Abstract
This review presents an update of the role of the renin-angiotensin system in normal pregnancy and pre-eclampsia. We have known for years that the circulatory renin-angiotensin system in pre-eclampsia is suppressed. We now know that the circulating renin-angiotensin system does not only have a vasoconstrictor arm, but also a vasodilator arm, which is upregulated in normal pregnancy; this balance is probably disturbed in pre-eclampsia. Recent studies show the importance of the local renin-angiotensin system in the uteroplacental unit for early placentation and regulation of placental blood flow. We discuss the possible role of autoantibodies against the AT1-receptor in pre-eclampsia and the suggestion that activation of the AT1-receptor in the placenta may lead to placental dysfunction and the clinical syndrome of pre-eclampsia.
Keywords: hypertension, physiology, pre-eclampsia, renin, angiotensin, placental insufficiency
Introduction
The renin-angiotensin system primarily regulates blood pressure, fluid and sodium homeostasis. As pregnancy is a state of marked vasodilation and increased plasma volume, it is evident that the renin-angiotensin system plays an important role in the establishment of these adaptations. More than 50 years ago it was noticed that the renin-angiotensin system is upregulated in pregnancy.1,2 It has been postulated that in preeclampsia this system must be further activated, as observations outside pregnancy showed that such activation can lead to hypertension. However, reduced plasma renin levels have been found consistently in pre-eclampsia.3 This finding has been poorly explained for years, despite extensive research and the realization that understanding this mechanism may give clues to the pathophysiology of pre-eclampsia.
Recently, unraveling of the s-Flt1 pathway, an anti-angiogenic factor leading to endothelial dysfunction, has given new directives for research into the renin-angiotensin system in pre-eclampsia. We now know that stimulation of the angiotensin-1 (AT1) receptor in the placenta may cause the release of s-Flt1 and other pathogenic substances. Moreover, circulating antibodies that stimulate the AT1-receptor have been discovered in pre-eclampsia. The additional new recognition that prorenin may have physiological effects raises new possibilities for a local role of this system in pre-eclampsia. The controversy of a ‘suppressed’ circulatory renin-angiotensin system in pre-eclampsia now seems to fit with a new model of the renin-angiotensin system in pregnancy.
Importance of The Renin-Angiotensin System In Pregnancy
The renin-angiotensin system plays an important role in the maternal haemodynamic adaptations to normal pregnancy. Pregnancy is characterized by an increase in plasma volume and marked vasodilation. The renin-angiotensin system is highly activated in order to increase sodium retention and hence plasma volume and thereby maintain blood pressure in the setting of vasodilation. We will first focus on the role of the traditional renin-angiotensin system in pregnancy, including angiotensin II and aldosterone. Subsequently, we will discuss new peptides of the renin-angiotensin system, such as angiotensin 1-7, that counterbalance the actions of the traditional system. After the role of the circulating renin-angiotensin system in the maternal haemodynamic adaptations to pregnancy, we will focus on the role of the local renin-angiotensin system in the development and function of the placenta. A timeline of the most important findings over the years is given in Figure 1.
Figure 1.
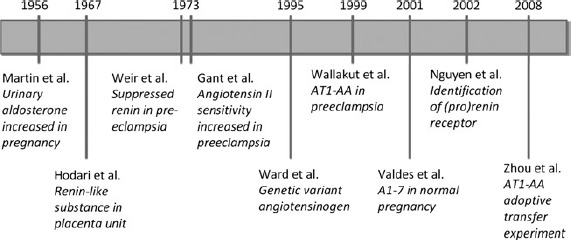
Timeline of important findings concerning the renin-angiotensin system and pre-eclampsia
The traditional renin-angiotensin system
In pregnancy, all factors of the renin-angiotensin system are upregulated, including angiotensinogen, renin, angiotensin I, angiotensin II and aldosterone.4 An overview of the components of the renin-angiotensin system is presented in Figure 2. Oestrogens stimulate the production of angiotensinogen by the liver. The kidneys, ovaries and placenta contribute to the production of circulating prorenin, while active renin is mainly produced by the kidneys.5,6 The levels of circulating prorenin are much higher than levels of active renin in pregnancy. Traditionally, prorenin has been considered as an ‘inert’ enzyme that only has a role once converted to active renin but it is now known that there are independent actions of prorenin through stimulation of its own (pro)renin receptor.7,8 The exact role of prorenin in pregnancy still has to be established, but it seems that binding to the (pro)renin receptor activates intracellular signalling pathways directly and may contribute to angiotensin II formation independent of the circulating active renin concentration.9-12
Figure 2.
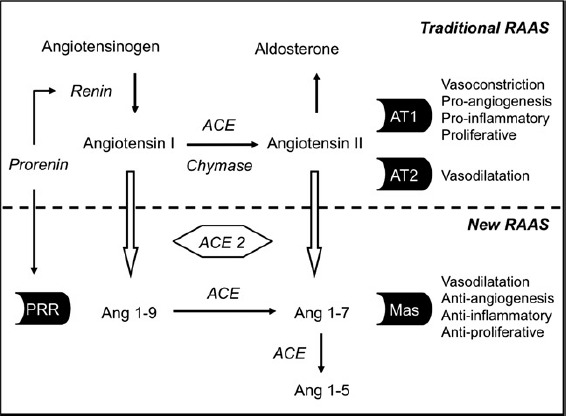
Overview of the traditional and new renin-angiotensin systems (RAAS)
Angiotensin II is a potent vasoconstrictor; however, in pregnancy marked vasodilation is observed despite high angiotensin II levels. Abdul-Karim and Assalin13 were among the first to notice that pregnancy is a state of relative vascular insensitivity to angiotensin II. Pregnant women require about double the dose of angiotensin II infusion to reach an equal blood pressure increase.14 This effect is apparent even within the first 12 weeks of pregnancy and there is also some refractoriness to the sodium and urate retaining effects of angiotensin II at this early stage.15 Recent research shows that the AT1-receptor is usually present in a monomeric form and is partly inactivated in normal pregnancy by, for example, reactive-oxygen species (ROS).16 Angiotensin II is not only a vasoactive substance, but also has angiogenic, inflammatory and proliferative functions. Angiotensin II has a high affinity to bind the AT1-receptor; however, it can also bind to the AT2-receptor which has counter regulatory actions.
High circulating aldosterone leads to sodium retention in the cortical collecting duct in the kidneys and subsequently an increase in plasma volume.17 Although the vascular responsiveness to angiotensin II is reduced in pregnancy, the adrenal responsiveness is not. On the contrary, there is a dissociation of the renin and aldosterone levels, in such a way that aldosterone levels are higher than expected for the renin levels compared with that in non-pregnant women.18,19 This implies that either the adrenal sensitivity to angiotensin II is increased or other factors contribute to the release of aldosterone by the adrenal glands. Earlier studies showed that non-angiotensin factors such as adrenocorticotropic hormone do contribute to the release of aldosterone in pregnancy.20 Despite the upregulation of the renin-angiotensin system in pregnancy, the system seems to ‘reset’ and still responds adequately to high- or low-salt diets, saline loading or upright posture.3,21,22
The ‘new’ renin-angiotensin system
Although we have always thought of the renin-angiotensin system as ‘defending’ the vasodilatory state of normal pregnancy, it is now possible that some newly discovered factors of the renin-angiotensin system may contribute to this vasodilation directly. Angiotensin II, with its main actions through the AT1-receptor, was considered the most important biologically active end-product of the renin-angiotensin system. However, further cleavage of this octapeptide is possible, leading to formation of a heptapeptide called angiotensin 1- 7. Angiotensin 1-7 has its own Mas-receptor that exerts actions counterbalancing the traditional renin-angiotensin system.23,24 The main effects are vasodilation, antiangiogenesis, anti-inflammation and anti-proliferation. Besides this, angiotensin 1-7 may contribute to the regulation of plasma volume as it possibly serves as an aquaretic, increasing water diuresis.25
Recent studies have shown that in pregnancy not only the traditional vasoconstrictor arm, but also the vasodilator arm of the renin-angiotensin system is upregulated. High angiotensin 1-7 levels are found in serum and urine of pregnant compared with non-pregnant women.26,27 This led to postulate that the balance between these two arms of the renin-angiotensin system is important and, in normal pregnancy, favours vasodilation. Blockade of the vasoconstrictor pathway by an angiotensin-converting enzyme (ACE)-inhibitor in pregnancy leads to a drop in blood pressure that is larger than in non-pregnant women,28 implying a fair degree of dependence on the vasoconstrictor arm of the renin-angiotensin system in normal pregnancy to ‘defend’ against the actions of the vasodilator arm.
In summary, both arms of the renin-angiotensin system contribute to the maintenance of blood pressure in pregnancy and the increase in plasma volume. An overview of the effects of the circulating renin-angiotensin system in pregnancy is presented in Figure 3a.
Figure 3.
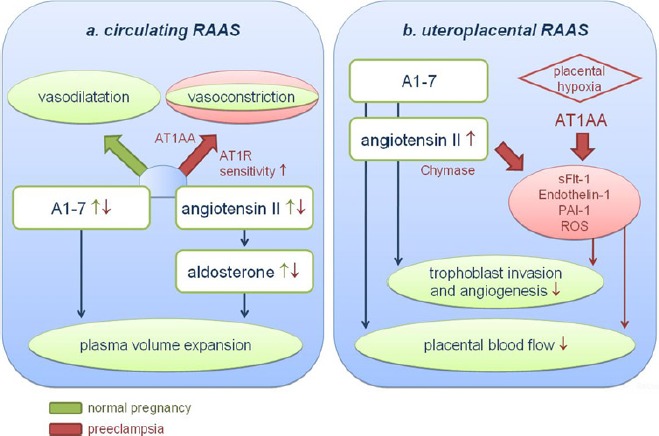
Main actions of the ‘new’ renin-angiotensin system (RAAS) during pregnancy and preeclampsia in (a) the circulating system and (b) the uteroplacental unit. (a) In normal pregnancy the renin-angiotensin system is up regulated and contributes to the establishment of plasma volume expansion. The balance between circulating A1-7 and angiotensin II favours vasodilation. In pre-eclampsia the renin-angiotensin system is down regulated. Relatively low A1-7 levels in the presence of AT1-AA and increased AT1-receptor sensitivity may result in a shift favouring vasoconstriction.(b) In normal pregnancy both A1-7 and angiotensin II contribute to the regulation of trophoblast invasion and angiogenesis. In pre-eclampsia, over activation of the AT1-receptor in the uteroplacental unit by AT1-AA or angiotensin II may cause the release of pathogenic substances, leading to impaired placentation and reduced placental blood flow
The local uteroplacental renin-angiotensin system
Besides the circulating renin-angiotensin system, there is a complete local renin-angiotensin system in the uteroplacental unit.29,30 A local renin-angiotensin system can be found in many organs, such as the heart, brain and adipose tissue; we have only begun to discover its role in many regulatory processes such as angiogenesis, inflammation and proliferation.
In the placenta, the renin-angiotensin system is found in cytotrophoblast, decidua and endothelium.31,32 The renin- angiotensin system has both secretory and vasoactive actions in the placenta. A lot of work to explore the role of the uteroplacental renin-angiotensin system in pregnancy is either done in animal models or in vitro using placental material, limiting immediate implications for human pregnancy. Nevertheless, these studies showed that stimulation of the AT1-receptor on trophoblast cells can induce the release of many regulatory factors, such as estradiol, endothelin-1, placental growth factor and ROS.33-36 AT1-receptors are highly abundant in vessels on the fetal side of the placenta and both angiotensin II and angiotensin 1-7 may directly influence placental blood flow.37-39 On the maternal side, the invasion of trophoblast cells into spiral arteries is induced at least in part by activation of AT1-receptors.40 Activation of mineralocorticoid receptors by maternal aldosterone also appears to be required for trophoblast growth and normal placental function.41 It therefore seems that the uteroplacental renin-angiotensin system plays an important role in trophoblast invasion, angiogenesis and placental blood flow (Figure 3b).
The Renin-Angiotensin System In Pre-Eclampsia
Pre-eclampsia is characterized by vasoconstriction and reduced plasma volume compared with normal pregnancy.42,43 Contrary to what would be expected in a state of primary volume reduction, the circulating renin-angiotensin system is suppressed in pre-eclampsia almost down to levels seen in non-pregnant women.44 Plasma active renin, angiotensin II and aldosterone concentrations are all lower in pre-eclampsia.45 However, total renin concentration (active renin and prorenin together) is similar compared with normal pregnancy, meaning that there is a relatively high prorenin in preeclampsia.46 This was always thought to mean that part of the pathophysiology of pre-eclampsia was the inability to convert prorenin to active renin but now that we know that prorenin has biological actions on its own, this opens up the possibility that there is a direct role for prorenin in pre-eclampsia in stimulating local angiotensin production.
Studies suggest that both urinary and plasma angiotensin 1- 7 levels are reduced in pre-eclampsia compared with normal pregnancy and that the balance between angiotensin II and angiotensin 1-7 may be disturbed.26,27 This would favour the vasoconstrictor arm of the renin-angiotensin system, perhaps leading to hypertension despite ‘low’ circulating levels of angiotensin II and aldosterone.
Moreover, in pre-eclampsia the vascular responsiveness to angiotensin II is increased.14 Many explanations have been proposed for this observation, including impaired vasodilation as a consequence of reduced prostacyclin production or enhanced vasoconstriction due to endothelin or thromboxane A2.47-49 Although these explanations remain plausible, it has been recently found that the monomeric AT1-receptor that is usually present in normal pregnancy is present in an heteromeric form in pre-eclampsia; this receptor appears insensitive to inactivation and highly sensitive to binding by angiotensin II.16
Thus, as depicted in Figure 3a, the relatively low angiotensin 1-7 levels in combination with the increased sensitivity to angiotensin II and altered AT-1 receptor conformation means that the renin-angiotensin system favours vasoconstriction in pre-eclampsia.
A few longitudinal studies have observed that the circulating renin-angiotensin system is suppressed mainly during the clinical phase of pre-eclampsia but not in the preclinical phase.42,50,51 This suggests that the suppressed circulating renin may be secondary somehow to the clinical syndrome of pre-eclampsia. This is the opposite to that expected in the face of volume contraction, but it is possible that there is enhanced intra-renal renin sensitivity to feedback suppression by angiotensin II or that impaired renal prostacyclin production contributes to impaired renin stimulation in pre-eclampsia;52 this remains conjecture for now. The regulation of renin release by physiological stimulation or suppression (such as salt loading, head-up tilt test and ambulation) is still intact in pre-eclampsia, meaning that renin release has been ‘reset’ around this new low level.3,22
Another possibility is that the low level of angiotensin peptides or aldosterone in pre-eclampsia is caused by a deficiency in enzymatic pathways. Reduced activity of the 18-methyloxidase component of aldosterone synthase has recently been described in pre-eclampsia.53
Despite lower active renin and aldosterone levels in preeclampsia, the dissociation between plasma renin activity and aldosterone levels remains and may be even larger than in normal pregnancy.19,54 This relatively enhanced sensitivity of the adrenal cortex did not seem to be explained by non-angiotensin factors.20,55,56 There must be another factor involved in this regulatory process, perhaps a placental-derived substance which is capable of inhibiting renal active renin release while increasing the vascular and adrenal sensitivity to angiotensin.4 It is also possible that the newly discovered autoantibodies to the AT1-receptor fulfil this role. Interestingly, a recent study observed that the aldosterone-to-renin ratio correlated with an increase in vascular resistance in the uterine artery.57
Autoantibodies to the AT1-receptor
Wallukat et al.58 were among the first in the late 1990s to notice the presence of AT1-receptor autoantibodies (AT1-AA) in preeclampsia. They developed a bioassay in which plasma of patients with pre-eclampsia stimulated cardiomyocytes of rats to contract and this action was blocked by an AT1-receptor antagonist. After this, several studies explored the possible role of these autoantibodies in pre-eclampsia. AT1-AA have been reported in as many as 95% of women with pre-eclampsia and seems to correlate with disease severity.59 Strong evidence supporting a central role for AT1-AA came from an adoptive transfer experiment, in which serum of pre-eclamptic women induced features of the pre-eclampsia syndrome in pregnant mice.60 Immunization of pregnant rats with AT1-AA induces sensitivity to angiotensin II and high blood pressure, growth restriction and placental dysfunction.61 AT1-AA can be produced by the placenta in response to placental ischaemia.62,63 This led to the theory that AT1-AA are capable of inducing features of pre-eclampsia in the mother and placental dysfunction and growth restriction in the fetus. Clearly many more studies are needed before we can be certain about the prevalence, physiological and clinical significance of these antibodies in preeclampsia but such studies will be of interest.
AT1-receptor activation and placental dysfunction
In contrast to what is found in the circulation, the uteroplacental renin-angiotensin system is upregulated in pre-eclampsia (Figure 3b). Chymase, the main factor converting AI to angiotensin II in the placenta,49 is further upregulated in preeclampsia.64 In the placenta, the levels of prorenin, renin and angiotensin II are higher in pre-eclampsia than normal pregnancy.65, 66 This local angiotensin II together with AT1-AA may lead to over activation of AT1-receptors. Animal models and in vitro studies show that stimulation of AT1-receptors induces the release of pathogenic factors that have been implicated in the pathogenesis of pre-eclampsia.67 On trophoblast cells, the AT1-AA stimulate the AT1-receptor to release several pathogenic substances, such as sFlt1, endothelin-1, PAI-1 and ROS/NADPH.40,68-70 AT1-receptor stimulation by AT1-AA appears to cause vasoconstriction on the fetal side of the placenta, which may lead to reduced placental blood flow.71 Angiotensin II-induced sFlt-1 secretion leads to reduced trophoblast invasion in vitro.72
Further evidence supporting the importance of the renin- angiotensin system in the aetiology of pre-eclampsia was suggested by several genetic variants of components of this system that are found to be associated with pre-eclampsia. After discovery of a molecular variant in angiotensinogen by Ward et al.73 and Procopciuc et al.,74 several polymorphisms in the genes encoding for angiotensinogen, ACE and the AT1-receptor were found in relation to pre-eclampsia and even fetal genetic variations in these genes may play a role. However, these findings have not been consistent and as such a genetic predisposition to pre-eclampsia due to an altered renin angiotensin system seems unlikely. Rather, there is likely to be one or more non-genetic factors that alter the regulation of this system in pre-eclampsia.
Several epidemiological studies have indicated that preeclampsia has lifelong cardiovascular implications.75, 76 The plasma values for active renin and aldosterone do not differ from parous controls in the years postpartum,77 but the increased sensitivity to angiotensin II remains.78 Moreover, the presence of AT1-AA do not regress completely after pregnancy and have been observed in the postpartum period.79 Higher levels of soluble Flt-1, although lower than during the clinical phase of pre-eclampsia, are still observed and respond to angiotensin II infusion.78, 79 Thus, alterations in the renin-angiotensin system may contribute to the increased risk of cardiovascular disease in later life in women with a history of pre-eclampsia. Whether these alterations were induced by the pre-eclampsia event or whether they are intrinsic to the woman predisposed to preeclampsia will no doubt be a direction of future research.
Future Perspectives
Our knowledge of the renin-angiotensin system in pregnancy and pre-eclampsia has increased substantially as summarized in Table 1. Still, several questions remain for future research. First, although it is tempting to consider the AT1-receptor autoantibodies as the ‘missing link’ in pre-eclampsia, explaining the suppression of the circulating renin-angiotensin system with at the same time over activation of AT1-receptors, more research is necessary to confirm the importance of this pathway in humans. For example, a study in Mexican women did not support the presence of autoantibodies in pre-eclampsia, suggesting racial differences in the circulating factors involved.80 AT1-AA are also present in cases of fetal growth restriction without pre-eclampsia and a direct relationship with sFlt-1 levels has not been confirmed in humans.63,81, 82 So far, we do not know what exact epitope causes the production of these agonistic AT1-receptor autoantibodies.
Table 1.
Summary of the RAAS in normal pregnancy and pre-eclampsia
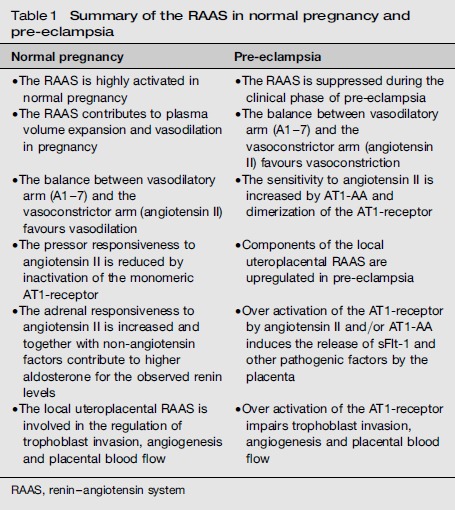
| Normal pregnancy | Pre-eclampsia |
|---|---|
| • The RAAS is highly activated in normal pregnancy | • The RAAS is suppressed during the clinical phase of preeclampsia |
| • The RAAS contributes to plasma volume expansion and vasodilation in pregnancy | • The balance between vasodilatory arm (A1-7) and the vasoconstrictor arm (angiotensin II) favours vasoconstriction |
| • The balance between vasodilatory arm (A1-7) and the vasoconstrictor arm (angiotensin II) favours vasodilation | • The sensitivity to angiotensin II is increased by AT1-AA and dimerization of the AT1-receptor |
| • The pressor responsiveness to angiotensin II is reduced by inactivation of the monomeric AT1-receptor | • Components of the local uteroplacental RAAS are upregulated in preeclampsia |
| • The adrenal responsiveness to angiotensin II is increased and together with non-angiotensin factors contribute to higher aldosterone for the observed renin levels | • Over activation of the AT1-receptor by angiotensin II and/or AT1-AA induces the release of sFlt-1 and other pathogenic factors by the placenta |
| • The local uteroplacental RAAS is involved in the regulation of trophoblast invasion, angiogenesis and placental blood flow | • Over activation of the AT1-receptor impairs trophoblast invasion, angiogenesis and placental blood flow |
RAAS, renin-angiotensin system
Secondly, the interplay between pre-eclampsia and the fetal circulating renin-angiotensin system has not received as much attention, although the renin-angiotensin system clearly plays a regulatory role in the development of many fetal tissues. The amniotic fluid contains prorenin in high amounts, about 10 times the concentration in plasma from pregnancy,6 suggesting that there is an important local role for the renin angiotensin system in fetal development and wellbeing. We know from toxicology studies that fetal kidney development is often impaired by the use of ACE- or AT1-receptor blockers in pregnancy.83 Dysregulation of the fetal renin-angiotensin system, induced by a low protein diet in pregnant rats, leads to smaller kidneys and 30-40% less functional nephrons.84 AT1 autoantibodies cross the placenta and may have a direct negative influence on fetal growth and the development of the kidneys and liver.39, 85 More research is necessary to explore the role of the fetal renin-angiotensin system in growth restriction and pre-eclampsia.
Finally, postulating that the AT1-receptor plays an important role in the pathophysiology of pre-eclampsia and placental dysfunction, the question arises whether blockage of this receptor or stimulation of its counterbalancing pathway may be of therapeutic benefit. The use of ACE-receptor blockers is strongly discouraged in pregnancy, as it is related to fetal kidney dysfunction and oligo/anhydramnios in the second half of pregnancy and the same observations are made for specific AT1-receptor blockers.83 New therapeutic options may become available in the future; currently promising is the development of agents that promote the production of angiotensin 1-7, such as ACE2 therapy or A1-7 agonists.24
In summary, our thinking around the renin-angiotensin system in pregnancy and pre-eclampsia has changed greatly over the past decade. It now seems likely that overactivation of the AT1-receptor, whether stimulated directly by angiotensin II or AT1-AA or indirectly by prorenin, can cause the release of pathogenic factors by the placenta that probably lead to impaired trophoblast invasion and reduced placental blood flow. Subsequently, this may cause the maternal features of preeclampsia and the typical ‘suppression’ of the circulatory maternal renin-angiotensin system in this condition. Reduced levels of circulating renin, aldosterone and angiotensin II remain among the most consistently observed pathophysiological findings in pre-eclampsia; research that uncovers the mechanism behind these findings is likely to contribute to our understanding of the development of this enigmatic disorder.
Declarations
Competing interests: None declared.
Funding: None.
Guarantor: MB.
Contributorship: JS researched the literature and drafted the manuscript, MB revised and edited the manuscript.
Acknowledgements: None.
REFERENCES
- 1.Martin JD, Mills IH. Aldosterone excretion in normal and toxaemic pregnancies. Br Med J 1956; 2: 571–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JJ, Davies DL, Doak PB, Lever AF, Robertson JI. Plasma-renin in normal pregnancy. Lancet 1963; 2: 900–1 [DOI] [PubMed] [Google Scholar]
- 3.Brown MA, Gallery ED, Ross MR, Esber RP. Sodium excretion in normal and hypertensive pregnancy: a prospective study. Am J Obstet Gynecol 1988; 159: 297–307 [DOI] [PubMed] [Google Scholar]
- 4.Brown MA, Wang J, Whitworth JA. The renin-angiotensin-aldosterone system in pre-eclampsia. Clin Exp Hypertens 1997; 19: 713–26 [DOI] [PubMed] [Google Scholar]
- 5.Brar HS, Do YS, Tam HB, et al. Uteroplacental unit as a source of elevated circulating prorenin levels in normal pregnancy. Am J Obstet Gynecol 1986; 155: 1223–6 [DOI] [PubMed] [Google Scholar]
- 6.Hsueh WA. Renin in the female reproductive system. Cardiovasc Drugs Ther 1988; 2: 473–7 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 2002; 109: 1417–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saris JJ, Hoen PA, Garrelds IM, et al. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension 2006; 48: 564–71 [DOI] [PubMed] [Google Scholar]
- 9.Pringle KG, Zakar T, Yates D, Mitchell CM, Hirst JJ, Lumbers ER. Molecular evidence of a (pro)renin/(pro)renin receptor system in human intrauterine tissues in pregnancy and its association with PGHS-2. J Renin Angiotensin Aldosterone Syst 2011; 12: 304–10 [DOI] [PubMed] [Google Scholar]
- 10.Batenburg WW, Krop M, Garrelds IM, et al. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens 2007; 25: 2441–53 [DOI] [PubMed] [Google Scholar]
- 11.Danser AH. Does prorenin exert angiotensin-independent effects in vivo? Hypertension 2009; 54: 1218–20 [DOI] [PubMed] [Google Scholar]
- 12.Elijovich F, Laffer CL. Detrimental effects of dual ACEI-ARB therapy: is the (pro)renin receptor the culprit? Kidney Int 2011; 80: 911–4 [DOI] [PubMed] [Google Scholar]
- 13.Abdul-Karim R, Assalin S. Pressor response to angiotonin in pregnant and nonpregnant women. Am J Obstet Gynecol 1961; 82: 246–51 [DOI] [PubMed] [Google Scholar]
- 14.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 1973; 52: 2682–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MA, Broughton Pipkin F, Symonds EM. The effects of intravenous angiotensin II upon blood pressure and sodium and urate excretion in human pregnancy. J Hypertens 1988; 6: 457–64 [DOI] [PubMed] [Google Scholar]
- 16.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med 2001; 7: 1003–9 [DOI] [PubMed] [Google Scholar]
- 17.West C, Zhang Z, Ecker G, Masilamani SM. Increased renal alpha-epithelial sodium channel (ENAC) protein and increased ENAC activity in normal pregnancy. Am J Physiol Regul Integr Comp Physiol 2010; 299: R1326–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberger MH, Kramer NJ, Petersen LP, Cleary RE, Young PC. Sequential changes in the renin-angiotensin-aldosterone systems and plasma progesterone concentration in normal and abnormal human pregnancy. Perspect Nephrol Hypertens 1976; 5: 263–9 [PubMed] [Google Scholar]
- 19.Brown MA, Zammit VC, Mitar DA, Whitworth JA. Renin-aldosterone relationships in pregnancy-induced hypertension. Am J Hypertens 1992; 5: 366–71 [DOI] [PubMed] [Google Scholar]
- 20.Brown MA, Thou ST, Whitworth JA. Stimulation of aldosterone by ACTH in normal and hypertensive pregnancy. Am J Hypertens 1995; 8: 260–7 [DOI] [PubMed] [Google Scholar]
- 21.Brown MA, Nicholson E, Gallery ED. Sodium-renin-aldosterone relations in normal and hypertensive pregnancy. Br J Obstet Gynaecol 1988; 95: 1237–46 [DOI] [PubMed] [Google Scholar]
- 22.Brown MA, Zammit VC, Adsett D. Stimulation of active renin release in normal and hypertensive pregnancy. Clin Sci (Lond) 1990; 79: 505–11 [DOI] [PubMed] [Google Scholar]
- 23.Brosnihan KB, Neves LA, Chappell MC. Does the angiotensin-converting enzyme (ACE)/ACE2 balance contribute to the fate of angiotensin peptides in programmed hypertension? Hypertension 2005; 46: 1097–9 [DOI] [PubMed] [Google Scholar]
- 24.Ferreira AJ, Santos RA, Bradford CN, et al. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension 2010; 55: 207–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyner J, Neves LA, Stovall K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) serves as an aquaretic by increasing water intake and diuresis in association with downregulation of aquaporin-1 during pregnancy in rats. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1073–80 [DOI] [PubMed] [Google Scholar]
- 26.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine 2002; 18: 239–45 [DOI] [PubMed] [Google Scholar]
- 27.Valdes G, Germain AM, Corthorn J, et al. Urinary vasodilator and vasoconstrictor angiotensins during menstrual cycle, pregnancy, and lactation. Endocrine 2001; 16: 117–22 [DOI] [PubMed] [Google Scholar]
- 28.August P, Mueller FB, Sealey JE, Edersheim TG. Role of renin-angiotensin system in blood pressure regulation in pregnancy. Lancet 1995; 345: 896–7 [DOI] [PubMed] [Google Scholar]
- 29.Li C, Ansari R, Yu Z, Shah D. Definitive molecular evidence of renin-angiotensin system in human uterine decidual cells. Hypertension 2000; 36: 159–64 [DOI] [PubMed] [Google Scholar]
- 30.Morgan T, Craven C, Ward K. Human spiral artery renin-angiotensin system. Hypertension 1998; 32: 683–7 [DOI] [PubMed] [Google Scholar]
- 31.Poisner AM. The human placental renin-angiotensin system. Front Neuroendocrinol 1998; 19: 232–52 [DOI] [PubMed] [Google Scholar]
- 32.Cooper AC, Robinson G, Vinson GP, Cheung WT, Broughton Pipkin F. The localization and expression of the renin-angiotensin system in the human placenta throughout pregnancy. Placenta 1999; 20: 467–74 [DOI] [PubMed] [Google Scholar]
- 33.Anton L, Merrill DC, Neves LA, Gruver C, Moorefield C, Brosnihan KB. Angiotensin II and angiotensin-(1-7) decrease sFlt1 release in normal but not preeclamptic chorionic villi: an in vitro study. Reprod Biol Endocrinol 2010; 8: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan P, Fu H, Zhang L, et al. Angiotensin II upregulates the expression of placental growth factor in human vascular endothelial cells and smooth muscle cells. BMC Cell Biol 2010; 11: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalenga MK, de Gasparo M, Thomas K, De Hertogh R. Angiotensin II induces human placental lactogen and pregnancy-specific beta 1-glycoprotein secretion via an angiotensin AT1 receptor. Eur J Pharmacol 1994; 268: 231–6 [DOI] [PubMed] [Google Scholar]
- 36.Kalenga MK, De Gasparo M, Thomas K, De Hertogh R. Angiotensin-II stimulates estradiol secretion from human placental explants through AT1 receptor activation. J Clin Endocrinol Metab 1995; 80: 1233–7 [DOI] [PubMed] [Google Scholar]
- 37.Benoit C, Gu Y, Zhang Y, Alexander JS, Wang Y. Contractility of placental vascular smooth muscle cells in response to stimuli produced by the placenta: roles of ACE vs. non-ACE and AT1 vs. AT2 in placental vessel cells. Placenta 2008; 29: 503–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knock GA, Sullivan MH, McCarthy A, Elder MG, Polak JM, Wharton J. Angiotensin II (AT1) vascular binding sites in human placentae from normal-term, preeclamptic and growth retarded pregnancies. J Pharmacol Exp Ther 1994; 271: 1007–15 [PubMed] [Google Scholar]
- 39.Laskowska M, Leszczynska-Gorzelak B, Oleszczuk J. Placental angiotensin II receptor AT1R in normotensive patients and its correlation between infant birth weight. Eur J Obstet Gynecol Reprod Biol 2003; 109: 166–70 [DOI] [PubMed] [Google Scholar]
- 40.Zhou CC, Ahmad S, Mi T, et al. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension 2008; 51: 1010–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gennari-Moser C, Khankin EV, Schuller S, et al. Regulation of placental growth by aldosterone and cortisol. Endocrinology 2011; 152: 263–71 [DOI] [PubMed] [Google Scholar]
- 42.Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 2006; 47: 203–8 [DOI] [PubMed] [Google Scholar]
- 43.Brown MA, Zammit VC, Mitar DM. Extracellular fluid volumes in pregnancy-induced hypertension. J Hypertens 1992; 10: 61–8 [DOI] [PubMed] [Google Scholar]
- 44.Hanssens M, Keirse MJ, Spitz B, van Assche FA. Angiotensin II levels in hypertensive and normotensive pregnancies. Br J Obstet Gynaecol 1991; 98: 155–61 [DOI] [PubMed] [Google Scholar]
- 45.Weir RJ, Brown JJ, Fraser R, et al. Plasma renin, renin substrate, angiotensin II, and aldosterone in hypertensive disease of pregnancy. Lancet 1973; 1: 291–4 [DOI] [PubMed] [Google Scholar]
- 46.Nicholson E, Gallery E, Brown M, Ross M, Jones M. Renin activation in normal and hypertensive human pregnancy. Clin Exp Hypertens 1987; 6: 453–64 [Google Scholar]
- 47.Gant NF, Worley RJ, Everett RB, MacDonald PC. Control of vascular responsiveness during human pregnancy. Kidney Int 1980; 18: 253–8 [DOI] [PubMed] [Google Scholar]
- 48.Rajagopalan S, Laursen JB, Borthayre A, et al. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension 1997; 30: 29–34 [DOI] [PubMed] [Google Scholar]
- 49.Benoit C, Zavecz J, Wang Y. Vasoreactivity of chorionic plate arteries in response to vasoconstrictors produced by preeclamptic placentas. Placenta 2007; 28: 498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.August P, Lenz T, Ales KL, et al. Longitudinal study of the renin-angiotensin-aldosterone system in hypertensive pregnant women: deviations related to the development of superimposed preeclampsia. Am J Obstet Gynecol 1990; 163: 1612–21 [DOI] [PubMed] [Google Scholar]
- 51.de Leon RG, de Melian EM, Coviello A, De Vito E. Prorenin concentration in the hypertensive disorders in pregnancy. Hypertens Pregnancy 2001; 20: 157–68 [DOI] [PubMed] [Google Scholar]
- 52.Brown MA, Reiter L, Rodger A, Whitworth JA. Impaired renin stimulation in pre-eclampsia. Clin Sci (Lond) 1994; 86: 575–81 [DOI] [PubMed] [Google Scholar]
- 53.Shojaati K, Causevic M, Kadereit B, et al. Evidence for compromised aldosterone synthase enzyme activity in preeclampsia. Kidney Int 2004; 66: 2322–8 [DOI] [PubMed] [Google Scholar]
- 54.Elsheikh A, Creatsas G, Mastorakos G, Milingos S, Loutradis D, Michalas S. The renin-aldosterone system during normal and hypertensive pregnancy. Arch Gynecol Obstet 2001; 264: 182–5 [DOI] [PubMed] [Google Scholar]
- 55.Lowe SA, Zammit VC, Mitar D, Macdonald GJ, Brown MA. Atrial natriuretic peptide and plasma volume in pregnancy-induced hypertension. Am J Hypertens 1991; 4: 897–903 [DOI] [PubMed] [Google Scholar]
- 56.Brown MA, Zammit VC, Mitar D, Whitworth JA. Controls of aldosterone in normal and hypertensive pregnancy: effect of metoclopramide. Hypertens Pregnancy 1993; 12: 37–51 [Google Scholar]
- 57.Kim EH, Lim JH, Kim YH, Park YW. The relationship between aldosterone to renin ratio and RI value of the uterine artery in the preeclamptic patient vs. normal pregnancy. Yonsei Med J 2008; 49: 138–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 1999; 103: 945–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 2010; 55: 386–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou CC, Zhang Y, Irani RA, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 2008; 14: 855–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wenzel K, Rajakumar A, Haase H, et al. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 2011; 58: 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 2008; 52: 1168–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walther T, Wallukat G, Jank A, et al. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension 2005; 46: 1275–9 [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Gu Y, Lewis DF, Alexander JS, Granger DN. Elevated plasma chymotrypsin-like protease (chymase) activity in women with preeclampsia. Hypertens Pregnancy 2010; 29: 253–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anton L, Merrill DC, Neves LA, et al. The uterine placental bed renin-angiotensin system in normal and preeclamptic pregnancy. Endocrinology 2009; 150: 4316–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh HJ, Rahman A, Larmie ET, Nila A. Raised prorenin and renin concentrations in pre-eclamptic placentae when measured after acid activation. Placenta 2004; 25: 631–6 [DOI] [PubMed] [Google Scholar]
- 67.Irani RA, Zhang Y, Zhou CC, et al. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension 2010; 55: 1246–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LaMarca B, Parrish M, Ray LF, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 2009; 54: 905–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dechend R, Viedt C, Muller DN, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 2003; 107: 1632–9 [DOI] [PubMed] [Google Scholar]
- 70.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig 2003; 10: 82–93 [DOI] [PubMed] [Google Scholar]
- 71.Yang X, Wang F, Chang H, et al. Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. J Hypertens 2008; 26: 1629–35 [DOI] [PubMed] [Google Scholar]
- 72.Zhou CC, Ahmad S, Mi T, et al. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res 2007; 100: 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ward K, Hata A, Jeunemaitre X, et al. A molecular variant of angiotensinogen associated with preeclampsia. Nat Genet 1993; 4: 59–61 [DOI] [PubMed] [Google Scholar]
- 74.Procopciuc LM, Caracostea G, Zaharie G, et al. Maternal/newborn genotype contribution of the renin-angiotensin system (Met235Thr, Thr174Met, I/ D-ACE, A2350G-ACE, A1166C-AT2R1, C3123A-AT2R2, 83A/G-REN) to the risk of pre-eclampsia: a Romanian study. J Renin Angiotensin Aldosterone Syst 2011; 12: 539–48 [DOI] [PubMed] [Google Scholar]
- 75.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007; 335: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008; 156: 918–30 [DOI] [PubMed] [Google Scholar]
- 77.Mangos G, Spaan J, Pirabhahar S, Brown M. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens 2012; 30: 351–58 [DOI] [PubMed] [Google Scholar]
- 78.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension 2010; 55: 1239–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hubel CA, Wallukat G, Wolf M, et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 2007; 49: 612–7 [DOI] [PubMed] [Google Scholar]
- 80.Leanos-Miranda A, Campos-Galicia I, Alvarez-Jimenez G, Isordia-Salas I, Rivera-Leanos R, Ulloa-Aguirre A. Stimulating autoantibodies against the angiotensin II type 1 receptor are not associated with preeclampsia in Mexican-Mestizo women. J Hypertens 2010; 28: 834–41 [DOI] [PubMed] [Google Scholar]
- 81.Verlohren S, Muller DN, Luft FC, Dechend R. Immunology in hypertension, preeclampsia, and target-organ damage. Hypertension 2009; 54: 439–43 [DOI] [PubMed] [Google Scholar]
- 82.Stepan H, Faber R, Wessel N, Wallukat G, Schultheiss HP, Walther T. Relation between circulating angiotensin II type 1 receptor agonistic autoantibodies and soluble fms-like tyrosine kinase 1 in the pathogenesis of preeclampsia. J Clin Endocrinol Metab 2006; 91: 2424–7 [DOI] [PubMed] [Google Scholar]
- 83.Alwan S, Polifka JE, Friedman JM. Angiotensin II receptor antagonist treatment during pregnancy. Birth Defects Res A Clin Mol Teratol 2005; 73: 123–30 [DOI] [PubMed] [Google Scholar]
- 84.Alwasel SH, Kaleem I, Sahajpal V, Ashton N. Maternal protein restriction reduces angiotensin II AT(1) and AT(2) receptor expression in the fetal rat kidney. Kidney Blood Press Res 2010; 33: 251–9 [DOI] [PubMed] [Google Scholar]
- 85.Irani RA, Zhang Y, Blackwell SC, et al. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. J Exp Med 2009; 206: 2809–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodari AA, Smeby R, Bumpus FM. A renin-like substance in the human placenta. Obstet Gynecol 1967; 29: 313–17 [PubMed] [Google Scholar]


