||
| No diabetes | 60.3 | 71.2 | 60.6 | 16.9 | 19.9 | 17.5 | 890.6 | 958.6 | 859.8 | |
| Diabetes | 64.1 | 75.7 | 64.4 | 17.7 | 20.8 | 18.3 | 871.9 | 938.4 | 841.8 | |
| Cause of ESRD | ||||||||||
| Diabetes | 62.4 | 74.7 | 63.8 | 17.2 | 20.5 | 18.1 | 835.3 | 913.9 | 821.8 | |
| Hypertension | 55.1 | 66.0 | 56.3 | 15.7 | 18.7 | 16.6 | 751.4 | 822.2 | 739.3 | |
| Other | 68.1 | 81.6 | 69.6 | 18.7 | 22.2 | 19.6 | 1111.1 | 1215.8 | 1093.3 | |
| U.S. Geographic Region | ||||||||||
| New England | 60.7 | 71.4 | 62.2 | 16.3 | 19.1 | 17.3 | 1088.3 | 1168.0 | 1069.7 | |
| Mideast | 47.9 | 56.3 | 49.0 | 13.7 | 16.1 | 14.5 | 840.3 | 901.8 | 826.0 | |
| Great Lakes | 74.1 | 87.1 | 75.8 | 20.7 | 24.3 | 21.9 | 1075.3 | 1153.9 | 1056.9 | |
| Plains | 71.0 | 83.5 | 72.7 | 18.7 | 22.0 | 19.8 | 989.8 | 1062.2 | 972.9 | |
| Southeast | 63.9 | 75.2 | 65.4 | 18.5 | 21.8 | 19.6 | 876.6 | 940.8 | 861.6 | |
| Southwest | 59.5 | 70.0 | 60.9 | 15.8 | 18.6 | 16.7 | 696.8 | 747.8 | 684.9 | |
| Rocky Mountain | 78.4 | 92.3 | 80.3 | 20.7 | 24.3 | 21.9 | 1187.2 | 1274.1 | 1166.9 | |
| Far West | 59.5 | 70.0 | 60.9 | 15.5 | 18.2 | 16.4 | 817.4 | 877.3 | 803.5 | |
rates presented per 100 person-days observed
all 2011 vs. 2007 and 2014 vs. 2011 comparsions statistically significatly different based on 95% CI
Overall, rates of opioid quantity, days supply, and MME were highest among HD patients who were between 35 and 50 years old, female, white, non-Hispanic, dual-eligible, retired and disabled, diabetic, or had ESRD from causes other than diabetes or hypertension. Respectively, non-Hispanic and dual-eligible patients received total opioid MMEs at 1.8 and 1.6-fold the rate of their Hispanic (2014; 888mg vs. 486mg) and non-eligible counterparts (2014; 1037mg vs.631mg). HD patients in the Rocky Mountain region experienced the highest rates of opioid quantity (2014; 80.3 pills), days supply (2014; 21.9 days), and total MME (2014; 1167mg) compared to other regions of the United States. Incidence rates of opioid quantity and days supply were similarly high in the Rocky Mountain region. Despite having lower incidence rates of opioid quantity and days supply, patients in New England received opioid MME at a higher rate than patients in the Great Lakes (2014; 1070mg vs 1056mg).
Opioid prescribing among HD patients compared to general U.S. population
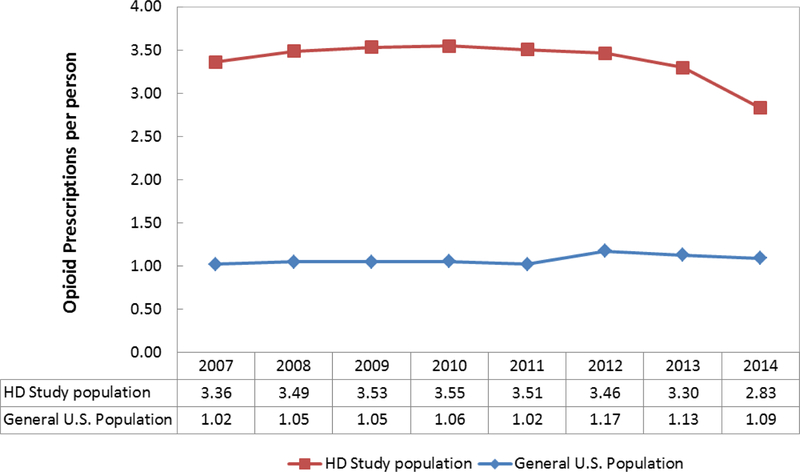
Between 2007 and 2014, patients undergoing HD received 3.2-fold more opioid prescriptions per person compared to the general U.S. population (Figure 1). HD patients received approximately 3.5 opioid prescriptions per person from 2007 through 2012. Opioid prescriptions per person declined earlier and more rapidly among HD patients compared to the general U.S. population. Between 2012 and 2014, opioid prescriptions per HD patient declined from 3.6 to 2.8 prescriptions per person (18%). During this same time period, prescriptions per person in the general U.S. population declined from 1.17 to 1.09 (7%).
Figure 1.
Opioid prescriptions per person comparing hemodialysis study population and general U.S. population, by year (2007–2014) (N=484,745)
Sources: USRDS, IQVIA National Prescription Audit, Kaiser Family Foundation
Opioid prescribing by molecule
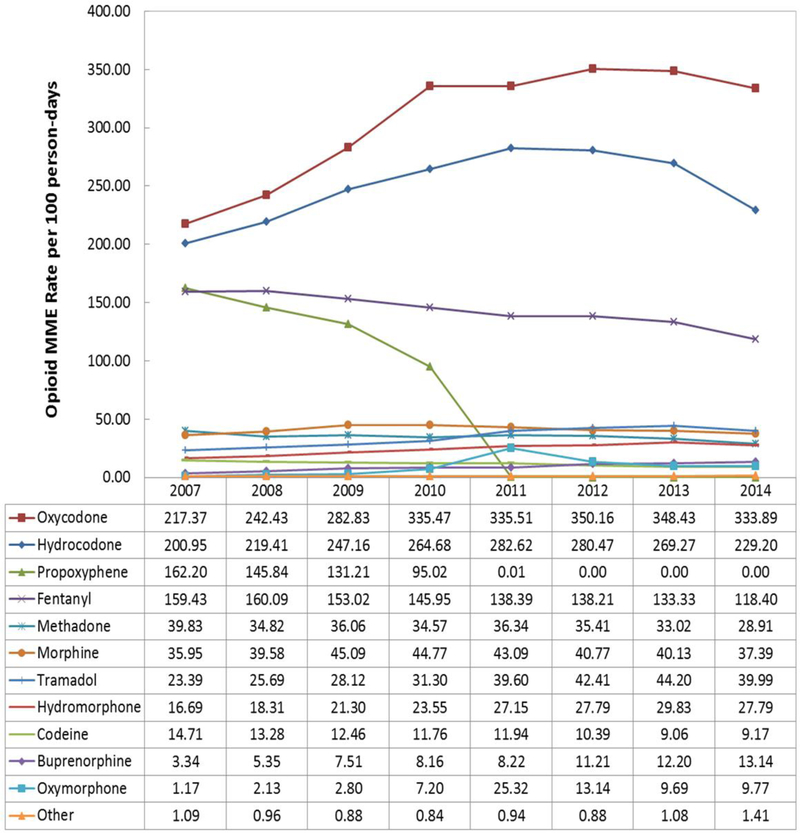
In 2007 the most commonly prescribed opioids among patients undergoing HD were oxycodone, hydrocodone, propoxyphene, and fentanyl (Figure 2). From 2007 through 2011, rates of oxycodone and hydrocodone prescribing increased 54% and 41%, respectively. During this time period, rates of propoxyphene prescribing decreased 100% due to market withdrawal and fentanyl prescribing decreased by 13%. After 2011, rates of oxycodone prescribing remained stable, whereas rates of hydrocodone dispensing decreased. By 2014, HD patients where prescribed 333.9 MME of oxycodone per 100 person-days and 229.2 MME of hydrocodone per 100 person-days.
Figure 2.
Opioid MME Rate per 100 person-days, by year (2007–2014) (N=484,745)
Source: USRDS
Sensitivity analysis
Overall rates of opioid prescriptions, quantity, days supply, and MME per 1000 person-days among the 270,210 patients who began HD and MPAB coverage on the same day were consistent the results from our main analysis presented in Appendix Table 3.
DISCUSSION
In this national study of patients undergoing HD, we found 63% of patients received at least one opioid prescription in 2010. By 2014, half of all HD patients received an opioid prescription.
Overall rates of opioid prescriptions, quantity, days supply, and total MME peaked between 2010 and 2012 then declined until 2014. Rates of MME were 1.8-fold and 1.6-fold higher among non- Hispanic and dual-eligible HD patients, respectively. Despite an 18% decline between 2012 and 2014, HD patients received three-fold more opioid prescriptions per person compared to the general U.S. population. Thus, while opioid prescribing among individuals undergoing HD has begun to decline, these patients continue to receive large quantities of opioids, and there are large differences in the rates of opioid prescribing based on patient ethnicity and income status.
Our results are consistent with results from two prior retrospective studies examining opioid prescribing among HD patients and suggest over half of these patients receive opioids.17,18 We built upon these previous findings by using data though 2014 and multiple prescribing measures which allowed us to comprehensively examine temporal changes in the incidence rate of opioid prescribing among HD patients. Results from prior studies suggest opioid prescribing increased among HD patients though 2010, however our results indicate rates of prescriptions, quantity, days supply and MME have declined since 2010, with the most substantial declines occurring between 2013 and 2014. Despite these recent declines, opioid use in this population still remains high. In 2014, HD patients 36–50 years old received sufficient opioids to take 25 pills every 30 days.
The findings from our study and at least one other prior study33 demonstrate opioid prescribing has declined in both the HD and general U.S. population since 2010, respectively. However, using national projections of opioid dispensing, we found on average HD patients receive 3.2- fold more opioid prescriptions per patient than the general U.S. population. This difference in prescribing is consistent with a comparison between our findings and data from the 2015 National Survey on Drug Use and Health (NSDUH),34 which suggests a higher proportion of HD patients received opioids compared to the general U.S. population (2014: 50% vs. 2015: 38%). In addition to a higher prevalence of pain and comorbidities, the lack of widely accepted guidelines on how to manage pain in patients with renal impairment may be contributing to these higher levels of opioid prescribing. Given the lack of evidence supporting opioid use for chronic pain in the general population,35 clinicians and patients should carefully consider the risks associated with opioid analgesics when selecting a pain management approach.
There are some notable limitations to our study. First, the USRDS does not capture prescriptions filled by HD patients who were not enrolled in Medicare or Part D. Although this is a common limitation in studies of ESRD36 and the vast majority of HD patients enroll in Medicare and Part D, rates of opioid prescribing may differ among privately insured HD patients. Second, data from Part D does not capture opioid prescriptions filed outside of Part D insurance plans (i.e. cash prescriptions). Third, we compared opioid prescriptions claims from Part D to national projections of opioid dispensing, thus the two estimates may not be directly comparable. Other data sets that may have provided a more direct comparison, such as Medicare data, would not have provided visibility of patients less than 65 years of age without ESRD. Given the IQVIA data captures approximately 88% of retail pharmacy claims for all payers prior to projection and our interest in comparing to the general U.S. population, we believed these data provided the best available comparison. Fourth, short-acting opioids are typically prescribed as needed. Therefore, the days supply on a pharmacy claim may not truly reflect the time period a patient had an opioid available to them. Finally, there is variation in the reporting quality of variables captured in the CMS 2728 form.37,38 To mitigate this, we limited our analysis to sociodemographic variables on the CMS 2728 form that are required to be reported or had little (<1%) to no missing values. Our analysis also had several strengths. First, we used a national registry with detailed information on patients’ dialysis treatment, sociodemographic characteristics, Medicare and Part D coverage, and prescription drug fills. Second, the vast majority of ESRD patients enroll in Medicare and approximately 80% of these also enroll in Part D, which makes our findings applicable to almost the entire population of ESRD patients. Third, a variety of factors contribute to changes in the total amount of opioid dispensed, thus we examined multiple measures of prescribing. Finally, we provided context to our findings by comparing the number of opioid prescriptions per patient in our study sample to the general U.S. population.
In this longitudinal cohort study of nearly a half a million HD patients, opioid prescribing declined earlier and more rapidly among HD patients than in the general U.S. population. Despite this, HD patients continue to receive three-fold more opioids than the average American. Efforts designed to curb over-utilization in the HD population might consider targeting middle- aged, non-Hispanic, and dual-eligible patients. In the context of a nation-wide opioid epidemic, identification of high-risk subgroups and clinician caution when prescribing opioids is essential to reducing morbidity and mortality associated with these products in this already vulnerable population.
Acknowledgments
Funding sources
Matthew Daubresse is supported by the NIH Clinical Research and Epidemiology in Diabetes and Endocrinology Training Grant (T32) awarded to Johns Hopkins University. Mara McAdams- DeMarco is supported by NIH grants R01AG055781, R01DK114074, and K01AG043501.
Deidra Crews is supported by NIH grant U01MD010550. Dorry Segev is supported by NIH grant K24DK101828. These funding sources had no role in the design and conduct of the study, analysis or interpretation of the data; and preparation or final approval of the manuscript prior to publication.
Appendix Table 1. Opioids included in analysis
| Buprenorphine |
| Butorphanol |
| Codeine |
| Dihydrocodeine |
| Fentanyl |
| Hydrocodone |
| Hydromorphone |
| Levorphanol |
| Meperidine |
| Methadone |
| Morphine |
| Opium |
| Oxycodone |
| Oxymorphone |
| Pentazocine |
| Propoxyphene |
| Tapentadol |
| Tramadol |
Appendix Table 2. Proportion of hemodialysis patients receiving opioids and total opioids supplied, by year (2007–2014) (N=484,745)
| Total HD patients | Patients with at least one opioid Rx* (%) | Total Opioids (in thousands) | ||||
|---|---|---|---|---|---|---|
| Prescriptions | Quantity** | Days supply | MME† | |||
| 2007 | 163,558 | 62.4 | 550 | 28,370 | 7,891 | 403,510 |
| 2008 | 167,675 | 62.5 | 585 | 30,901 | 8,728 | 432,866 |
| 2009 | 172,789 | 62.8 | 611 | 34,304 | 9,394 | 476,609 |
| 2010 | 179,621 | 63.2 | 638 | 37,365 | 10,286 | 516,444 |
| 2011 | 186,741 | 62.4‡ | 655 | 39,534 | 10,935 | 510,203 |
| 2012 | 192,136 | 60.9‡ | 665 | 40,911 | 11,434 | 531,519 |
| 2013 | 202,775 | 60.2‡ | 669 | 41,686 | 11,828 | 546,223 |
| 2014 | 208,807 | 53.7‡ | 591 | 37,694 | 10,793 | 510,786 |
restricted to patients observed for full calendar year
pills, patches, sublingual films
morphine milligram equivalents (mg)
statistically significant difference from prior year based on 95% CI
Appendix Table 3. Overall rates of opioid prescriptions, quantity, days supply and MME among hemodialysis patients, by year (2007–2014) (N=484,745)*
| Prescriptions | Quantity† | Days supply | MME‡ | |
|---|---|---|---|---|
| 2007 | 1.2 | 61.5 | 17.1 | 874.8 |
| 2008 | 1.2 § | 64.7§ | 18.3§ | 906.7§ |
| 2009 | 1.2 § | 69.7§ | 19.1§ | 967.7§ |
| 2010 | 1.2 | 72.5§ | 20.0§ | 1002.4§ |
| 2011 | 1.2 § | 73.5§ | 20.3§ | 948.4§ |
| 2012 | 1.2 § | 73.1§ | 20.4§ | 950.1§ |
| 2013 | 1.1 § | 70.9§ | 20.1§ | 929.5§ |
| 2014 | 1.0 § | 62.6§ | 17.9§ | 848.4§ |
rates presented per 100 person-days observed
pills, patches, sublingual films
morphine milligram equivalents (mg)
statistically significant difference from prior year based on 95% CI
Appendix Table 4. Opioid prescription rates by sociodemographic characteristics*
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| 18–35 | 1.3 | 1.4 | 1.4 | 1.4 | 1.4 | 1.3 | 1.3 | 1.1† |
| 36–50 | 1.5 | 1.6 | 1.6 | 1.6 | 1.6 | 1.5 | 1.4 | 1.2† |
| 51–65 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.2 | 1.2 | 1.0† |
| ≥66 | 0.9 | 0.9 | 1.0 | 1.0 | 0.9 | 0.9 | 0.9 | 0.7† |
| Sex | ||||||||
| Female | 1.3 | 1.3† | 1.4 | 1.4 | 1.3 | 1.3 | 1.3† | 1.1† |
| Male | 1.1 | 1.1† | 1.1 | 1.1 | 1.1 | 1.1 | 1.0† | 0.9† |
| Race | ||||||||
| Black | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.2 | 1.2 | 1.0† |
| White | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.1 | 1.0† |
| Native American/Asian | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.6† |
| Other/Unknown | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.9 | 0.9 | 0.8† |
| Ethnicity | ||||||||
| Hispanic | 0.8 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.8 | 0.7† |
| Non-Hispanic | 1.2 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.2 | 1.0† |
| Other/Unknown | 1.5 | 1.5 | 1.6 | 1.6 | 1.5 | 1.5 | 1.4 | 1.2† |
| Dual-Eligible | ||||||||
| Non-Eligible | 0.9 | 0.9† | 0.9 | 0.9 | 0.9 | 0.9 | 0.9† | 0.8† |
| Dual-Eligible | 1.4 | 1.4† | 1.5 | 1.5 | 1.4 | 1.4 | 1.4† | 1.2† |
| Employment | ||||||||
| Unemployed | 1.3 | 1.4 | 1.4 | 1.4 | 1.3 | 1.3 | 1.2 | 1.1† |
| Full or Part-time | 0.9 | 0.9 | 1.0 | 0.9 | 0.9 | 0.9 | 0.9 | 0.7† |
| Retired | 0.9 | 1.0 | 1.0 | 1.0 | 0.9 | 0.9 | 0.9 | 0.8† |
| Retired Disabled | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.4 | 1.2† |
| Other | 1.1 | 1.1 | 1.2 | 1.1 | 1.1 | 1.1 | 1.0 | 0.9† |
| Diabetes | ||||||||
| No Diabetes | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.1 | 1.1 | 0.9† |
| Diabetes | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.2 | 1.2 | 1.0† |
| Cause of ESRD | ||||||||
| Diabetes | 1.2 | 1.3† | 1.3 | 1.3 | 1.2 | 1.2† | 1.2† | 1.0† |
| Hypertension | 1.1 | 1.1† | 1.1 | 1.1 | 1.1 | 1.1† | 1.1† | 0.9† |
| Other | 1.3 | 1.3† | 1.3 | 1.3 | 1.3 | 1.3† | 1.2† | 1.0† |
| U.S. Geographic Region | ||||||||
| New England | 1.1 | 1.2 | 1.2 | 1.2 | 1.2 | 1.1 | 1.1 | 1.0† |
| Mideast | 0.9 | 0.9 | 1.0 | 0.9 | 0.9 | 0.9 | 0.9 | 0.8† |
| Great Lakes | 1.4 | 1.5 | 1.5 | 1.5 | 1.4 | 1.4 | 1.4 | 1.2† |
| Plains | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.3 | 1.2† |
| Southeast | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.2 | 1.1† |
| Southwest | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.0 | 0.9† |
| Rocky Mountain | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.4 | 1.2† |
| Far West | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.0 | 0.9† |
rates presented per 100 person-days observed;
statistically significant difference from prior year based on 95% CI
Footnotes
Disclosure statement
Dr. Alexander is Chair of the FDA’s Peripheral and Central Nervous System Advisory Committee; serves on the Advisory Board of MesaRx Innovations; holds equity in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and serves as a member of OptumRx’s P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. Remaining authors have no conflicts of interest to report.
REFERENCES
- 1.U.S. Agency for Health Care Policy and Research. Acute Pain Management: Operative or Medical Procedures and Trauma. (AHCPR Pub. No. 92–0032) Rockville, MD, U.S. Department of Health and Human Services, 1992. [Google Scholar]
- 2.American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 4 Glenview, IL: American Pain Society; 1999. [Google Scholar]
- 3.American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 1995;274:1874–80. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO’s Pain Relief Ladder (2009). Available at: www.who.int/cancer/palliative/painladder/en/ (Accessed January 2, 2017).
- 5.Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers---United States, 1999−−2008. MMWR Morb Mortal Wkly Rep 2011. November 4;60(43):1487–92. [PubMed] [Google Scholar]
- 6.Daubresse M, Chang HY, Yu Y, Viswanathan S, Shah ND, Stafford RS, Kruszewski SP, Alexander GC.Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med Care 2013. October;51(10):870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in Commercial and Medicaid insurance plans: The TROUP study. Pain 2008;138:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martell BA, O’Connor PG, Kerns RD, et al. Systematic Review: Opioid Treatment for Chronic Back Pain: Prevalence, Efficacy, and Associations with Addiction. Annals of Internal Medicine 2007;146:116–27. [DOI] [PubMed] [Google Scholar]
- 9.Chou R, Clark E, Helfand M. Comparative Efficacy and Safety of Long-Acting Oral Opioids for Chronic Non-Cancer Pain: A systematic review. Journal of Pain and Symptom Management 2003;26;1026–48. [DOI] [PubMed] [Google Scholar]
- 10.Manchikanti L, Singh A. Therapeutic opioids: A ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 2008; 11: S63–S88. [PubMed] [Google Scholar]
- 11.Davison SN: Pain in hemodialysis patients: Prevalence, etiology, severity, and analgesic use. Am J Kidney Dis 42: 1239–1247, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Murtagh FE, Eddington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Smyth B, Jones C, Saunders J. Prescribing for patients on dialysis. Aust Prescr 2016. February;39(1):21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niscola P, Scaramucci L, Vischini G, et al. The use of major analgesics in patients with renal dysfunction. Curr Drug Targets 2010. June;11(6):752–8. [DOI] [PubMed] [Google Scholar]
- 15.Wyne A1, Rai R, Cuerden M, Clark WF, Suri RS. Opioid and benzodiazepine use in end- stage renal disease: a systematic review. Clin J Am Soc Nephrol 2011. February;6(2):326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailie GR, Mason NA, Bragg-Gresham JL, Gillespie BW, Young EW: Analgesic prescription patterns among hemodialysis patients in the DOPPS: Potential for underprescription. Kidney Int 65: 2419–2425, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Butler AM, Kshirsagar AV, Brookhart MA. Opioid use in the US hemodialysis population. Am J Kidney Dis 2014. January;63(1):171–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW. Opioid Prescription, Morbidity, and Mortality in United States Dialysis Patients. J Am Soc Nephrol 2017. December;28(12):3658–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang HY, Lyapustina T, Rutkow L, Daubresse M, Richey M, Faul M, Stuart EA, Alexander GC.Impact of prescription drug monitoring programs and pill mill laws on high-risk opioid prescribers: A comparative interrupted time series analysis. Drug Alcohol Depend 2016. August 1;165:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyapustina T, Rutkow L, Chang HY, Daubresse M, Ramji AF, Faul M, Stuart EA, Alexander GC.Effect of a “pill mill” law on opioid prescribing and utilization: The case of Texas. Drug Alcohol Depend 2016. February 1;159:190–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutkow L, Chang HY, Daubresse M, Webster DW, Stuart EA, Alexander GC.Effect of Florida’s Prescription Drug Monitoring Program and Pill Mill Laws on Opioid Prescribing and Use. JAMA Intern Med 2015. October;175(10):1642–9 [DOI] [PubMed] [Google Scholar]
- 22.Daubresse M, Gleason PP, Peng Y, Shah ND, Ritter ST, Alexander GC.Impact of a drug utilization review program on high-risk use of prescription controlled substances. Pharmacoepidemiol Drug Saf 2014. April;23(4):419–27. [DOI] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. FDA Drug Safety Communication: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit; Boxed Warning Will Highlight Potential for Severe Liver Failure (2011) Available at: https://www.fda.gov/Drugs/DrugSafety/ucm239821.htm (Accessed January 2, 2017).
- 24.Drug Enforcement Administration. Schedules of Controlled Substances: Placement of Tramadol Into Schedule IV (2014). Available at: https://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0702.htm (Accessed January 2, 2017).
- 25.National Institute on Drug Abuse (2014). America’s Addiction to Opioids: Heroin and Prescription Drug Abuse Available at: https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse (Accessed January 2, 2017).
- 26.Pezalla EJ, Rosen D, Erensen JG, Haddox JD, Mayne TJ. Secular trends in opioid prescribing in the USA. J Pain Res 2017. February 14;10:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser Family Foundation. Population Distribution by Age Available at: https://www.kff.org/other/state-indicator/distribution-by-age/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D (Accessed January 2, 2017).
- 28.Centers for Disease Control. Opioid Overdose Data Resources Available at: https://www.cdc.gov/drugoverdose/resources/data.html (Accessed January 2, 2017).
- 29.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin. J. Pain, 24 (2008), pp. 521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson R, Brown T. The Use of Bureau of Economic Analysis (BEA) Areas and Regions for Representing Geographic Variation Available at: https://www.nij.gov/topics/drugs/markets/adam/documents/wilson-brown-paper.pdf (Accessed January 2, 2017).
- 31.Bureau of Economic Analysis. BEA regions Available at: https://www.bea.gov/regional/docs/regions.cfm (Accessed January 2, 2017).
- 32.Austin PC, Hux JE. A brief note on overlapping confidence intervals. J Vasc Surg 2002. July;36(1):194–5. [DOI] [PubMed] [Google Scholar]
- 33.Pezalla EJ, Rosen D, Erensen JG, Haddox JD, Mayne TJ. Secular trends in opioid prescribing in the USA. J Pain Res 2017. February 14;10:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017. September 5;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 35.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA 2016. April 19;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAdams-DeMarco MA, Bae S, Chu N, Gross AL, et al. Dementia and Alzheimer’s Disease among Older Kidney Transplant Recipients. J Am Soc Nephrol 2017. May;28(5):1575–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggers PW. CMS 2728: what good is it?. Clin J Am Soc Nephrol 2010. November;5(11):1908–9. [DOI] [PubMed] [Google Scholar]
- 38.Longenecker JC, Coresh J, Klag MJ, Levey AS, Martin AA, Fink NE, Powe NR. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol 2000. March;11(3):520–9. [DOI] [PubMed] [Google Scholar]