Abstract
Background
The aim of this study was to determine the prevalence of neurological disorders and their associated correlates and relations with clinical and behavioural problems among children and adolescents with HIV/AIDS (CA-HIV).
Methods
This study involved a sample of 1070 CA-HIV/caregiver dyads who were evaluated at their 6-month follow-up visit as part of their participation in the longitudinal study, ‘Mental health among HIV infected CHildren and Adolescents in KAmpala and Masaka, Uganda (the CHAKA study)’. Participants completed an extensive battery of measures that included a standardized DSM-5- referenced rating scale, the parent version (5–18 years) of the Child and Adolescent Symptom Inventory-5 (CASI-5). Using logistic regression, we estimated the prevalence of neurological disorders and characterised their associations with negative clinical and behavioural factors.
Results
The overall prevalence of at least one neurological disorders was 18.5% (n = 198; 95% CI, 16.2–20.8). Enuresis / encopresis was the most common (10%), followed by motor/vocal tics (5.3%); probable epilepsy was the least prevalent (4%). Correlates associated with neurological disorders were in two domains: socio-demographic factors (age, ethnicity and staying in rural areas) and HIV-related factors (baseline viral load suppression). Enuresis/encopresis was associated with psychiatric comorbidity. Neurological disorders were associated with earlier onset of sexual intercourse (adjusted OR 4.06, 95% CI 1.26–13.1, P = 0.02).
Conclusions
Neurological disorders impact lives of many children and adolescents with HIV/AIDS. There is an urgent need to integrate the delivery of mental and neurological health services into routine clinical care for children and adolescents with HIV/AIDS in sub-Saharan Africa.
Electronic supplementary material
The online version of this article (10.1186/s12888-019-2023-9) contains supplementary material, which is available to authorized users.
Keywords: Neurological disorders, Children/adolescents, HIV, Prevalence, Correlates
Background
Children and adolescents infected with HIV/AIDS (CA-HIV) are at increased risk of developmental and neuropsychological disturbances owing to both the direct and indirect effects of the HIV virus [1, 2]. HIV has direct neurotoxic effects on brain structures involved in the regulation of emotion, behavior, and cognition, this effect is thought to result primarily from HIV’s ability to induce inflammatory factors that cause neuronal cell damage and eventual cell death [1]. The indirect effects of HIV include social stressors, poverty, illness and trauma on morbidity and mortality [1, 2]. Previous research suggests that HIV-1 mutations differ by subtype, which may lead to increased resistance to anti-retroviral therapy (ART) thus resulting in persistence of neurological and neurodevelopmental disorders among CA-HIV receiving ART [2].
In sub Saharan Africa, with the exception of epilepsy, few studies have attempted to determine the prevalence of neurologic disease among children and adolescents and therefore characterize their clinical correlates [1]. In one study conducted in East Africa, approximately two-thirds of new patients presenting to a child and adolescent psychiatry service had epilepsy [3], and seizures are also common among individuals with HIV/AIDS [4]. Although neurological disorders are reported to be associated with HIV/AIDS [1, 2, 5], no such studies have been conducted among CA-HIV in Uganda. The primary objectives of the present study were to (a) document the prevalence of neurological disorders among a large sample of CA-HIV attending rural and urban HIV clinics in Uganda, (b) characterize the relation of neurological disorders with a wide range of commonly studied clinical correlates, (c) establish the co-occurrence of neurological and psychiatric disorders, and (d) investigate the relation of neurological disorders with important indices of functioning.
Methods
Participants
The study sample comprises 1070 CA-HIV and their caregivers who were evaluated at their 6-month follow-up visit as part of their participation in the Mental health among HIV infected CHildren and Adolescents in KAmpala and Masaka, Uganda (CHAKA) study. Details about the study design, eligibility criteria and study procedures of CHAKA are described in a previous publication [6]. Of the initial 1339 CA-HIV/ caregiver dyads interviewed at baseline, 269 (20.0%) were not included in the 6 months analyses for various reasons including: 3 had incomplete records and the remaining 266 CA-HIV did not turn up for the 6 months follow-up interview despite various attempts to reach them. Some of these later returned for their 12-month interview reporting that they had temporarily transferred out of the study clinic.
Measures
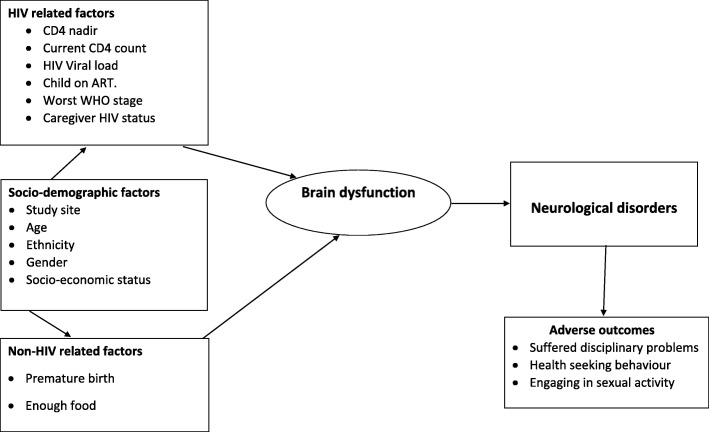
The assessment battery comprised locally adapted standardised psychosocial instruments described in earlier publications [6, 7] that have previously been used in the USA [8–10]. Study variables reported in this paper are described in Additional file 1 and are arranged according to the conceptual framework depicted in Fig. 1.
Fig. 1.
Conceptual framework for clinical correlates of neurological disorders
Epilepsy
Probable clinical epilepsy was based on a score of yes to the following item: Has this child/adolescent ever suffered repeated seizures or convulsions without fever being present?
Tic and elimination disorders
Motor tics and vocal tics and elimination disorders (enuresis and encopresis) were established using a clinically practical, DSM-5-referenced, behaviour rating scale, the Child and Adolescent Symptom Inventory-5 (CASI-5) [10], which was previously used to study emotional and behavioral disorders among CA-HIV in the United States [9, 11]. The items in the CASI-5 are based on symptom statements that appear in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Informants indicate whether these symptoms occur, never, sometimes, often, or very often. CASI-5 items can be scored in several different ways, and in the present study we used the symptom count cut-off score for these items. Symptom count cut-off score indicates whether child/adolescent has the prerequisite number of symptoms necessary for a DSM-5 diagnosis, which for this study was a rating of often or very often.
Psychiatric disorders
The CASI-5 also provides scoring algorithms for estimating psychiatric diagnoses. For purposes of this paper, we generated clinical cutoff scores for 12 psychiatric disorders, which we categorised as either internalising or externalising. The internalising disorder category included generalised anxiety disorder, social anxiety disorder, separation anxiety disorder, specific phobia disorder, panic disorder, post-traumatic stress disorder (PTSD), major depressive episode (MDD and dysthymia). Externalising disorders included attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), conduct disorder, and callous unemotional. To receive a clinical cutoff score, a CA-HIV had to meet both DSM-based symptom count criteria for a specific disorder as well as impairment cutoff criteria. The latter is a rating of often or very often to the question of whether symptoms interceded with social, academic, or chore-related functioning. The CASI-5 was administered by trained psychiatric nurse research assistants.
Sexual intercourse
Sexual intercourse was a response of yes to the question, Have you ever had sex? Possible responses were yes/no, this question was asked only to adolescents.
Statistical analysis
Prevalence estimates and 95% confidence intervals for the three neurological outcomes of enuresis/encopresis, motor or vocal tics and probable epilepsy are reported overall and by age and gender category. Using a conceptual framework (Fig. 1) based on both HIV- and non-HIV-related factors hypothesized to be associated with neurological disorders, we investigated several widely studied variables. Associations between each of the neurological disorders evaluated at the 6-month follow-up period, baseline socio-demographic and HIV-related factors were undertaken using logistic regression models. Multivariable models were selected based on backward elimination, and models were fitted sequentially to include significant factors from each category at p < 0.1. The final model included the pre-defined design variable of study site, age and sex and all significant factors from the previous models.
The prevalence and 95% confidence intervals of comorbidities between neurological disorders as well as externalising and internalising disorders were estimated. We investigated associations of neurological disorders with the following outcomes using logistic regression: social outcomes as evidenced by disciplinary actions in school, any missed days at school during the last term, and onset of sexual intercourse (yes/no; adolescents only); and health-related outcomes such as any visit to the health unit in the last month; any hospital admissions in the last 6 months, and non-adherence to ART defined as any missed ART in the last 3 days (yes/no). For each outcome, associations were assessed using logistic regression models, adjusted for age, sex, study site and ethnicity. In accordance with previously published work from the CHAKA study, adjustment was made for CD4 cell count, visits to the health unit, and hospital admissions and for HIV ART status for the adherence outcome. Similarly, we used 5% p-values for these descriptive exploratory analyses because it is not clear how many comparisons should be corrected for and precautions taken in interpretation due to multiple comparisons. We used Bonferroni correction adjusted p-values by multiplying the observed p value from the significance tests by the number of tests k, for each of the analyses with psychiatric symptom outcomes [26, 27]. Then if any kP is less than 0.05 the differences in the two groups (with versus those without neurological disorders) are significant at the 0.05 level.
Results
Characteristics of study participants
Overall, the study included 677 (63.3%) children aged 5–11 years and 393 (36.7%) adolescents aged 12–17 years. The urban and rural study sites contributed equally. There were slightly more females (52%) than males (48%). With regard to HIV illness parameters, 88% had CD4 counts equal or greater than 350 cells/μL, and most (95%) of the participants were receiving ART (Additional File 2).
Prevalence of neurological disorders
One hundred ninety eight participants had at least one neurological disorder (prevalence 18.5%). The prevalence of neurological disorders was 10% for enuresis/encopresis, 5.3% for motor/vocal tics, and 4% for probable epilepsy (Table 1). Of the 198 with any neurological disorder, 189 (17.7%) had at most one disorder [tics (n = 51, enuresis/encopresis (n = 102), and epilepsy (n = 36)], and 9 (0.8%) had at most two disorders [tics and enuresis/encopresis (n = 2), tics and epilepsy (n = 4), enuresis/encopresis and epilepsy (n = 3)]. The prevalence of any neurological disorder was 18% in males and 19% in females. The prevalence of probable epilepsy among children and adolescent was similar (4.0% vs. 4.1%), and enuresis/encopresis was higher among children than adolescents (14.0% vs. 3.1%), whereas motor/vocal tics were more frequent among adolescents than children (11% vs. 2%). Marginal gender differences in prevalence of probable epilepsy in females versus males (5% vs 3%) and in enuresis/encopresis in females versus males (9% vs 11%), whereas motor/vocal tics were more common in females versus males (7% vs 4%).
Table 1.
Prevalence and 95% CI of neurological disorders based on symptom counts cut-off scores of the CASI-5
| Subgroup | N | Prevalence enuresis/encopresis n (%) 95% CI | Prevalence motor/vocal tics | Prevalence epilepsy | Prevalence any neurological disorder |
|---|---|---|---|---|---|
| Children | 677 | 95 (14.0%) (11.4, 16.7) | 14 (2.1%) (1.0, 3.1) | 27 (4.0%) (2.5, 5.5) | 131 (19.4%) (16.4, 22.3) |
| Adolescents | 393 | 12 (3.1%) (1.3, 4.8) | 43 (10.9%) (7.8, 14.0) | 16 (4.1%) (2.1, 6.0) | 67 (17.1%) (13.3, 20.8) |
| Female | 550 | 48 (8.7%) (6.4, 11.1) | 37 (6.7%) (4.6, 8.8) | 26 (4.7%) (3.0, 6.5) | 106 (19.3%) (16.0, 22.6) |
| Male | 520 | 59 (11.3%) (8.6, 14.1) | 20 (3.9%) (2.2, 5.5) | 17 (3.3%) (1.7, 4.8) | 92 (17.7%) (14.4, 21.0) |
| Overall | 1070 | 107 (10%) (8.1, 11.8) | 57 (5.3%) (4.0, 6.7) | 43 (4.0%) (2.8, 5.2) | 198 (18.5%) (16.2, 20.8) |
Correlates of elimination disorders
Factors associated with enuresis/encopresis symptom count cut-off scores in the multivariable model were younger age (aOR 0.79, 95% CI 0.73–0.85, P < 0.001), ethnicity (being a non-Muganda with aOR of 0.56, CI 0.33–0.95, P = 0.03), living in rural areas (aOR 2.25, CI 1.42–3.54, P = 0.4), and less viral load suppression at baseline (aOR 1.75, CI 1.08–2.83, P = 0.02) (Additional file 3).
Clinical correlates of motor and vocal tics)
CA-HIV socio-demographic variables associated with motor/vocal tics symptom count cut off scores in the multivariable model were increasing age (aOR 1.28, CI 1.17–1.40, P < 0.001) and ethnicity (being non-Muganda; aOR 0.33, CI 0.14–0.76, P = 0.01) adjusted for site and sex (Additional file 3).
Clinical correlates of probable epilepsy
We found ethnicity marginally associated with probable epilepsy (being a non-Muganda with aOR of 1.87, CI 0.98–3.57, P = 0.06), adjusted for age, sex and site. None of the other factors was associated with probable epilepsy (p > 0.1) (Additional file 3).
Association with psychiatric symptoms
CA-HIV with enuresis/encopresis had a higher risk for co-occurring externalising disorder (18.7%) and internalising disorder (36.4%) (p < 0.001, and p < 0.001, respectively) (Table 2). All the other associations between neurological disorders and psychiatric comorbidities were not significant.
Table 2.
Comorbidities between neurological disorders with externalising and internalising psychiatric disorders
| N = 1070 | Enuresis/ Encopresis n(%) CI, p-value | Motor/vocal tics | Epilepsy | Any neurological disorder |
|---|---|---|---|---|
| n | 107 | 57 | 43 | 198 |
| Externalising disorder | 20 (18.7%) | 5 (8.8%) | 4 (9.3%) | 28 (14.1) |
| (12.4, 27.3) | (3.6, 19.5) | (3.6, 19.5) | (9.9, 19.7) | |
| P < 0.001 | P = 0.9 | P = 0.9 | P = 0.01 | |
| Internalising disorder | 39 (36.4%) | 14 (24.6%) | 14 (32.6%) | 63 (31.8%) |
| (27.8, 46.0) | (15.0, 37.4) | (20.1, 25.2) | (25.7, 38.7) | |
| P < 0.001 | P = 0.8 | P = 0.1 | P = 0.001 |
Externalising includes; any ADHD, ODD, CD, callous unemotional by symptom frequency, or clinically significant or interferes with function
Internalising; MDD, persistent dysthymia, GAD, panic disorder, specific phobia, PTSD, any social anxiety, separation anxiety,
Association with social and academic functioning
Having any neurological disorder was associated with an earlier onset of sexual intercourse (adjusted OR 4.06, 95% CI 1.26–13.1, P = 0.02). The relation of neurological disorders with the other social or academic outcomes were not significant (Table 3).
Table 3.
Association between neurological disorders and adverse outcomes
| Outcomes | Enuresis/ Encopresis aOR, p-valuea | Motor/ Vocal TICS aOR, p-valuea | Epilepsy aOR, p-valuea | Any neurological disorder aOR, p-valuea |
|---|---|---|---|---|
| Any poor social functioning (Suffered disciplinary measures ie suspension, dismissal)f | 0.73 (0.16, 3.27) | 0.71 (0.23, 2.15) | 1.10 (0.43, 2.77) | 1.06 (0.31, 3.61) |
| P = 0.7 | P = 0.5 | P = 0.8 | P = 0.9 | |
| Any days missed at school in the last termf | 0.89 (0.59, 1.35) | 0.93 (0.67, 1.30) | 1.35 (0.99, 1.85) | 1.17 (0.78, 1.74) |
| P = 0.6 | P = 0.7 | P = 0.06 | P = 0.4 | |
| Any visit to the health unit in the last monthc,f | O.44 (0.17, 1.13) | 0.57 (0.30, 1.06) | 0.98 (0.59, 1.63) | 1.39 (0.76, 2.56) |
| P = 0.09 | P = 0.08 | P = 0.9 | P = 0.3 | |
| Any admission to hospital in last 6 monthsc,6 | 0.66 (0.15, 2.91) | 0.94(0.34, 2.60) | 0.85 (0.34, 2.13) | 1.29 (0.44, 3.79) |
| P = 0.6 | P = 0.9 | P = 0.7 | P = 0.6 | |
| Early onset of sexual intercourseb,e,f | N/A | 0.78 (0.26, 2.30) | 1.87 (0.56, 6.21) | 4.06 (1.26, 13.1) |
| P = 0.7 | P = 0.3 | P = 0.02 | ||
| Any non-adherence to ART in last 3 daysb,d,f | N/A | 0.78 (0.33, 1.85) | 1.21 (0.46, 3.21) | 1.60 (0.55, 4.66) |
| P = 0.6 | P = 0.7 | P = 0.4 |
aAdjusted for age, sex, study site and ethnicity
bonly asked in adolescents,
cAdjusted for CD4 cell count at baseline
dNon adherence to ART adjusted for CD4 count at baseline and whether CA-HIV on ART
N/A – outcome does not vary
eBonferroni adjusted p value is 0.02*3 = 0.06
fBonferroni adjusted p value is > 0.1
Discussion
This study aimed to determine the prevalence of neurological disorders among CA-HIV in Uganda and their association with commonly studied mental health clinical correlates, associated psychiatric comorbidities, and functioning. The prevalence of at least one neurological disorder among CA-HIV was 18.5%, which is consistent with findings from previous studies that suggest a high prevalence of neurological disorders among CA-HIV [2, 4].
The prevalence of probable epilepsy in this study was 4%, which is within the range reported in prior studies both in the West and in South Africa. Kellinghaus and colleagues (2008) found that among HIV positive adult patients attending a neurological clinic in Germany, 6.1% had epilepsy [12]. Pascual-Sedano and colleagues (1999) reported a 3% prevalence for new-onset seizures during a one year follow-up period among an adult HIV cohort in Spain [13]. In one of the few studies in this area from sub-Saharan Africa, Samai and colleagues (2013) noted that among HIV positive youth in South Africa, 7.6% had epilepsy [14].
The prevalence of enuresis/encopresis in this study was 10%, and we were unable to locate any other studies in the extant literature about these problems among CA-HIV for purposes of comparison. A rate of enuresis of 5–9% [15–17] and that of encopresis of 1–3% [15–17] have been reported among general population samples of children. In this study, the prevalence for motor/vocal tics was 5.3%, a figure within the 4–12% range reported among children in the general population [18].
In this study, the only variable that was marginally associated with probable epilepsy was ethnicity. Belonging to an ethnic group that was not indigenous to the study area conferred an increased risk for epilepsy. Ethnicity could be associated with epilepsy for two reasons. Firstly, ethnicity may be a pointer to the genetic underpinnings of epilepsy [19]. Secondly, ethnicity may be a marker for socio-economic disadvantage with respondents who were not indigenous to the study area being more likely to experience medical complications while growing up that are associated with both social disadvantage and epilepsy such as birth trauma, other central nervous system infections (including complicated malaria), and traumatic brain injury [20].
In this study, enuresis/encopresis were significantly associated with younger age and marginally higher among males than in females. Both enuresis and encopresis have previously been reported to decrease with increasing age [16, 21, 22] and to be higher among males than among females [21, 22]. In this study, enuresis/encopresis was significantly associated with living in a rural area. Living in a rural area was probably an index of low socio-economic status. Indeed, in a Turkish study [16], enuresis was reported to be associated with low maternal education and with low monthly income, indices of poor socio-economic status. Ethnicity was significantly associated with enuresis/encopresis in this study. As previously discussed under probable epilepsy, the association between enuresis/encopresis and ethnicity may be underlined by the same reasons namely, as a pointer to an underlying genetic underpinning for enuresis/encopresis [23], secondly, as a marker for socio-economic disadvantage. The association between enuresis/encopresis and ‘less HIV viral load suppression’ observed in this study may be due to the direct neurotoxic effect of HIV or/ and to the increased psychological distress associated with a worse HIV clinical state. Lastly, enuresis/encopresis were the only neurological disorders significantly associated with both externalising and internalising psychiatric disorders. Previous research has reported that 20–40% of all children with enuresis have additional comorbid psychiatric disorders which include the externalising disorders of attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) and the internalising disorder of depression [21]. In a large community study, children with encopresis were reported to have increased rates of the externalising disorders of ADHD and ODD and the internalising disorders of separation anxiety, specific phobia and generalised anxiety disorders [24].
In this study motor/vocal tics were more common in females than in males. Scahill and colleagues (2014) in a review involving eleven community studies reported great variation in the female to male ratio of motor/vocal tics ranging from 1 to 1, 1 to 4, through to 1 to 10 [25]. The factors significantly associated with motor/ vocal tics in this study included increasing age and ethnicity. Metzger and colleagues (2012) observed that as children progress into young adulthood, tics often go into remission [18]. The significant association between motor/vocal tics and ethnicity as discussed under probable epilepsy is probably underlined by two factors, a genetic underpinning [26] and social disadvantage.
Having any neurological disorder in this study was associated with an earlier onset of sexual intercourse. The reasons for this association were not immediately clear, however this relationship could be mediated through psychiatric comorbidity [6].
This study has several strengths that include a large sample size, comprehensive assessment battery, and cross-cultural orientation, however, there were also several limitations. Firstly, it is not possible to comment on the casual direction of obtained associations given the cross-sectional design. Secondly, neurological disorders were assessed using single-item questions which increased the risk of false positives. Thirdly, because we did not have a comparable sample of sero-negative youth from the same geographic areas, it is not possible to know whether relations between neurological and putative mental health factors and functional outcomes are influenced by HIV status. This does not, however, detract from the clinical implications of our findings for CA-HIV. Lastly, by design, the present study focused on CA-HIV living in Uganda, and owing to considerable cultural variation in East Africa, our results may not be generalizable to other countries in the region.
Conclusions
In summary, approximately 18% of CA-HIV living in Uganda met DSM-5 symptom count plus impairment criteria for neurological disorder. Risk factors of neurological disorders among CA-HIV as hypothesised in the conceptual framework fell under two broad domains of HIV related factors (less viral load suppression at baseline) and socio-demographic factors (age, gender, ethnicity and residence in the rural area). However, the association between neurological disorders on one hand and ethnicity and externalising disorders on the other seem to suggest a genetic vulnerability component. The association between ‘having a neurological disorder’ and earlier age of sexual intercourse, may have important implications for clinical management, quality of life, long-term outcome and possible disease transmission. Moving forward, there is a definite need to integrate neurological/mental health services into routine HIV care for youth in low-and middle-income settings such as those in sub-Saharan Africa. This should include the development of cost effective assessment and treatment strategies that have high probability of success in challenging interventional settings.
Additional files
Data collection tools for the study. (DOCX 39 kb)
Characteristics of study participants. (DOCX 15 kb)
Correlates of neurological disorders. (DOCX 41 kb)
Acknowledgments
The authors wish to thank the managers of the five study sites (Lubowa Joint Clinical Research Centre, Nsambya homecare department Children’s HIV Care clinic; Nsambya hospital, the Children’s clinic at The AIDS Support Organisation; TASO Masaka, Uganda Cares / Masaka Regional Referral Hospital and Kitovu Mobile AIDS Organisation, Masaka) for permitting the study to be conducted at their specialised HIV/AIDS clinics. The authors extend appreciation to the Medical Research Council, Uganda (MRC, Uganda) for funding and facilitating the study. Special gratitude is extended to the staff working at the five specialised HIV/AIDS clinics where the study was conducted. Appreciation is extended to the diligent work of research assistants. Appreciation is extended to the participants for their time and trust.
Funding
This study was funded by an MRC/DfID grant awarded to Professor Eugene Kinyanda after winning an African Leadership Award; MRC African Research Leaders MR/L004623/1 - Mental health among HIV infected CHildren and Adolescents in KAmpala, Uganda (CHAKA).
Availability of data and materials
All data and materials in this manuscript, additional files and figures attached are freely available with no restrictions.
Abbreviations
- ADD
Attention deficit disorder
- ADHD
Attention - deficit / hyperactivity disorder
- AIDS
Acquired immune deficiency syndrome
- APA
American psychiatric association
- ART
Anti-retroviral therapy
- CA-HIV
Children and adolescents infected with HIV/AIDS
- CASI-4R
Child adolescent symptom inventory-4Revised
- CBCL
Child behavioural checklist
- CD
Conduct disorder
- CDC
Centre for disease control and prevention
- CHAKA
stands for the study ‘Mental health among HIV infected CHildren and Adolescents in KAmpala and Masaka, Uganda’
- CTX
Cotrimoxazole
- DISC-IV
Diagnostic interview schedule for children version IV
- GAD
Generalised anxiety disorder
- HIV
Human immunodeficiency virus
- MDD
Major depressive disorder
- ODD
Oppositional defiant disorder
- PD
Psychiatric disorder
- YI-4R
Youth Inventory-4Revised
Authors’ contributions
RSM, EK, GZR, KDG and VP have made substantial contributions to conception, design, acquisition of data, drafting the manuscript, revising it critically and gave the final approval of this version to be published. SKM did the analysis and interpretation of data. Each author participated sufficiently in this work and takes public responsibility for appropriate portions of the content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study obtained ethical approvals from the Uganda Virus Research Institute’s Research and Ethics Committee (Ref: GC/127/14/04/459), the Ethics Committee of the London School of Hygiene and Tropical Medicine and the Uganda National Council of Science and Technology (Research Registration number: HS 1601). The study obtained consent from parents/ guardians of the CA-HIV and assent from CA-HIV. Participants found to have a psychiatric disorder were provided with psycho-education and referred to their local mental health departments.
Consent for publication
Not applicable.
Competing interests
Dr. Gadow is shareholder in Checkmate Plus, publisher of the Child and Adolescent Symptom Inventory-5. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Richard Stephen Mpango, Phone: +256 772 592504, Phone: +256 702 592504, Email: richmpan@gmail.com, Email: Richard.Mpango@mrcuganda.org.
Godfrey Zari Rukundo, Phone: 0772663671, Email: grukundo@must.ac.ug.
Sylvia Kiwuwa Muyingo, Email: Sylvia.Muyingo@mrcuganda.org.
Kenneth D. Gadow, Phone: 631-638-1549, Email: kenneth.gadow@stonybrook.edu
Vikram Patel, Email: Vikram_Patel@hms.harvard.edu.
Eugene Kinyanda, Phone: 0788461950, Email: ekinyanda@hotmail.com, Email: Eugene.kinyanda@mrcuganda.org.
References
- 1.Benton TD, Lachman A, Seedat S. HIV/aids. Addressing the mental health needs of affected children and families. Geneva: International Association for Child and Adolescent Psychiatry and allied professions 2013; 2013. [Google Scholar]
- 2.Boivin MJ, Ruisenor-Escudero H, Familiar-Lopez I. CNS impact of perinatal HIV infection and early treatment: the need for behavioral rehabilitative interventions along with medical treatment and care. Curr HIV/AIDS Rep. 2016;13(6):318–327. doi: 10.1007/s11904-016-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen JP, Baig B, Abbo C, Baheretibeb Y. Child and adolescent mental health in sub-Saharan Africa: a perspective from clinicians and researchers. Bjpsych International 2016;13 (2). [DOI] [PMC free article] [PubMed]
- 4.Bearden D, Steenhoff AP, Dlugos DJ, Kolson D, Mehta P, Kessler S, et al. Early antiretroviral therapy is protective against epilepsy in children with human immunodeficiency virus infection in Botswana. J acquired immune deficiency syndromes (1999) 2015;69(2):193–199. doi: 10.1097/QAI.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benton TD. Psychiatric considerations in children and adolescents with HIV/AIDS. Child Adolesc Psychiatr Clin N Am. 2010;19(2):387–400. doi: 10.1016/j.chc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Mpango RS, Kinyanda E, Rukundo GZ, Levin J, Gadow KD, Patel V. Prevalence and correlates for ADHD and relation with social and academic functioning among children and adolescents with HIV/AIDS in Uganda. BMC psychiatry. 2017;17(1):336. doi: 10.1186/s12888-017-1488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mpango RS, Kinyanda E, Rukundo GZ, Gadow KD, Patel V. Cross-cultural adaptation of the Child and adolescent symptom Inventory-5 (CASI-5) for use in central and South-Western Uganda: the CHAKA project. Trop Dr. 2017;47(4):347–354. doi: 10.1177/0049475517724688. [DOI] [PubMed] [Google Scholar]
- 8.Gadow KD, Chernoff M, Williams PL, Brouwers P, Morse E, Heston J, et al. Co-occuring psychiatric symptoms in children perinatally infected with HIV and peer comparison sample. Journal of developmental and behavioral pediatrics : JDBP. 2010;31(2):116–128. doi: 10.1097/DBP.0b013e3181cdaa20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachman S, Chernoff M, Williams P, Hodge J, Heston J, Gadow KD. Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Archives of pediatrics & adolescent medicine. 2012;166(6):528–535. doi: 10.1001/archpediatrics.2011.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadow KD, Sprafkin J. Child & Adolescent Symptom Inventory-5 (Ages 5 to 18 Years) Stony Brook, NY: Checkmate Plus.; 2013 a,b. https://www.checkmateplus.com/product/casi5.htm].
- 11.Gadow KD, Kaat AJ, Lecavalier L. Relation of symptom-induced impairment with other illness parameters in clinic-referred youth. J child psychology and psychiatry, and allied disciplines. 2013;54(11):1198–1207. doi: 10.1111/jcpp.12077. [DOI] [PubMed] [Google Scholar]
- 12.Kellinghaus C, Engbring C, Kovac S, Moddel G, Boesebeck F, Fischera M, et al. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure. 2008;17(1):27–33. doi: 10.1016/j.seizure.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Pascual-Sedano B, Iranzo A, Marti-Fabregas J, Domingo P, Escartin A, Fuster M, et al. Prospective study of new-onset seizures in patients with human immunodeficiency virus infection: etiologic and clinical aspects. Arch Neurol. 1999;56(5):609–612. doi: 10.1001/archneur.56.5.609. [DOI] [PubMed] [Google Scholar]
- 14.Samia P, Petersen R, Walker KG, Eley B, Wilmshurst JM. Prevalence of seizures in children infected with human immunodeficiency virus. J Child Neurol. 2013;28(3):297–302. doi: 10.1177/0883073812446161. [DOI] [PubMed] [Google Scholar]
- 15.Bower WF, Moore KH, Shepherd RB, Adams RD. The epidemiology of childhood enuresis in Australia. Br J Urol. 1996;78(4):602–606. doi: 10.1046/j.1464-410X.1996.13618.x. [DOI] [PubMed] [Google Scholar]
- 16.Unalacak M, Sögüt A, Aktunc E, Demircan N, Altin R. Enuresis noctuma: Prevalence and risk factors among school age children in northwest Turkey 2004.
- 17.Sureshkumar P, Jones M, Caldwell PH, Craig JC. Risk factors for nocturnal enuresis in school-age children. J Urol. 2009;182(6):2893–2899. doi: 10.1016/j.juro.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 18.Metzger H, Wanderer S, V. VR. Tic disorders. 2012. In: IACAPAP ( International Association for Child and Adolescent Psychiatry and Allied Professions ) e-Textbook of Child and Adolescent Mental Health 2012 [Internet]. Geneva.
- 19.Berkovic SF. Genetics of epilepsy in clinical practice. Epilepsy currents. 2015;15(4):192–196. doi: 10.5698/1535-7511-15.4.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ba-Diop A, Marin B, Druet-Cabanac M, Ngoungou EB, Newton CR, Preux PM. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13(10):1029–1044. doi: 10.1016/S1474-4422(14)70114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Gontard A. Enuresis. In: IACAPAP (International Association for Child and Adolescent Psychiatry and allied professions ) e-textbook of Child and adolescent mental health [internet]. Geneva; 2012.
- 22.von Gontard A. Encopresis. In: IACAPAP (International Association for Child and Adolescent Psychiatry and allied professions ) e-textbook of Child and adolescent mental health [internet]. Geneva; 2012.
- 23.von Gontard A, Schaumburg H, Hollmann E, Eiberg H, Rittig S. The genetics of enuresis: a review. J Urol. 2001;166(6):2438–2443. doi: 10.1016/S0022-5347(05)65611-X. [DOI] [PubMed] [Google Scholar]
- 24.Joinson C, Heron J, Butler U, von Gontard A. Psychological differences between children with and without soiling problems. Pediatrics. 2006;117(5):1575–1584. doi: 10.1542/peds.2005-1773. [DOI] [PubMed] [Google Scholar]
- 25.Scahill L, Specht M, Page C. The prevalence of tic disorders and clinical characteristics in children. J obsessive-compulsive and related disorders. 2014;3(4):394–400. doi: 10.1016/j.jocrd.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer HS. Tourette’s syndrome: from behaviour to biology. Lancet Neurol. 2005;4(3):149–159. doi: 10.1016/S1474-4422(05)70018-1. [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. Bmj. 1995;310(6973):170. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data collection tools for the study. (DOCX 39 kb)
Characteristics of study participants. (DOCX 15 kb)
Correlates of neurological disorders. (DOCX 41 kb)
Data Availability Statement
All data and materials in this manuscript, additional files and figures attached are freely available with no restrictions.