Abstract
Background:
Genetic polymorphisms of drug metabolizing enzymes can substantially modify the pharmacokinet-ics of a drug and eventually its efficacy or toxicity; however, inferring a patient’s drug metabolizing capacity merely from his or her genotype can lead to false prediction. Non-genetic host factors (age, sex, disease states) and environmental factors (nutrition, co-medication) can transiently alter the enzyme expression and activities resulting in genotype-phenotype mis-match. Although valproic acid is a well-tolerated anticonvulsant, pediatric patients are particularly vulnerable to valproate in-jury that can be partly attributed to the age-related differences in metabolic pathways.
Methods:
CYP2C9 mediated oxidation of valproate, which is the minor metabolic pathway in adults, appears to become the principal route in children. Genetic and non-genetic variations in CYP2C9 activity can result in significant inter- and intra-individual differences in valproate pharmacokinetics and valproate induced adverse reactions.
Results:
The loss-of-function alleles, CYP2C9*2 or CYP2C9*3, display significant reduction in valproate metabolism in children; furthermore, low CYP2C9 expression in patients with CYP2C9*1/*1 genotype also leads to a decrease in valproate metabolizing capacity. Due to phenoconversion, the homozygous wild genotype, expected to be translated to CYP2C9 en-zyme with normal activity, is transiently switched into poor (or extensive) metabolizer phenotype.
Conclusion:
Novel strategy for valproate therapy adjusted to CYP2C9-status (CYP2C9 genotype and CYP2C9 expression) is strongly recommended in childhood. The early knowledge of pediatric patients’ CYP2C9-status facilitates the optimization of valproate dosing which contributes to the avoidance of misdosing induced adverse reactions, such as abnormal blood lev-els of ammonia and alkaline phosphatase, and improves the safety of children’s anticonvulsant therapy.
Keywords: Valproic acid, epilepsy, psychiatric disorders, CYP2C9 genotype, CYP2C9 expression, personalized medication, pediatric patients
1. INTRODUCTION
A patient’s drug metabolizing capacity highly influences his or her response to a drug; therefore, genetic variations or alterations in the expression and activities of drug metabolizing enzymes can substantially modify the pharmacokinetics of a drug and eventually its efficacy or toxicity [1]. The routine clinical practice generally applies symptom driven drug therapy with or without blood concentration guided dosing; however, the inter-individual variability in drug metabolism calls for personalized medication primarily for drugs with narrow therapeutic index [2, 3]. The identification of genetic and non-genetic factors that can potentially affect the pharmacokinetics of a particular drug is a prerequisite of tailored pharmacotherapy [4, 5]. Although in vitro pharmacokinetic and enzyme mapping studies provide useful information about the metabolic pathways and the enzymes responsible for the biotransformation of a drug, the clinical significance of these factors must be verified [6-8]. The first step is to identify the major enzyme(s) involved in the biotransformation of a particular drug at clinically relevant concentration. Clinical studies generally focus on the functional impact of genetic variations in drug metabolizing enzymes; however, regulation of enzyme expression and inhibition of enzyme function are also of high importance for predicting the fate of a drug and the clinical outcome of drug therapy. For such an approach, a reliable diagnostic tool is required to estimate a patient’s capability for drug metabolism and elimination. The characterization of patients’ drug metabolizing capacity can facilitate more precise prediction of the fate of a drug and can contribute to the improvement of drug therapy.
2. CYTOCHROME P450 (CYP) ENZYMES
CYP enzymes, the key players in the metabolism of most drugs, convert lipophilic compounds to more polar metabolites which are readily excreted [9]. In humans, 57 functional CYP genes have been identified; however, only 10 to 12 enzymes belonging to the CYP1, CYP2 and CYP3 enzyme families are involved in the metabolism of the majority of drugs, whereas others are responsible for endobiotic biotransformation (e.g. steroids, prostaglandins, fatty acids, retinoic acid) [10, 11]. Although the overlapping substrate specificities of the drug metabolizing CYPs could ensure a balanced metabolic rate, many drugs display great inter-individual and intra-individual variations in their pharmacokinetics [12]. It can be attributed to several facts: i) many drugs are metabolized at therapeutic concentrations only by one or two CYP enzymes [13], ii) the expression and activities of CYP enzymes display substantial, even more than 100-fold inter-individual variability [14], iii) loss-of-function or gain-of-function mutations in CYP genes result in permanent poor or extensive metabolism, whereas non-genetic (internal or environmental) factors can modify drug metabolizing capacity, evoking transient poor or extensive metabolism [1, 13, 15]. Plenty of CYP genetic variants have been identified, and the clinical relevance for some of them has been clearly evidenced [13, 15, 16]. CYP genotype determines the potential for the expression of functional or non-functional enzymes; thus, the heritable genetic polymorphisms can explain some inter-individual differences in drug metabolism. However, non-genetic host factors (age, sex, disease states) and environmental factors (nutrition, medication, smoking, alcohol consumption) can alter the expression and activities of CYP enzymes [17]. Due to phenoconversion, homozygous wild genotype, expected to be translated to CYP enzyme with normal activity, can be transiently switched into poor or extensive metabolizer phenotype [1]. Consequently, both the CYP genotype and the current CYP expression must be considered for the estimation of a patient’s drug metabolizing capacity. CYPtestTM, a complex diagnostic system has been introduced for the estimation of patients’ drug metabolizing capacity by CYP genotyping and by CYP expression in leukocytes [14]. Although hepatic CYP activities are the best for characterization of a patient’s drug metabolizing status, liver biopsies are generally not available, whereas peripheral blood is a more easily accessible sample. First CYP genotyping for loss-of-function or gain-of-function mutations is carried out by hydrolysis single nucleotide polymorphism assay (TaqMan assay) and allelic discrimination, then CYP expression is quantified in peripheral leukocytes by quantitative real-time PCR analysis. CYP mRNA levels in leukocytes isolated from peripheral blood were demonstrated to provide information about hepatic CYP activities in those subjects who do not carry polymorphic alleles. The knowledge of a patient’s CYP-status may facilitate tailoring of drug therapy by the choice of the appropriate drug and dosage.
3. POLYMORPHIC METABOLISM OF VALPROIC ACID
Valproic acid is one of the first choices of antiepileptic therapy, and is successfully applied for treatment of both generalized and partial seizures [18]. Furthermore, it is effectively used in the adjuvant therapy of psychiatric disorders, such as schizophrenia and bipolar disorder [19-21]. It is well-tolerated by most of the patients, and serious side effects, including bone marrow suppression, coagulopathy, hepatotoxicity, hyperammonemic encephalopathy and bone metabolic disorders rarely occur [22-25]. Pediatric patients appear to be more vulnerable to valproic acid induced injury than adults; the risk of serious adverse reactions is significantly increased in children younger than 2 years of age, especially who have pre-existing neurologic or other medical defects or who are under multiple anticonvulsant therapy [25-27]. Although the mechanism of valproic acid induced adverse reactions is not clearly understood, the cytotoxicity and mitochondrial dysfunction are attributed to the parent compound and some of its unsaturated metabolites [28-31].
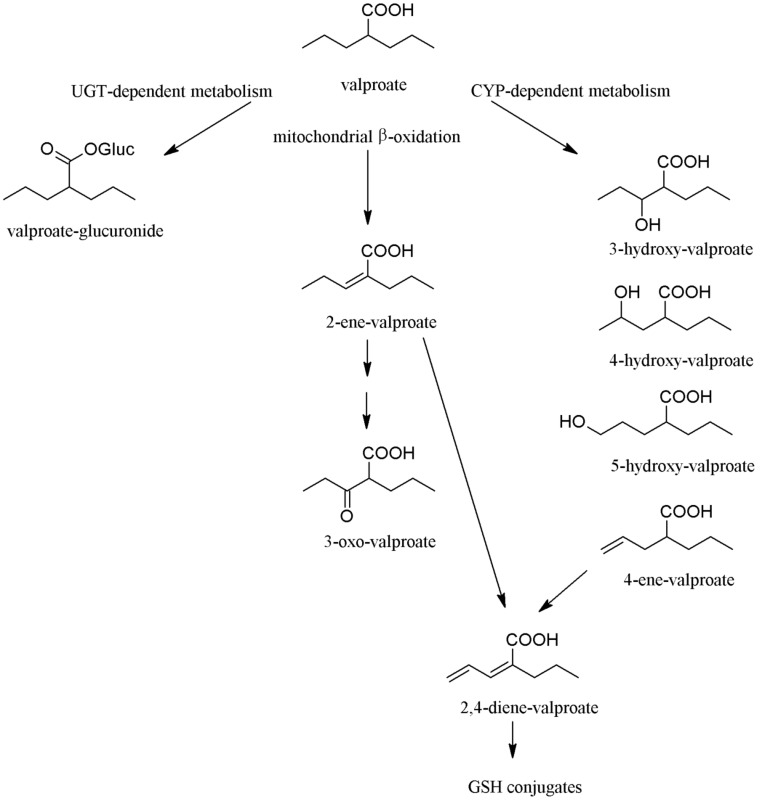
Valproic acid is a simple, branched short-chain carboxylic acid, which is extensively metabolized in the liver, and less than 3% of the administered dose is excreted as unchanged parent compound. Biotransformation of valproic acid results in several hydroxylated, unsaturated and conjugated metabolites (Fig. 1) [32]. In adults on monotherapy, conjugation with glucuronic acid is the major metabolic pathway, and 30-50% of the dose is converted to valproate-glucuronide. Mitochondrial β-oxidation, forming 2-ene-valproate and 3-keto-valproate, accounts for approximately 40% of the dose, whereas the minor metabolic pathway catalysed by CYP enzymes eliminates 10-15% of the dose, producing hydroxylated metabolites (3-, 4-, and 5-hydroxy-valproate metabolites) and 4-ene-valproate [31, 33, 34]. CYP2C9 has been demonstrated to be the main catalyst of CYP-mediated hydroxylation and desaturation, while CYP2A6 and CYP2B6 display minor contribution to valproic acid metabolism [34]. In mitochodria, the 4-ene metabolite is further desaturated to 2,4-diene-valproate which is conjugated with glutathione [32].
Fig. (1).
Metabolic pathways of valproic acid. In adults, the majority of valproate dose is eliminated via glucuronidation and mitochondrial β-oxidation pathways, and about 15-20% of the dose is metabolized by CYP enzymes. In contrast, valproate metabolism is shifted towards CYP-dependent route in children. Abbreviations: UGT: UDP-glucuronyl transferase, GSH: glutathion.
In contrast to the ratio of valproate metabolic routes in adults, the CYP-catalysed oxidation can become the principal pathway in those special cases when glucuronidation or mitochondrial β-oxidation pathways are compromised or poorly developed, for example, in children. The expression of UDP-glucuronyl transferases is known to be developmentally regulated [35, 36]; the enzymes responsible for glucuronidation of valproic acid are poorly expressed in infants, and their activities slightly increase, however, they are under the adult levels, sometimes 10-15 years of age. Therefore, the formation of valproate-glucuronide is poor in pediatric patients, primarily in children younger than 2 years of age [37]. Furthermore, valproic acid and/or some of its metabolites inhibit mitochondrial β-oxidation [31, 38]. On the other hand, CYP expression and activities in children exceed adult levels, and decrease to the adult activities by puberty [36, 39]. Consequently, larger proportion of the administered valproic acid dose is metabolized by CYP enzymes in children than in adults. Shifting the metabolic pathways in children can account for the age-related differences in the incidence of valproic acid induced adverse reactions.
CYP2C9, primarily responsible for hydroxylation and 4-ene desaturation of valproic acid, is a polymorphic enzyme with the highest frequency of CYP2C9*2 and CYP2C9*3 alleles in Caucasian white populations [15, 40]. The prevalence of these two alleles displays inter-ethnic differences. CYP2C9*2 is almost absent in Asian and African populations, whereas it is present in 10-19% of Europeans. The frequency of CYP2C9*3 is 3-6% in Asians, 1-5% in Africans and 6-9% in Europeans [15, 41, 42]. The CYP2C9*2 and CYP2C9*3 allelic variants result in substantial decrease in CYP2C9 enzyme activity due to reduced interaction with NADPH-CYP-reductase or with CYP2C9 substrate, respectively [43-45]. These loss-of-function mutations of CYP2C9 have been demonstrated to be less active in valproate metabolism than the wild type CYP2C9*1 allele [46]. CYP2C9*2 and CYP2C9*3 alleles thus lead to genetically determined poor metabolism of valproic acid; however, the poor metabolizer phenotype can transiently occur in patients carrying wild CYP2C9 genotype (CYP2C9*1/*1). Non-genetic factors, such as age, co-morbidities, nutrition or co-medication, can modulate a patient’s valproate metabolizing capacity by reducing (or increasing) CYP2C9 expression, resulting in weaker (or more extensive) metabolizer phenotype. This genotype-phenotype mismatch may give rise to more poor metabolizers than it would be predicted from CYP2C9 genotype [1].
4. CYP2C9-STATUS GUIDED VALPROIC ACID THERAPY
4.1. Valproate Blood Concentration as a Function of CYP2C9
Predicting a patient’s drug metabolizing phenotype is highly complex, requiring the basic knowledge of his or her genotypes of drug metabolizing enzyme(s). However, inferring the valproate metabolizing phenotype from CYP2C9 genotype may lead false prediction because non-genetic factors can significantly alter drug metabolizing phenotype. In patients carrying the loss-of-function CYP2C9*3 allele, Tan et al. [47] have demonstrated somewhat higher valproate plasma concentrations normalized by the dose and the bodyweight than in those with two wild type alleles (3.9±0.4 (μg/ml)/(mg dose/kg bw) vs 3.4±0.4 (μg/ml)/(mg dose/kg bw) in patients carrying CYP2C9*1/*3 and CYP2C9*1/*1, respectively). The CYP2C9*3 evoked slight increase in valproate levels may be attributed to the facts that Tan et al. [47] took neither the CYP2C9 expression nor the age-related differences in valproic acid metabolism into account. CYP2C9*1/*1 carriers are basically assumed to have functional CYP2C9 enzyme and therefore to be extensive metabolizers; however, CYP2C9 genotype can be converted to a drug metabolizing phenotype different from that would be predicted from the genotype. We have reported that pediatric patients with CYP2C9*1/*1 genotype expressing CYP2C9 at low level displayed significantly higher valproate concentrations (5.13±1.2 (μg/ml)/(mg dose/kg bw)) than normal CYP2C9 expressers (2.12±0.5 (μg/ml)/(mg dose/kg bw), P<0.0001) [48]. While, no statistically significant differences in valproic acid concentrations were found between the patients with heterozygous genotypes carrying any of the polymorphic CYP2C9 alleles (4.33-5.54±1.2 (μg/ml)/(mg dose/kg bw)) and those low CYP2C9 expressers with two functional alleles (CYP2C9*1/*1). Consistently, valproic acid concentration in children with homozygous wild CYP2C9 genotype was influenced by CYP2C9 expression, which is considered to be a further evidence for phenoconversion of CYP2C9*1/*1.
Phenoconversion of CYP2C9*1/*1 genotype can be the consequence of various factors, such as disease, hormonal status, nutrition or co-medication. Multi-drug therapy with valproic acid and antiepileptic drugs known to be CYP2C9 inducers is expected to increase CYP2C9 expression that eventually leads to enhanced valproate clearance. The concomitant treatment of patients with valproate and phenytoin, phenobarbital or carbamazepine has been reported to increase valproate metabolism comparing to the patients on valproate monotherapy [49, 50]. Due to the CYP2C9 inducer co-administration, the formation of the hepatotoxic 4-ene-valproate was observed to be elevated in patients [50, 51], which can explain the higher risk of hepatotoxicity in those subjects on polytherapy with CYP2C9 inducers [25, 52]. In our recent study involving pediatric patients (<15 years) with epilepsy, the ratio of low CYP2C9 expressers was unusually high (57.9%), approximately 4-fold higher than in healthy adults (13.3%) (Fig. 2A and 2B). The substantial alteration in CYP2C9 expression was not attributed to co-medication, since no drug therapy was applied at the time of blood sampling. It was rather considered to be the consequence of some suppressive factors released during epileptic seizure. In the anamnesis of low CYP2C9 expresser children, the prevalence of seizures 72 hr before CYPtesting was significantly higher than in normal or high expressers (24/44 vs 3/32, P<0.0001). In epileptic patients, substantial increase of pro-inflammatory cytokine release has been observed following seizures [53, 54], and the down-regulation of CYP expression is proposed for the mechanism of cytokine action [55]. The down-regulation of CYP2C9 mediated by the pro-inflammatory IL-6 and IL-1β is the consequence of the repression of pregnane X receptor and constitutive androstane receptor [56, 57]. These nuclear receptors transcriptionally regulate the expression of several drug metabolizing enzymes, including CYP2C9 [58-61].
Fig. (2).
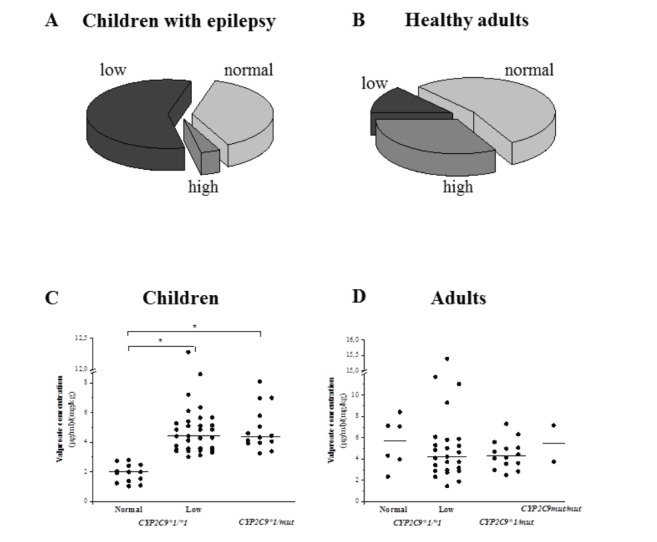
Impact of epilepsy and age on CYP2C9 function. Epileptic seizures negatively influence CYP2C9 expression in patients (A) comparing to healthy subjects (B). CYP2C9 mRNA levels were determined in leukocytes that can reflect the hepatic CYP2C9 activity. More than half of the pediatric patients with epilepsy (44/76) expressed CYP2C9 at low level, whereas the ratio of CYP2C9 low expressers in healthy subjects was less than one fifth (15/113). Association between steady-state serum concentrations of valproate and CYP2C9-status was demonstrated in children (N=76) (C), but not in adults (N=47) (D). The serum concentrations normalized by the dose and the bodyweight were determined at least four weeks after the beginning of valproate therapy. Solid line means the median of the groups, while * means the significant difference (P<0.01). Abbreviations: mut: loss-of-function CYP2C9 allele (CYP2C9*2 or CYP2C9*3), Low: low CYP2C9 expressers, Normal: normal CYP2C9 expressers.
Age-related changes in valproate pharmacokinetics can be explained by distinct priority of metabolic pathways in children and adults [37, 62]. The glucuronidation and mitochondrial β-oxidation of valproic acid as the major metabolic routes in adults appear to be shifted towards CYP2C9 dependent metabolism in children. A significant association between CYP2C9-status (determined by CYP2C9 genotype and CYP2C9 expression) and valproate serum concentrations has been demonstrated in pediatric patients [48]. In our recent follow-up study with adult patients (>18 years), no association was observed between valproate pharmacokinetics and patients’ CYP2C9-status (Fig. 2C and D). The different priority of CYP2C9-dependent and CYP2C9-independent metabolism in pediatric and adult patients may contribute to the age-related differences in the incidence of valproate induced adverse reactions. Therefore, the identification of the genetic and non-genetic factors, influencing valproate metabolizing capacity, can facilitate the optimization of valproate therapy.
4.2. CYP2C9-status Guided Valproic Acid Therapy in Children
Valproic acid is a drug with narrow therapeutic range, and the plasma concentration for optimal therapeutic effect is between 40 and 100 μg/ml. In symptom driven antiepileptic therapy, low dosages (daily dose of 10-15 mg/kg bodyweight) are initiated, and the target doses are subsequently titrated until the optimal clinical response is achieved [23, 27]. Therapeutic monitoring of valproate is advised; assaying valproate plasma concentrations is recommended four weeks after the beginning of valproate therapy and later if drug-interactions, lack of therapeutic effect or overdose-related adverse effects are suspected.
The novel findings that the steady-state valproate concentrations in pediatric patients are in strong association with the patients’ CYP2C9-status determined by the genetic variability of CYP2C9 and by CYP2C9 expression, provided the basic tool for personalized antiepileptic therapy [48]. CYP2C9-status guided valproic acid therapy can be recommended for children with at least one or two wild type CYP2C9 alleles (CYP2C9*1/*1, CYP2C9*1/*2 or CYP2C9*1/*3) (Fig. 3). Normal dose can be applied for the normal CYP2C9 expressers carrying CYP2C9*1/*1; reduced dose is proposed for the children with heterozygous genotypes (CYP2C9*1/*2 or CYP2C9*1/*3) or for low CYP2C9 expressers, whereas the dose of valproic acid is suggested to be increased in high expresser patients with CYP2C9*1/*1 genotype. For children with two loss-of-function CYP2C9 alleles (CYP2C9*2/*2, CYP2C9*3/*3 or CYP2C9*2/*3), an alternative antiepileptic (non-valproate) therapy is recommended because of the potential for poor valproate metabolism [63]. In a neonate patient under valproate therapy, the serious adverse effects, such as hyperammonemia, increased serum alkaline phosphatase and bone marrow depression, were attributed to reduced ability to metabolize valproate [63]. As a consequence of two loss-of-function CYP2C9 alleles (CYP2C9*3/*3), the exaggerated blood concentration of valproate was likely to manifest the toxic symptoms.
Fig. (3).
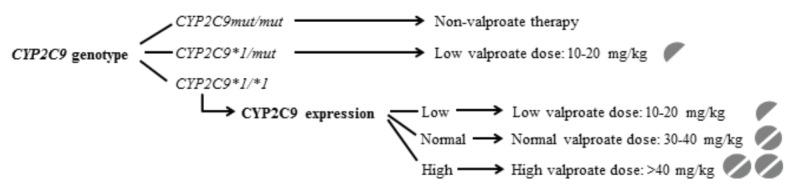
CYP2C9-status guided personalized valproic acid therapy in children. Abbreviations: mut: loss-of-function CYP2C9 allele (CYP2C9*2 or CYP2C9*3), Low: low CYP2C9 expressers, Normal: normal CYP2C9 expressers, High: high CYP2C9 expressers.
Special attention has been focused on the age-related differences in the incidence of adverse effects associated with valproate therapy, which may be attributed to developmental variations in valproate metabolism [30, 37, 62, 64]. Valproic acid itself and some of its unsaturated metabolites are assumed to be responsible for the side effects, such as hematologic disorders or hepatotoxicity [24, 30, 65]. Assigning a prominent role in valproate metabolism to CYP2C9 in pediatric patients, the patients’ CYP2C9-status may account for the predisposition of some adverse reactions, and both loss-of-function mutations in the CYP2C9 gene and low CYP2C9 expression can be considered to be the risk factors for undesired side effects [48]. The benefit of CYP2C9-status guided valproate therapy over symptom driven therapy has been demonstrated in children [66]. CYP2C9-status adapted valproate dosing significantly reduced the ratio of patients with blood concentrations out of the therapeutic range, and substantially decreased the incidence of hyperammonemia and of abnormal serum levels of alkaline phosphatase. Hyperammonemia is considered to be associated with high valproate doses or with exaggerated blood concentrations of valproate [66-69]. The increase in blood ammonia level, frequently observed in the patients under symptom driven valproate therapy (17%), always accompanied with extreme serum levels of alkaline phosphatase and with secondary symptoms, such as nausea, somnolence, fatigue, consciousness or behaviour disturbances [66, 70]. Therefore, personalized valproic acid therapy adjusted to the patients’ CYP2C9-status is strongly recommended, because it can improve the safety of antiepileptic therapy in one of the most vulnerable patient population [66, 70].
CONCLUSION
As a consequence of the prominent role of CYP2C9 in valproic acid metabolism in pediatric patients, the identification of genetic and non-genetic factors that can modify CYP2C9 activity is required for the improvement of the safety of valproate therapy. The novel strategy of personalized anticonvulsant therapy comprises the following steps: i) determination of patient’s CYP2C9-status by revealing the allelic variants in CYP2C9 gene and the current CYP2C9 expression; ii) initiation of non-valproate therapy for patients with two loss-of-function CYP2C9 alleles; iii) initiation of CYP2C9 adapted valproate dosing for patients with one or two wild type alleles (CYP2C9*1); iv) follow-up assaying of CYP2C9 expression in patients carrying CYP2C9*1/*1 genotype, and modification of valproate dosing if necessary. Tailored valproate therapy adjusted to the pediatric patients’ CYP2C9-status can reduce the risk of exaggerated valproate concentrations and misdosing induced serious adverse reactions.
Acknowledgements
The authors are indebted to Tímea Zentai and Mária Szabó for their skillful assistance in this study. The study was supported by the grants from the National Research, Development and Innovation Fund (OTKA K104459, K104738 and VEKOP-2.3.3-15-2017-00014).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Shah R.R., Smith R.L. Addressing phenoconversion: the Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015;79(2):222–240. doi: 10.1111/bcp.12441. [http://dx.doi.org/10.1111/bcp.12441]. [PMID: 24913012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squassina A., Manchia M., Manolopoulos V.G., Artac M., Lappa-Manakou C., Karkabouna S., Mitropoulos K., Del Zompo M., Patrinos G.P. Realities and expectations of pharmacogenomics and personalized medicine: impact of translating genetic knowledge into clinical practice. Pharmacogenomics. 2010;11(8):1149–1167. doi: 10.2217/pgs.10.97. [http://dx.doi.org/10.2217/pgs.10.97]. [PMID: 20712531]. [DOI] [PubMed] [Google Scholar]
- 3.Gervasini G., Benítez J., Carrillo J.A. Pharmacogenetic testing and therapeutic drug monitoring are complementary tools for optimal individualization of drug therapy. Eur. J. Clin. Pharmacol. 2010;66(8):755–774. doi: 10.1007/s00228-010-0857-7. [http://dx.doi.org/10.1007/s00228-010-0857-7]. [PMID: 20582584]. [DOI] [PubMed] [Google Scholar]
- 4.Shah R.R., Shah D.R. Personalized medicine: is it a pharmacogenetic mirage? Br. J. Clin. Pharmacol. 2012;74(4):698–721. doi: 10.1111/j.1365-2125.2012.04328.x. [http://dx.doi.org/10.1111/j.1365-2125.2012.04328.x]. [PMID: 22591598]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sim S.C., Kacevska M., Ingelman-Sundberg M. Pharmacogenomics of drug-metabolizing enzymes: a recent update on clinical implications and endogenous effects. Pharmacogenomics J. 2013;13(1):1–11. doi: 10.1038/tpj.2012.45. [http://dx.doi.org/10.1038/tpj.2012.45]. [PMID: 23089672]. [DOI] [PubMed] [Google Scholar]
- 6.Lipscomb J.C., Poet T.S. In vitro measurements of metabolism for application in pharmacokinetic modeling. Pharmacol. Ther. 2008;118(1):82–103. doi: 10.1016/j.pharmthera.2008.01.006. [http://dx.doi.org/10.1016/j.pharmthera.2008.01. 006]. [PMID: 18374419]. [DOI] [PubMed] [Google Scholar]
- 7.Wilk-Zasadna I., Bernasconi C., Pelkonen O., Coecke S. Biotransformation in vitro: An essential consideration in the quantitative in vitro-to-in vivo extrapolation (QIVIVE) of toxicity data. Toxicology. 2015;332:8–19. doi: 10.1016/j.tox.2014.10.006. [http://dx.doi.org/10.1016/j.tox. 2014.10.006]. [PMID: 25456264]. [DOI] [PubMed] [Google Scholar]
- 8.Tóth K., Sirok D., Kiss Á., Mayer A., Pátfalusi M., Hirka G., Monostory K. Utility of in vitro pharmacokinetic data in prediction of in vivo hepatic clearance of psychopharmacons. Microchem. J. 2018;136:193–199. [http://dx.doi.org/10.1016/j.microc.2016.10.028]. [Google Scholar]
- 9.Cederbaum A.I. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015;4:60–73. doi: 10.1016/j.redox.2014.11.008. [http://dx.doi.org/10.1016/j.redox. 2014.11.008]. [PMID: 25498968]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis D.F. 57 varieties: the human cytochromes P450. Pharmacogenomics. 2004;5(3):305–318. doi: 10.1517/phgs.5.3.305.29827. [http://dx.doi.org/10.1517/phgs. 5.3.305.29827]. [PMID: 15102545]. [DOI] [PubMed] [Google Scholar]
- 11.Zanger U.M., Turpeinen M., Klein K., Schwab M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 2008;392(6):1093–1108. doi: 10.1007/s00216-008-2291-6. [http://dx.doi.org/10.1007/s00216-008-2291-6]. [PMID: 18695978]. [DOI] [PubMed] [Google Scholar]
- 12.Guengerich F.P. Cytochrome P450 Enzymes Cytochrome P450, Structure, Mechanism, and Biochemistry; P.R, F.P., Ed. Dordrecht: Springer; 2015. pp. 523–785. [http://dx.doi.org/10.1007/978-3-319-12108-6_9] [Google Scholar]
- 13.Zhou S.F., Liu J.P., Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 2009;41(2):89–295. doi: 10.1080/03602530902843483. [http://dx.doi.org/10.1080/03602530902843483]. [PMID: 19514967]. [DOI] [PubMed] [Google Scholar]
- 14.Temesvári M., Kóbori L., Paulik J., Sárváry E., Belic A., Monostory K. Estimation of drug-metabolizing capacity by cytochrome P450 genotyping and expression. J. Pharmacol. Exp. Ther. 2012;341(1):294–305. doi: 10.1124/jpet.111.189597. [http://dx.doi.org/10.1124/jpet.111.189597]. [PMID: 22262920]. [DOI] [PubMed] [Google Scholar]
- 15.Zanger U.M., Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013;138(1):103–141. doi: 10.1016/j.pharmthera.2012.12.007. [http://dx.doi.org/10.1016/j.pharmthera.2012.12.007]. [PMID: 23333322]. [DOI] [PubMed] [Google Scholar]
- 16.Samer C.F., Lorenzini K.I., Rollason V., Daali Y., Desmeules J.A. Applications of CYP450 testing in the clinical setting. Mol. Diagn. Ther. 2013;17(3):165–184. doi: 10.1007/s40291-013-0028-5. [http://dx.doi.org/10.1007/ s40291-013-0028-5]. [PMID: 23588782]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rendic S., Guengerich F.P. Update information on drug metabolism systems--2009, part II: summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr. Drug Metab. 2010;11(1):4–84. doi: 10.2174/138920010791110917. [http://dx.doi.org/10.2174/138920010791110917]. [PMID: 20302566]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16(10):669–694. doi: 10.2165/00023210-200216100-00003. [http://dx.doi.org/10.2165/00023210-200216100-00003]. [PMID: 12269861]. [DOI] [PubMed] [Google Scholar]
- 19.Peterson G.M., Naunton M. Valproate: a simple chemical with so much to offer. J. Clin. Pharm. Ther. 2005;30(5):417–421. doi: 10.1111/j.1365-2710.2005.00671.x. [http:// dx.doi.org/10.1111/j.1365-2710.2005.00671.x]. [PMID: 16164485]. [DOI] [PubMed] [Google Scholar]
- 20.Chiu C.T., Wang Z., Hunsberger J.G., Chuang D.M. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol. Rev. 2013;65(1):105–142. doi: 10.1124/pr.111.005512. [http://dx. doi.org/10.1124/pr.111.005512]. [PMID: 23300133]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng P.T., Chen Y.W., Chung W., Tu K.Y., Wang H.Y., Wu C.K., Lin P.Y. Significant effect of valproate augmentation therapy in patients with schizophrenia: A meta-analysis study. Medicine (Baltimore) 2016;95(4):e2475. doi: 10.1097/MD.0000000000002475. [http://dx.doi.org/10.1097/ MD.0000000000002475]. [PMID: 26825886]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sztajnkrycer M.D. Valproic acid toxicity: overview and management. J. Toxicol. Clin. Toxicol. 2002;40(6):789–801. doi: 10.1081/clt-120014645. [http://dx. doi.org/10.1081/CLT-120014645]. [PMID: 12475192]. [DOI] [PubMed] [Google Scholar]
- 23.Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16(10):695–714. doi: 10.2165/00023210-200216100-00004. [http://dx.doi.org/10.2165/00023210-200216100-00004]. [PMID: 12269862]. [DOI] [PubMed] [Google Scholar]
- 24.Chateauvieux S., Morceau F., Dicato M., Diederich M. Molecular and therapeutic potential and toxicity of valproic acid. J. Biomed. Biotechnol. 2010;2010:479364. doi: 10.1155/2010/479364. [http://dx.doi.org/10.1155/ 2010/479364]. [PMID: 20798865]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanau R.M., Neuman M.G. Adverse drug reactions induced by valproic acid. Clin. Biochem. 2013;46(15):1323–1338. doi: 10.1016/j.clinbiochem.2013.06.012. [http://dx. doi.org/10.1016/j.clinbiochem.2013.06.012]. [PMID: 23792104]. [DOI] [PubMed] [Google Scholar]
- 26.König S.A., Siemes H., Bläker F., Boenigk E., Gross-Selbeck G., Hanefeld F., Haas N., Köhler B., Koelfen W., Korinthenberg R., Kurek E., Lenard H-G., Penin H., Penzien J.M., Schünke W., Schultze C., Stephani U., Stute M., Traus M., Weinmann H-M., Scheffner W. Severe hepatotoxicity during valproate therapy: an update and report of eight new fatalities. Epilepsia. 1994;35(5):1005–1015. doi: 10.1111/j.1528-1157.1994.tb02546.x. [http://dx.doi.org/10.1111/j.1528-1157.1994.tb02546.x]. [PMID: 7925143]. [DOI] [PubMed] [Google Scholar]
- 27.Guerrini R. Valproate as a mainstay of therapy for pediatric epilepsy. Paediatr. Drugs. 2006;8(2):113–129. doi: 10.2165/00148581-200608020-00004. [http://dx.doi.org/10. 2165/00148581-200608020-00004]. [PMID: 16608372]. [DOI] [PubMed] [Google Scholar]
- 28.Kochen W., Schneider A., Ritz A. Abnormal metabolism of valproic acid in fatal hepatic failure. Eur. J. Pediatr. 1983;141(1):30–35. doi: 10.1007/BF00445664. [http://dx.doi.org/10.1007/BF00445664]. [PMID: 6416845]. [DOI] [PubMed] [Google Scholar]
- 29.Fisher E., Siemes H., Pund R., Wittfoht W., Nau H. Valproate metabolites in serum and urine during antiepileptic therapy in children with infantile spasms: abnormal metabolite pattern associated with reversible hepatotoxicity. Epilepsia. 1992;33(1):165–171. doi: 10.1111/j.1528-1157.1992.tb02301.x. [http://dx.doi.org/10.1111/j.1528-1157.1992.tb02301.x]. [PMID: 1733752]. [DOI] [PubMed] [Google Scholar]
- 30.Siemes H., Nau H., Schultze K., Wittfoht W., Drews E., Penzien J., Seidel U. Valproate (VPA) metabolites in various clinical conditions of probable VPA-associated hepatotoxicity. Epilepsia. 1993;34(2):332–346. doi: 10.1111/j.1528-1157.1993.tb02419.x. [http://dx.doi.org/10.1111/j.1528-1157.1993. tb02419.x]. [PMID: 8453944]. [DOI] [PubMed] [Google Scholar]
- 31.Silva M.F., Aires C.C., Luis P.B., Ruiter J.P., IJlst L., Duran M., Wanders R.J., Tavares de Almeida I. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: a review. J. Inherit. Metab. Dis. 2008;31(2):205–216. doi: 10.1007/s10545-008-0841-x. [http://dx.doi.org/10. 1007/s10545-008-0841-x]. [PMID: 18392741]. [DOI] [PubMed] [Google Scholar]
- 32.Abbott F.S., Anari M.R. In: Chemistry and Biotransformation. Milestones in Drug Therapy: Valproate; Löscher, W. Verlag B., editor. Basel: 1999. pp. 47–75. [Google Scholar]
- 33.Sadeque A.J., Fisher M.B., Korzekwa K.R., Gonzalez F.J., Rettie A.E. Human CYP2C9 and CYP2A6 mediate formation of the hepatotoxin 4-ene-valproic acid. J. Pharmacol. Exp. Ther. 1997;283(2):698–703. [PMID: 9353388]. [PubMed] [Google Scholar]
- 34.Kiang T.K., Ho P.C., Anari M.R., Tong V., Abbott F.S., Chang T.K. Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol. Sci. 2006;94(2):261–271. doi: 10.1093/toxsci/kfl096. [http://dx.doi.org/10.1093/toxsci/kfl096]. [PMID: 16945988]. [DOI] [PubMed] [Google Scholar]
- 35.Strassburg C.P., Strassburg A., Kneip S., Barut A., Tukey R.H., Rodeck B., Manns M.P. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50(2):259–265. doi: 10.1136/gut.50.2.259. [http://dx.doi.org/10.1136/gut.50.2.259]. [PMID: 11788570]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ginsberg G., Hattis D., Sonawane B., Russ A., Banati P., Kozlak M., Smolenski S., Goble R. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol. Sci. 2002;66(2):185–200. doi: 10.1093/toxsci/66.2.185. [http://dx.doi.org/10.1093/toxsci/66.2.185]. [PMID: 11896285]. [DOI] [PubMed] [Google Scholar]
- 37.Reith D.M., Andrews J., Parker-Scott S., Eadie M.J. Urinary excretion of valproate metabolites in children and adolescents. Biopharm. Drug Dispos. 2000;21(8):327–330. doi: 10.1002/bdd.247. [http://dx.doi.org/10. 1002/bdd.247]. [PMID: 11514952]. [DOI] [PubMed] [Google Scholar]
- 38.Ponchaut S., van Hoof F., Veitch K. In vitro effects of valproate and valproate metabolites on mitochondrial oxidations. Relevance of CoA sequestration to the observed inhibitions. Biochem. Pharmacol. 1992;43(11):2435–2442. doi: 10.1016/0006-2952(92)90324-c. [http://dx.doi.org/10.1016/0006-2952(92)90324-C]. [PMID: 1610408]. [DOI] [PubMed] [Google Scholar]
- 39.Stewart C.F., Hampton E.M. Effect of maturation on drug disposition in pediatric patients. Clin. Pharm. 1987;6(7):548–564. [PMID: 3319364]. [PubMed] [Google Scholar]
- 40.Zhou S.F., Zhou Z.W., Huang M. Polymorphisms of human cytochrome P450 2C9 and the functional relevance. Toxicology. 2010;278(2):165–188. doi: 10.1016/j.tox.2009.08.013. [http://dx.doi.org/10.1016/j.tox.2009.08.013]. [PMID: 19715737]. [DOI] [PubMed] [Google Scholar]
- 41.Kurose K., Sugiyama E., Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: implications in the clinical trials for novel drug development. Drug Metab. Pharmacokinet. 2012;27(1):9–54. doi: 10.2133/dmpk.dmpk-11-rv-111. [http://dx.doi.org/10.2133/dmpk.DMPK-11-RV-111]. [PMID: 22123129]. [DOI] [PubMed] [Google Scholar]
- 42.Hirota T., Eguchi S., Ieiri I. Impact of genetic polymorphisms in CYP2C9 and CYP2C19 on the pharmacokinetics of clinically used drugs. Drug Metab. Pharmacokinet. 2013;28(1):28–37. doi: 10.2133/dmpk.dmpk-12-rv-085. [http:// dx.doi.org/10.2133/dmpk.DMPK-12-RV-085]. [PMID: 23165865]. [DOI] [PubMed] [Google Scholar]
- 43.Gotoh O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J. Biol. Chem. 1992;267(1):83–90. [PMID: 1730627]. [PubMed] [Google Scholar]
- 44.Crespi C.L., Miller V.P. The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH: cytochrome P450 oxidoreductase. Pharmacogenetics. 1997;7(3):203–210. doi: 10.1097/00008571-199706000-00005. [http://dx.doi.org/10.1097/00008571-199706000-00005]. [PMID: 9241660]. [DOI] [PubMed] [Google Scholar]
- 45.Wei L., Locuson C.W., Tracy T.S. Polymorphic variants of CYP2C9: mechanisms involved in reduced catalytic activity. Mol. Pharmacol. 2007;72(5):1280–1288. doi: 10.1124/mol.107.036178. [http://dx.doi.org/10.1124/ mol.107.036178]. [PMID: 17686967]. [DOI] [PubMed] [Google Scholar]
- 46.Ho P.C., Abbott F.S., Zanger U.M., Chang T.K. Influence of CYP2C9 genotypes on the formation of a hepatotoxic metabolite of valproic acid in human liver microsomes. Pharmacogenomics J. 2003;3(6):335–342. doi: 10.1038/sj.tpj.6500210. [http://dx.doi.org/10.1038/sj.tpj.6500210]. [PMID: 14597963]. [DOI] [PubMed] [Google Scholar]
- 47.Tan L., Yu J.T., Sun Y.P., Ou J.R., Song J.H., Yu Y. The influence of cytochrome oxidase CYP2A6, CYP2B6, and CYP2C9 polymorphisms on the plasma concentrations of valproic acid in epileptic patients. Clin. Neurol. Neurosurg. 2010;112(4):320–323. doi: 10.1016/j.clineuro.2010.01.002. [http:// dx.doi.org/10.1016/j.clineuro.2010.01.002]. [PMID: 20089352]. [DOI] [PubMed] [Google Scholar]
- 48.Tóth K., Bűdi T., Kiss Á., Temesvári M., Háfra E., Nagy A., Szever Z., Monostory K. Phenoconversion of CYP2C9 in epilepsy limits the predictive value of CYP2C9 genotype in optimizing valproate therapy. Per. Med. 2015;12(3):199–207. doi: 10.2217/pme.14.82. [http://dx.doi. org/10.2217/pme.14.82]. [PMID: 29771647]. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka E. Clinically significant pharmacokinetic drug interactions between antiepileptic drugs. J. Clin. Pharm. Ther. 1999;24(2):87–92. doi: 10.1046/j.1365-2710.1999.00201.x. [http://dx.doi.org/10.1046/j.1365-2710.1999.00201.x]. [PMID: 10380060]. [DOI] [PubMed] [Google Scholar]
- 50.Amini-Shirazi N., Ghahremani M.H., Ahmadkhaniha R., Mandegary A., Dadgar A., Abdollahi M., Shadnia S., Pakdaman H., Kebriaeezadeh A. Influence of CYP2C9 polymorphism on metabolism of valproate and its hepatotoxin metabolite in Iranian patients. Toxicol. Mech. Methods. 2010;20(8):452–457. doi: 10.3109/15376516.2010.497977. [http://dx. doi.org/10.3109/15376516.2010.497977]. [PMID: 20602621]. [DOI] [PubMed] [Google Scholar]
- 51.Levy R.H., Rettenmeier A.W., Anderson G.D., Wilensky A.J., Friel P.N., Baillie T.A., Acheampong A., Tor J., Guyot M., Loiseau P. Effects of polytherapy with phenytoin, carbamazepine, and stiripentol on formation of 4-ene-valproate, a hepatotoxic metabolite of valproic acid. Clin. Pharmacol. Ther. 1990;48(3):225–235. doi: 10.1038/clpt.1990.144. [http://dx.doi.org/10.1038/clpt.1990.144]. [PMID: 2119269]. [DOI] [PubMed] [Google Scholar]
- 52.Star K., Edwards I.R., Choonara I. Valproic acid and fatalities in children: a review of individual case safety reports in VigiBase. PLoS One. 2014;9(10):e108970. doi: 10.1371/journal.pone.0108970. [http://dx.doi.org/10.1371/ journal.pone.0108970]. [PMID: 25302991]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu N., Di Q., Hu Y., Zhang Y.F., Su L.Y., Liu X.H., Li L.C. A meta-analysis of pro-inflammatory cytokines in the plasma of epileptic patients with recent seizure. Neurosci. Lett. 2012;514(1):110–115. doi: 10.1016/j.neulet.2012.02.070. [http://dx.doi.org/10.1016/j.neulet.2012.02.070]. [PMID: 22402188]. [DOI] [PubMed] [Google Scholar]
- 54.Uludag I.F., Bilgin S., Zorlu Y., Tuna G., Kirkali G. Interleukin-6, interleukin-1 beta and interleukin-1 receptor antagonist levels in epileptic seizures. Seizure. 2013;22(6):457–461. doi: 10.1016/j.seizure.2013.03.004. [http://dx.doi. org/10.1016/j.seizure.2013.03.004]. [PMID: 23566695]. [DOI] [PubMed] [Google Scholar]
- 55.Aitken A.E., Morgan E.T. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab. Dispos. 2007;35(9):1687–1693. doi: 10.1124/dmd.107.015511. [http://dx.doi.org/10.1124/dmd.107.015511]. [PMID: 17576808]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pascussi J.M., Gerbal-Chaloin S., Pichard-Garcia L., Daujat M., Fabre J.M., Maurel P., Vilarem M.J. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem. Biophys. Res. Commun. 2000;274(3):707–713. doi: 10.1006/bbrc.2000.3219. [http://dx.doi.org/10. 1006/bbrc.2000.3219]. [PMID: 10924340]. [DOI] [PubMed] [Google Scholar]
- 57.Pascussi J.M., Dvorák Z., Gerbal-Chaloin S., Assenat E., Maurel P., Vilarem M.J. Pathophysiological factors affecting CAR gene expression. Drug Metab. Rev. 2003;35(4):255–268. doi: 10.1081/dmr-120026394. [http:// dx.doi.org/10.1081/DMR-120026394]. [PMID: 14705859]. [DOI] [PubMed] [Google Scholar]
- 58.Monostory K., Pascussi J.M. Regulation of drug-metabolizing human cytochrome P450s. Acta Chim. Slov. 2008;55:20–37. [Google Scholar]
- 59.Pascussi J.M., Gerbal-Chaloin S., Duret C., Daujat-Chavanieu M., Vilarem M.J., Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu. Rev. Pharmacol. Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [http:// dx.doi.org/10.1146/annurev.pharmtox.47.120505.105349]. [PMID: 17608617]. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi K., Hashimoto M., Honkakoski P., Negishi M. Regulation of gene expression by CAR: an update. Arch. Toxicol. 2015;89(7):1045–1055. doi: 10.1007/s00204-015-1522-9. [http://dx.doi.org/10.1007/s00204-015-1522-9]. [PMID: 25975989]. [DOI] [PubMed] [Google Scholar]
- 61.Prakash C., Zuniga B., Song C.S., Jiang S., Cropper J., Park S., Chatterjee B. Nuclear Receptors in drug metabolism, drug response and drug interactions. Nucl. Receptor Res. 2015;2:101178. doi: 10.11131/2015/101178. [http://dx.doi.org/10.11131/2015/101178]. [PMID: 27478824]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson G.D. Children versus adults: pharmacokinetic and adverse-effect differences. Epilepsia. 2002;43(Suppl. 3):53–59. doi: 10.1046/j.1528-1157.43.s.3.5.x. [http:// dx.doi.org/10.1046/j.1528-1157.43.s.3.5.x]. [PMID: 12060006]. [DOI] [PubMed] [Google Scholar]
- 63.Nagy A., Bűdi T., Temesvári M., Szever Z., Szabó P.T., Monostory K. Adverse events in a newborn on valproate therapy due to loss-of-function mutations in CYP2C9. Epilepsy Behav. Case Rep. 2015;4:86–87. doi: 10.1016/j.ebcr.2015.08.006. [http://dx.doi.org/10.1016/j.ebcr.2015. 08.006]. [PMID: 26543813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dreifuss F.E., Langer D.H. Hepatic considerations in the use of antiepileptic drugs. Epilepsia. 1987;28(Suppl. 2):S23–S29. doi: 10.1111/j.1528-1157.1987.tb05768.x. [http:// dx.doi.org/10.1111/j.1528-1157.1987.tb05768.x]. [PMID: 3121292]. [DOI] [PubMed] [Google Scholar]
- 65.Scheffner D., König S., Rauterberg-Ruland I., Kochen W., Hofmann W.J., Unkelbach S. Fatal liver failure in 16 children with valproate therapy. Epilepsia. 1988;29(5):530–542. doi: 10.1111/j.1528-1157.1988.tb03757.x. [http://dx. doi.org/10.1111/j.1528-1157.1988.tb03757.x]. [PMID: 3137017]. [DOI] [PubMed] [Google Scholar]
- 66.Bűdi T., Tóth K., Nagy A., Szever Z., Kiss Á., Temesvári M., Háfra E., Garami M., Tapodi A., Monostory K. Clinical significance of CYP2C9-status guided valproic acid therapy in children. Epilepsia. 2015;56(6):849–855. doi: 10.1111/epi.13011. [http://dx.doi.org/10.1111/epi. 13011]. [PMID: 25967074]. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto Y., Takahashi Y., Suzuki E., Mishima N., Inoue K., Itoh K., Kagawa Y., Inoue Y. Risk factors for hyperammonemia associated with valproic acid therapy in adult epilepsy patients. Epilepsy Res. 2012;101(3):202–209. doi: 10.1016/j.eplepsyres.2012.04.001. [http://dx.doi.org/10.1016/ j.eplepsyres.2012.04.001]. [PMID: 22542569]. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto Y., Takahashi Y., Imai K., Mishima N., Yazawa R., Inoue K., Itoh K., Kagawa Y., Inoue Y. Risk factors for hyperammonemia in pediatric patients with epilepsy. Epilepsia. 2013;54(6):983–989. doi: 10.1111/epi.12125. [http://dx.doi.org/10.1111/epi.12125]. [PMID: 23409971]. [DOI] [PubMed] [Google Scholar]
- 69.Tseng Y.L., Huang C.R., Lin C.H., Lu Y.T., Lu C.H., Chen N.C., Chang C.C., Chang W.N., Chuang Y.C. Risk factors of hyperammonemia in patients with epilepsy under valproic acid therapy. Medicine (Baltimore) 2014;93(11):e66. doi: 10.1097/MD.0000000000000066. [http://dx.doi. org/10.1097/MD.0000000000000066]. [PMID: 25192484]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monostory K., Bűdi T., Tóth K., Nagy A., Szever Z., Kiss Á., Temesvári M., Háfra E., Tapodi A., Garami M. In response: Commentary on clinical significance of CYP2C9-status-guided valproic acid therapy in children. Epilepsia. 2016;57(8):1339–1340. doi: 10.1111/epi.13451. [http://dx.doi.org/10.1111/epi.13451]. [PMID: 27485380]. [DOI] [PubMed] [Google Scholar]