Abstract
A primigravida was referred with hyperthyroidism in early pregnancy from longstanding Graves’ disease treated with propylthiouracil. She had selective elevation of free tri-iodothyronine (fT3) levels, low normal free tetra-iodothyronine (fT4) and suppressed thyroid-stimulating hormone (TSH). Given her symptoms of thyrotoxicosis and elevated TSH receptor antibodies, therapy was tailored towards maintaining clinical and biochemical euthyroidism. However the fetus developed a goitre secondary to hypothyroidism. This case highlights the dilemmas in managing maternal T3 toxicosis while aiming for a high normal fT4 to prevent fetal hypothyroidism including the role of fetal ultrasound monitoring and amniocentesis.
Keywords: Grave's disease, pregnancy, fetal goitre, fetal hypothyroidism, maternal T3 toxicosis, maternal thyrotoxicosis
Introduction
Fetal and neonatal thyroid disturbances are well described in association with maternal Graves’ disease.1 The most common disturbances are neonatal thyrotoxicosis induced by thyrotropin receptor-stimulating antibodies and hypothyroidism from maternal antithyroid drugs or thyrotropin receptor inhibiting antibodies (TRiAbs) which bind to the thyroid-stimulating hormone (TSH) receptor and prevent TSH action.1,2 We describe an unusual case of maternal tri-iodothyronine (T3) toxicosis with neonatal hypothyroidism.
CASE
A 32-year-old primigravida was referred to our centre at seven weeks gestation, with a 15-year history of Graves’ disease for which she had refused definitive treatment. At presentation she was clinically hyperthyroid with tachycardia, tremors and a prominent goitre with an audible bruit, but no ophthalmopathy. She was taking 50 mg propylthiouracil (PTU) thrice daily. The thyroid function tests (TFTs) suggested T3 toxicosis with free T4 (fT4) of 7 nmol/L, free T3 (fT3) of 11.8 pmol/L and TSH < 0.01 mU/L (Table 1). She had an admission for threatened miscarriage at nine weeks gestation. Maternal TSH receptor antibodies (TRAbs) were positive at 14 IU/L. At 27 weeks, the TFT results improved (Table 1) and the PTU dose was reduced to 50 mg twice daily.
Table 1.
Maternal TFT trends through pregnancy with the increased T3 and low normal T4
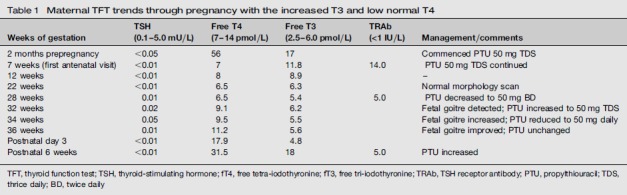
| Weeks of gestation | TSH (0.1-5.0 mU/L) | Free T4 (7-14 pmol/L) | Free T3 (2.5-6.0 pmol/L) | TRAb (<1 IU/L) | Management/comments |
|---|---|---|---|---|---|
| 2 months prepregnancy | <0.05 | 56 | 17 | Commenced PTU 50 mg TDS | |
| 7 weeks (first antenatal visit) | <0.01 | 7 | 11.8 | 14.0 | PTU 50 mg TDS continued |
| 12 weeks | <0.01 | 8 | 8.9 | - | |
| 22 weeks | <0.01 | 6.5 | 6.3 | Normal morphology scan | |
| 28 weeks | 0.01 | 6.5 | 5.4 | 5.0 | PTU decreased to 50 mg BD |
| 32 weeks | 0.02 | 9.1 | 6.2 | Fetal goitre detected; PTU increased to 50 mg TDS | |
| 34 weeks | 0.05 | 9.5 | 5.5 | Fetal goitre increased; PTU reduced to 50 mg daily | |
| 36 weeks | 0.01 | 11.2 | 5.6 | Fetal goitre improved; PTU unchanged | |
| Postnatal day 3 | <0.01 | 17.9 | 4.8 | ||
| Postnatal 6 weeks | <0.01 | 31.5 | 18 | 5.0 | PTU increased |
TFT, thyroid function test; TSH, thyroid-stimulating hormone; fT4, free tetra-iodothyronine; fT3, free tri-iodothyronine; TRAb, TSH receptor antibody; PTU, propylthiouracil; TDS, thrice daily; BD, twice daily
However, an obstetric ultrasound at 32 weeks demonstrated a normally grown fetus with a goitre (Figure 1) with a thyroid circumference (TC) of 82 mm (>95th centile). There were no other ultrasound features to suggest fetal hypothyroidism or hyperthyroidism. Maternal hyperthyroidism had recurred with symptoms and marginal increase in T3 (Table 1) at this time and hence the PTU was increased to 50 mg thrice daily. However the progress scan at 34 weeks revealed an increase in size of the fetal thyroid (TC 92 mm) and absence of distal femoral ossification now suggesting fetal hypothyroidism. Invasive testing was recommended and since the mother refused cord blood sampling, amniocentesis was performed. The amniotic fluid TSH level was 1.93 (0.1-0.5) mU/L and fT4 was 10 (5.1-9.0) pmol/L. These results were consistent with fetal hypothyroidism and hence PTU was decreased to 50 mg daily. A 36 weeks maternal scan revealed a decrease in goitre size (TC 72 mm) amniotic fluid TSH level had improved (TSH 0.19).
Figure 1.
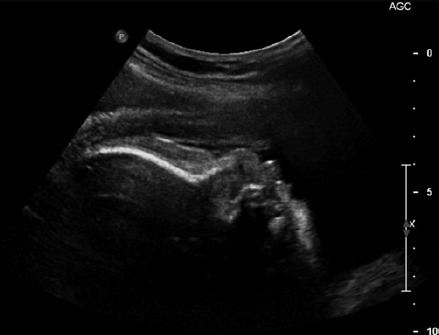
Ultrasound image showing the fetal goitre with extended neck
Fetal distress with bradycardia was identified at a routine 37 weeks follow-up scan, resulting in an emergency caesarean section with uncomplicated maternal recovery. The neonate was delivered with a birth weight of 3.3 kg and APGAR scores of 5 and 9 at one and five minutes, respectively. A metabolic acidaemia was reported on the cord gases and the baby was admitted to the neonatal intensive care unit. No goitre was noted on clinical examination. At four hours of age, the neonatal TSH was 182 with fT4 of 13.7 (12-36) pmol/L. Thyroxine replacement was commenced at 10 μg/kg and neonatal TFT improved back into normal range after one week of therapy. Thyroxine was discontinued at three months with normal development milestones over the next 12 months. Maternal hyperthyroidism recurred at the six week follow-up visit requiring increased PTU doses. This was subsequently changed to carbimazole at a current dose of 10 mg daily.
Discussion
Graves’ disease occurs in approximately 0.1-0.4% of pregnancies and the current guidelines recommend treating maternal hyperthyroidism to keep the fT4 in the upper third of the reference range.1 It is also known that fetal thyroid function correlates with maternal fT4 rather than the dose of antithyroid drugs (ATDs).3 However, to the best of our knowledge, there are no guidelines or literature on managing T3 toxicosis in pregnancy.
Our patient had clinical thyrotoxicosis with elevated free T3, significantly raised TRAbs and a threatened miscarriage in early pregnancy, despite a low normal fT4. The TFTs improved in late second trimester as is usually the case permitting a dose reduction of PTU. Recurrence of maternal hyperthyroid symptoms and detection of the fetal goitre at 32 weeks were suspicious for fetal thyrotoxicosis. Moreover, maternal TRAbs of greater than 5 IU/L seem to have a 100% sensitivity and 76% specificity for neonatal thyrotoxicosis independent of maternal thyroid function.4
Ultrasound scores based on fetal heart rate, movements, bone maturation and Doppler vascularization of the fetal goitre may help to differentiate fetal hypothyroidism from thyrotoxicosis and there have been attempts to standardize these findings.5,6 These features were unfortunately not evident on our initial scan at 32 weeks, confusing the decision further. However, later detection of delayed ossification of the distal femoral epiphysis coupled with increase in goitre size after increased PTU suggested fetal hypothyroidism.
Although fetal blood sampling is the gold standard to assess fetal thyroid status, amniotic fluid sampling has been shown to be useful for detecting fetal hypothyroidism.7 Reducing the dose of ATDs is the initial step in treatment and if this does not suffice then intra-amniotic thyroxine can be administered with benefit as evidenced by case reports in literature.8,9
Despite the improvement in the goitre and amniotic fluid TSH, the baby experienced severe bradycardia warranting emergency delivery. There was no evidence of abruption or other pathology of the maternal-fetal placental unit to explain this event. The elevated TSH at birth is difficult to interpret in the context of fetal distress with bradycardia and therefore an exaggerated TSH surge in the first 24-48 hours after birth.10 Nevertheless, treatment with thyroxine was prudent to avoid the consequences of neonatal hypothyroidism. It is also encouraging to note that the baby has developed with normal milestones and only required three months of thyroxine replacement suggesting the hypothyroidism was transient.
The risks for fetal hypothyroidism in our case were the low normal maternal fT4, but more so the use of antithyroid drugs. It is known that the affinity of the thyroid hormone transporters expressed in the placenta is similar for free T4 and free T3, except for MCT 10 and LAT1, which have higher affinity for T3. However the deiodinase enzyme D3 which inactivates T4 and T3 to rT3/T2 by inner ring deiodination, has much greater expression and activity in the placenta and D3 has a higher affinity for free T3. Thus T3 may preferentially be prevented from reaching the fetus as a protective measure in normal physiology.11 Therefore, the elevated fT3 will not compensate for the low fT4.
Given the maternal thyrotoxicosis, TRiAb was less likely to be responsible. It appears that aiming for high normal fT4 levels despite elevated fT3 may have fetal benefits in reducing the risk of hypothyroidism, though this needs to be weighed against the risk of continuation of the pregnancy as in our case. The recent American thyroid association (ATA) guidelines recommend against routinely measuring T3 during pregnancy and to avoid aiming for normal T3 levels given the associated risk of elevated fetal TSH at birth, but T3 toxicosis is mentioned as an exception to this suggestion.12 Close fetal ultrasound monitoring with amniocentesis or fetal blood sampling to guide therapy is probably necessary in situations with discordant thyroid function tests.
Declarations
Competing interests: None declared.
Funding: Not applicable.
Ethical approval: Written patient consent was obtained.
Guarantor: VS.
Contributorship: VS, HDM, JN and SGP were all involved with clinical management and literature research for the case. VS and HDM wrote up the initial draft and all authors have reviewed and edited the manuscript.
Acknowledgements: Maternal Fetal Medicine, Mater Health Services.
REFERENCES
- 1.Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2007; 92: S1–47 [DOI] [PubMed] [Google Scholar]
- 2.Mandel SJ, Cooper DS. The use of antithyroid drugs in pregnancy and lactation. J Clin Endocrinol Metab 2001; 86: 2354–59 [DOI] [PubMed] [Google Scholar]
- 3.Momotani N, Noh J, Oyanagi H, Ishikawa N, Ito K. Antithyroid drug therapy for Graves’ disease during pregnancy. Optimal regimen for fetal thyroid status. N Engl J Med 1986; 315: 24–8 [DOI] [PubMed] [Google Scholar]
- 4.Peleg D, Cada S, Peleg A, Ben-Ami M. The relationship between maternal serum thyroid-stimulating immunoglobulin and fetal and neonatal thyrotoxicosis. Obstet Gynecol 2002; 99: 1040–3 [DOI] [PubMed] [Google Scholar]
- 5.Huel C, Guibourdenche J, Vuillard E, et al. Use of ultrasound to distinguish between fetal hyperthyroidism and hypothyroidism on discovery of a goiter. Ultrasound Obstet Gynecol 2009; 33: 412–20 [DOI] [PubMed] [Google Scholar]
- 6.Luton D, Le Gac I, Vuillard E, et al. Management of Graves’ disease during pregnancy: the key role of fetal thyroid gland monitoring. J Clin Endocrinol Metab 2005; 90: 6093–8 [DOI] [PubMed] [Google Scholar]
- 7.Singh PK, Parvin CA, Gronowski AM. Establishment of reference intervals for markers of fetal thyroid status in amniotic fluid. J Clin Endocrinol Metab 2003; 88: 4175–9 [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto H, Hashimoto K, Suehara N. Successful in utero treatment of fetal goitrous hypothyroidism: case report and review of the literature. Fetal Diagn Ther 2006; 21: 360–5 [DOI] [PubMed] [Google Scholar]
- 9.Van Loon AJ, Derksen JT, Bos AF, Rouwe CW. In utero diagnosis and treatment of fetal goitrous hypothyroidism, caused by maternal use of propylthiouracil. Prenat Diagn 1995; 15: 599–604 [DOI] [PubMed] [Google Scholar]
- 10.Buyukgebiz A. Newborn screening for congenital hypothyroidism. J Pediatr Endocrinol Metab 2006; 19: 1291–8 [DOI] [PubMed] [Google Scholar]
- 11.Patel J, Landers K, Li H, Mortimer RH, Richard K. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab 2011; 22: 164–70 [DOI] [PubMed] [Google Scholar]
- 12.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21: 1081–125 [DOI] [PMC free article] [PubMed] [Google Scholar]


