Abstract
Immune-mediated cerebellar ataxias (IMCAs), a clinical entity reported for the first time in the 1980s, include gluten ataxia (GA), paraneoplastic cerebellar degenerations (PCDs), anti-glutamate decarboxylase 65 (GAD) antibody-associated cerebellar ataxia, post-infectious cerebellitis, and opsoclonus myoclonus syndrome (OMS). These IMCAs share common features with regard to therapeutic approaches. When certain factors trigger immune processes, elimination of the antigen(s) becomes a priority: e.g., gluten-free diet in GA and surgical excision of the primary tumor in PCDs. Furthermore, various immunotherapeutic modalities (e.g., steroids, immunoglobulins, plasmapheresis, immunosuppressants, rituximab) should be considered alone or in combination to prevent the progression of the IMCAs. There is no evidence of significant differences in terms of response and prognosis among the various types of immunotherapies. Treatment introduced at an early stage, when CAs or cerebellar atrophy is mild, is associated with better prognosis. Preservation of the “cerebellar reserve” is neces-sary for the improvement of CAs and resilience of the cerebellar networks. In this regard, we emphasize the therapeutic prin-ciple of “Time is Cerebellum” in IMCAs.
Keywords: Cerebellar ataxias, immune-mediated cerebellar ataxias, prognosis, therapy, treatment, immunotherapy, gluten ataxia, paraneoplastic cerebellar degeneration, anti-GAD65Ab-associated cerebellar ataxia, post-infectious cerebellitis, opsoclonus myoclonus syndrome
1. Introduction
Immune-mediated neuronal dysfunction leading to cell death is a pathomechanism responsible for the development of cerebellar ataxia (CA) [1]. Immune-mediated cerebellar ataxias (IMCAs) characteristically encompass diverse autoimmune-based etiologies [1], such as gluten ataxia (GA) [2], paraneoplastic cerebellar degeneration (PCD) [3-8], anti-glutamate decarboxylase 65 antibody-associated cerebellar ataxia (anti-GAD65Ab-associated CA) [9], post-infectious cerebellitis, and opsoclonus myoclonus syndrome (OMS). The therapeutic response is thought to vary in particular according to the etiology of IMCAs [1-9]. To date, there are virtually no large-scale randomized studies on therapeutic strategies. Indeed, most published studies are retrospective in design or case reports. Accordingly, only fragmented information on treatment is available to the clinicians.
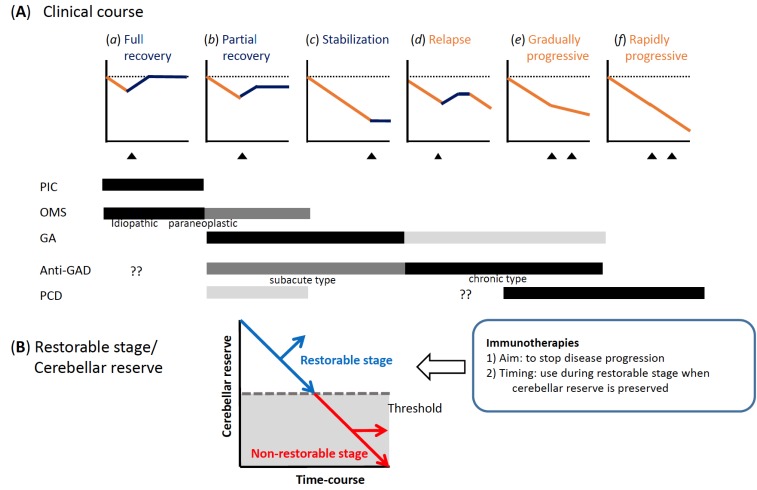
We have proposed recently a classification of these immunologically divergent etiologies [10] and we have also suggested therapeutic guidelines based on available reports [11]. Furthermore, we also discussed the existence of treatable and non-treatable stages that are commonly observed in IMCAs, and, hence, proposed the importance of early immunotherapy during the time when “cerebellar reserve”, defined as preservation of the capacity of the intact tissue for compensation and restoration, is still sufficient [12-14].
Recent studies have described new findings with regard to the pathogenesis and clinical trials in IMCAs. Many cell- and antibody-mediated immune mechanisms have been described, which can collectively provide a rationale for new and early treatments. Large-scale studies have reported recently the prevalence of the clinical subtypes [15, 16]. Furthermore, accumulated case reports suggest possible effective outcomes following the use of combinations of immunotherapies. However, apart from these clinical aspects of IMCAs, the majority of these ataxias remain underdiagnosed since the progressive forms of IMCAs mimic the profiles of degenerative CA. Unfortunately, delay in clinical intervention is associated with loss of therapeutic opportunities. This is also explained by the fact that the cerebellum is a privileged site of neuronal loss in a large number of diseases, often delaying the diagnosis.
The present review not only expands our previous studies [10-14], but also aims to outline updated therapeutic guidelines for IMCAs according to their etiology based on very recent findings and to stress a common therapeutic principle in IMCAs on the basis of the notion of the cerebellar reserve. For this purpose, this review is arranged in four chapters (or sections) from basic principles to the clinic. We first discuss certain issues related to the classification based on a large-scale study [15]. Secondly, we review the general principles underlying IMCAs. Some cytokines and autoantibodies, which impair the cellular activities in the cerebellum, could be possible molecular targets for therapeutics in the near future. Thirdly, we review the therapies that have been tried in five representative IMCAs (gluten ataxia, PCDs, anti-GAD65Ab-associated CA, post-infectious cerebellitis, and OMS). The pathogenic mechanisms and prognostic factors of each disorder still remain uncertain and debated. We outline an updated framework for possible first-line therapies. Since there is no definite consensus on the therapeutic interventions for PCDs and anti-GAD65Ab-associated CA, we also survey treatments described in published case reports. Finally, we review the physiological mechanisms underlying “cerebellar reserve”. Since the cerebellar circuitry is well known for its inherent ability of self-organization, the currently available as well as future immunotherapies should optimize/promote the neural tissue capacity of this part of the brain for self-repair. By using the motto of “Time is Cerebellum”, we stress the importance of early diagnosis and treatment of IMCAs.
2. CLASSIFICATION
2.1. History
Historically, J.M. Charcot was the first to describe immune-mediated CAs [1]. In 1868, he described the features of multiple sclerosis (MS), the relapse and remission of symptoms, such as intention tremor, scanning speech and nystagmus, together with motor paralysis and optic neuritis [17]. The next historical milestone was the description by B. Brouwer in 1919 of the association of CAs with malignancies [18]. The association between CA and many types of neoplasms, including breast, uterine, and ovarian cancers, small cell lung carcinoma, and Hodgkin’s lymphoma, has been largely documented during the last three decades. Interestingly, specific autoantibodies have been identified in several categories of neoplasms [3-8], including anti-Yo, anti-Hu, anti-Tr, anti-CV2, anti-Ri, anti-Ma2, and anti-VGCC [3-8].
Around the turn of the millennium, several reports about GA [2] and anti-GAD65Ab-associated CA [9] were among the main turning points in the field. Both conditions are characterized by the association of autoantibodies, absent or relatively mild cerebellar atrophy when compared with the clinical severity. Together with “classical diseases”, such as MS and PCDs, the clinical category of IMCAs has now been established in ataxiology [1, 10, 11, 16].
2.2. Proposal of Classification
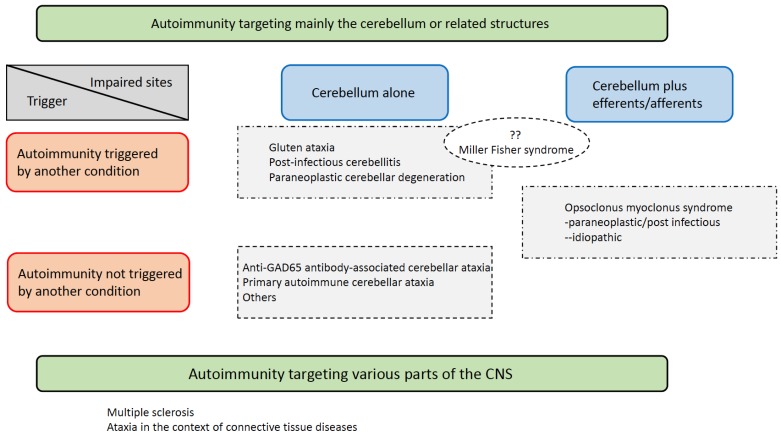
There is no definite consensus on the classification of IMCAs. In our consensus paper in 2016 [10], we proposed a classification based on two criteria: the main autoimmune target and the definite antigen that triggers the immune response. These two factors reflect the autoimmune mechanism in a particular pathology. First, IMCAs can be classified into two groups based on whether the cerebellum is the main target for the immune attack (Table 1) [10]. For example, in MS and CAs in the context of connective tissue diseases, such as lupus erythematosus, the cerebellum is only one of multiple autoimmune targets [10]. For the second group in which the cerebellum is the main autoimmune target, two subtypes can be distinguished: one subtype includes diseases in which autoimmunity is triggered by another well-defined disease or condition (e.g., GA, post-infectious cerebellitis, Miller Fisher syndrome, and PCDs), and a second subtype in which autoimmunity is not triggered by another disease or condition (e.g., anti-GAD65Ab-associated CA) [10].
Table 1.
List of representative auto antibodies to cerebellar antigens in paraneoplastic cerebellar degenerations.
|
Well Characterized Autoantibodies,
Suggestive of a Specific Disease |
Autoantibodies Found in Different Conditions,
Suggestive of Autoimmune Mechanisms |
||
|---|---|---|---|
| Anti-TG 2, 6 | Gluten ataxia | Anti-VGCC (P/Q type) | PCD (SCLC), PACA |
| Anti-Yo | PCD (breast, uterus and ovarian cancers) | Anti-Homer-3 | Neoplasm*, PIC, PACA |
| Anti-Hu | PCD (SCLC) | Anti-GluRδ2 | PIC, PACA |
| Anti-CV2 | PCD (SCLC, thymoma) | Anti-GAD65 (low titer) | PACA |
| Anti-Ri | PCD, paraneoplastic OMS (breast cancer) |
Less well characterized autoantibodies Reported only in a few patients with cerebellar ataxias |
|
| Anti-Ma2 | PCD (Testis and lung cancers) | ||
| Anti-GAD65 (high titer) | Anti-GAD65 antibody-associated ataxia | PCA-2 | PCD (rare) |
| Partially characterized autoantibodies | Anit-glycine R | Paraneoplastic OMS (rare) | |
| Anti-mGluR1 | Neoplasm* | ||
| Anti-Tr | PCD (Hodgkin’s lymphoma) | Anti-Ca/ARHGAP26 | Neoplasm*, unknown |
| Anti-CARP VIII | Neoplasm* | ||
| Anti-PKCγ | Neoplasm* | ||
| Anti-Nb/AP3B2 | Unknown | ||
| Anti-Sj/ITPR1 | Unknown | ||
| Anti-LGl1 | Unknown | ||
| Anti-CASPR-2 | Unknown | ||
| Anti-neurochondrin | Unknown | ||
*Association was reported only in a few patients with neoplasm. Unknown conditions might be PACA.
Abbreviations: PCD; paraneoplastic cerebellar degeneration, PIC; post-infectious cerebellitis, PACA; Primary autoimmune cerebellar ataxia, SCLC; small cell lung cancer.
2.3. Prevalence
There have been only a few large-scale studies on the prevalence of CAs. The study by Hadjivassiliou et al. [15] is the only large-scale published study on the etiology of CAs, with a survey of 1,500 UK patients with progressive ataxia. The authors reported that 33% of the patients had a genetic disorder, although some did not show evident family history, and 11% of the patients had multiple systemic atrophy (MSA). Apart from the above 44% of the cohort with degenerative CAs, 30% had definite IMCAs; 25% had GA, 3% had PCD, 2% had anti-GAD65Ab-associated CA, 1% had post-infectious cerebellitis, and <1% had OMS.
Interestingly, the authors classified 24% of the patients with idiopathic sporadic ataxias. This category included primary autoimmune cerebellar ataxia (PACA) [19]. CAs were diagnosed as PACA when immune-mediated mechanisms were suspected, though the clinical profiles of the patients did not match any of the known diseases in IMCAs. Thus, the group was heterogeneous and probably included several subcategories.
2.4. Open Problems in Classification
In the present classification, we carry on the framework of two coordinates that we used in our previous classification published in 2016; a target tissue/organ and an autoimmunity trigger [10], since these two factors can reflect the pathological nature of each autoimmune etiology. However, we revised the following points in our previous classification (Table 1).
2.4.1. Cerebellar Efferents/afferents as Target of Autoimmunity
Here we discuss not only the pure cerebellar type but also the cerebellar efferent/afferent type (Fig. 1). In this regard, OMS are added to the list as an efferent type. It is assumed that autoimmune reactions disrupt the cerebellar cortex-mediated control on the cerebellar nuclei (the sole site of projections outside the cerebellum; cerebellar efferents), resulting in the clinical appearance of neurological symptoms. In Miller Fisher syndrome, the origin of ataxia is attributed to the cerebellum [10, 15]. However, the possible involvement of the spino-cerebellar afferents as the target of autoimmunity cannot be ruled out [10].
Fig. (1).
Classification of immune-mediated cerebellar ataxias (IMCAs). (The color version of the figure is available in the electronic copy of the article).
2.4.2. Primary Autoimmune Cerebellar Ataxia (PACA)
Another revision concerns PACA. PACA is indicated when CA is elicited by an autoimmune reaction in the cerebellum [e.g., association with HLA DQ2, autoimmune diseases, and autoantibodies (anti-thyroid antibodies and autoantibodies that target the cerebellum), and response to immunotherapy)], and the clinical features do not match the defined etiology [15]. The high frequency of idiopathic CAs in the above-discussed study [15] indicates that the etiology of autoimmune-mediated CAs in some patients is still unknown and requires further definition. This may be the reason for the underestimated rate and overlooked diagnosis of IMCAs in daily clinical practice. To gain clinical attention, PACA could be utilized for tentative diagnosis indicating IMCAs. Further advancements in immune profiles might allow the establishment of a new clinical entity from tentative classification. Alternatively, PACA could be presented as an entity of a spectrum that also includes other phenotypes (Table 1).
This spectrum may also be used for patients with Hashimoto’s encephalopathy (HE). In the original classification, HE was classified as an independent entity [10]. HE was reported to have a good response to steroids and to be associated with anti-thyroid Abs [10, 20, 21]. However, the recently reported association with other autoantibodies (e.g., low titer of anti-GAD Abs or anti-gliadin Abs) [11] and heterogeneous physiological actions in the CSF [22] render a single entity unlikely. Thus, these patients could be described as patients with good response to corticosteroids [11, 23] and classified as one phenotype in PACA.
2.4.3. Diversity of Autoantibodies
Table 1 lists representative autoantibodies associated with IMCAs. From a diagnostic point of view, these autoantibodies against cerebellar targets can be classified into two groups; disease-specific and -nonspecific autoantibodies. The former include the well-characterized autoantibodies associated with gluten sensitivity (TG2 and TG6 antibodies) for GA, the onconeural antibodies for PCDs, such as anti-Yo, Hu, CV2, Ri, and Ma-2 antibodies, the high-titer of anti-GAD65 antibody for anti-GAD65 Ab-associated CA, and anti-Ri antibody for OMS [3, 6-8, 10, 11]. However, the association with nonspecific autoantibodies indicates only the presence of an autoimmune reaction. This category includes anti-VGCC, Homer-3, GluR-delta2, and low-titer of anti-GAD65 antibodies. Since these autoantibodies are characteristically associated with different etiologies, the diagnosis should be considered based on comprehensive clinical profiles.
PCA-2, anti- mGluR CA/ARHGAP26, CARP VIII, PKCγ, Nb/AP3B2, Sj/ITPR1, LGl1, CASPR-2, and neurochondrin antibodies were reported only in a few patients [11, 24, 25], and therefore, are less well characterized. More antibodies and their target antigen molecules will probably be added to this list.
3. Autoimmune Pathogenesis as a Possible Therapeutic Target
3.1. Cell-mediated Autoimmune Mechanisms
3.1.1. CD4+ T Cells
Studies on experimental autoimmune encephalomyelitis (EAE) suggest possible T cell-mediated autoimmune mechanisms, specifically involving CD4+ T cells [26]. Naïve CD4+ T cells (Th 0 cells) are activated by antigens presented by major histocompatibility complex (MHC) class II molecules on antigen-presenting cells (APC) and differentiate into Th1 and Th17 cells in the periphery. They infiltrate the CNS, where they start to orchestrate an inflammatory cascade by secreting cytokines (e.g., IFN-γ by Th1 and IL-17 by Th17), leading to demyelination or cell death [26].
Regulatory T cells (Treg) are a population of differentiated CD4+ T cells that maintain self-tolerance, inhibit autoimmunity, and act as critical negative modulators of inflammation in various autoimmune diseases [27]. Thus, dysfunction of Treg is one of mechanisms that precedes and leads to cell-mediated autoimmunity [27].
The primary genetic associations with human leukocyte antigen (HLA) class II in MS, whose gene products present to CD4+ T cells, suggest possible pathogenic roles of CD4+ T cells in MS [28]. Likewise, in anti-GAD65 Ab associated CA, it is reported that GAD65 stimulates proliferation of CD4+ T cells in the periphery [29].
3.1.2. CD8+ T Cells
CD8+ T cells, which recognized MHC class I molecules expressed on nucleated cells, also play cytotoxic roles in MS under a facilitation by CD4+ T cells [28]. CD8+ T cells are observed in MS plaque, outnumbering CD4+ T cells 3-to 10-fold and the degree of axon damages are correlated with the number of CD8+ T cells and macrophages rather than CD4+ T cells [28]. Notably, clinical therapeutic trials using monoclonal antibodies specifically against CD4+ T cells or their cytokines have failed to elicit therapeutic benefits, unlike therapies targeting broad spectrum T cells [28]. It is therefore hypothesized that CD8+ T cells are crucial in the autoimmunity of MS, but that CD4+ T cells determine whether the disease progresses or halts after an initial attack [28]. Consistently, in some subtypes of IMCAs, CD8+ T cells are reported to increase in the CSF [24] and infiltrate in the cerebellum with macrophages [30], which suggests common roles of CD8+ T cells in cell-mediated pathogenesis in IMCAs. However, detailed mechanisms remain unclear. Autoreactive CD8+ T cells are assumed to be due to a failure in elimination during thymic negative selection, either as a result of weak expression or poor binding of certain self-peptides to the predisposing HLA molecules [28].
3.2. Relevance of Autoantibodies and Possible Pathogenic Mechanisms
The targets of autoantibodies in IMCAs include nuclear, intracellular, and extracellular antigens. While the mechanisms that trigger the formation of autoantibodies remain obscure, several environmental factors, including viral infection, exposure to toxic chemicals, diet, [31] and diseases, such as cancer [32], have been suggested to trigger the formation of autoantibodies. Indeed, many cancer-associated antibodies are directed against self-antigens [33]. Matzinger (2002) proposed the “danger” model, where the immune system is activated by danger signals emitted from injured tissues [34]. Autoantigens, either presented to the immune system in association with inflammatory signals, or after modification caused by inflammation, or through prolonged exposure, may trigger an autoimmune response [33].
Autoantibodies can also be found in healthy individuals, although their levels are usually low [35-38], suggesting that autoantibody titers, rather than their presence, is related to disease development. However, even the presence of high autoantibody titers is not necessarily associated with disease [39, 40]. Characteristically, autoantibodies in healthy subjects are restricted to the periphery, while in IMCAs autoantibodies are also present in the CSF [41, 42], permitting access of the autoantibodies to neuronal targets [43]. Furthermore, while autoantibodies in healthy individuals may recognize the same protein, they often recognize different antibody epitopes, suggesting a different underlying autoimmune response [44].
Based on the intracellular or nuclear localization of many autoantigens in IMCAs, it has often been argued that the autoantibodies do not have a pathological role and are mere markers of an autoimmune response. However, the dogma that neurons cannot take up antibodies, has long been disputed in several studies. The uptake of immunoglobulins by a wide range of living cells both ex vivo and in vivo, often with pathogenic consequences, has been demonstrated unambiguously in experimental studies [45-49]. Specifically, Purkinje cells incorporate both host and non-host immunoglobulins in rat organotypic cultures [45], and kappa and lambda light chains were clearly detected in Purkinje cells of a patient with multiple myeloma [50]. Internalized IgGs have been confirmed in the rat cerebellum [51], autopsies of human cerebellar tissue [52], after interventricular injection of human IgGs in guinea pigs [53] and after peripheral administration of human IgGs in rats [49]. Human monoclonal GAD65Ab are taken up by cultured neurons [54] and can be detected in CA1 interneurons and Purkinje cells after injection in the medial septum/diagonal band and ipsilateral interpositus nucleus, respectively [55, 56]. Moreover, the internalized monoclonal GAD65Ab elicits behavioral abnormalities [55, 56]. The pathogenic roles of autoantibodies have been experimentally characterized not only in anti-GAD65Ab-associated CAs but also in PCD (see chapter III).
In conclusion, recent studies have elucidated certain aspects of the intricately intertwined cell- and autoantibody- mediated autoimmune mechanisms. Divergent cell- and autoantibody-mediated mechanisms are duplicated in the development of the disease. These mechanisms could be potentially suitable targets for the design of molecular therapeutics aimed at preventing an autoimmune attack in the cerebellum.
4. Available Treatment Options for Each Subtype of IMCAs
4.1. Gluten Ataxia
4.1.1. Diagnosis
GA is characterized by autoimmunity based on gluten-sensitivity. GA affects mostly women in their 40-50s (mean age; 48 years), and show either chronic or insidious onset, sometimes associated with sensorimotor axonal neuropathy, focal myoclonus, palatal tremor, gluten-sensitive enteropathy/gastrointestinal symptoms [1, 2, 10, 11, 16]. Enteropathy is seen in half of the patients [1, 2, 10, 11, 16]. Almost all patients with GA present clinically with posture and gait ataxia, whereas limb ataxia, dysarthria, and nystagmus are observed in only about 60 to 70% of the patients [1, 2, 10, 11, 16]. Association with autoimmune diseases is common, such as thyroiditis, type 1 diabetes mellitus, and pernicious anemia [1, 2, 10, 11, 16].
The HLA type DQ2 is detected in 70% of the patients. Anti-gliadin (IgG and IgA), anti-transglutaminase 2 (TG2) and anti-TG6 antibodies are detected in the sera and CSF of patients with GA. In a few cases, oligoclonal bands are detected in the CSF [1, 2, 10, 11, 16]. Anti-TG6 antibody has been shown to be specific to the neurological manifestations of gluten sensitivity [16]. Since the clinical course of GA is similar to that of degenerative CA, differentiating GA from the latter is important (Table 2).
Table 2.
Clinical profiles of gluten ataxia and anti-GAD65 Ab-associated cerebellar ataxia.
| Gluten Ataxia | Anti-GAD65 Ab-associated Cerebellar Ataxia | |
|---|---|---|
| Clinical profile | ||
| Time course | Insidious and chronic | Insidious and chronic or subacute |
| Age and sex | 50s, female (55%) | 60s, female (mostly) |
| Main symptoms of cerebellar involvement |
Gait ataxia. Limb ataxia and nystagmus are mild and less frequent (60-70%) | Gait ataxia. Limb ataxia and nystagmus are mild and less frequent (60-70%) |
| Associated neurological symptoms | Cortical myoclonus, neuropathy | Stiff person syndrome, epilepsy |
| Abnormality in cerebrospinal fluid | Generally none | Sometimes; CSF oligoclonal bands |
| Cerebellar atrophy on MRI | Present depending on duration of ataxia. The vermis is primarily involved. The degree of atrophy is mild relative to ataxia |
Present depending on duration of ataxia The vermis is primarily involved. The degree of atrophy is mild relative to ataxia |
|
Clues for diagnosis Neurological features suggestive the need for further investigations on autoimmunity: • 40-60s, female • Gait ataxia with dominant vermian atrophy • Atrophy on MRI is milder relative to the severity of ataxia | ||
| Gluten Ataxia | Anti-GAD65Ab-associated Cerebellar Ataxia | |
| Autoimmune background | ||
| Trigger of autoimmunity | Gluten ingestion | Unknown |
| HLA | Type DQ2 or DQ8 | - |
| Well characterized autoantibodies | Anti-gliadin (IgG/IgA) Anti-TG2, TG6 |
Anti-GAD65Ab (high titer) usually exceeds the levels seen in type 1 diabetes mellitus by 100-fold |
| Associated autoimmune diseases | Coeliac disease (47%) | Type 1 diabetes mellitus, autoimmune thyroid diseases, pernicious anemia |
4.1.2. Types of Therapies that have been Tested so far
A gluten-free diet is considered an effective therapy for GA, based on avoidance of antigens that can trigger immune-mediated mechanisms, similar to the strategy used in celiac disease [57].
4.1.3. Prognosis
Hadjivassiliou et al. [57] confirmed the benefits of gluten-free diet in 43 patients with GA. The authors compared prospectively the clinical courses of patients who adhered to gluten-free diet and the control group whose patients refused the diet. The two groups were matched for baseline conditions (age, duration of symptoms, and severity of CA). Patients of the treatment group showed improvement in the subjective clinical impression score and the performance test score (the Finger-to-Nose test latency and tapping), compared with patients of the control group. Furthermore, the improvement in CAs was associated with a decrease in the titer of anti-gliadin Ab. The response to the gluten- free diet was similar between patients with and without enteropathy.
A long-term observational study analyzed the prognostic factors involved in the response to gluten-free diet [11, 16, 57]. The study comprised 371 patients with GA, including 74% with mild ataxia (ability to walk unaided), 16% with moderate ataxia (inability to walk without support), and 10% with severe ataxia (wheelchair use). Strict gluten-free diet was applied for one year, which was confirmed by various serological tests showing elimination of gluten sensitivity. Clinical improvement correlated significantly with severity of cerebellar atrophy and was evident in patients with mild ataxia. Gluten-free diet halted and stabilized CAs, but did not improve clinical symptoms in some patients with severe ataxia.
In contrast to the above study, another work described lack of clinical response to gluten-free diet [58]. The authors argued that these patients were treated with intravenous immunoglobulins (IVIg), and all showed a satisfactory response coupled with clinical and serological improvements. However, the most common cause of lack of response to gluten-free diet is poor adherence to the diet or hypersensitivity to gluten, in which a small amount of gluten still present in the usual gluten-free food, causes a strong immune-mediated response, including cerebellar damage. Interestingly, the titers of anti-gliadin antibodies are persistently high in these treatment-failure cases [59, 60].
Thus, serological tests for gluten sensitivity should be conducted when gluten-free diet is ineffective. In patients with persistently high titers of anti-gliadin antibodies, strict adherence or wheat-free diet should be considered before switching to immunotherapy. MR spectroscopy can also be an effective tool to check strict adherence to gluten-free diet [61]. The authors reported that the N-acetylaspartate (NAA)/ creatine (Cr) area ratio from the cerebellar vermis increased in 62 out of 63 (98%) patients on strict gluten free diet, in 9 of 35 (26%) patients on gluten free diet but positive antibodies, and in only 1 of 19 (5%) patients not on gluten free diet. [61]. If it is not possible to control this kind of ataxia with gluten-free diet, maintenance therapy of IVIg or immunosuppressants (e.g., mycophenolate mofetil, cyclosporin, and cyclophosphamide) is recommended [11, 16].
Myoclonic ataxia is a subtype associated with refractory celiac disease [62]. Despite strict gluten-free diet, myoclonus, CAs and malabsorption characteristically persist in such patients. Immunosuppression with mycophenolate mofetil is reported to improve CAs and malabsorption [62].
4.1.4. Pathogenesis
Studies in coeliac disease suggest that a diet component is a potential environmental risk, leading to activation of autoimmunity [63]. Digested gluten peptides are cross-linked and deaminated by TG2, leading to the creation of an immunostimulatory epitope for HLA-DQ2 or -DQ8 on antigen-presenting cells. These epitopes are presented to CD4+ T cells, from which cytokines are released to facilitate the production of antibodies against gliadin and TG. Similar mechanisms are assumed to operate in GA. Anti-TG antibodies target tissue transglutaminase TG2, which is present in most cells, and TG6, which is abundantly expressed in the CNS [64]. A pathogenic role for anti-TG antibodies was suggested when anti-TG2 antibody isolated from celiac patients induced neuronal cell apoptosis in cell cultures [65]. Subsequent studies showed that intraventricular injection of anti-TG2 antibody induced ataxia in mice [66]. TG2 activity is upregulated through calcium binding, and anti-TG2 antibody cloned from celiac patients preferably bind to the calcium-activated enzyme conformation [67], suggesting that anti-TG2 antibody may play a pathogenic role by maintaining TG2 in its enzymatically active conformation. This hypothesis is supported by the presence of aberrant cerebral TG activity and protein aggregates in affected brain areas of patients with neurodegenerative diseases [68]. These findings provide a rationale for the removal of gluten. On the other hand, the mechanisms of cell-mediated autoimmunity remain to be clarified.
4.1.5. Recommended Therapeutic Strategy
Gluten-free diet is the first line of therapy for GA. Since the response is dependent on the severity of CAs and extent of cerebellar atrophy, early intervention is recommended, at a stage when the immune cascade has not caused a substantial cerebellar neuronal loss beyond a threshold (see the concept of cerebellar reserve). The therapeutic strategy is based on the avoidance of the antigen.
Poor adherence to the gluten-free diet or hypersensitivity to gluten does not eliminate the trigger to the autoimmune cascade, leading to disease progression. Titers of anti-gliadin antibodies should be monitored for poor adherence and assessment of sensitivity.
In cases of hypersensitivity, a wheat-free diet should be considered, followed by treatment with immunosuppressants (e.g., mycophenolate mofetil, cyclosporin, and cyclophosphamide).
4.2. Paraneoplastic Cerebellar Degeneration (PCD)
4.2.1. Diagnosis
PCD is characterized by an autoimmune response triggered by a neoplasm. It tends to show an acute or subacute time-course at the onset [3-8]. The cerebellar syndrome may be preceded by prodromal symptoms such as nausea, vomiting, and dizziness, resembling a viral infection-related disease [4]. Subsequently, patients notice gait instability, which is followed by limb ataxia, dysarthria and nystagmus (pancerebellar ataxia), usually within weeks [4]. The CSF frequently shows signs of inflammation, which include moderate lymphocytic pleocytosis, increased protein concentrations, high IgG index, and CSF-specific oligoclonal bands [4].
Many patients with PCD are positive for onconeuronal antibodies [4, 6-8] listed in Table 1, though some are seronegative for these antibodies [5, 10]. Since subacute CAs are listed as classical symptoms in the 2004 diagnostic criteria [3], the diagnosis of cancer, which develops within 5 years of diagnosis of CA, or appearance of well-characterized onconeuronal antibodies (anti-Yo, anti-Hu, anti-CV2, anti-Ri, anti-MA2) helps to establish a definite diagnosis of PCDs [3, 4].
It should be noted that CA is the first manifestation of neoplasm in 70% of the patients [4]. Of these cases, systemic investigation of the neoplasm is necessary.
4.2.2. Types of Therapies that have been Tested so far
Once the diagnosis of PCD is confirmed, the patient is generally scheduled for surgical excision of the neoplasm as soon as possible [4, 11, 69, 70]. Surgery is combined with radiotherapy and/or chemotherapy, depending on the type of neoplasm, in order to prevent systemic metastasis and remove antigens at the origin of the immune-mediated attacks [4, 11, 69, 70]. Such approach is reported to significantly prolong survival time [4, 69-71]. In addition to the above strategy, various kinds of immunotherapies (e.g., single drug therapy, or combinations of corticosteroids, IVIg, plasmapheresis, immunosuppressants, and rituximab) have been used.
4.2.3. Prognosis
4.2.3.1. Poor Prognosis
Prognosis of PCDs is generally poor [4, 69-71]. The response to immunotherapy following removal of the neoplastic lesion is often disappointing [4, 69-71]. Peterson et al [72] examined the efficacy of various immunosuppressive therapies in patients with anti-Yo antibody. Of the 22 patients who received plasmapheresis, only one showed moderate sustained clinical benefits while four other patients showed mild but transient improvement. Corticosteroids were tried in 17 patients, of whom only one patient showed a satisfactory clinical response. All 4 patients who were treated with cyclophosphamide showed no clinical improvement. Furthermore, Rojas et al. [73] reported no benefits in 23 patients with PCDs who received combinations of these therapies.
Two long-term studies reported short survival times in patients with PCDs. Candler et al. [74] examined the clinical course of 63 patients with paraneoplastic neurological diseases, including 13 patients with PCDs. These patients received various kinds of anti-neoplasm therapies, followed or not by one or several combinations of immunotherapies (prednisolone, IVIg, and/or plasmapheresis). Three of the 13 (23%) patients with PCDs died before the final follow-up, and the mean survival time from onset of neurological symptoms was 42 months (95% CI 32-52). In patients with paraneoplastic neurological diseases, 17 of the 63 (27%) patients died and the mean survival time was 43 months. The cause of death was not described in their study. On the other hand, Keime-Guibert et al. [75] examined the outcome of long-term follow up of 16 patients with PCDs (10 patients with anti-Hu antibody and six patients with anti-Yo antibody). After surgical removal of tumors, various types of immunotherapies were tried, including one to nine cycles of the combination of intravenous methylprednisolone, IVIg, and cyclophosphamide. None of the 16 patients who underwent the above protocol showed clinical improvement (more than 1 point change in Rankin Score), although 3 patients showed stabilization (lack of change in the score after three courses of the therapies). Fourteen of the 16 (88%) patients died during the follow-up, and the cause of death was neoplasm-related in 9 patients, neurological deficits in 3, and unknown in the remaining 2 patients. The median survival time from the first therapy was 10.2 months (range 2-38). Based on the above data, it is considered that fewer than 10% of patients with PCDs are likely to respond to immunotherapy [11].
4.2.3.2. Benign Prognostic Factors and Types of PCD
Although there are only a few studies on the therapeutic benefits in patients with PCDs, we have surveyed the prognostic factors commonly observed in responders.
4.2.3.3. Autoantibodies
Shams’ili et al. [76] examined the relation between the effectiveness of treatment and the associated antibodies in their study of 50 patients with PCDs. Patients positive for anti-Ri antibody showed the best response to anti-neoplasm and anti-immune therapies. Three of the 6 patients showed satisfactory clinical responses, and 5 (83%) patients were able to walk at the time of the last-follow up or before death. In contrast, only 4 of the 19 (21%) patients positive for anti-Yo antibody and only 4 of the 16 (25%) patients positive for anti-Hu antibody could walk unaided after the completion of treatment. Furthermore, the survival time from diagnosis was significantly longer in patients positive for anti-Tr antibody (median >113 months), compared with >69 months in patients with anti-Ri antibody, 13 months in patients with anti-Yo antibody, and 7 months in those with anti-Hu antibody. While the survival time seems longer in patients with anti-Tr antibody, these patients suffer severe disability or dependence. A long survival time is also sometimes recorded in patients with anti-CV2 antibody, provided the underlying cancer is controlled.
4.2.3.4. Other Benign Prognostic Factors
Table 3 summarizes the findings in published case reports of patients with PCDs who showed good response to immunotherapy [77-96]. These articles were selected for review based on the following process; (1) we searched for articles using key words of ‘Paraneoplastic cerebellar degeneration’ and ‘Treatment’ published between 1993-2017 in PubMed database, and (2) among those identified, we selected English-language articles that described the effectiveness of immunotherapeutic in their abstracts. Some of these articles had already been reviewed elsewhere [11].
Table 3.
Beneficial effects of immunotherapy on cerebellar ataxia with paraneoplastic cerebellar degeneration. Summary of 20 studies.
| Age/Gender | Associated Neoplasm/Treatment | Delay | Autoantibodies/MRI | Immunotherapy | Outcome/follow-up Period (Months) | |
|---|---|---|---|---|---|---|
| Moll et al. (1993) | ||||||
| 44/F | Breast cancer. Surg, chemo | 13 days | Not identified. No atrophy | plasmapheresis IVIg | Gait with assistance → gait without assistance 8 | |
| Stark et al. (1995) | ||||||
| 61/F | Ovarian tube cancer. Surg, chemo | 2.5 m | Not identified. Mild atrophy | oral PSL (no effect), CS, CP | Gait with wheel chair →gait with assistance. 54 | |
| 51/F | Ovarian cancer. Surg | 24 days | Not identified. ND | CP | Moderate ataxic gait → minimal ataxic gait. 24 | |
| David et al. (1996) | ||||||
| 81/F | Ovarian cancer. No cancer therapy | 2 m | Anti-Yo Ab. Mild atrophy | plasmapheresis, IVIg | Gait with assistance → gait no assistance. 2 (symptom deterioration 3 m later) | |
| Blaes et al. (1999) | ||||||
| patient 1: 62/F | Peritoneal carcinomatosis. Chemo | ND | Anti-Yo Ab. ND | IVIg | Marked reduction of ataxia. 24 | |
| Batocchi et al. (1999) | ||||||
| 47/F | Pelvic endometroid cancer. Surg, chemoradio | 6 m | Anti-Yo Ab. Mild atrophy | CP | ? → gait with wheel chair, 21 | |
| Mowzoon & Bradley (2000) | ||||||
| 56/F | Unknown | 19 m | Not identified. Mild atrophy | IVIg, oral PSL, CP | Gait with wheel chair → gait with cane, 18 | |
| Shams’ili et al. (2006) | ||||||
| patient 4: 48/F | Ovarian cancer. Surg | 1 m | Anti-Yo. ND | Rituximab | Modified Rankin scale 4→3, 16 | |
| Taniguchi et al. (2006) | ||||||
| 53/M | Hogkin's lymphoma, Chemoradio | immediate | Anti-Tr Ab. ND | IVIg, plasmapheresis | Improvement in standing and gait disturbances | |
| Geromin et al. (2006) | ||||||
| 17/F | Hogkin's lymphoma, Chemoradio | ND | Anti-Tr Ab. Mild atrophy | IVIg | Severe nystagmus, dysarthria → mild nystagmus, dysarthria, 120 | |
| Phuphanich & Brock (2007) | ||||||
| 72/F | Papillary carcinoma (extraovarian origin). chemo | 12 m | Anti-Yo Ab. Atrophy | IVIg | ? → able to swallow, 18 | |
| 54/F | Ovarian cancer. Surg, chemo | 6 m | Anti-Yo Ab. ND | IVIg | Gait with wheel chair → gait with assistance, Unknown | |
| Thöne et al. (2008) | ||||||
| 86/F | Ovarian cancer. No cancer therapy | 5 m | Anti-Yo Ab. No atrophy | mPSL, oral PSL, CP | Gait with assistance → gait with walking frame, 4 | |
| Esposito et al. (2008) | ||||||
| 48/F | Lung small cell carcinoma. Chemoradio | 12 m | Anti-Hu Ab. No atrophy | Rituximab | ICARS 40→11, 24 | |
| Schessl et al. (2011) | ||||||
| 72/F | Ovarian cancer. Surg, chemo | ND | Anti-Yo Ab. No atrophy | IVIg, oral PSL, rituximab | Gait with assistance decrease in falling, 120 | |
| Age/Gender | Associated Neoplasm/Treatment | Delay | Autoantibodies/MRI | Immunotherapy | Outcome/follow-up Period (Months) | |
| Shimazu et al. (2012) | ||||||
| 55/M | Lymphoma. Chemo | 3 m | Not identified Mild atrophy | Rituximab | SARA 30→1, 15 | |
| Yeo et al. (2012) | ||||||
| 10/M | Hodgkin’s lymphoma. Chemoradio | 2 years | Anti-Tr Ab. ND | Rituximab, IVIg | Gait with a walker → gait with assistance, 36 | |
| Lakshmaiah et al. (2013) | ||||||
| 68/M | Lymphoma. Chemo | 2 m | Not identified. Mild atrophy | Rituximab | There was a significant improvement in cerebellar signs, 8 | |
| Bhargava et al. (2014) | ||||||
| 44/F | Ovarian cancer. Surg | ~10 m | Anti Yo Ab. Mild atrophy | IVIg | Modified Rankin scale 4→2 2 | |
| Mitchell et al. (2015) | ||||||
| 75/F | Lung cancer. Surg | 3 m | Anti-Ri Ab. No atrophy | Plasmapheresis, IVIg | Nearly complete clinical recovery, 24 | |
| Zhu et al. (2016) | ||||||
| 68/M | Bladder cancer. Surg | 5 days | Not identified. No atrophy | mPSL | Barthel index of activities of daily living, 35→85 | |
| Gungor et al. (2017) | ||||||
| 11/F | Hodgkin’s lymphoma. Chemo | 1 wk | Anti-Tr Ab. No atrophy | Plasmapheresis, IVIg | Gait with assistance → gait without assistance, Unknown | |
Abbreviations: Surg: surgery, chemo: chemotherapy, chemoradio: chemoradiotherapy, mPSL; intravenous methylprednisolone, oral PSL; oral prednisolone, IVIg; intravenous immunoglobulins, CS: cyclosporine, CP: cyclophosphamide, ND: Not described.
The treatments received by the 22 responders described in 20 articles included intravenous methylprednisolone (n=1), IVIg (n=5), cyclophosphamide (n=2), rituximab (n=4), oral prednisolone + IVIg + cyclophosphamide (n=1), intravenous methylprednisolone + oral prednisolone + cyclophosphamide (n=1), oral prednisolone + IVIg + rituximab (n=1), oral prednisolone + cyclosporine + cyclophosphamide (n=1), IVIg + plasmapheresis (n=5), and IVIg + rituximab (n=1). There appears to be no significant difference in the outcome considering different types of immunotherapy. Of these reports, Voltz [71] proposed a regime of immunotherapy consisting of one course of intravenous methylprednisolone, followed by 1 or 2 weeks of IVIg in case of lack of improvements, and plasmapheresis or cyclophosphamide in case of no efficacy in the following 1 or 2 weeks. On the other hand, Dalmau and Rosenfeld [4] suggested that the combination of IVIg or plasmapheresis with cyclophosphamide could be effective in a subgroup of patients. Notably, rituximab was reported to be effective in patients with B cell lymphoma (for example, Hodgkin lymphoma) [89-91]. In these cases, R-CHOP therapy should be beneficial for both lymphoma and CAs.
In the here described patients (Table 3), there were no significant differences in the latency to treatment and severity of CAs at the start of therapy. Latency was short (less than 1 month between the onset of CAs and the start of immunotherapy) in 5 of the 22 (23%) patients, moderate (more than 1 month but less than 3 months) in 6 (27%) patients, and long (more than 3 months) in 8 (36%) patients. On the other hand, Berzero et al. [97] recently reported therapeutic benefits after early IVIg (within 3 months of onset of neurological symptoms) in 17 patients with paraneoplastic neurological diseases, including 1 patient with PCDs. The median survival time was 25.6 months, which was longer than that reported in the study of Keime-Guibert et al. [75]. Importantly, their data showed only one patient with neurological disorders-related symptoms. Similar tendency was observed also in the study by Shams’ili et al. [83].
The severity of CA before therapy was considered moderate in all 22 patients and 11 patients were unable to walk without assistance.
MRI studies showed no cerebellar atrophy in 7 of the examined 16 patients (44%) and mild cerebellar atrophy in 8 patients (50%) (Table 3). However, since PCDs are often defined as CAs with no initial MRI evidence of atrophy [3], further control studies are necessary.
4.2.4. Pathogenesis
4.2.4.1. PCDs Associated with Anti-Yo and Anti-Hu Antibodies
Both Yo (cdr2) and Hu proteins are intracellular antigens [5, 98]. It is assumed that cell-mediated mechanisms are the main autoimmune mechanisms underlying PCDs associated with anti-Yo and anti-Hu antibodies [4, 98]. Passive transfer experiments using onconeuronal antibodies targeting intracellular antigens and immunization using protein or DNA failed to induce ataxic symptoms in animals [99-101]. High proportions of cdr2- or Hu-specific T cells are present in blood of patients with anti-Yo (cdr2) antibody or anti-Hu antibody, respectively [102-104]. These results suggest that PCDs are mediated by T-cell immune response towards an autoantigen recognized by onconeuronal Ab [5]. Consistently, autopsy studies of patients with anti-Yo antibody showed near total loss of Purkinje neurons and gliosis [105]. Infiltration of CD8+ T cells in the cerebellum is another feature, with appearance of cytotoxic T cells proximal to damaged neurons [105]. Collaboration of CD4+ and CD8+ T cells is necessary to induce inflammation, where CD4+ T cells exhibit a Th1 phenotype, and secrete IFN-γ and TNF-α [106]. Histochemical examination showed lack of both IgG deposits and B cells infiltration [98].
However, a direct pathogenic effect was demonstrated for anti-Yo antibody, which is internalized by Purkinje cells and associated with subsequent cell death [107, 108]. Okano et al. [107] proposed a pathogenic role for anti-Yo antibody targeting Cdr2. Cdr2 encodes a leucine zipper motif that
interacts with another leucine zipper motif on the nuclear transcription factor c-Myc. Cdr2 regulates the level of c-Myc in the nucleus and c-Myc associated signaling activities. Okano et al [107] also demonstrated that Cdr2-specific anti-Yo antibodies inhibit the interaction between Cdr2 and c-Myc, possibly allowing excess of c-Myc to enter the nucleus and disrupt cell cycle signaling. Similarly, the binding of anti-Yo antibody to Cdr2 may prevent interaction with mortality factor-like protein MRGX and/or NFkb and consequently alter transcriptional activity and lead to cell apoptosis [108].
4.2.4.2. PCD Associated with VGCC Antibody
P/Q type voltage-gated calcium channels (VGCC) are membrane proteins on Purkinje cells known to determine the activity and survival of neurons [98, 109]. PCD patients positive for anti-VGCC antibody show diffuse loss of Purkinje cells and low number of VGCC [109]. Administration of Abs triggers ataxic movements in mice with an epitope-dependence [110]. Thus, autoimmunity against VGCC is considered to be mediated by VGCC autoantibody [109, 110].
4.2.5. Recommended Therapeutic Strategy
Immediate attention to the treatment of neoplasm should be the first objective of treatment.
Immediate induction of immunotherapy using corticosteroids (intravenous methylprednisolone or oral prednisolone), IVIg, plasmapheresis, immunosuppressants, or rituximab, either alone or in different combinations. There are no significant differences in the response to these types of immunotherapies.
The anti-neoplasm therapies and immunotherapies have no benefits in most cases, with a relatively short median survival time (10.2 to 43 months), although retrospective studies on a few responders have identified some prognostic factors (for example, anti-Tr or anti-Ro antibody).
4.3. Anti-GAD65Ab-associated Cerebellar Ataxia
4.3.1. Diagnosis
Anti-GAD65Ab-associated CA is characterized by CAs associated with high-titer anti-GAD65 antibody. The disease has a predilection for women in their 50-60s (mean age; 58 years) [9-11, 111]. The onset is subacute or chronic, and the phenotype is sometimes associated with stiff-person syndrome and epilepsy [9-11, 111]. Association with type 1 diabetes mellitus is frequently observed, and most patients have high serum and CSF titers of anti-GAD65Ab. Almost all patients with anti-GAD65Ab-associated CA present clinically with posture and gait ataxia, whereas limb ataxia, dysarthria, and nystagmus are observed in only about 60 to 70% of the patients [9-11, 111]. The ataxic patients characteristically have high titers of anti-GAD65Ab in serum (10 to 100-fold, compared with patients with type 1 diabetes mellitus) and in CSF [9-11, 111]. The CSF shows CSF-specific oligoclonal band in 70% of the patients, although protein concentration is normal. No cell proliferation is observed in most patients [9]. The clinical course in anti-GAD65Ab-associated CA is sometimes similar to that of degenerative CA, and therefore differential diagnosis between these two diseases is important (Table 2).
4.3.2. Types of Therapies that have been Tested so far
Combination immunotherapies (e.g., one or the combination of corticosteroids, IVIg, plasmapheresis, immunosuppressants, and rituximab) have been attempted [10, 111], though there are no published large-scale studies, mainly due to the rarity of this form of CA.
4.3.3. Prognosis
The clinical factors that influence prognosis of anti-GAD65Ab-associated CA have not been identified yet. To identify such factors, we divided the patients based on the mode of onset. The onset was defined as subacute, when the CA reached its nadir or required neurological assessment within 3 months [111], and as chronic when the above criterion was not satisfied [111].
4.3.3.1. Benign Prognostic Factors: Analysis of Short-term Follow-up Studies
Table 4 summarizes the findings of previous short-term follow up studies on patients with anti-GAD65Ab-associated CA [43, 112-127]. These publications were identified by searching the 1993-2017 PubMed database using the key words “anti-GAD antibody” and “cerebellar ataxia”. Among them, the case reports that provided details of the clinical course were selected. Some of these articles had already been reviewed elsewhere [11]. The results obtained from 22 patients of 27 trials showed definite tendency in the induction and response to maintenance therapies, although meaningful statistical analysis could not be performed due to the small number of patients.
Table 4.
Summary of 17 studies on the effects of various immunotherapies in patients with anti-GAD65Ab-associated CA.
| Age/Gender |
Delay
Subtype |
GAD Abs
MRI |
Induction / Maintenance Therapy |
Outcome Estimation
Follow up Period (Months) |
Levels of GAD Abs | |||
|---|---|---|---|---|---|---|---|---|
| Ishida et al. (1998) | ||||||||
| 66/F | 7 months. Chronic | 77,000 (U/ml). Atrophy | oral PSL / oral PSL, plasma exchange | Ataxias: slight recovery Subsequent progression of ataxia. Death 4 years after first therapy. Low response | Decreased | |||
| Abele et al. (1999) | ||||||||
| 68/F | 18 years Chronic | >1,000 (U/l) Atrophy | 1) IVIg 2) IVIg / none | ICARS: 59→50 ICACRS: 50→48 Low response (for 3 months) | No change | |||
| Takenoshita et al. (2001) | ||||||||
| 72/F | 2 years Chronic | 95,500(U/ml) ND | IVIg / none | Ataxias: unchanged No change (for 3 months) |
No change | |||
| Kono et al. (2001) | ||||||||
| 66/F | 10 months Chronic | > 50,000(U/ml) Normal | plasmapheresis+IVIg /none | Ataxias: unchanged No change (follow up ?) |
Decreased | |||
| Rüegg et al. (2002) | ||||||||
| 62/F | 1 year Chronic | 321 (U/l) Mild atrophy | IVIg / none | Gait ataxia: unchanged No change (for 1 month) |
ND | |||
| Matsumoto et al. (2002) | ||||||||
| 63/F | 1 month Subacute | 10,400(U/ml) Normal | 1) plasmapheresis 2) IVIg / none | Gait with aid→gait without aid (relapsed after 3 weeks) Prominent gait ataxia: unchanged No change (for 1 month) |
Decreased | |||
| Lauria et al. (2003) | ||||||||
| 66/F | 5 months Chronic | 531,000 (U/l) Mild atrophy | [First therapy] mPSL+IVIg / oral PSL [Second therapy] mPSL / oral PSL+CP | ICARS: 60→10 Low response (relapsed after 12 months) ICARS: 36→8 High response (for 6 months) |
Decreased Decreased |
|||
| Birand et al. (2006) | ||||||||
| 38/F | 33 months Chronic? | 6,472 (U/ml) Atrophy | mPSL / none | ICARS: 61→51 Low response (18 months) |
||||
| McFarland et al. (2006) | ||||||||
| 70/M | 9 months Chronic | 10,018 (U/ml) Normal | 1) mPSL 2) plasma exchange 3) mPSL / azathioprine |
ICARS: 78→20 (relapse after 2 months) ICARS: 29→30 ICARS: 30→6 High response (for 12 months from azathioprine medication) |
Decreased Decreased Decreased | |||
| Kim et al. (2006) | ||||||||
| 40/F +Stiff person syndrome |
4 months Chronic | 92,680(U/ml) ND | 1) mPSL 2) mPSL / oral PSL | ICARS: 31→20 (relapse after 4 months) ICARS: 23→11 High response (for 5 month) |
Decreased Decreased | |||
| Rakocevic et al. (2006) | ||||||||
| 30/M | 1 year Chronic | 2,413(nmol/L) Normal | IVIg / none | Ataxias: unchanged No change (follow up ?) |
ND | |||
| Vulliemoz et al. (2007) | ||||||||
| 58/M +Epilepsy |
2 months Subacute | 1/8000 Normal | mPSL / oral PSL, azathioprine |
Gait with aid→gait without aid High response (for 8 months) |
ND | |||
| Age/Gender | Delay Subtype |
GAD Abs MRI |
Induction / Maintenance Therapy |
Outcome Estimation Follow up Period (Months) |
Levels of GAD Abs | |||
| Chang et al. (2007) | ||||||||
| 56/F +Cognition failure |
3 months Subacute | 1,752 (nmol/l) Normal | mPSL / oral PSL, plasma exchange + plasma exchange + mPSL (monthly for 9 months) + mycophenolate mofefill /none | Gait with aid→gait without aid High response (for 15 months) | Decreased | |||
| Bonnan et al. (2008) | ||||||||
| 38/F | 12 months Chronic | >30,000 (U/ml) ND | mPSL × 6+plasma exchange / none | ICARS: 60→52 Low response (36 months) |
Fluctuated | |||
| 45/F | 12 months Chronic | 9,565(U/ml) ND | mPS L × 6 / azathioprine+ periodic IVIg |
ICARS: 8→12 Progression (31 months) |
Fluctuated | |||
| 75/F | 10 months Chronic | 302/(U/ml) ND | mPSL × 6 / none | ICARS: 16→14 No change (12 months) |
Fluctuated | |||
| Georgieva and Parton (2014) | ||||||||
| 45/M +epilepsy |
7 years Chronic | Strongly positive Mild atrophy | [First therapy] IVIg / azathioprine [Second therapy] IVIg / azathioprine [Third therapy] plasma exchange / azathioprine [Fourth therapy] IVIg / azathioprine [Fifth therapy] plasma exchange / azathioprin |
ICARS: 8→4.5 Low response (relapsed after 10 weeks) ICARS: 7.5→5.5 Low response (relapsed after 10 weeks) ICARS 15→6 Low response (relapsed after 12 weeks) ICARS: 12.5→10 Low response (relapsed after 8 weeks) ICARS 12→8 Low response (relapsed after 8 weeks) |
ND | |||
| Planche et al. (2014) | ||||||||
| 72/F | 6 months Subacute | >250 (U/ml) Normal | 1) IVIg×2 2) rituximab / none |
Ataxias: progressive ICARS: 22→10 High response (for 14 months) |
Increased Decreased | |||
| 73/F | 3 years Chronic? | >221 (U/ml) Normal | 1) IVIg×2 2) rituximab / IVIg×3 |
Ataxias: slight recovery ICARS: 22→21 Low response (for 17 months) |
ND No change |
|||
| 65/M | 3 years Chronic? | >1,000 (U/ml) Atrophy | 1) IVIg 2) IVIg+rituximab+CP / none |
Ataxias: progressive ICARS: 35→43 Progressed (for 16 months) |
No change | |||
| Kuchling et al. (2014) | ||||||||
| 74/F | 6 months Subacute | > 2,000 IU/ml Normal | immunoabsorption+ rituximab / none | Gait with aid→gait without aid High response (for 1 month) |
ND | |||
| 76/F | 6 years Subacute | > 2,000 IU/ml Normal | immunoabsorption+ rituximab / none | Prominent gait ataxia: unchanged No change (for 1 month) | ND | |||
Summary of data of 27 clinical trials that included 22 patients from 17 studies.
Abbreviations: SPS: Stiff person syndrome, mPSL; intravenous methylprednisolone, oral PSL; oral prednisolone, IVIg; intravenous immunoglobulins, CP: Cyclophosphamide, ND: Not described.
First, there appears to be no significant difference in the response to different types of immunotherapies used for patients with anti-GAD65Ab-associated CA. High responsiveness was observed following induction with intravenous methylprednisolone (3 trials), intravenous methylprednisolone + plasmapheresis (1 trial), IVIg + rituximab (1 trial), and plasmapheresis + rituximab (1 trial). Second, the outcome appears to correlate with the type of onset (subacute or chronic onset) and the degree of cerebellar atrophy. High responsiveness to immunotherapies was observed in 4 of the 6 trials with subacute onset (67%), but only in 3 of 21 patients with chronic onset (14%). Improvement after immunotherapies was observed in none of the 4 trials involving patients with evident cerebellar atrophy (marked neuronal loss), compared to improvement in 1 of the 8 trials involving patients with mild atrophy (12%) and in 5 of the 10 trials involving patients with no atrophy (50%).
4.3.3.2. Benign Prognostic Factors: Analysis of Long-term Follow-up Studies
Arińo et al (2014) examined the long-term effects of immunotherapies in 25 patients with anti-GAD65Ab-associated CA [111]. The median duration of treatment was 5.4 years (3.1-10.3 years). Using the modified Rankin Sale (mRS), they considered immunotherapy to be effective when the score improved by more than 1 point. Compared with the International Cooperative Ataxia Rating Scale (ICARS) used in most of the short-term follow-up studies, improvement of more than 1 point in the modified Rankin Scale means significant change in daily lives.
Importantly, CAs progressed in five patients who received no immunotherapy. One of them became bedridden (mRS=5), and two other patients became incapable of attending their bodily needs without aid or to walk without assistance (mRS=4). Thus, anti-GAD65Ab-associated CA has a progressive nature.
The induction immunotherapies used in 20 patients in the above study included IVIg (n=10), intravenous methylprednisolone (n=9; methylprednisolone alone, n=4; methylprednisolone + IVIg; n=4, methylprednisolone + rituximab, n=1), and oral prednisolone (n=1). Seventeen of these 20 patients received further maintenance immunotherapies; 6 were treated with IVIg for a median duration of 56.2 months (24.4-121.5 months), while 11 patients received oral immunosuppressants, and one or a combination of prednisolone, azathioprine or mycophenolate mofetil. The authors did not report the superiority of any particular type of immunotherapy. Using multivariate analysis, they found that age, sex, mRS at diagnosis, evidence of cerebellar atrophy on MRI, association with type 1 diabetes mellitus or other autoimmune diseases, were similar between responders and non-responders to the treatment.
On the other hand, there were significant differences between the subacute and chronic onset groups. The immunotherapies improved CAs in 5 of 7 (71%) patients with subacute onset. Three of these five responders showed no disabilities in daily lives (mRS=1); they were able to perform all usual activities although their movements were ataxic. In contrast, only 2 of 13 (15%) patients with the chronic type showed improvement following immunotherapies. In spite of induction and maintenance immunotherapy, 3 patients still required help in daily living activities, although they were able to walk unaided (mRS=3), and 4 patients found it difficult to attend to their bodily needs without aid or to walk without assistance (mRS=4).
Anti-GAD65Ab-associated CA sometimes shows a plateau clinical-course, instead of linear progressive time-course [1]. Even in these cases, clinicians should continue immunosuppressive therapy.
4.3.3.3. Summary of Short- and Long-term Follow-up Studies
Considered together, it is clear that the results of short- and long-term follow-up studies are similar. Patients with subacute onset showed relatively good response to these therapies compared to patients with chronic onset. Short-term follow-up studies have identified cerebellar atrophy as a high risk factor for disability, though this finding was not observed in the long-term follow-up studies. There appears to be no significant differences in the response to prednisolone, IVIg and plasmapheresis.
4.3.4. Pathogenesis
4.3.4.1. Anti-GAD65Ab-associated CA
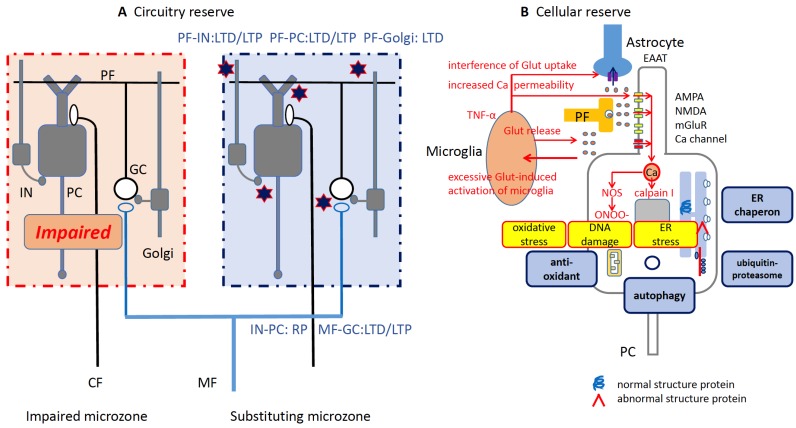
It has been considered to be unlikely that anti-GAD65 antibody has a pathogenic role because of GAD65’s cytoplasmic location and its antibody’s association with other conditions, such as type 1 diabetes mellitus, CA, and SPS [128]. However, several recent studies documented clearly the pathogenic roles of anti-GAD65 antibody in the development of CAs [13, 14, 56]. Anti-GAD65Ab accesses GAD65, located on the cytosolic surface of GABA-containing vesicles, in GABAergic neurons terminals, and such antigen-antibody interaction interferes with the packaging of GABA into the synaptic vesicles and shuffling of the synaptic clefts [56], resulting in a decrease of GABA release [129-132]. It is assumed that the antigen might be temporally exposed during exocytosis, providing a chance for binding with the antibodies [133, 134]. Since a disinhibition of the dentate-thalamo-cortical pathway (an extrication of the interneuron-induced inhibition on Purkinje cells) generates an initiation of movements with an exact timing [135], any impairment at GABA synapses can cause critical deficit in cerebellar timing coordination, leading to CAs. Consistently, administration of patients’ CSF and monoclonal GAD65Ab mimicking epitope specificity of GAD65Ab in CA patients elicits impairment of cerebellar control of the motor cortex [136] and ataxic movements in experimental animals [56].
The above mentioned pathogenic actions were proved using the following experimental designs; (1) the pathogenic actions of patients’ CSF diminish after absorption of anti-GAD65 antibody and (2) monoclonal GAD65 antibody with pathologic epitope specificity mimicked the patients’ CSF effects, and (3) no pathogenic effects were observed in slice preparations from GAD65 knockout mice [13, 14, 56]. These findings show that the binding of GAD65 by GAD65 antibodies impairs GABA release, leading to the development of cerebellar dysfunction.
Importantly, the anti-GAD65Ab elicits these pathogenic actions in an epitope-specific fashion [13, 14, 56], and only anti-GAD65Ab with a distinct epitope specificity characteristic for CA patients interferes with GABA release mechanisms, while anti-GAD65Ab epitope specificities associated with type 1 diabetes mellitus have no effects on GABAergic neurotransmission [13, 14, 56]. Furthermore, differences in neurological symptoms can be explained by differences in epitope specificity. It is assumed that the cerebello-cerebral controls on timing necessitates exact timing in cerebellar GABAergic neuronal activities, that is, an exact timing of GABA release, whereas in the cerebello-spinal loop, the descending tracts are under less-phasic inhibition by the cerebellum, which requires GABA synthesis [13]. Consistent with this assumption, cerebellar administration of CSF obtained from CA and SPS patients elicits opposite effects, mimicking CA- and SPS-like symptoms, respectively [44]. This strict dependence of induced symptoms on anti-GAD65Ab epitope specificity may explain why no correlation between the overall anti-GAD65Ab titer and severity of symptoms has been observed [137]. It should be emphasized here that the above results do not exclude the involvement of cell-mediated autoimmunity.
4.3.5. Recommended Therapeutic Strategies
Induction therapy (e.g., intravenous methylprednisolone, IVIg, immunosuppressants, plasma exchange, and/or rituximab, either alone or in combination) is recommended during the early stage of CAs. There are no significant differences in the response to each type of these immunotherapies. A decrease in anti-GAD65Ab levels can be utilized as therapeutic index.
Maintenance therapy (e.g., oral prednisolone, IVIg, immunosuppressants, and/or rituximab, alone or in combination) is also recommended. Since anti-GAD65Ab-associated CA exhibits a chronic time-course, the efficacy and therapeutic dose of prednisolone should be monitored carefully to avoid side effects.
4.4. Post-infectious Cerebellitis
4.4.1. Diagnosis
Infectious-induced cerebellar dysfunction is classified into two categories; (1) inflammation caused by direct invasion of viral or bacterial microorganisms, and (2) inflammation induced by immune mechanisms triggered by the infection [138]. Some authors termed the former as acute cerebellitis (AC) [139] and the latter as post- or para-infectious cerebellitis, post-infectious cerebellar ataxia, or acute cerebellar ataxia (ACA) [138, 140].
Cerebellitis affects mainly younger children after a variety of illnesses, usually viral infection, most commonly varicella [138, 139]. It is reported that 0.05% of children with varicella infection develop CAs [141]. Other viral infections include EB virus, Coxsackie virus, influenza A and B virus, parainfluenza virus, measles, mumps, and rubella [10]. Cerebellitis is also associated with bacterial infections, such as diphtheria, pertussis, typhoid, Legionnaires, leptospirosis, and mycoplasma.
Connolly et al. [142] described in detail the clinical features based on examination of 73 patients. The age distribution was skewed to younger children with 60% of the children between 2-4 years. Furthermore, 57% of the children were boys. With regard to background infections, 25% of the children had varicella, 52% had other viral infections, and 3% of the patients had post-infectious cerebellitis related to immunization. Cases associated with EB virus, mycoplasma and vaccination-related cerebellitis were more frequent in older children. The mean latency from the prodromal illness to the onset of CAs was 9.9±7.9 days, whereas 19% of the patients did not exhibit any infection before the onset of CAs. The most recognized clinical signs in CAs of post-infectious cerebellitis were gait ataxia, with 24% of the children reported inability to walk. Nystagmus was present in 14% and cranial nerve palsies (e.g., abducens or facial nerve) in 7%. On the other hand, the study showed that patients with CAs of post-infectious cerebellitis did not present with fever, meningeal signs, raised intracranial pressure or extracerebellar symptoms, such as temporary clouding of consciousness, seizures, extremely altered mental status (e.g., extreme irritability), or cerebral focal signs. Rather, the presence of these clinical signs is suggestive of inflammation induced directly by infection, rather than inflammation induced by autoimmunity [139]. However, mild behavioral changes, such as mild irritability, hyperactivity, moodiness, and whining was sometimes noted by parents, which correlated with the degree of CAs. Laboratory tests in post-infectious cerebellitis showed an inflammatory state in the CSF with no involvement of cerebellar atrophy [142]. MRI, which was conducted in only 8 patients, showed no atrophy or abnormal intensity area, except for one child who exhibited a hypersignal area in the cerebellar hemisphere with T2-weighted resonance. In the CSF, pleocytosis (roughly equal numbers of granulocytes and lymphocytes; 75%, lymphocyte predominance; 20%) and high CSF/serum IgG index were observed in half of the children. Oligoclonal bands were present in 10-17% of the children.
Adult patients with cerebellitis exhibited different clinical features, as reported in the study of Klockgether et al. [141] on 11 adult patients (mean age, 40.7±15.2 years). The prodromal phase varied from 1 to 8 weeks. The most common IgM in the CSF was EBV-VCA antibody and 73% of the patients showed cerebellar oculomotor disturbances, such as impaired smooth pursuit, reduced optokinetic nystagmus, impaired suppression of the vestibule-ocular reflex by fixation of a stationary target, nystagmus on lateral gaze, and saccadic hypermetria. Extracerebellar clinical signs, such as temporary clouding of consciousness, unilateral sensory disturbance and facial nerve palsy were present in 36% of the patients. CSF examination showed lymphocytic pleocytosis in 91% and high IgG in 67%, whereas the IgG index was normal and oligoclonal bands were not present in all subjects. Other clinical signs and symptoms in adults were similar to those observed in children.
4.4.2. Types of Therapies that have been Tested so far
Since cerebellitis is self-limiting, close monitoring of the patient and follow-up form the basis of clinical management. When the CA persists or progresses, a combination of immunotherapies has been considered [138]. Some infectious diseases (caused by bacterial agents) require the fast administration of antibiotics.
4.4.3. Prognosis
4.4.3.1. Pediatric Cases
In one large study of 60 pediatric cases, 43 (72%) patients who were followed for more than 4 months showed complete recovery from CAs [142]. Full gait recovery required about 2 months in most cases. Persistent gait ataxia was observed only in children without a preceding infection or children whose preceding illness was a nonspecific respiratory or gastrointestinal viral illness. Behavioral changes were associated with CAs, although the severity was only mild. On the other hand, 15 (25%) of these children experienced transient behavioral or intellectual difficulties, such as mild irritability, hyperactivity, moodiness, and whining, whereas only 3 (5%) patients demonstrated persistent learning problems.
4.4.3.2. Adult Cases
In one study of 11 adult patients with CAs, full recovery was documented in 9 (81%) [137], whereas the other two patients who were older than 60 years showed persistent, though non-progressive, CAs, associated with a great imbalance in stance and gait [141]. Both elderly patients showed cerebellar atrophy on MRI.
4.4.4. Pathogenesis
The good prognosis associated with this type of CAs limits pathological investigations. MRI investigation in one pediatric case of a 5-year-old patient with oligoclonal band showed a lesion suggestive of demyelination in the cerebellar peduncle [143]. The author concluded that the mechanism of post-infectious cerebellitis is similar to that of MS/acute disseminated encephalomyelitis. Another case of an adult patient who developed CA after EBV infection was found positive for serum IgG and IgM antibodies that were reactive to Purkinje cells [144]. While these findings suggest autoimmune mechanisms, further large scale studies are needed to confirm the role of such mechanisms.
4.4.5. Recommended Therapeutic Strategies
Close observation and checking for stabilization and improvement (for instance following surgical intervention in case of edema of the posterior fossa) are recommended. Antibiotics may be required.
Only when the symptoms persist, immunotherapies using corticosteroids, IVIg, immunosuppressants, and/or plasma exchange, either alone or in combination, are indicated.
4.5. Opsoclonus Myoclonus Syndrome (OMS)
4.5.1. Diagnosis
OMS is characterized by subacute onset of opsoclonus (i.e., repeated, random and rapid eye movements in both horizontal and vertical directions) and action myoclonus [16, 145-147]. Since most patients show CAs, it is sometimes termed opsoclonus myoclonus ataxia syndrome [16]. OMS affects primarily children under the age of two years, and half of the patients have paraneoplastic syndrome, usually due to neuroblastoma [16]. Sine other etiologies are post-infectious and idiopathic, OMS is classified into three types; paraneoplastic, post-infection and idiopathic [16].
On the other hand, adult OMS is rare, affecting mostly women (55%) in their 40-60s (mean age; 54 years) in paraneoplastic OMS, and 30-50s (mean age; 38 years) in idiopathic OMS [145]. The most common symptom at onset is acute vertigo (22% in paraneoplastic OMS and 47% in idiopathic OMS), though some patients present with encephalopathy (13% in paraneoplastic OMS and 7% in idiopathic OMS) and behavior changes (about 5% in both). Characteristically, autoimmune background is low compared with GA and anti-GAD65Ab-associated CA (3% in paraneoplastic OMS and 11% in idiopathic OMS) [145]. MRI is usually negative for cerebellar or brain stem atrophy [145-147]. The CSF shows abnormalities (high leukocyte count and protein content) in 24% of paraneoplastic OMS patients and 42% of idiopathic patients [145]. Onconeuronal antibodies are detected in 11% of the patients, mostly anti-Ri antibody in breast cancer [145], as listed in Table 1. Other autoantibodies, such as anti-glycine receptor antibody, are detected in few patients with cancers [145]. Thus, the clinical profile is different in paraneoplastic OMS compared with idiopathic OMS.
4.5.2. Types of Therapies that have been Tested so far
In the case of paraneoplastic OMS, patients have been scheduled for surgical excision of the neoplasm as soon as possible [145, 146]. After the neoplasm therapy, immunotherapies (e.g., singly therapy or a combination of corticosteroids, IVIg, plasmapheresis, immunosuppressants, and rituximab) have been tried [16, 145-147]. On the other hand, post-infectious OMS is usually self-limiting and shows good prognosis, while idiopathic OMS shows spontaneous recovery in some patients [16]. Similar immunotherapies used in paraneoplastic OMS have been tried in non-paraneoplastic OMS, especially in patients who do not show improvement and those with progressive opsoclonus and myoclonus [16, 145-147]. At the time of writing of this review, there are no published large-scale studies probably due to the rarity of this form of CA.
The involuntary movements of the opsoclonus and the action myoclonus interfere with the daily lives of patients. Thus, symptomatic therapies using clonazepam or baclofen have also been described [146].
4.5.3. Prognosis
The prognosis was compared between paraneoplastic and idiopathic OMS in a few retrospective studies. Battler et al. [147] examined outcomes in 24 patients [paraneoplastic associated with small cell lung carcinoma, non-small cell lung cancer, breast carcinoma, gastric adenocarcinoma, and kidney carcinoma (total n=10) and idiopathic (n=14)]. Paraneoplastic patients showed two different clinical courses; despite treatment with corticosteroids and/or IVIg, five patients whose cancers was not removed died, while eight patients, whose cancer was removed, showed complete or partial improvement of neurological symptoms. On the other hand, 8 of the 10 patients with idiopathic OMS showed good outcome and monophasic time-course; five patients showed full recovery, and the other two patients, who showed self-limiting disease, showed good response to corticosteroids and/or IVIg. The other 2 patients with idiopathic OMS showed relapse, which was controlled by immunotherapy.
Klaas et al. [146] reported similar good outcome for idiopathic OMS. They examined the outcomes of 19 adult patients (paraneoplastic OMS, n=3, idiopathic OMS, n=16) with long-term follow-up (1-187 months). Of the 19, 18 patients received immunotherapy (methylprednisolone alone, n=3, IVIg alone, n=1, methylprednisolone +IVIg, n=11; methylprednisolone +plasmapheresis, n=2, methylprednisolone + IVIg + plasmapheresis, n=2). Although their study did not detect differences between paraneoplastic and idiopathic, 16 patients showed good outcome (remission in 13, and improvement in 3).
Amangué et al. [145] examined the therapeutic benefits in a large-scale study of 81 adult patients (paraneoplastic OMS, n=38, idiopathic OMS, n=43) with long-term follow-up (>3 months). Of the 81 patients, 70 received immunotherapy [corticosteroids alone, n=27, IVIg alone, n=10, corticosteroids + IVIg, n=24, corticosteroid +IVIg + plasmapheresis, n=9, while in addition to the latter, 6 patients also received azathioprine (n=4), rituximab (n=1), or mycophenolate mofetil (n=1)]. Ten other patients were managed by close observation only. Good outcome, defined by a modified Rankin Score ≤2, was observed in 39% of patients with paraneoplastic OMS and 84% of patients with idiopathic OMS, and relapse was recorded in 24% of patients with paraneoplastic OMS compared with 7% of patients with idiopathic OMS. The above two findings suggest good outcome in idiopathic OMS. Some of the untreated patients appeared to belong to idiopathic OMS and develop self-limiting disease. No correlation was observed between the type of immunotherapy and outcome.
Taken together, idiopathic OMS seems to be self-limiting at least in some cases, although the condition exhibits a progressive nature; complete remission with immunotherapy is the most common outcome and is the most common feature [146]. In this regard, more than half of the patients with paraneoplastic OMS show poor outcome. However, when compared with prognosis in PCDs, that of paraneoplastic OMS is better; more patients showed good response to immunotherapies with better overall survival.
Pranazatelli et al. [148] administered B cell depletion therapy using a combination of rituximab with corticosteroids and IVIg in 12 children with OMS. These therapies rapidly diminish the opsoclonus and myoclonus. All patients became ambulatory but two children subsequently showed relapse. A 93% reduction in high CSF B cell count was reported at 6 months post-treatment. Although background differences between pediatric and adult cases should be taken into account, this strategy is promising.
Hadjivassiliou [16] stressed the need for implementation of a therapeutic strategy in adult OMS, including survey for malignancy first using onconeuronal antibodies and PET. After ruling out malignancy, he proposed the following strategies [16]; (1) close observation for stabilization or improvement even without any intervention, (2) the use of gabapentin, especially in patients with prominent opsoclonus and action myoclonus, and (3) immunotherapy, especially in patients with progressive condition. This could be the administration of corticosteroids as the first line, followed by other combinations of immunotherapies, in which rituximab is promising.
4.5.4. Pathogenesis
Not only paraneoplastic and post infectious OMS but also idiopathic OMS are considered to be autoimmune-mediated for the following reasons [16, 145, 146]; (1) autoantibodies, especially anti-Ri antibody, are associated with breast cancer [145, 146], (2) CSF exhibits expansion of CD19+ B cells and gamma-delta T cell subsets [149], and (3) autopsy shows perivascular collection of lymphocytes [148]. Unlike PCD, neither cell loss nor extensive T cell infiltration is characteristically evident in paraneoplastic OMS [150, 151].
Immune mechanisms appear to disrupt adequate controls by the cerebellar cortex on the cerebellar nuclei (fastigial nucleus and dentate nucleus) in opsoclonus and action myoclonus, respectively. A recent physiological study demonstrated that hyper-activation of fastigial nucleus neurons, which terminate on excitatory burst neurons, underlies the generation of opsoclonus [152]. It is assumed that the net output from the cerebellar cortex cannot appropriately inhibit the fastigial nucleus, resulting in facilitation of excitatory burst neurons responsible for the chaotic saccade [152]. On the other hand, the mechanisms of action myoclonus remain uncertain. In analogy with the opsoclonus, a similar hyper-activation in the dentate-thalamo-cortical tract could be operational, which may be caused by malfunction of cerebellar cortical inhibition of the dentate nucleus. Overflow of the cerebellar output to the motor cortex can unstabilize its excitability, in which even subthreshold inputs can easily activate the motor cortex, generating the action myoclonus. In consistence with this assumption, electrophysiological studies showed that the myoclonus is of cortical excitability origin in OMS associated with gluten sensitivity [62] and functional MRI studies have shown low correlation between the cerebellum and motor cortex [153].
It should be acknowledged again that autoimmunity does not only target the cerebellar cortex, but also the cerebellar cortex-mediated modulation of cerebellar nuclei.
4.5.5. Recommended Therapeutic Strategies
-
Therapeutic strategies vary according to the etiology of OMS.
Association with neoplasm should be considered first, and necessary investigations should be conducted to determine the stage of malignancy. In case of paraneoplastic OMS, treatment of the neoplasm should be followed subsequently by immunotherapy.
Patients with post-infectious OMS should be observed closely, looking for stabilization and improvement, since this subtype often shows full recovery.
Patients with idiopathic OMS should also be observed closely, checking for stabilization and improvement. The appearance of progressive symptoms warrant commencement of immunotherapy.
Corticosteroids (intravenous methylprednisolone) are recommended as the first line of induction immunotherapy. When the clinical course exhibits a progressive nature, other combinations of IVIg, plasmapheresis, immunosuppressants, and/or rituximab should be considered. Rituximab is assumed a promising agent.
The use of gabapentin is recommended for patients with opsoclonus and action myoclonus who show disturbance of daily living.
5. Common Therapeutic Rationale for Various Types of IMCAs
5.1. The “Restorable Stage/cerebellar Reserve” Concept for Understanding the Clinical Course of IMCAs
Detailed analysis of the reported patients suggests a characteristic time-related clinical course that depends to a large extent on the etiology (Fig. 2) [11]. Considerations about the time-related clinical course of IMCAs led us to propose two novel concepts in the field of ataxiology: the “restorable stage” and “cerebellar reserve” [12-14] (Fig. 2). The “restorable stage” is defined as the time period during which the recovery capacity of the cerebellum is still intact. Within the “restorable stage”, proper therapies can improve CAs. In contrast, beyond the “restorable stage”, i.e., “non-restorable stage”, any therapy is less likely to show any clinical effect. Such capacity in turn correlates with the “cerebellar reserve”, which is defined as the capacity of the cerebellum for compensation and restoration. By introducing these two concepts, “restorable stage” and “cerebellar reserve”, the time course of each type of IMCAs can be better explained [12, 13, 14] (Fig. 2).
Fig. (2).
(A) Clinical time-course is classified into six patterns; (a) full recovery type, (b) partial recovery type, (c) stabilization type, (d) relapse type, (e) gradually progressive type, and (f) rapidly progressive type. Triangles: time of commencement of immunotherapy. Possible clinical courses for each subtype are schematically indicated below. Black: common course, gray: occasional course, light gray: rare course. PCI: post-infectious cerebellitis, OMS: opsoclonus myoclonus syndrome, GA: gluten ataxia, PCD: paraneoplastic cerebellar degeneration, Anti-GAD: anti-GAD65Ab-associated CA. (B) Schematic diagram of decline of cerebellar function and the concept of “restorable stage” and “cerebellar reserve”. Proper therapies can restore cerebellar function in patients with the cerebellum at the “restorable stage”, meaning the presence of sufficient cerebellar functional reserve. (The color version of the figure is available in the electronic copy of the article).
In the nonresponsive type of IMCAs, especially PCDs, the underlying autoimmune mechanisms damage cerebellar neurons (in both the cerebellar cortex and nuclei) and neural circuitry [4], and can potentially result in loss of the “cerebellar reserve”. Consequently, such patients show progressive CAs.
On the other hand, in the responsive type of IMCAs (e.g., post-infectious cerebellitis, GA and subacute type of anti-GAD65Ab-associated CA), disease progression can be controlled by immunotherapy.
When early therapeutic intervention halts the progression of the disease, the stage will be still within the “restorable stage”. These patients show good clinical outcome after immunotherapy. “Cerebellar reserve” is a factor which can diminish the ataxic symptoms.
In post-infectious cerebellitis, the autoimmune attack halts naturally. Since the stage will be within the “restorable stage”, the disease shows a self-limiting nature.
Importantly, any delay in treatment is potentially a missed opportunity to take advantage of the “cerebellar reserve”. If the disease stage is beyond the “restorable stage”, improvement in CA is not expected even in a potentially responsive case. It is conceivable that even after halting disease progression by immunotherapy, CAs will persist and continue to impair the daily lives of patients.
These classifications suggest an order in chaotic pathologies.
These rationales are based on a physiological test that defines the ratio of feed-forward control to feed-back control [10]. In this test, normal healthy subjects complete a tracking task using feed-forward control operated by the cerebro-cerebellar loop. In patients with degenerative CAs and evident cerebellar atrophy, the tracking movements are guided under feed-back control [10]. These results are rational since the predictive feed-forward control cannot be utilized in patients with diffuse cerebellar damage. In such patients, the cerebrum controls the tracking in a compensatory feed-back fashion, by which smooth movements are impossible. In contrast, in patients with early IMCAs and no or mild cerebellar atrophy, the tracking task can be achieved by feed-forward control though it is not perfect [10]. That is, although the tracking task can be poor in both patient groups, the control mode is different. These results suggest that cerebellar functions are still preserved at the early stage of IMCAs, i.e., “restorable stage”.
5.2. Quantification of the “Cerebellar Reserve”
Prognostic factors suggest that patients with no or mild cerebellar atrophy respond well to immunotherapy and show improvement in CA (Table 5). Undoubtedly, the extent of atrophy on MRI can be used for rough estimations of “cerebellar reserve”. Interestingly, a recent report described the use of MR spectroscopy for quantitative estimation of cerebellar neuronal function [61]. The authors reported an increase in the N-acetylaspartate (NAA)/creatine (Cr) ratio, which represents the numerical value of cerebellar cellular activities, after immunotherapy [61], suggesting that MR spectroscopy can be a useful tool for the assessment of the cerebellar reserve.
Table 5.
Prognostic factors for GA, paraneoplastic cerebellar degeneration, and anti-GAD65Ab-associated CA.
| Gluten Ataxia |
Paraneoplastic Cerebellar
Degenerations |
Anti-GAD65
b -Associated Cerebellar Ataxia |
Post-infectious
Cerebellitis |
Opsoclonus
Myoclonus Syndrome |
|
|---|---|---|---|---|---|
| Cerebellar atrophy | Mild or no atrophy | Unknown | Mild or no atrophy | No atrophy | - (Originally, atrophy is absent.) |
| Latency from onset to diagnosis | Not determined | Early intervention? | Subacute | Not assessed | Not assessed |
| Others | Strict gluten-free diet |
Association of anti-Tr Ab or anti-Ri Ab | - | Children | Idiopathic type |
Functionally, noninvasive cerebellar stimulation using transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) is another promising tool not only to promote recovery but also to assess the residual circuitry. Activation of the cerebellum 5-6 msec before cerebral stimuli results in inhibition of motor cortex excitability, a process termed cerebellar brain inhibition (CBI) [154]. The CBI is explained by Purkinje cell-mediated inhibition of the cerebello-cerebral pathway [155]; since the motor cortex is facilitated by the dentate-thalamo-cerebral pathway, activation of inhibitory Purkinje cells reduces this facilitation [155]. Furthermore, by changing stimulus parameters, the stimuli can in turn activate interneurons so as to depress Purkinje cells, resulting in facilitation of the dentate-thalamo-cerebral pathway and increase in motor cortex excitability [155]. Thus, noninvasive cerebellar stimulation can also be used as a tool for assessment of preserved neurons in the cerebellar neural circuitry.
Thus, it seems that the combination of imaging and noninvasive cerebellar stimulation can be used for quantitative assessment of the “cerebellar reserve”. Specific studies are warranted.
5.3. Synaptic Plasticity and Convergence of Redundant Information; “Circuitry Reserve”
The cerebellar cortex is divided into numerous functional units. Purkinje cells located within a narrow slit extending in rostro-caudal direction act as a functional unit (a microzone) [156, 157]. The microzone is located in the cerebro-cerebellar loop, spino-cerebellar loop, and vestibulo-cerebellar loop [158]. The microzone in the cerebro-cerebellar loop coordinates timing in muscle activities in a predictive fashion [158], and its malfunction leads to the development of limb ataxia [159]. The cerebellar cortical neural circuits are characteristically endowed with dynamic and flexible features compared with other CNS areas.
First, there are many forms of synaptic plasticity in the cerebellar circuits. For example, synaptic plasticity is elicited activity-dependently by combinations of two inputs to the Purkinje cells, parallel fibers and climbing fiber inputs; conjunctive inputs of parallel fibers and climbing fibers induce long-term depression (LTD) on parallel fibers-Purkinje cells synapses [156]. The classic Marr-Albus-Ito theory hypothesized that error signals conveyed by climbing fibers eliminate inadequate parallel fiber inputs to Purkinje cells [156], resulting in an update of the internal model, a neural machinery for predictive recognition of errors in outputs and predictive formation of motor commands for desirable outputs [160]. While a consensus on this assumption has not been obtained yet, new motor commands can be acquired in a microzone through learning processes using many forms of synaptic plasticity [161]. The co-existing synaptic plasticity is illustrated in Fig. 3 [161].
Fig. (3).
A schematic diagram of the neural circuitry and cellular reserves underlying cerebellar reserve. (A) A schematic diagram of the neural circuitry in the cerebellar cortex. PC: Purkinje cell, GC: granule cell, IN: molecular layer interneurons; Golgi: Golgi cells; MF: mossy fibers, PF: parallel fibers, CF: climbing fibers. Two cellular mechanisms constitute the cerebellar reserve; (1) Multiple forms of synaptic plasticity and (2) Convergence and divergence of mossy fiber inputs. When one microzone is pathologically impaired, the reserve makes it possible to substitute this function with another microzone. LTD: long-term depression, LTP: long-term potentiation, RP: rebound potentiation. Excitatory neurons are shown with a white soma, whereas inhibitory neurons are shown with a black soma. (B) Pathology of excitotoxicity, which causes increased calcium entry. The extracellular invasion, excitotoxicity, is characterized by enhancement through microglia-induced neuroinflammation in a positive-feedback fashion. The excitotoxicity in turn forms a chained cascade leading to lethal statuses (indicated by yellow boxes); (1) oxidative stress, (2) ER stress, and 3) DNA damage. Importantly, the cells are protected against these stresses by (1) antioxidant agents and (2) ER chaperon, ubiquitin-dependent proteasome, and autophagy (indicated by blue boxes). Thus, the lethal statuses, which are beyond these protective capacities, elicit cell death. The protective capacities consist of cellular reserve, cellular ability for compensation and restoration. Glut: glutamate. (The color version of the figure is available in the electronic copy of the article).
Second, the mossy fibers-granule cells-parallel fibers system conveys redundant information to the microzone. The peripheral sensory information and the central commands or central cognitive information are conveyed to the cerebral cortex through mossy fibers [156, 157]. Surprisingly large banks of external and internal (of cerebral cortex origin) information are conveyed to the cerebellar cortex. A single mossy fiber axon projects to the cerebral cortex with expansive mediolateral direction, resulting in innervation of multiple microzones [162]. The mossy fibers terminate on the granule cells in the microzone and, in turn, axons of the granule cells make contacts on Purkinje cell dendrites [156, 157]. Consequently, huge information can converge into a single microzone. In the ordinary mode, the majority of parallel fiber inputs on Purkinje cells are silent synapses [163] and only a few of them are utilized, which provides abundant reserve capacities. It should be acknowledged also that the number of granule cells is massive; their number is half of the number of CNS neurons [164]. About 60% of neurons are located in the cerebellum, although the cerebellum constitutes only 10% of the brain mass [164]. This anatomical/structural peculiarity of granule cells means abundant reserve capacity.
Under these conditions, disruption of one microzone and loss of its function is rapidly compensated for by other microzones through the employment of synaptic plasticity and deserialization of unutilized information (Fig. 3). In this regard, synaptic plasticity and convergence of redundant information constitute capacities for compensation and restoration at the neural circuitry level. Provided these capacities of “circuitry reserve” are preserved, “cerebellar reserve” can be clinically preserved.
5.4. From Functional Disorder to Cell Death; Positive-feedback Fashion Acceleration versus “Cellular Reserve”
What are the factors that can determine the switching from “restorable stage” to “non-restorable stage”, or in other words, disruption of “cerebellar reserve”? Recent physiological studies of anti-GAD65Ab-associated CA might provide a clue to this question. In this etiology, there is a switching from functional disorder to cell death. As discussed in a previous chapter, anti-GAD65Ab suppresses the release of GABA at cerebellar inhibitory synapses, resulting in impairment of cerebellar motor control, ultimately leading to the development of CAs [13, 56]. However, in the advanced stage, this functional suppression of GABA release is followed by complete loss of cerebellar neurons [129]. The following cascade reactions are assumed to take place during this process [13, 14]; (1) low GABA release attenuates heterosynaptic inhibition of glutamate release, resulting in increased glutamate levels, (2) excess glutamate stimulates microglia, resulting in glutamate release from microglia [165], presumably through system xc(-), an antiporter exchanging extracellular cystine for intracellular glutamate [166] (3) the activated microglia also release TNF-α, a neuroinflammatory cytokine [167], which suppresses the uptake of glutamate by astrocytes [167], and potentiates Ca2+ permeability of AMPA and NMDA receptors on neurons [168, 169], (4) the combination of these changes results in accumulation of glutamate in the extracellular space, and (5) finally, Ca2+ influx excess results in stimulation of calpain I and nNOS [170]. These molecules damage the DNA and enhance the formation of ONOO- (nitrosative stress), respectively, leading to ER stress. Thus, a decrease in GABA release can accelerate the positive feedback loop, leading to hyperexcitability of cerebellar neurons. When the “cellular reserve”, which protects against these lethal cascades, diminishes in size, cell death is accelerated. Under conditions of positive feed-back loop fashion accelerations, “cellular reserve” will be easily disrupted, indicating the need for early treatment before such accelerations.
It is not certain whether progression from functional disorder to cell death occurs also in other types of IMCAs. However, consistent with our hypothesis, any functional impairment (e.g., synaptic dysfunction or deranged signal flow within the cerebellar neuronal circuitry) that precedes degenerative cell loss, can advance to clinically-evident CA in degenerative CAs [171, 172]. Taken together, the “restorable stage” corresponds to the period of functional disorders and the period of minimal cell loss.
In this regard, in the responsive type of IMCAs, such as post-infectious cerebellitis, idiopathic OMS, and GA (Fig. 2), switching will occur slowly and the underlying immune mechanisms can also be controlled. Consequently, patients with these subtypes show no or only mild cerebellar atrophy, and exhibit improvement of CAs using preserved “cerebellar reserve”. In contrast, for IMCAs patients who do not respond to treatment, such as those with PCDs (Fig. 2), the process tilts towards the switching point and the underlying autoimmunity is difficult to control. Fulminant autoimmunity can be due to one or more of the following mechanisms; (1) downregulation of regulatory T cells, (2) persistent autoimmune response to the malignancy, (3) structural modification of certain intracellular proteins in cancer cells, which can lead to stimulation of the immune system, and (4) death of cancer cells with subsequent release of intracellular antigens followed by exposure to the immune system [32]. On the other hand, anti-GAD65Ab-associated CA and paraneoplastic OMS are positioned somewhere in the middle of these two groups.
Conclusion
Systematic review of currently available treatments for IMCAs identified a common therapeutic rationale for the different types of IMCAs. In IMCAs, immunotherapy is designed to stop disease progression within the “restorable stage” or while “cerebellar reserve” is still preserved (Table 6). Especially, GA and the subacute type of anti-GAD65Ab-associated CA, which can potentially respond to immunotherapy, can benefit from immunotherapy, particularly when such treatment is used within the “restorable stage” [173]. For early treatment interventions, early diagnosis is necessary. When cerebellar atrophy is mild compared with the severity of CAs, IMCA should be considered in the differential diagnosis.
Table 6.
Reported first line immunotherapy for each subtype of IMCAs.
| Gluten ataxia |
| Induction and maintenance therapies: strict gluten-free diet |
| (In case of no improvement and negative gluten related antibodies, immunosuppressants or IVIg) |
| Paraneoplastic cerebellar degeneration |
| Quick removal of neoplasm must be the first objective of treatment Induction therapy as soon as possible: mPSL, IVIg, immunosuppressants, or/and plasma exchange Discussion according associated Abs |
| Maintenance therapy: continuous oral PSL, IVIg, immunosuppressants |
| Anti-GAD Abs associated cerebellar ataxia |
| Induction therapy: mPSL, IVIg, immunosuppressants, plasma exchange, or/and rituximab |
| Maintenance therapy: continuous oral PSL, IVIg, immunosuppressants, or/and rituximab |
| Post-infectious cerebellitis |
| Since cerebellitis is self-limiting, close monitoring of the patient and follow-up form the basis of clinical management. |
| (When the CA persists, a combination of immunotherapies has been considered) |
| Opsoclonus myoclonus syndrome |
| 1. Neoplastic type; Quick removal of neoplasm must be the first objective of treatment |
| 2 Post-infectious and idiopathic types; OMS should be observed closely, looking for stabilization and improvement |
Abbreviations: Antibodies; mPSL: intravenous methylprednisolone; oral PSL: oral prednisolone; IVIg: intravenous immunoglobulins; CAs: cerebellar ataxias; OMS; opsoclonus myoclonus syndrome.
Acknowledgements
Mario Manto is supported by the FNRS and the Fonds Erasme.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Hadjivassiliou M. Immune-mediated acquired ataxias. Handb. Clin. Neurol. 2012;103:189–199. doi: 10.1016/B978-0-444-51892-7.00011-5. [http://dx.doi.org/10.1016/B978-0-444-51892-7.00011-5]. [PMID: 21827889]. [DOI] [PubMed] [Google Scholar]
- 2.Hadjivassiliou M., Grünewald R.A., Chattopadhyay A.K., Davies-Jones G.A., Gibson A., Jarratt J.A., Kandler R.H., Lobo A., Powell T., Smith C.M. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet. 1998;352(9140):1582–1585. doi: 10.1016/s0140-6736(98)05342-2. [http://dx.doi.org/10.1016/S0140-6736(98)05342-2]. [PMID: 9843103]. [DOI] [PubMed] [Google Scholar]
- 3.Graus F., Delattre J.Y., Antoine J.C., Dalmau J., Giometto B., Grisold W., Honnorat J., Smitt P.S., Vedeler Ch., Verschuuren J.J., Vincent A., Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. [http://dx.doi.org/10.1136/jnnp. 2003.034447]. [PMID: 15258215]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J., Rosenfeld M.R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327–340. doi: 10.1016/S1474-4422(08)70060-7. [http://dx.doi.org/10.1016/ S1474-4422(08)70060-7]. [PMID: 18339348]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducray F., Demarquay G., Graus F., Decullier E., Antoine J.C., Giometto B., Psimaras D., Delattre J.Y., Carpentier A.F., Honnorat J. Seronegative paraneoplastic cerebellar degeneration: the PNS Euronetwork experience. Eur. J. Neurol. 2014;21(5):731–735. doi: 10.1111/ene.12368. [http://dx.doi.org/10.1111/ene.12368]. [PMID: 24471811]. [DOI] [PubMed] [Google Scholar]
- 6.Jarius S., Wildemann B. Medusa-head ataxia’: the expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 1: Anti-mGluR1, anti-Homer-3, anti-Sj/ITPR1 and anti-CARP VII. J. Neuroinflammation. 2015 doi: 10.1186/s12974-015-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarius S., Wildemann B. Medusa-head ataxia’: the expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 2: Anti-PKC-gamma, anti-GluR-delta2, anti-Ca/ ARHGAP26 and anti-VGCC. J. Neuroinflammation. 2015 doi: 10.1186/s12974-015-0357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarius S., Wildemann B. Medusa-head ataxia’: the expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 3: Anti-Yo/CDR2, anti-Nb/AP3B2, PCA-2, anti-Tr/DNER, other antibodies, diagnostic pitfalls, summary and outlook. J. Neuroinflammation. 2015 doi: 10.1186/s12974-015-0358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honnorat J., Saiz A., Giometto B., Vincent A., Brieva L., de Andres C., Maestre J., Fabien N., Vighetto A., Casamitjana R., Thivolet C., Tavolato B., Antoine J., Trouillas P., Graus F. Cerebellar ataxia with anti-glutamic acid decarboxylase antibodies: study of 14 patients. Arch. Neurol. 2001;58(2):225–230. doi: 10.1001/archneur.58.2.225. [http:// dx.doi.org/10.1001/archneur.58.2.225]. [PMID: 11176960]. [DOI] [PubMed] [Google Scholar]
- 10.Mitoma H., Adhikari K., Aeschlimann D., Chattopadhyay P., Hadjivassiliou M., Hampe C.S., Honnorat J., Joubert B., Kakei S., Lee J., Manto M., Matsunaga A., Mizusawa H., Nanri K., Shanmugarajah P., Yoneda M., Yuki N. Consensus Paper: Neuroimmune mechanisms of cerebellar ataxias. Cerebellum. 2016;15(2):213–232. doi: 10.1007/s12311-015-0664-x. [http://dx.doi.org/10.1007/s12311-015-0664-x]. [PMID: 25823827]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitoma H., Hadjivassiliou M., Honnorat J. Guidelines for treatment of immune-mediated cerebellar ataxias. Cerebellum Ataxias. 2015;2:14. doi: 10.1186/s40673-015-0034-y. [http://dx.doi.org/10.1186/s40673-015-0034-y]. [PMID: 26561527]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitoma H., Manto M. The physiological basis of therapies for cerebellar ataxias. Ther. Adv. Neurol. Disorder. 2016;9(5):396–413. doi: 10.1177/1756285616648940. [http://dx.doi.org/10.1177/1756285616648940]. [PMID: 27582895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitoma H., Manto M., Hampe C.S. Pathogenic roles of glutamic acid decarboxylase 65 autoantibodies in cerebellar ataxias. J. Immunol. Res. 2017;2017:2913297. doi: 10.1155/2017/2913297. [http://dx.doi.org/10.1155/2017/ 2913297]. [PMID: 28386570]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitoma H., Manto M., Hampe C.S. Immune-mediated cerebellar ataxias: from bench to bedside. Cerebellum Ataxias. 2017;4:16. doi: 10.1186/s40673-017-0073-7. [http://dx.doi.org/10.1186/s40673-017-0073-7]. [PMID: 28944066]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadjivassiliou M., Martindale J., Shanmugarajah P., Grünewald R.A., Sarrigiannis P.G., Beauchamp N., Garrard K., Warburton R., Sanders D.S., Friend D., Duty S., Taylor J., Hoggard N. Causes of progressive cerebellar ataxia: prospective evaluation of 1500 patients. J. Neurol. Neurosurg. Psychiatry. 2017;88(4):301–309. doi: 10.1136/jnnp-2016-314863. [http://dx.doi.org/10.1136/jnnp-2016-314863]. [PMID: 27965395]. [DOI] [PubMed] [Google Scholar]
- 16.Hadjivassiliou M. Advances in therapies of cerebellar disorders: Immune-mediated ataxias. CNS Neurol. Disord. Drug Targets. 2017 doi: 10.2174/1871527317666171221110548. [http://dx.doi.org/10.2174/1871527317666171221110548]. [PMID: 29268693]. [DOI] [PubMed] [Google Scholar]
- 17.Charcot J.M. Séance du 14 mars. CR Soc. Biol. (Paris) 1868;20:13. [Google Scholar]
- 18.Brouwer B. Beitrag zur Kenntnis der chronischen diffusen Kleinhirnerkrankungen. Neurol. Zentralbl. 1919;38:674–682. [Google Scholar]
- 19.Hadjivassiliou M., Boscolo S., Tongiorgi E., Grünewald R.A., Sharrack B., Sanders D.S., Woodroofe N., Davies-Jones G.A. Cerebellar ataxia as a possible organ-specific autoimmune disease. Mov. Disord. 2008;23(10):1370–1377. doi: 10.1002/mds.22129. [http://dx.doi.org/10.1002/ mds.22129]. [PMID: 18546342]. [DOI] [PubMed] [Google Scholar]
- 20.Shaw P.J., Walls T.J., Newman P.K., Cleland P.G., Cartlidge N.E. Hashimoto’s encephalopathy: a steroid-responsive disorder associated with high anti-thyroid antibody titers--report of 5 cases. Neurology. 1991;41(2 (Pt 1)):228–233. doi: 10.1212/wnl.41.2_part_1.228. [http://dx.doi.org/10.1212/ WNL.41.2_Part_1.228]. [PMID: 1992366]. [DOI] [PubMed] [Google Scholar]
- 21.Matsunaga A., Ikawa M., Fujii A., Nakamoto Y., Kuriyama M., Yoneda M. Hashimoto’s encephalopathy as a treatable adult-onset cerebellar ataxia mimicking spinocerebellar degeneration. Eur. Neurol. 2013;69(1):14–20. doi: 10.1159/000342217. [http://dx.doi.org/10.1159/000342217]. [PMID: 23128836]. [DOI] [PubMed] [Google Scholar]
- 22.Mitoma H., Yoneda M., Saitow F., Suzuki H., Matsunaga A., Ikawa M., Mizusawa H. Presynaptic dysfunction caused by CSF from a patient with ataxic form of Hashimoto’s encephalopathy. Neurol. Clin. Neurosci. 2014 [http://dx.doi.org/10.1111/ ncn3.105]. [Google Scholar]
- 23.Hilberath J.M., Schmidt H., Wolf G.K. Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT): case report of reversible coma and status epilepticus in an adolescent patient and review of the literature. Eur. J. Pediatr. 2014;173(10):1263–1273. doi: 10.1007/s00431-014-2391-6. [http://dx.doi.org/10.1007/s00431-014-2391-6]. [PMID: 25084973]. [DOI] [PubMed] [Google Scholar]
- 24.Melzer N., Golombeck K.S., Gross C.C., Meuth S.G., Wiendl H. Cytotoxic CD8+ T cells and CD138+ plasma cells prevail in cerebrospinal fluid in non-paraneoplastic cerebellar ataxia with contactin-associated protein-2 antibodies. J. Neuroinflammation. 2012;9:160. doi: 10.1186/1742-2094-9-160. [http://dx.doi.org/10.1186/1742-2094-9-160]. [PMID: 22759321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miske R., Gross C.C., Scharf M., Golombeck K.S., Hartwig M., Bhatia U. Neurochondrin is a neural target antigen in autoimmune cerebellar degeneration. Neurol. Neuroimmunol. Neuroinflamm. 2016;3:e255. doi: 10.1212/NXI.0000000000000307. [http://dx.doi.org/10.1212/NXI.0000000000000255]. [PMID: 27458598]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostami A., Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013;333(1-2):76–87. doi: 10.1016/j.jns.2013.03.002. [http://dx.doi.org/10.1016/j.jns.2013.03.002]. [PMID: 23578791]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danikowski K.M., Jayaraman S., Prabhakar B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflammation. 2017;14(1):117. doi: 10.1186/s12974-017-0892-8. [http://dx.doi.org/10.1186/s12974-017-0892-8]. [PMID: 28599652]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friese M.A., Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann. Neurol. 2009;66(2):132–141. doi: 10.1002/ana.21744. [http://dx.doi.org/ 10.1002/ana.21744]. [PMID: 19743458]. [DOI] [PubMed] [Google Scholar]
- 29.Costa M., Saiz A., Casamitjana R., Castañer M.F., Sanmartí A., Graus F., Jaraquemada D. T-cell reactivity to glutamic acid decarboxylase in stiff-man syndrome and cerebellar ataxia associated with polyendocrine autoimmunity. Clin. Exp. Immunol. 2002;129(3):471–478. doi: 10.1046/j.1365-2249.2002.01931.x. [http://dx.doi.org/10.1046/j.1365-2249.2002.01931. x]. [PMID: 12197888]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iorio R., Damato V., Evoli A., Gessi M., Gaudino S., Di Lazzaro V., Spagni G., Sluijs J.A., Hol E.M. Clinical and immunological characteristics of the spectrum of GFAP autoimmunity: a case series of 22 patients. J. Neurol. Neurosurg. Psychiatry. 2018;89(2):138–146. doi: 10.1136/jnnp-2017-316583. [http://dx.doi.org/10.1136/jnnp-2017-316583]. [PMID: 28951498]. [DOI] [PubMed] [Google Scholar]
- 31.Vojdani A. A Potential Link between Environmental Triggers and Autoimmunity. Autoimmune Dis. 2014;2014:437231. doi: 10.1155/2014/437231. [http://dx. doi.org/10.1155/2014/437231]. [PMID: 24688790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaenker P., Gray E.S., Ziman M.R. Autoantibody Production in Cancer--The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmun. Rev. 2016;15(5):477–483. doi: 10.1016/j.autrev.2016.01.017. [http://dx.doi.org/10.1016/j.autrev.2016.01.017]. [PMID: 26827909]. [DOI] [PubMed] [Google Scholar]
- 33.Bei R., Masuelli L., Palumbo C., Modesti M., Modesti A. A common repertoire of autoantibodies is shared by cancer and autoimmune disease patients: Inflammation in their induction and impact on tumor growth. Cancer Lett. 2009;281(1):8–23. doi: 10.1016/j.canlet.2008.11.009. [http:// dx.doi.org/10.1016/j.canlet.2008.11.009]. [PMID: 19091462]. [DOI] [PubMed] [Google Scholar]
- 34.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [http://dx.doi.org/10.1126/science. 1071059]. [PMID: 11951032]. [DOI] [PubMed] [Google Scholar]
- 35.de Vlam K., De Keyser F., Verbruggen G., Vandenbossche M., Vanneuville B., D’Haese D., Veys E.M. Detection and identification of antinuclear autoantibodies in the serum of normal blood donors. Clin. Exp. Rheumatol. 1993;11(4):393–397. [PMID: 8403584]. [PubMed] [Google Scholar]
- 36.Satoh M., Chan E.K., Ho L.A., Rose K.M., Parks C.G., Cohn R.D., Jusko T.A., Walker N.J., Germolec D.R., Whitt I.Z., Crockett P.W., Pauley B.A., Chan J.Y., Ross S.J., Birnbaum L.S., Zeldin D.C., Miller F.W. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64(7):2319–2327. doi: 10.1002/art.34380. [http://dx.doi.org/10.1002/art. 34380]. [PMID: 22237992]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sørgjerd E.P., Thorsby P.M., Torjesen P.A., Skorpen F., Kvaløy K., Grill V. Presence of anti-GAD in a non-diabetic population of adults; time dynamics and clinical influence: results from the HUNT study. BMJ Open Diabetes Res. Care. 2015;3(1):e000076. doi: 10.1136/bmjdrc-2014-000076. [http://dx.doi.org/10.1136/bmjdrc-2014-000076]. [PMID: 26157582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caglar E., Ugurlu S., Ozenoglu A., Can G., Kadioglu P., Dobrucali A. Autoantibody frequency in celiac disease. Clinics (São Paulo) 2009;64(12):1195–1200. doi: 10.1590/S1807-59322009001200009. [http://dx.doi.org/10.1590/S1807-59322009001200009]. [PMID: 20037707]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hampe C.S., Maitland M.E., Gilliam L.K., Phan T.H., Sweet I.R., Radtke J.R., Bota V., Ransom B.R., Hirsch I.B. High titers of autoantibodies to glutamate decarboxylase in type 1 diabetes patients: epitope analysis and inhibition of enzyme activity. Endocr. Pract. 2013;19(4):663–668. doi: 10.4158/EP12318.OR. [http://dx.doi.org/10.4158/EP12318. OR]. [PMID: 23512385]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chéramy M., Hampe C.S., Ludvigsson J., Casas R. Characteristics of in-vitro phenotypes of glutamic acid decarboxylase 65 autoantibodies in high-titre individuals. Clin. Exp. Immunol. 2013;171(3):247–254. doi: 10.1111/cei.12026. [http://dx.doi.org/10.1111/cei.12026]. [PMID: 23379430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura Y., Nakajima H., Hosokawa T., Yamane K., Ishida S., Kimura F. Acute cerebellar ataxia associated with anti-glutamic acid decarboxylase antibodies mimicking miller fisher syndrome. Intern. Med. 2018;57(2):269–271. doi: 10.2169/internalmedicine.9190-17. [http://dx.doi.org/10.2169/ internalmedicine.9190-17]. [PMID: 29093402]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saiz A., Blanco Y., Sabater L., González F., Bataller L., Casamitjana R., Ramió-Torrentà L., Graus F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(Pt 10):2553–2563. doi: 10.1093/brain/awn183. [http://dx.doi.org/10.1093/brain/awn183]. [PMID: 18687732]. [DOI] [PubMed] [Google Scholar]
- 43.Takenoshita H., Shizuka-Ikeda M., Mitoma H., Song S., Harigaya Y., Igeta Y., Yaguchi M., Ishida K., Shoji M., Tanaka M., Mizusawa H., Okamoto K. Presynaptic inhibition of cerebellar GABAergic transmission by glutamate decarboxylase autoantibodies in progressive cerebellar ataxia. J. Neurol. Neurosurg. Psychiatry. 2001;70(3):386–389. doi: 10.1136/jnnp.70.3.386. [http://dx.doi.org/10.1136/jnnp.70.3.386]. [PMID: 11181864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manto M.U., Hampe C.S., Rogemond V., Honnorat J. Respective implications of glutamate decarboxylase antibodies in stiff person syndrome and cerebellar ataxia. Orphanet J. Rare Dis. 2011;6:3. doi: 10.1186/1750-1172-6-3. [http://dx.doi.org/10.1186/1750-1172-6-3]. [PMID: 21294897]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill K.E., Clawson S.A., Rose J.W., Carlson N.G., Greenlee J.E. Cerebellar Purkinje cells incorporate immunoglobulins and immunotoxins in vitro: implications for human neurological disease and immunotherapeutics. J. Neuroinflammation. 2009;6:31. doi: 10.1186/1742-2094-6-31. [http://dx.doi.org/10.1186/1742-2094-6-31]. [PMID: 19874605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alarcon-Segovia D., Ruiz-Argüelles A., Llorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol. Today. 1996;17(4):163–164. doi: 10.1016/s0167-5699(96)90258-3. [PMID: 8871346]. [DOI] [PubMed] [Google Scholar]
- 47.Greenlee J.E., Clawson S.A., Hill K.E., Wood B.L., Tsunoda I., Carlson N.G. Purkinje cell death after uptake of anti-Yo antibodies in cerebellar slice cultures. J. Neuropathol. Exp. Neurol. 2010;69(10):997–1007. doi: 10.1097/NEN.0b013e3181f0c82b. [http://dx.doi.org/10.1097/NEN.0b013e3181 f0c82b]. [PMID: 20838245]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenlee J.E., Clawson S.A., Hill K.E., Wood B., Clardy S.L., Tsunoda I., Carlson N.G. Anti-Yo antibody uptake and interaction with its intracellular target antigen causes Purkinje cell death in rat cerebellar slice cultures: a possible mechanism for paraneoplastic cerebellar degeneration in humans with gynecological or breast cancers. PLoS One. 2015;10(4):e0123446. doi: 10.1371/journal.pone.0123446. [http://dx.doi.org/10. 1371/journal.pone.0123446]. [PMID: 25885452]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenlee J.E., Burns J.B., Rose J.W., Jaeckle K.A., Clawson S. Uptake of systemically administered human anticerebellar antibody by rat Purkinje cells following blood-brain barrier disruption. Acta Neuropathol. 1995;89(4):341–345. doi: 10.1007/BF00309627. [http://dx.doi.org/10.1007/ BF00309627]. [PMID: 7610765]. [DOI] [PubMed] [Google Scholar]
- 50.Borges L.F., Busis N.A. Intraneuronal accumulation of myeloma proteins. Arch. Neurol. 1985;42(7):690–694. doi: 10.1001/archneur.1985.04060070084021. [http://dx.doi.org/ 10.1001/archneur.1985.04060070084021]. [PMID: 3925934]. [DOI] [PubMed] [Google Scholar]
- 51.Fabian R.H., Ritchie T.C. Intraneuronal IgG in the central nervous system. J. Neurol. Sci. 1986;73(3):257–267. doi: 10.1016/0022-510x(86)90150-4. [http://dx.doi.org/10. 1016/0022-510X(86)90150-4]. [PMID: 3522806]. [DOI] [PubMed] [Google Scholar]
- 52.Fishman P.S., Farrand D.A., Kristt D.A. Internalization of plasma proteins by cerebellar Purkinje cells. J. Neurol. Sci. 1990;100(1-2):43–49. doi: 10.1016/0022-510x(90)90011-b. [http://dx.doi.org/10.1016/0022-510X(90)90011-B]. [PMID: 1708409]. [DOI] [PubMed] [Google Scholar]
- 53.Graus F., Illa I., Agusti M., Ribalta T., Cruz-Sanchez F., Juarez C. Effect of intraventricular injection of an anti-Purkinje cell antibody (anti-Yo) in a guinea pig model. J. Neurol. Sci. 1991;106(1):82–87. doi: 10.1016/0022-510x(91)90198-g. [http://dx.doi.org/10.1016/0022-510X(91)90198-G]. [PMID: 1779243]. [DOI] [PubMed] [Google Scholar]
- 54.Hampe C.S., Petrosini L., De Bartolo P., Caporali P., Cutuli D., Laricchiuta D., Foti F., Radtke J.R., Vidova V., Honnorat J., Manto M. Monoclonal antibodies to 65kDa glutamate decarboxylase induce epitope specific effects on motor and cognitive functions in rats. Orphanet J. Rare Dis. 2013;8:82. doi: 10.1186/1750-1172-8-82. [http://dx.doi. org/10.1186/1750-1172-8-82]. [PMID: 23738610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vega-Flores G., Rubio S.E., Jurado-Parras M.T., Gómez-Climent M.Á., Hampe C.S., Manto M., Soriano E., Pascual M., Gruart A., Delgado-García J.M. The GABAergic septohippocampal pathway is directly involved in internal processes related to operant reward learning. Cereb. Cortex. 2014;24(8):2093–2107. doi: 10.1093/cercor/bht060. [http://dx.doi.org/10.1093/cercor/bht060]. [PMID: 23479403]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manto M., Honnorat J., Hampe C.S., Guerra-Narbona R., López-Ramos J.C., Delgado-García J.M., Saitow F., Suzuki H., Yanagawa Y., Mizusawa H., Mitoma H. Disease-specific monoclonal antibodies targeting glutamate decarboxylase impair GABAergic neurotransmission and affect motor learning and behavioral functions. Front. Behav. Neurosci. 2015;9:78. doi: 10.3389/fnbeh.2015.00078. [http:// dx.doi.org/10.3389/fnbeh.2015.00078]. [PMID: 25870548]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hadjivassiliou M., Davies-Jones G.A.B., Sanders D.S., Grünewald R.A. Dietary treatment of gluten ataxia. J. Neurol. Neurosurg. Psychiatry. 2003;74(9):1221–1224. doi: 10.1136/jnnp.74.9.1221. [http://dx.doi.org/ 10.1136/jnnp.74.9.1221]. [PMID: 12933922]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Souayah N., Chin R.L., Brannagan T.H., Latov N., Green P.H.R., Kokoszka A., Sander H.W. Effect of intravenous immunoglobulin on cerebellar ataxia and neuropathic pain associated with celiac disease. Eur. J. Neurol. 2008;15(12):1300–1303. doi: 10.1111/j.1468-1331.2008.02305.x. [http://dx.doi.org/10.1111/j.1468-1331.2008.02305.x]. [PMID: 19049545]. [DOI] [PubMed] [Google Scholar]
- 59.Hadjivassiliou M., Grünewald R.A., Davies-Jones G.A.B. Gluten sensitivity as a neurological illness. J. Neurol. Neurosurg. Psychiatry. 2002;72(5):560–563. doi: 10.1136/jnnp.72.5.560. [http://dx.doi.org/10.1136/jnnp.72.5.560]. [PMID: 11971034]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hadjivassiliou M., Sanders D.S., Woodroofe N., Williamson C., Grünewald R.A. Gluten ataxia. Cerebellum. 2008;7(3):494–498. doi: 10.1007/s12311-008-0052-x. [http://dx.doi.org/10.1007/s12311-008-0052-x]. [PMID: 18787912]. [DOI] [PubMed] [Google Scholar]
- 61.Hadjivassiliou M., Grünewald R.A., Sanders D.S., Shanmugarajah P., Hoggard N. Effect of gluten-free diet on cerebellar MR spectroscopy in gluten ataxia. Neurology. 2017;89(7):705–709. doi: 10.1212/WNL.0000000000004237. [http://dx.doi.org/10.1212/WNL.0000000000004237]. [PMID: 28724585]. [DOI] [PubMed] [Google Scholar]
- 62.Sarrigiannis P.G., Hoggard N., Aeschlimann D., Sanders D.S., Grünewald R.A., Unwin Z.C., Hadjivassiliou M. Myoclonus ataxia and refractory coeliac disease. Cerebellum Ataxias. 2014;1:11. doi: 10.1186/2053-8871-1-11. [http://dx.doi.org/10.1186/2053-8871-1-11]. [PMID: 26331035]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schuppan D., Junker Y., Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137(6):1912–1933. doi: 10.1053/j.gastro.2009.09.008. [http://dx.doi.org/10.1053/j.gastro.2009.09.008]. [PMID: 19766641]. [DOI] [PubMed] [Google Scholar]
- 64.Thomas H., Beck K., Adamczyk M., Aeschlimann P., Langley M., Oita R.C., Thiebach L., Hils M., Aeschlimann D. Transglutaminase 6: a protein associated with central nervous system development and motor function. Amino Acids. 2013;44(1):161–177. doi: 10.1007/s00726-011-1091-z. [http://dx.doi.org/10.1007/s00726-011-1091-z]. [PMID: 21984379]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cervio E., Volta U., Verri M., Boschi F., Pastoris O., Granito A., Barbara G., Parisi C., Felicani C., Tonini M., De Giorgio R. Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology. 2007;133(1):195–206. doi: 10.1053/j.gastro.2007.04.070. [http://dx.doi.org/10.1053/j.gastro.2007. 04.070]. [PMID: 17631142]. [DOI] [PubMed] [Google Scholar]
- 66.Boscolo S., Lorenzon A., Sblattero D., Florian F., Stebel M., Marzari R., Not T., Aeschlimann D., Ventura A., Hadjivassiliou M., Tongiorgi E. Anti transglutaminase antibodies cause ataxia in mice. PLoS One. 2010;5(3):e9698. doi: 10.1371/journal.pone.0009698. [http://dx.doi.org/10.1371/ journal.pone.0009698]. [PMID: 20300628]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iversen R., Di Niro R., Stamnaes J., Lundin K.E., Wilson P.C., Sollid L.M. Transglutaminase 2-specific autoantibodies in celiac disease target clustered, N-terminal epitopes not displayed on the surface of cells. J. Immunol. 2013;190(12):5981–5991. doi: 10.4049/jimmunol.1300183. [http://dx.doi.org/10.4049/jimmunol.1300183]. [PMID: 23690478]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ricotta M., Iannuzzi M., Vivo G.D., Gentile V. Physio-pathological roles of transglutaminase-catalyzed reactions. World J. Biol. Chem. 2010;1(5):181–187. doi: 10.4331/wjbc.v1.i5.181. [http://dx.doi.org/10.4331/wjbc. v1.i5.181]. [PMID: 21541002]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Höftberger R., Rosenfeld M.R., Dalmau J. Update on neurological paraneoplastic syndromes. Curr. Opin. Oncol. 2015;27(6):489–495. doi: 10.1097/CCO.0000000000000222. [http://dx.doi.org/10.1097/CCO.0000000000000222]. [PMID: 26335665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Demarquay G., Honnorat J. Clinical presentation of immune-mediated cerebellar ataxia. Rev. Neurol. (Paris) 2011;167(5):408–417. doi: 10.1016/j.neurol.2010.07.032. [http://dx.doi.org/10.1016/j.neurol.2010.07.032]. [PMID: 21055784]. [DOI] [PubMed] [Google Scholar]
- 71.Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol. 2002;1(5):294–305. doi: 10.1016/s1474-4422(02)00135-7. [http://dx.doi.org/10.1016/S1474-4422(02)00135-7]. [PMID: 12849427]. [DOI] [PubMed] [Google Scholar]
- 72.Peterson K., Rosenblum M.K., Kotanides H., Posner J.B. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology. 1992;42(10):1931–1937. doi: 10.1212/wnl.42.10.1931. [http://dx.doi.org/10.1212/WNL.42.10.1931]. [PMID: 1407575]. [DOI] [PubMed] [Google Scholar]
- 73.Rojas I., Graus F., Keime-Guibert F., Reñé R., Delattre J.Y., Ramón J.M., Dalmau J., Posner J.B. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55(5):713–715. doi: 10.1212/wnl.55.5.713. [http://dx.doi.org/10.1212/WNL. 55.5.713]. [PMID: 10980743]. [DOI] [PubMed] [Google Scholar]
- 74.Candler P.M., Hart P.E., Barnett M., Weil R., Rees J.H. A follow up study of patients with paraneoplastic neurological disease in the United Kingdom. J. Neurol. Neurosurg. Psychiatry. 2004;75(10):1411–1415. doi: 10.1136/jnnp.2003.025171. [http://dx.doi.org/10.1136/jnnp.2003.025171]. [PMID: 15377687]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keime-Guibert F., Graus F., Fleury A., René R., Honnorat J., Broet P., Delattre J.Y. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti-Hu, anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J. Neurol. Neurosurg. Psychiatry. 2000;68(4):479–482. doi: 10.1136/jnnp.68.4.479. [http://dx.doi.org/10.1136/jnnp.68.4.479]. [PMID: 10727484]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shams’ili S., Grefkens J., de Leeuw B., van den Bent M., Hooijkaas H., van der Holt B., Vecht C., Sillevis Smitt P. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126(Pt 6):1409–1418. doi: 10.1093/brain/awg133. [http://dx.doi.org/10.1093/brain/awg133]. [PMID: 12764061]. [DOI] [PubMed] [Google Scholar]
- 77.Moll J.W.B., Henzen-Logmans S.C., Van der Meché F.G., Vecht C.H. Early diagnosis and intravenous immune globulin therapy in paraneoplastic cerebellar degeneration. J. Neurol. Neurosurg. Psychiatry. 1993;56(1):112. doi: 10.1136/jnnp.56.1.112. [http://dx.doi.org/10.1136/ jnnp.56.1.112]. [PMID: 8429313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stark E., Wurster U., Patzold U., Sailer M., Haas J. Immunological and clinical response to immunosuppressive treatment in paraneoplastic cerebellar degeneration. Arch. Neurol. 1995;52(8):814–818. doi: 10.1001/archneur.1995.00540320098016. [http://dx.doi.org/10.1001/archneur.1995.00540320098016]. [PMID: 7639633]. [DOI] [PubMed] [Google Scholar]
- 79.David Y.B., Warner E., Levitan M., Sutton D.M., Malkin M.G., Dalmau J.O. Autoimmune paraneoplastic cerebellar degeneration in ovarian carcinoma patients treated with plasmapheresis and immunoglobulin. A case report. Cancer. 1996;78(10):2153–2156. [http://dx.doi.org/10.1002/(SICI)1097-0142(19961115)78:10<2153:AID-CNCR16>3.0.CO;2-Y]. [PMID: 8918408]. [PubMed] [Google Scholar]
- 80.Blaes F., Strittmatter M., Merkelbach S., Jost V., Klotz M., Schimrigk K., Hamann G.F. Intravenous immunoglobulins in the therapy of paraneoplastic neurological disorders. J. Neurol. 1999;246(4):299–303. doi: 10.1007/s004150050350. [http://dx.doi.org/10.1007/s004150050350]. [PMID: 10367699]. [DOI] [PubMed] [Google Scholar]
- 81.Batocchi A.P., De Rosa G., Evoli A., Tonali P., Greggi S., Scambia G., Salerno G., Tonali P. Positive response to therapy in a patient with a seropositive paraneoplastic cerebellar degeneration and an endometrioid carcinoma of the vesicovaginal septum. J. Neurol. Neurosurg. Psychiatry. 1999;67(3):412–413. doi: 10.1136/jnnp.67.3.412. [http://dx. doi.org/10.1136/jnnp.67.3.412]. [PMID: 10577031]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mowzoon N., Bradley W.G. Successful immunosuppressant therapy of severe progressive cerebellar degeneration and sensory neuropathy: a case report. J. Neurol. Sci. 2000;178(1):63–65. doi: 10.1016/s0022-510x(00)00353-1. [http:// dx.doi.org/10.1016/S0022-510X(00)00353-1]. [PMID: 11018251]. [DOI] [PubMed] [Google Scholar]
- 83.Shams’ili S., de Beukelaar J., Gratama J.W., Hooijkaas H., van den Bent M., van ’t Veer M., Sillevis Smitt P. An uncontrolled trial of rituximab for antibody associated paraneoplastic neurological syndromes. J. Neurol. 2006;253(1):16–20. doi: 10.1007/s00415-005-0882-0. [http://dx.doi.org/ 10.1007/s00415-005-0882-0]. [PMID: 16444604]. [DOI] [PubMed] [Google Scholar]
- 84.Taniguchi Y., Tanji C., Kawai T., Saito H., Marubayashi S., Yorioka N. A case report of plasmapheresis in paraneoplastic cerebellar ataxia associated with anti-Tr antibody. Ther. Apher. Dial. 2006;10(1):90–93. doi: 10.1111/j.1744-9987.2006.00348.x. [http://dx.doi.org/10.1111/j.1744-9987. 2006.00348.x]. [PMID: 16556143]. [DOI] [PubMed] [Google Scholar]
- 85.Geromin A., Candoni A., Marcon G., Ferrari S., Sperotto A., De Luca S., Fanin R. Paraneoplastic cerebellar degeneration associated with anti-neuronal anti-Tr antibodies in a patient with Hodgkin’s disease. Leuk. Lymphoma. 2006;47(9):1960–1963. doi: 10.1080/10428190600678082. [http:// dx.doi.org/10.1080/10428190600678082]. [PMID: 17065013]. [DOI] [PubMed] [Google Scholar]
- 86.Phuphanich S., Brock C. Neurologic improvement after high-dose intravenous immunoglobulin therapy in patients with paraneoplastic cerebellar degeneration associated with anti-Purkinje cell antibody. J. Neurooncol. 2007;81(1):67–69. doi: 10.1007/s11060-006-9198-x. [http://dx.doi.org/10. 1007/s11060-006-9198-x]. [PMID: 16773214]. [DOI] [PubMed] [Google Scholar]
- 87.Thöne J., Hohaus A., Lamprecht S., Bickel A., Erbguth F. Effective immunosuppressant therapy with cyclophosphamide and corticosteroids in paraneoplastic cerebellar degeneration. J. Neurol. Sci. 2008;272(1-2):171–173. doi: 10.1016/j.jns.2008.04.020. [http://dx.doi.org/10.1016/j.jns.2008. 04.020]. [PMID: 18632117]. [DOI] [PubMed] [Google Scholar]
- 88.Esposito M., Penza P., Orefice G., Pagano A., Parente E., Abbadessa A., Bonavita V. Successful treatment of paraneoplastic cerebellar degeneration with Rituximab. J. Neurooncol. 2008;86(3):363–364. doi: 10.1007/s11060-007-9479-z. [http://dx.doi.org/10.1007/s11060-007-9479-z]. [PMID: 17924058]. [DOI] [PubMed] [Google Scholar]
- 89.Schessl J., Schuberth M., Reilich P., Schneiderat P., Strigl-Pill N., Walter M.C., Schlotter-Weigel B., Schoser B. Long-term efficiency of intravenously administered immunoglobulin in anti-Yo syndrome with paraneoplastic cerebellar degeneration. J. Neurol. 2011;258(5):946–947. doi: 10.1007/s00415-010-5859-y. [http://dx.doi.org/10.1007/s00415-010-5859-y]. [PMID: 21174114]. [DOI] [PubMed] [Google Scholar]
- 90.Shimazu Y., Minakawa E.N., Nishikori M., Ihara M., Hashi Y., Matsuyama H., Hishizawa M., Yoshida S., Kitano T., Kondo T., Ishikawa T., Takahashi R., Takaori-Kondo A. A case of follicular lymphoma associated with paraneoplastic cerebellar degeneration. Intern. Med. 2012;51(11):1387–1392. doi: 10.2169/internalmedicine.51.7019. [http://dx.doi.org/ 10.2169/internalmedicine.51.7019]. [PMID: 22687848]. [DOI] [PubMed] [Google Scholar]
- 91.Yeo K.K., Walter A.W., Miller R.E., Dalmau J. Rituximab as potential therapy for paraneoplastic cerebellar degeneration in pediatric Hodgkin disease. Pediatr. Blood Cancer. 2012;58(6):986–987. doi: 10.1002/pbc.23314. [http://dx.doi.org/10.1002/pbc.23314]. [PMID: 22532986]. [DOI] [PubMed] [Google Scholar]
- 92.Lakshmaiah K.C., Viveka B.K., Anil K.N., Saini M.L., Sinha S., Saini K.S. Gastric diffuse large B cell lymphoma presenting as paraneoplastic cerebellar degeneration: case report and review of literature. J. Egypt. Natl. Canc. Inst. 2013;25(4):231–235. doi: 10.1016/j.jnci.2013.07.001. [http://dx.doi.org/10.1016/j.jnci.2013.07.001]. [PMID: 24207096]. [DOI] [PubMed] [Google Scholar]
- 93.Bhargava A., Bhushan B., Kasundra G.M., Shubhakaran K., Pujar G.S., Banakar B. Response to abdominal hysterectomy with bilateral salpingo-oophorectomy in postmenopausal woman with anti-yo antibody mediated paraneoplastic cerebellar degeneration. Ann. Indian Acad. Neurol. 2014;17(3):355–357. doi: 10.4103/0972-2327.138528. [http://dx.doi.org/ 10.4103/0972-2327.138528]. [PMID: 25221413]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitchell A.N., Bakhos C.T., Zimmerman E.A. Anti-Ri-associated paraneoplastic brainstem cerebellar syndrome with coexisting limbic encephalitis in a patient with mixed large cell neuroendocrine lung carcinoma. J. Clin. Neurosci. 2015;22(2):421–423. doi: 10.1016/j.jocn.2014.06.103. [http://dx.doi.org/10.1016/j.jocn.2014.06.103]. [PMID: 25443085]. [DOI] [PubMed] [Google Scholar]
- 95.Zhu Y., Chen S., Chen S., Song J., Chen F., Guo H., Shang Z., Wang Y., Zhou C., Shi B. An uncommon manifestation of paraneoplastic cerebellar degeneration in a patient with high grade urothelial, carcinoma with squamous differentiation: A case report and literature review. BMC Cancer. 2016;16:324. doi: 10.1186/s12885-016-2349-3. [http://dx. doi.org/10.1186/s12885-016-2349-3]. [PMID: 27209351]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gungor S., Kilic B., Arslan M., Ozgen U., Dalmau J. Hodgkin’s lymphoma associated with paraneoplastic cerebellar degeneration in children: a case report and review of the literature. Childs Nerv. Syst. 2017;33(3):509–512. doi: 10.1007/s00381-016-3284-y. [http://dx.doi.org/10.1007/s00381-016-3284-y]. [PMID: 27796550]. [DOI] [PubMed] [Google Scholar]
- 97.Berzero G., Karantoni E., Dehais C., Ducray F., Thomas L., Picard G. Early intravenous immunoglobulin treatment in paraneoplastic neurological syndromes with onconeural antibodies. 2017. [DOI] [PMC free article] [PubMed]
- 98.Lancaster E., Dalmau J. Neuronal autoantigens--pathogenesis, associated disorders and antibody testing. Nat. Rev. Neurol. 2012;8(7):380–390. doi: 10.1038/nrneurol.2012.99. [http://dx.doi.org/10.1038/nrneurol.2012.99]. [PMID: 22710628]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanaka K., Tanaka M., Igarashi S., Onodera O., Miyatake T., Tsuji S. Trial to establish an animal model of paraneoplastic cerebellar degeneration with anti-Yo antibody. 2. Passive transfer of murine mononuclear cells activated with recombinant Yo protein to paraneoplastic cerebellar degeneration lymphocytes in severe combined immunodeficiency mice. Clin. Neurol. Neurosurg. 1995;97(1):101–105. doi: 10.1016/0303-8467(95)00006-6. [http://dx.doi.org/10.1016/0303-8467(95)00006-6]. [PMID: 7788964]. [DOI] [PubMed] [Google Scholar]
- 100.Sillevis Smitt P.A., Manley G.T., Posner J.B. Immunization with the paraneoplastic encephalomyelitis antigen HuD does not cause neurologic disease in mice. Neurology. 1995;45(10):1873–1878. doi: 10.1212/wnl.45.10.1873. [http://dx.doi.org/10.1212/WNL.45.10.1873]. [PMID: 7477985]. [DOI] [PubMed] [Google Scholar]
- 101.Carpentier A.F., Rosenfeld M.R., Delattre J.Y., Whalen R.G., Posner J.B., Dalmau J. DNA vaccination with HuD inhibits growth of a neuroblastoma in mice. Clin. Cancer Res. 1998;4(11):2819–2824. [PMID: 9829748]. [PubMed] [Google Scholar]
- 102.Albert M.L., Austin L.M., Darnell R.B. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann. Neurol. 2000;47(1):9–17. [http://dx.doi.org/10.1002/1531-8249(200001)47:1<9:AID-ANA5 >3.0.CO;2-I]. [PMID: 10632096]. [PubMed] [Google Scholar]
- 103.Benyahia B., Liblau R., Merle-Béral H., Tourani J.M., Dalmau J., Delattre J.Y. Cell-mediated autoimmunity in paraneoplastic neurological syndromes with anti-Hu antibodies. Ann. Neurol. 1999;45(2):162–167. doi: 10.1002/1531-8249(199902)45:2<162::aid-ana5>3.0.co;2-r. [http://dx.doi.org/10.1002/1531-8249(199902) 45:2<162:AID-ANA5>3.0.CO;2-R]. [PMID: 9989617]. [DOI] [PubMed] [Google Scholar]
- 104.Rousseau A., Benyahia B., Dalmau J., Connan F., Guillet J.G., Delattre J.Y., Choppin J. T cell response to Hu-D peptides in patients with anti-Hu syndrome. J. Neurooncol. 2005;71(3):231–236. doi: 10.1007/s11060-004-1723-1. [http://dx.doi.org/10.1007/s11060-004-1723-1]. [PMID: 15735910]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Venkatraman A., Opal P. Paraneoplastic cerebellar degeneration with anti-Yo antibodies - a review. Ann. Clin. Transl. Neurol. 2016;3(8):655–663. doi: 10.1002/acn3.328. [http://dx.doi.org/10.1002/acn3.328]. [PMID: 27606347]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gebauer C., Pignolet B., Yshii L., Mauré E., Bauer J., Liblau R. CD4+ and CD8+ T cells are both needed to induce paraneoplastic neurological disease in a mouse model. OncoImmunology. 2016;6(2):e1260212. doi: 10.1080/2162402X.2016.1260212. [http://dx.doi.org/10.1080/2162402X.2016.1260212]. [PMID: 28344867]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okano H.J., Park W.Y., Corradi J.P., Darnell R.B. The cytoplasmic Purkinje onconeural antigen cdr2 down-regulates c-Myc function: implications for neuronal and tumor cell survival. Genes Dev. 1999;13(16):2087–2097. doi: 10.1101/gad.13.16.2087. [http://dx.doi.org/10.1101/gad.13. 16.2087]. [PMID: 10465786]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakai K., Kitagawa Y., Saiki S., Saiki M., Hirose G. Effect of a paraneoplastic cerebellar degeneration-associated neural protein on B-myb promoter activity. Neurobiol. Dis. 2004;15(3):529–533. doi: 10.1016/j.nbd.2003.11.003. [http://dx.doi.org/10.1016/j.nbd.2003.11.003]. [PMID: 15056460]. [DOI] [PubMed] [Google Scholar]
- 109.Fukuda T., Motomura M., Nakao Y., Shiraishi H., Yoshimura T., Iwanaga K., Tsujihata M., Eguchi K. Reduction of P/Q-type calcium channels in the postmortem cerebellum of paraneoplastic cerebellar degeneration with Lambert-Eaton myasthenic syndrome. Ann. Neurol. 2003;53(1):21–28. doi: 10.1002/ana.10392. [http://dx.doi.org/10.1002/ana.10392]. [PMID: 12509844]. [DOI] [PubMed] [Google Scholar]
- 110.Liao Y.J., Safa P., Chen Y.R., Sobel R.A., Boyden E.S., Tsien R.W. Anti-Ca2+ channel antibody attenuates Ca2+ currents and mimics cerebellar ataxia in vivo. Proc. Natl. Acad. Sci. USA. 2008;105(7):2705–2710. doi: 10.1073/pnas.0710771105. [http://dx.doi.org/10.1073/pnas.0710771105]. [PMID: 18272482]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ariño H., Gresa-Arribas N., Blanco Y., Martínez-Hernández E., Sabater L., Petit-Pedrol M., Rouco I., Bataller L., Dalmau J.O., Saiz A., Graus F. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol. 2014;71(8):1009–1016. doi: 10.1001/jamaneurol.2014.1011. [http://dx. doi.org/10.1001/jamaneurol.2014.1011]. [PMID: 24934144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ishida K., Mitoma H., Song S.Y., Uchihara T., Inaba A., Eguchi S., Kobayashi T., Mizusawa H. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann. Neurol. 1999;46(2):263–267. [http://dx. doi.org/10.1002/1531-8249(199908)46:2<263:AID-ANA19>3.0.CO;2-0]. [PMID: 10443895]. [PubMed] [Google Scholar]
- 113.Abele M., Weller M., Mescheriakov S., Bürk K., Dichgans J., Klockgether T. Cerebellar ataxia with glutamic acid decarboxylase autoantibodies. Neurology. 1999;52(4):857–859. doi: 10.1212/wnl.52.4.857. [http://dx.doi. org/10.1212/WNL.52.4.857]. [PMID: 10078741]. [DOI] [PubMed] [Google Scholar]
- 114.Kono S., Miyajima H., Sugimoto M., Suzuki Y., Takahashi Y., Hishida A. Stiff-person syndrome associated with cerebellar ataxia and high glutamic acid decarboxylase antibody titer. Intern. Med. 2001;40(9):968–971. doi: 10.2169/internalmedicine.40.968. [http://dx.doi.org/10.2169/internalmedicine. 40.968]. [PMID: 11579968]. [DOI] [PubMed] [Google Scholar]
- 115.Rüegg S., Stahl M., Bühlmann M., Dupont A., Lyrer P.A., Humbel R.L., Steck A.J. Cerebellar degeneration and polyglandular autoimmune syndrome with anti-glutamic acid decarboxylase antibodies. J. Neurol. 2002;249(3):348–350. doi: 10.1007/s004150200019. [http://dx.doi.org/10. 1007/s004150200019]. [PMID: 11993540]. [DOI] [PubMed] [Google Scholar]
- 116.Matsumoto S., Kusuhara T., Nakajima M., Ouma S., Takahashi M., Yamada T. Acute attacks and brain stem signs in a patient with glutamic acid decarboxylase autoantibodies. J. Neurol. Neurosurg. Psychiatry. 2002;73(3):345–346. doi: 10.1136/jnnp.73.3.345. [http://dx.doi.org/10.1136/ jnnp.73.3.345]. [PMID: 12185181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lauria G., Pareyson D., Pitzolu M.G., Bazzigaluppi E. Excellent response to steroid treatment in anti-GAD cerebellar ataxia. Lancet Neurol. 2003;2(10):634–635. doi: 10.1016/s1474-4422(03)00534-9. [http://dx.doi.org/10.1016/S1474-4422(03)00534-9]. [PMID: 14505586]. [DOI] [PubMed] [Google Scholar]
- 118.Birand B., Cabre P., Bonnan M., Olindo S., Smadja D. Rev. Med. Interne. 2006;27(8):616–619. doi: 10.1016/j.revmed.2006.04.006. [A new case of cerebellar ataxia with anti-GAD antibodies treated with corticosteroids and initially seronegative]. [http://dx.doi.org/10.1016/j.revmed.2006.04.006]. [PMID: 16797794]. [DOI] [PubMed] [Google Scholar]
- 119.McFarland N.R., Login I.S., Vernon S., Burns T.M. Improvement with corticosteroids and azathioprine in GAD65-associated cerebellar ataxia. Neurology. 2006;67(7):1308–1309. doi: 10.1212/01.wnl.0000238389.83574.be. [http://dx. doi.org/10.1212/01.wnl.0000238389.83574.be]. [PMID: 17030779]. [DOI] [PubMed] [Google Scholar]
- 120.Kim J.Y., Chung E.J., Kim J.H., Jung K.Y., Lee W.Y. Response to steroid treatment in anti-glutamic acid decarboxylase antibody-associated cerebellar ataxia, stiff person syndrome and polyendocrinopathy. Mov. Disord. 2006;21(12):2263–2264. doi: 10.1002/mds.21041. [http://dx.doi. org/10.1002/mds.21041]. [PMID: 17013903]. [DOI] [PubMed] [Google Scholar]
- 121.Rakocevic G., Raju R., Semino-Mora C., Dalakas M.C. Stiff person syndrome with cerebellar disease and high-titer anti-GAD antibodies. Neurology. 2006;67(6):1068–1070. doi: 10.1212/01.wnl.0000237558.83349.d0. [http://dx.doi.org/ 10.1212/01.wnl.0000237558.83349.d0]. [PMID: 17000981]. [DOI] [PubMed] [Google Scholar]
- 122.Vulliemoz S., Vanini G., Truffert A., Chizzolini C., Seeck M. Epilepsy and cerebellar ataxia associated with anti-glutamic acid decarboxylase antibodies. J. Neurol. Neurosurg. Psychiatry. 2007;78(2):187–189. doi: 10.1136/jnnp.2006.089268. [http://dx.doi.org/10.1136/jnnp.2006.089268]. [PMID: 17229747]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang C.C., Eggers S.D., Johnson J.K., Haman A., Miller B.L., Geschwind M.D. Anti-GAD antibody cerebellar ataxia mimicking Creutzfeldt-Jakob disease. Clin. Neurol. Neurosurg. 2007;109(1):54–57. doi: 10.1016/j.clineuro.2006.01.009. [http://dx.doi.org/10.1016/j.clineuro.2006.01.009]. [PMID: 16621241]. [DOI] [PubMed] [Google Scholar]
- 124.Bonnan M., Cabre P., Olindo S., Signate A., Saint-Vil M., Smadja D. Rev. Neurol. (Paris) 2008;164(5):427–433. doi: 10.1016/j.neurol.2008.02.032. [Steroid treatment in four cases of anti-GAD cerebellar ataxia]. [http://dx.doi. org/10.1016/j.neurol.2008.02.032]. [PMID: 18555874]. [DOI] [PubMed] [Google Scholar]
- 125.Georgieva Z., Parton M. Cerebellar ataxia and epilepsy with anti-GAD antibodies: treatment with IVIG and plasmapheresis. BMJ Case Rep. 2014;2014:bcr2013202314. doi: 10.1136/bcr-2013-202314. [http://dx.doi.org/10.1136/ bcr-2013-202314]. [PMID: 24419643]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Planche V., Marques A., Ulla M., Ruivard M., Durif F. Intravenous immunoglobulin and rituximab for cerebellar ataxia with glutamic acid decarboxylase autoantibodies. Cerebellum. 2014;13(3):318–322. doi: 10.1007/s12311-013-0534-3. [http://dx.doi.org/10.1007/s12311-013-0534-3]. [PMID: 24218114]. [DOI] [PubMed] [Google Scholar]
- 127.Kuchling J., Shababi-Klein J., Nümann A., Gerischer L.M., Harms L., Prüss H. GAD antibody-associated late-onset cerebellar ataxia in two female siblings. Case Rep. Neurol. 2014;6(3):264–270. doi: 10.1159/000369784. [http://dx.doi.org/10.1159/000369784]. [PMID: 25566057]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fouka P., Alexopoulos H., Akrivou S., Trohatou O., Politis P.K., Dalakas M.C. GAD65 epitope mapping and search for novel autoantibodies in GAD-associated neurological disorders. J. Neuroimmunol. 2015;281:73–77. doi: 10.1016/j.jneuroim.2015.03.009. [http://dx.doi.org/10.1016/j.jneuroim. 2015.03.009]. [PMID: 25867471]. [DOI] [PubMed] [Google Scholar]
- 129.Ishida K., Mitoma H., Wada Y., Oka T., Shibahara J., Saito Y., Murayama S., Mizusawa H. Selective loss of Purkinje cells in a patient with anti-glutamic acid decarboxylase antibody-associated cerebellar ataxia. J. Neurol. Neurosurg. Psychiatry. 2007;78(2):190–192. doi: 10.1136/jnnp.2006.091116. [http://dx.doi.org/10.1136/jnnp.2006.091116]. [PMID: 17119008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitoma H., Song S.Y., Ishida K., Yamakuni T., Kobayashi T., Mizusawa H. Presynaptic impairment of cerebellar inhibitory synapses by an autoantibody to glutamate decarboxylase. J. Neurol. Sci. 2000;175(1):40–44. doi: 10.1016/s0022-510x(00)00272-0. [http://dx.doi.org/10.1016/S0022-510X (00)00272-0]. [PMID: 10785255]. [DOI] [PubMed] [Google Scholar]
- 131.Mitoma H., Ishida K., Shizuka-Ikeda M., Mizusawa H. Dual impairment of GABAA- and GABAB-receptor-mediated synaptic responses by autoantibodies to glutamic acid decarboxylase. J. Neurol. Sci. 2003;208(1-2):51–56. doi: 10.1016/s0022-510x(02)00423-9. [http://dx.doi.org/10.1016/ S0022-510X(02)00423-9]. [PMID: 12639725]. [DOI] [PubMed] [Google Scholar]
- 132.Ishida K., Mitoma H., Mizusawa H. Reversibility of cerebellar GABAergic synapse impairment induced by anti-glutamic acid decarboxylase autoantibodies. J. Neurol. Sci. 2008;271(1-2):186–190. doi: 10.1016/j.jns.2008.04.019. [http://dx.doi.org/10.1016/j.jns.2008.04.019]. [PMID: 18534624]. [DOI] [PubMed] [Google Scholar]
- 133.Reetz A., Solimena M., Matteoli M., Folli F., Takei K., De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [http://dx.doi.org/10.1002/ j.1460-2075.1991.tb08069.x]. [PMID: 2022191]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Christgau S., Aanstoot H.J., Schierbeck H., Begley K., Tullin S., Hejnaes K., Baekkeskov S. Membrane anchoring of the autoantigen GAD65 to microvesicles in pancreatic beta-cells by palmitoylation in the NH2-terminal domain. J. Cell Biol. 1992;118(2):309–320. doi: 10.1083/jcb.118.2.309. [http://dx.doi.org/10.1083/jcb.118.2.309]. [PMID: 1321158]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ishikawa T., Tomatsu S., Tsunoda Y., Lee J., Hoffman D.S., Kakei S. Releasing dentate nucleus cells from Purkinje cell inhibition generates output from the cerebrocerebellum. PLoS One. 2014;9(10):e108774. doi: 10.1371/journal.pone.0108774. [http://dx.doi.org/10.1371/journal.pone.0108774]. [PMID: 25279763]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Manto M.U., Laute M.A., Aguera M., Rogemond V., Pandolfo M., Honnorat J. Effects of anti-glutamic acid decarboxylase antibodies associated with neurological diseases. Ann. Neurol. 2007;61(6):544–551. doi: 10.1002/ana.21123. [http://dx.doi.org/10.1002/ana.21123]. [PMID: 17600364]. [DOI] [PubMed] [Google Scholar]
- 137.Rakocevic G., Floeter M.K. Autoimmune stiff person syndrome and related myelopathies: understanding of electrophysiological and immunological processes. Muscle Nerve. 2012;45(5):623–634. doi: 10.1002/mus.23234. [http://dx.doi.org/10.1002/mus.23234]. [PMID: 22499087]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blumkin L., Pranzatelli M.R. Acquired ataxias, infectious and para-infectious. Handb. Clin. Neurol. 2012;103:137–146. doi: 10.1016/B978-0-444-51892-7.00007-3. [http://dx.doi.org/10.1016/B978-0-444-51892-7.00007-3]. [PMID: 21827885]. [DOI] [PubMed] [Google Scholar]
- 139.Sivaswamy L. Approach to acute ataxia in childhood: diagnosis and evaluation. Pediatr. Ann. 2014;43(4):153–159. doi: 10.3928/00904481-20140325-13. [http://dx.doi. org/10.3928/00904481-20140325-13]. [PMID: 24716559]. [DOI] [PubMed] [Google Scholar]
- 140.Sawaishi Y., Takada G. Acute cerebellitis. Cerebellum. 2002;1(3):223–228. doi: 10.1080/14734220260418457. [http://dx.doi.org/10.1080/14734220260418457]. [PMID: 12879984]. [DOI] [PubMed] [Google Scholar]
- 141.Klockgether T., Döller G., Wüllner U., Petersen D., Dichgans J. Cerebellar encephalitis in adults. J. Neurol. 1993;240(1):17–20. doi: 10.1007/BF00838440. [http://dx.doi.org/10.1007/BF00838440]. [PMID: 8380845]. [DOI] [PubMed] [Google Scholar]
- 142.Connolly A.M., Dodson W.E., Prensky A.L., Rust R.S. Course and outcome of acute cerebellar ataxia. Ann. Neurol. 1994;35(6):673–679. doi: 10.1002/ana.410350607. [http://dx.doi.org/10.1002/ana.410350607]. [PMID: 8210223]. [DOI] [PubMed] [Google Scholar]
- 143.Hayashi T., Ichiyama T., Kobayashi K. A case of acute cerebellar ataxia with an MRI abnormality. Brain Dev. 1994;16:488–490. doi: 10.1016/s0387-7604(89)80032-4. [PMID: 7695002]. [DOI] [PubMed] [Google Scholar]
- 144.Ito H., Sayama S., Irie S., Kanazawa N., Saito T., Kowa H., Haga S., Ikeda K. Antineuronal antibodies in acute cerebellar ataxia following Epstein-Barr virus infection. Neurology. 1994;44(8):1506–1507. doi: 10.1212/wnl.44.8.1506. [http://dx.doi.org/10.1212/WNL.44.8.1506]. [PMID: 8058157]. [DOI] [PubMed] [Google Scholar]
- 145.Armangué T., Sabater L., Torres-Vega E., Martínez-Hernández E., Ariño H., Petit-Pedrol M., Planagumà J., Bataller L., Dalmau J., Graus F. Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies. JAMA Neurol. 2016;73(4):417–424. doi: 10.1001/jamaneurol.2015.4607. [http://dx.doi.org/ 10.1001/jamaneurol.2015.4607]. [PMID: 26856612]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Klaas J.P., Ahlskog J.E., Pittock S.J., Matsumoto J.Y., Aksamit A.J., Bartleson J.D., Kumar R., McEvoy K.F., McKeon A. Adult-onset opsoclonus-myoclonus syndrome. Arch. Neurol. 2012;69(12):1598–1607. doi: 10.1001/archneurol.2012.1173. [http://dx.doi.org/10.1001/archneurol.2012.1173]. [PMID: 22986354]. [DOI] [PubMed] [Google Scholar]
- 147.Bataller L., Graus F., Saiz A., Vilchez J.J. Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus-myoclonus. Brain. 2001;124(Pt 2):437–443. doi: 10.1093/brain/124.2.437. [http://dx.doi.org/10.1093/brain/ 124.2.437]. [PMID: 11157570]. [DOI] [PubMed] [Google Scholar]
- 148.Pranzatelli M.R., Tate E.D., Swan J.A., Travelstead A.L., Colliver J.A., Verhulst S.J., Crosley C.J., Graf W.D., Joseph S.A., Kelfer H.M., Raju G.P. B cell depletion therapy for new-onset opsoclonus-myoclonus. Mov. Disord. 2010;25(2):238–242. doi: 10.1002/mds.22941. [http://dx.doi.org/10.1002/mds.22941]. [PMID: 20063398]. [DOI] [PubMed] [Google Scholar]
- 149.Pranzatelli M.R., Travelstead A.L., Tate E.D., Allison T.J., Moticka E.J., Franz D.N., Nigro M.A., Parke J.T., Stumpf D.A., Verhulst S.J. B- and T-cell markers in opsoclonus-myoclonus syndrome: immunophenotyping of CSF lymphocytes. Neurology. 2004;62(9):1526–1532. doi: 10.1212/wnl.62.9.1526. [http://dx.doi.org/10.1212/WNL.62.9. 1526]. [PMID: 15136676]. [DOI] [PubMed] [Google Scholar]
- 150.Young C.A., MacKenzie J.M., Chadwick D.W., Williams I.R. Opsoclonus-myoclonus syndrome: an autopsy study of three cases. Eur. J. Med. 1993;2(4):239–241. [PMID: 8261078]. [PubMed] [Google Scholar]
- 151.Mitoma H., Orimo S., Sodeyama N., Tamaki M. Paraneoplastic opsoclonus-myoclonus syndrome and neurofibrosarcoma. Eur. Neurol. 1996;36(5):322. doi: 10.1159/000117282. [PMID: 8864716]. [DOI] [PubMed] [Google Scholar]
- 152.Wong A.M.F., Musallam S., Tomlinson R.D., Shannon P., Sharpe J.A. Opsoclonus in three dimensions: oculographic, neuropathologic and modelling correlates. J. Neurol. Sci. 2001;189(1-2):71–81. doi: 10.1016/s0022-510x(01)00564-0. [http://dx.doi.org/10.1016/S0022-510X(01)00564-0]. [PMID: 11535236]. [DOI] [PubMed] [Google Scholar]
- 153.Chekroud A.M., Anand G., Yong J., Pike M., Bridge H. Altered functional brain connectivity in children and young people with opsoclonus-myoclonus syndrome. Dev. Med. Child Neurol. 2017;59(1):98–104. doi: 10.1111/dmcn.13262. [http://dx.doi.org/10.1111/dmcn.13262]. [PMID: 27658927]. [DOI] [PubMed] [Google Scholar]
- 154.Ugawa Y., Uesaka Y., Terao Y., Hanajima R., Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann. Neurol. 1995;37(6):703–713. doi: 10.1002/ana.410370603. [http://dx.doi.org/10.1002/ana.410370603]. [PMID: 7778843]. [DOI] [PubMed] [Google Scholar]
- 155.Ferrucci R., Bocci T., Cortese F., Ruggiero F., Priori A. Cerebellar transcranial direct current stimulation in neurological disease. Cerebellum Ataxias. 2016;3(1):16. doi: 10.1186/s40673-016-0054-2. [http://dx.doi.org/10. 1186/s40673-016-0054-2]. [PMID: 27595007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ito M. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 2006;78(3-5):272–303. doi: 10.1016/j.pneurobio.2006.02.006. [http://dx.doi.org/10.1016/j.pneurobio. 2006.02.006]. [PMID: 16759785]. [DOI] [PubMed] [Google Scholar]
- 157.Voogd J., Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1988;2:305–371. [PMID: 9735944]. [Google Scholar]
- 158.D’Angelo E., Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front. Neural Circuits. 2013;6:116. doi: 10.3389/fncir.2012.00116. [http://dx.doi.org/10.3389/ fncir.2012.00116]. [PMID: 23335884]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Manto M., Bower J.M., Conforto A.B., Delgado-García J.M., da Guarda S.N., Gerwig M., Habas C., Hagura N., Ivry R.B., Mariën P., Molinari M., Naito E., Nowak D.A., Oulad Ben Taib N., Pelisson D., Tesche C.D., Tilikete C., Timmann D. Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–487. doi: 10.1007/s12311-011-0331-9. [http://dx.doi.org/10.1007/s12311-011-0331-9]. [PMID: 22161499]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kawato M., Furukawa K., Suzuki R. A hierarchical neural-network model for control and learning of voluntary movement. Biol. Cybern. 1987;57(3):169–185. doi: 10.1007/BF00364149. [http://dx.doi.org/10.1007/ BF00364149]. [PMID: 3676355]. [DOI] [PubMed] [Google Scholar]
- 161.Schonewille M., Gao Z., Boele H.J., Veloz M.F., Amerika W.E., Simek A.A., De Jeu M.T., Steinberg J.P., Takamiya K., Hoebeek F.E., Linden D.J., Huganir R.L., De Zeeuw C.I. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70(1):43–50. doi: 10.1016/j.neuron.2011.02.044. [http://dx.doi.org/10.1016/j.neuron.2011.02.044]. [PMID: 21482355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu H.S., Sugihara I., Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J. Comp. Neurol. 1999;411(1):97–118. doi: 10.1002/(sici)1096-9861(19990816)411:1<97::aid-cne8>3.0.co;2-o. [http://dx.doi.org/10.1002/(SICI)1096-9861(19990816)411:1 <97:AID-CNE8>3.0.CO;2-O]. [PMID: 10404110]. [DOI] [PubMed] [Google Scholar]
- 163.Ekerot C.F., Jörntell H. Parallel fibre receptive fields of Purkinje cells and interneurons are climbing fibre-specific. Eur. J. Neurosci. 2001;13(7):1303–1310. doi: 10.1046/j.0953-816x.2001.01499.x. [http://dx.doi.org/10.1046/j.0953-816x.2001.01499.x]. [PMID: 11298790]. [DOI] [PubMed] [Google Scholar]
- 164.Colin F., Ris L., Godaux E. In: Neuroanatomy of the cerebellum. The cerebellum and its disorders; Manto, M. Pandolfo M., editor. Cambridge: Cambridge University Press; 2002. pp. 6–29. [Google Scholar]
- 165.Suzumura A. Neuron-microglia interaction in neuroinflammation. Curr. Protein Pept. Sci. 2013;14(1):16–20. doi: 10.2174/1389203711314010004. [http://dx.doi.org/10. 2174/1389203711314010004]. [PMID: 23544747]. [DOI] [PubMed] [Google Scholar]
- 166.Domercq M., Sánchez-Gómez M.V., Sherwin C., Etxebarria E., Fern R., Matute C. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J. Immunol. 2007;178(10):6549–6556. doi: 10.4049/jimmunol.178.10.6549. [http://dx.doi.org/10.4049/jimmunol. 178.10.6549]. [PMID: 17475885]. [DOI] [PubMed] [Google Scholar]
- 167.Mandolesi G., Musella A., Gentile A., Grasselli G., Haji N., Sepman H., Fresegna D., Bullitta S., De Vito F., Musumeci G., Di Sanza C., Strata P., Centonze D. Interleukin-1β alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J. Neurosci. 2013;33(29):12105–12121. doi: 10.1523/JNEUROSCI.5369-12.2013. [http:// dx.doi.org/10.1523/JNEUROSCI.5369-12.2013]. [PMID: 23864696]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Olmos G., Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014;2014:861231. doi: 10.1155/2014/861231. [http://dx.doi.org/10.1155/2014/861231]. [PMID: 24966471]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yuste J.E., Tarragon E., Campuzano C.M., Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015;9:322. doi: 10.3389/fncel.2015.00322. [http://dx.doi.org/10.3389/fncel. 2015.00322]. [PMID: 26347610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fujikawa D.G. The role of excitotoxic programmed necrosis in acute brain injury. Comput. Struct. Biotechnol. J. 2015;13:212–221. doi: 10.1016/j.csbj.2015.03.004. [http://dx.doi.org/10.1016/j.csbj.2015.03.004]. [PMID: 25893083]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chopra R., Shakkottai V.G. The role for alterations in neuronal activity in the pathogenesis of polyglutamine repeat disorders. Neurotherapeutics. 2014;11(4):751–763. doi: 10.1007/s13311-014-0289-7. [http://dx.doi.org/10.1007/ s13311-014-0289-7]. [PMID: 24986674]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Watson L.M., Wong M.M., Becker E.B. Induced pluripotent stem cell technology for modelling and therapy of cerebellar ataxia. Open Biol. 2015;5(7):150056. doi: 10.1098/rsob.150056. [http://dx.doi.org/10.1098/rsob. 150056]. [PMID: 26136256]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mitoma H., Manto M., Hampe C.S. Time is cerebellum. Cerebellum. 2018;17(4):387–391. doi: 10.1007/s12311-018-0925-6. [http://dx.doi.org/10.1007/s12311-018-0925-6]. [PMID: 29460203]. [DOI] [PMC free article] [PubMed] [Google Scholar]