Abstract
Atypical fibroxanthoma (AFX) is a rare mesenchymal tumor with predominance in older male patients located mainly in chronically UV-exposed skin. Differentiation from clinically more aggressive pleomorphic dermal sarcoma (PDS) is still under debate and immunohistochemical markers are not available yet. An immunohistochemical study, including 41 cases of AFX was conducted to investigate the expression of 3q encoded oncogene SEC62 in AFX and determine the associations with histomorphologic, clinical and viral parameters. Our cohort displayed a mean of 79.9 years at the onset of the disease. In total, 90.2% (37/41) AFXs were located in the head and neck area, whereas, four were located at the extremities (9.7%). Tumor diameter ranged between 0.06 and 40 cm2 with a mean of 5.7 cm2. SEC62 expression was markedly increased in lesional tissue compared with the adjacent healthy squamous epithelium. We found significantly higher expression of SEC62 in cases of AFX with tumor necrosis. Tendency of higher Sec62-IRS-scores were found for tumors with higher Clark levels and a tumor size >5 cm2. Sec62 is involved in endoplasmic reticulum stress tolerance and cell migration, and has been identified as a novel prognostic marker for non-small cell lung cancer as well as head and neck squamous cell carcinoma. For the first time, to the best of our knowledge, we suggest a role of 3q oncogene SEC62 in AFX and discuss a potential prognostic relevance in cases of disputable AFX with unfavorable histomorphologic features and may initiate a discussion on Sec62 serving as discriminating marker between AFX and PDS.
Keywords: atypical fibroxanthoma, SEC62, oncogenesis, prognosis, Merkel cell polyomavirus
Introduction
Atypical fibroxanthoma (AFX) is a comparably rare dermal neoplasm with only 3,000 cases reported in the literature so far (1). This tumor predominantly develops at sun-exposed areas of the human body with emphasis on the head and neck region, and typically affects more men than women with a median age above 60 years (1,2). While AFX can often show a large extension in the superficial skin layers, an invasion of deeper structures e.g., blood or lymph vessels, subcutaneous muscles or peripheral nerves is usually not found resulting in a classification as benign or semi-malignant tumor (3,4). Hence, the prognosis of AFX patients is excellent with a median 20-year disease-specific survival rate of 97.8% (1). After its first description in 1963, much effort was spent to better understand the tumorigenesis and molecular biology of this tumor entity including immunohistochemical analyses, electron microscopy, comparative genomic hybridization and next generation sequencing (5,6). However, the pathogenesis of AFX is still unclear with keratinocytes, fibroblasts and myofibroblasts having been discussed as potential cells of origin (7,8). Since this tumor shows a highly heterogeneous histological structure with tumor cells ranging from spindle and epithelioid to multinucleated cells and a variably structured extracellular matrix, the histological diagnosis is challenging with undifferentiated pleomorphic sarcoma, malignant fibrous histiocytoma, dedifferentiated squamous cell carcinoma, dermatofibrosarcoma protuberance and leiomyosarcoma as differential diagnoses (1,9). Immunohistochemical markers that can help to differentiate AFX from other tumor entities are CD99, S-100, CD34, cytokeratin, desmin, CD10, vimentin, HMB-45, CD68 and p63 (8–12). However, a disease-specific marker as well as a prognostic marker indicating a higher risk of recurrence or distant metastasis is still missing (1,3,4). In 2010 an association to Merkel Cell Polyomavirus was detected in 17% of all AFXs examined (13). Importance of this finding has to be further elucidated.
The SEC62 gene located at chromosomal region 3q26.2 encodes for a transmembrane protein of the endoplasmic reticulum (ER) that forms a heterotrimeric complex with the protein translocation pore and intracellular calcium channel Sec61 as well as Sec63 (14,15). Under physiological conditions, Sec62 is involved in the posttranslational transport of short secretory and transmembrane precursor proteins, possibly through its direct interaction with Sec61 and the ribosome (16–18). Apart from protein transport, Sec62 was shown to influence the passive calcium efflux through the Sec61 channel into the cytosol in an inhibitory manner (19–22). An amplification of the SEC62 encoding region 3q26.2 as well as an overexpression of the SEC62 gene was observed in various cancer entities including head and neck cancer (23,24), prostate cancer (25), esophageal cancer (26), cervical cancer (27,28), ovarian cancer (29) and non-small cell lung cancer (30). For NSCLC and HNSCC, high SEC62 expression level was a predictor of poor clinical outcome and significantly correlated with a positive lymph node status (31–33). In hepatocellular cancer, high SEC62 expression levels were correlated with a higher risk of recurrence after surgical treatment (34). Beneath its role as a prognostic biomarker, Sec62 was shown to influence tumor cell biology by stimulating cancer cell migration, invasion and enabling tumor cells to recover from ER stress by a mechanism called ‘recovER-phagy’ (21,35–39). These effects can explain how tumor cells profit from an increased SEC62 expression level and might be responsible for the poor prognosis of SEC62 overexpressing tumors. Based on the finding that the stimulation of cancer cell migration by Sec62 is probably mediated through its influence on the calcium homeostasis at the ER, the calmodulin antagonist trifluoperazine (TFP) could be identified as a potent agent to antagonize the calcium effect of Sec62 and thereby inhibiting Sec62 mediated cancer cell migration (21). Hence, TFP represents a promising agent for an antimetastatic therapy in SEC62 overexpressing tumors.
As for AFX, there exist neither reliable immunohistochemical markers enabling discrimination from other related sarcomatoid tumors nor prognostic biomarkers indicating a higher risk of recurrence or distant metastasis, we investigated in our study the expression of 3q oncogene SEC62 in 41 AFX cases and correlated the SEC62 expression level with the patients' clinical and viral data and the pathological characteristics of the tumors.
Materials and methods
General
Investigations were performed after approval by a local Human Investigations Committee, approval no. 281/10 (Ethikkomission der Ärztekammer des Saarlandes).
Patient characteristics and tissue samples
AFXs were retrieved from the histopathology archives of the department of dermatology. A period from 2006 to 2016 was investigated. Inclusion criteria for the study were availability of slides and blocks as well as tumors treated surgically by excision with curative intention. A total of 41 AFXs of 40 patients were investigated in this study. The following clinical and histopathologic features were evaluated: Sex, age and size, mitotic count, presence of necrosis, ulceration, vascular invasion as well as invasion depth and Clark level. Follow-up information was obtained from hospital medical records of the referring clinicians. Details of clinicopathological characteristics are summarized in Table I. Detailed histopathologic data is given in Table II.
Table I.
Clinical data of all patients enrolled in the present study.
| Patient no. | Age at time of diagnosis | Sex | Localization | Tumor diameter | Relapse | Distant metastases | Death |
|---|---|---|---|---|---|---|---|
| 1 | 63 | M | Head | 4.00 | No | No | No |
| 2 | 78 | M | Head | N.A. | No | No | No |
| 3 | 83 | M | Upper extremity | 1.00 | No | No | Yes |
| 4 | 84 | F | Head | 1.00 | No | No | No |
| 5 | 81 | M | Face | 0.06 | No | No | No |
| 6 | 77 | M | Head | 0.75 | No | No | No |
| 7 | 85 | M | Head | 4.00 | No | No | No |
| 8 | 81 | M | Head | 0.49 | No | No | Yes |
| 9 | 65 | M | Head | N.A. | No | No | No |
| 10 | 81 | M | Head | 1.00 | No | No | No |
| 11 | 83 | F | Head | N.A. | No | No | No |
| 12 | 78 | M | Head | 1.00 | No | No | Yes |
| 13 | 65 | M | Head | 1.65 | No | No | No |
| 14 | 81 | M | Head | N.A. | No | No | No |
| 15 | 81 | F | Head | 3.00 | No | No | No |
| 16 | 78 | M | Upper extremity | 4.00 | Yes | No | No |
| 17 | 79 | M | Head | 0.25 | No | No | No |
| 18 | 79 | M | Head | 2.50 | No | No | Yes |
| 19 | 84 | M | Head | N.A. | No | No | No |
| 20 | 72 | F | Lower extremity | 1.00 | No | No | No |
| 21 | 87 | M | Head | N.A. | No | No | No |
| 22 | 90 | F | Head | 4.00 | Yes | No | No |
| 23 | 92 | F | Head | 5.00 | No | No | No |
| 24 | 79 | M | Head | N.A. | No | No | No |
| 25 | 87 | M | Head | 40.00 | No | No | No |
| 26 | 80 | F | Face | 9.00 | No | No | No |
| 27 | 88 | M | Head | 25.00 | Yes | No | Yes |
| 28 | 79 | M | Head | 9.00 | No | No | No |
| 29 | 78 | M | Head | N.A. | No | No | No |
| 30 | 76 | M | Head | 5.75 | No | No | No |
| 31 | 80 | M | Head | 22.00 | No | No | No |
| 32 | 89 | M | Head | 9.00 | No | No | No |
| 33 | 87 | M | Head | 3.00 | No | No | No |
| 34 | 83 | M | Head | 9.00 | No | No | No |
| 35 | 84 | M | Head | N.A. | No | No | Yes |
| 36 | 78 | M | Head | 5.00 | No | No | No |
| 37 | 73 | M | Face | 0.32 | No | No | No |
| 38 | 72 | M | Lower extremity | 2.70 | No | No | No |
| 39 | 62 | M | Head | 1.00 | No | No | No |
| 40 | 88 | M | Head | N.A. | No | No | No |
| 41 | 89 | F | Face | 1.00 | No | No | No |
M, male; F, female; N.A., not applicable.
Table II.
Histomorphologic data of all atypical fibroxanthomas of the present study.
| Patient no. | Vertical tumor depth, mm | Clark level | Necrosis | Ulceration | Mitotic count | Vascular invasion | MCPyV DNA | SEC62 Immunoscore |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.35 | 5 | Yes | Yes | 8.70 | No | + | 9.0 |
| 2 | 2.68 | 3 | No | Yes | 3.55 | No | – | 2.6 |
| 3 | 2.20 | 3 | No | No | 2.40 | No | + | 6.0 |
| 4 | 2.90 | 4 | No | Yes | 4.40 | No | – | 8.3 |
| 5 | 2.20 | 5 | No | No | 1.50 | No | – | N.A. |
| 6 | 2.40 | 4 | No | No | 2.70 | No | – | N.A |
| 7 | 2.16 | 3 | No | Yes | 4.60 | No | – | 5.6 |
| 8 | 5.70 | 2 | No | Yes | 5.90 | No | – | 4.0 |
| 9 | 2.16 | 3 | No | No | 4.00 | No | – | 4.3 |
| 10 | 7.20 | 5 | Yes | Yes | 4.80 | No | – | 1.0 |
| 11 | 8.00 | 5 | No | Yes | N.A. | No | – | 6.3 |
| 12 | 3.10 | 4 | No | No | 4.20 | No | – | 11.0 |
| 13 | 6.90 | 4 | No | No | 4.80 | No | – | 9.3 |
| 14 | 4.08 | 5 | No | Yes | 5.70 | No | – | 10.0 |
| 15 | 7.50 | 5 | No | Yes | 7.50 | No | – | 8.0 |
| 16 | 5.80 | 5 | No | Yes | 5.70 | No | – | 8.0 |
| 17 | 1.29 | 4 | No | No | 2.40 | No | – | 8.6 |
| 18 | 4.80 | 4 | Yes | Yes | 1.10 | No | – | 12.0 |
| 19 | 14.00 | 2 | No | Yes | 8.90 | No | – | 10.0 |
| 20 | 2.40 | 2 | No | Yes | 3.70 | No | – | 6.6 |
| 21 | 6.52 | 5 | No | Yes | 1.00 | No | – | 3.6 |
| 22 | 5.28 | 5 | No | Yes | 5.70 | No | – | 7.3 |
| 23 | 2.60 | 4 | No | Yes | 3.80 | No | + | 8.0 |
| 24 | 4.08 | 4 | Yes | Yes | 5.60 | No | + | 11.0 |
| 25 | 40.00 | 5 | Yes | Yes | N.A. | No | – | 10.0 |
| 26 | 4.80 | 5 | No | Yes | 50.40 | No | – | 7.0 |
| 27 | 2.90 | 4 | No | Yes | 5.10 | No | – | 9.0 |
| 28 | 4.08 | 4 | No | No | 6.10 | No | – | 6.0 |
| 29 | 2.68 | 3 | No | Yes | 3.50 | No | – | 6.6 |
| 30 | 9.50 | 4 | No | No | 5.60 | No | – | 11.0 |
| 31 | 5.80 | 4 | No | Yes | 3.70 | No | – | 9.0 |
| 32 | 7.20 | 5 | No | Yes | 8.40 | No | – | 6.0 |
| 33 | 7.68 | 5 | No | No | 7.00 | No | – | 11.0 |
| 34 | 4.27 | 3 | Yes | Yes | 10.20 | No | – | 12.0 |
| 35 | 4.17 | 5 | No | Yes | 7.90 | No | – | 6.6 |
| 36 | 6.50 | 5 | No | Yes | 4.90 | No | – | 6.6 |
| 37 | 4.80 | 4 | No | Yes | 3.30 | No | – | N.A. |
| 38 | 1.90 | 3 | No | No | 3.60 | No | – | N.A. |
| 39 | 4.30 | 5 | No | No | 2.50 | No | – | N.A. |
| 40 | 6.40 | 5 | No | Yes | 3.80 | No | – | N.A. |
| 41 | 2.00 | 3 | No | No | 4.80 | No | – | N.A. |
N.A., not applicable.
Immunohistochemical analysis
FFPE tissue sections were obtained and used for immunohistochemical staining of Sec62. After omitting the first three 10-µm sections, consecutive 4-µm sections were obtained using a Leica RM 2235 rotary microtome (Leica Microsystems, Wetzlar, Germany), transferred onto Superfrost Ultra Plus microscope slides (Menzel-Gläser, Braunschweig, Germany) and dried in an incubator at 37°C overnight. Upon deparaffinization, heat-induced epitope retrieval was performed by incubation in 10 mM citrate buffer (pH 6.0) at 95°C for 30 min. Unspecific protein binding sites were blocked with 3% BSA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in PBS for 30 min at room temperature. Subsequently, primary antibody incubation was performed using an affinity-purified polyclonal rabbit anti-peptide antibody directed against the C terminus of human Sec62 (self-made). For each staining series, a specimen taken from a subcutaneously grown tumor in mice after local injection of UM-SCC1 cells (SEC62 overexpressing cell line) was used as positive control as well as negative controls by omitting the primary antibody. Visualization was performed using the REAL™ detection system Alkaline Phosphatase (Dako Agilent Technologies, Glostrup, Denmark), according to the manufacturer's instructions, and the slides were counterstained with hematoxylin (Dako Agilent Technologies). Sec62-immunoreactivity was evaluated using an immunoreactive score (IRS) according to Remmele and Stegner (40) with values ranging from 0 to 12. All immunohistochemical stainings were valued by three experienced examiners including one dermatopathologist and the mean values of the three scorings were used for statistical analysis. 34/41 cases were available for immunohistochemistry with sec62.
Specific detection of mitoses was performed using phosphohistone H3 (pHH3; polyclonal antibody, Cell Marque® no. 369A) at a dilution of 1:100 (pre-treatment for 30 min in a steamer) in accordance with the manufacturer's protocol. Mitotic figures labeled with pHH3 were twice counted in 10 high-power-fields within the ‘hot spot’ of the tumour and mean of mitoses was calculated.
MCPyV-DNA PCR
MCPyV-DNA-specific PCR was performed for all FFPE tissue specimens (n=41). DNA was extracted from the FFPE tissue samples using a QIAamp DNA FFPE Tissue kit (Qiagen N.V., Hilden, Germany) according to the manufacturer's instructions. MCPyV-DNA-PCR was performed with the LightCycler 2.0 instrument (Roche Diagnostics GmbH, Mannheim, Germany) using MCPyV-specific primers LT3F (5′-ttg tct cgc cag cat tgt ag-3′) and LT3R (5′-ata tag ggg cct cgt caa cc-3′) described by Feng et al (41). Cycling conditions were 94°C for 3 min, followed by 45 PCR cycles with denaturation at 94°C for 45 sec, annealing at 58°C for 30 sec, elongation at 72°C for 45 sec and finished by a last elongation at 72°C for 15 min. After amplification, the PCR products (10 µl) were separated on 2% agarose gels and visualized by ethidium bromide staining. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) PCR served as an internal control and was performed in parallel for each sample as described previously by Sperling et al (42).
Statistical analysis
Beside descriptive statistical analyses (frequencies, mean and standard deviation) the comparison of groups was performed with the Mann-Whitney U test resp. Kruskal-Wallis-Test. The analyses were executed with SPSS v. 17.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical and pathological characteristics of patients
Details of clinicopathological characteristics are summarized in Table I. Detailed histopathologic data is given in Table II. 33 male and 8 female patients were included in this study. Age at time of diagnosis ranged from 62 to 92 years, with a mean of 79.9 years. Clinical information on exact localization of tumors was available in all tumors. 90.2% (37/41) of tumors were located in the head and neck area, while 4 where located at the extremities (9.7%). Tumor diameter ranged from 0,06 to 40 cm2 with a mean of 5.7 cm2.
One female patient developed 2 separate tumors, when she was 90 years old and the second when she was 92 of age. Relapse of AFXs was seen in three patients (7.3%), distant metastases did not occur in any patient of this cohort. 6/40 (14.6%) patients died within observation period. Due to low number of cases we did not differentiate between the several morphologic variants of AFX, that have been described (spindle-cell nonpleomorphic AFX, clear-cell AFX, pigmented, myxoid, osteoclast-like giant cell rich AFX keloidal and granular cell AFX)43. Invasion depth of all tumors ranged from 1.29 to 40 mm with a mean of 5.6 mm. In analogy to Clark Level (CL) in malignant melanoma (CL I-melanoma in situ, CL II-infiltration of the upper part of the papillary dermis, CL III-expansion of melanoma cells into the papillary dermis and upper reticular dermis, CL IV-infiltration of the reticular dermis and CL V-infiltration of subcutis44) we analyzed invasion of anatomic levels of the skin: CL I is not applicable per definitionem as AFXs are primary dermal tumors. CL II was seen in 7% (3/41), CL III was observed in 19% (8/41). Thirteen cases showed CL IV (31%) and 41% (17/41) of the cases displayed extension to subcutaneous tissue. Tumor necrosis was observed in 14.3% (6/41), ulceration of the overlying epidermis was seen in 69% (28/40). Vascular invasion was seen in none of the cases of this cohort. Merkel Cell Polyomavirus (MCPyV)-DNA was detected in 4 cases (9.7%). As a trend, three of these cases were male with ulcerated tumors and one patient was female. One of these 4 patients died within observation period. A mean of 5.9 mitoses could be detected in 10 hotspot areas of every tumor.
Expression analysis of SEC62 oncogene
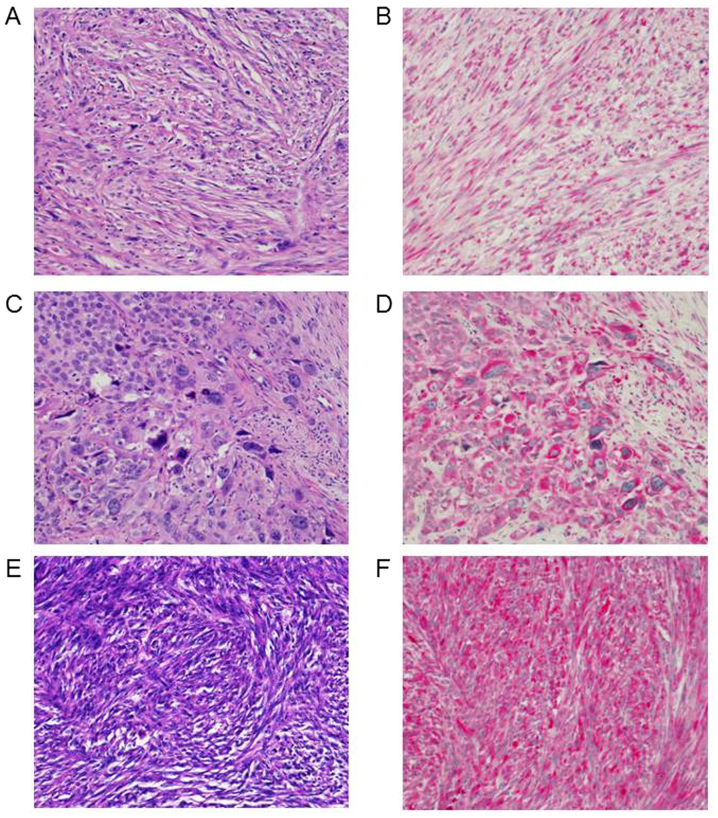
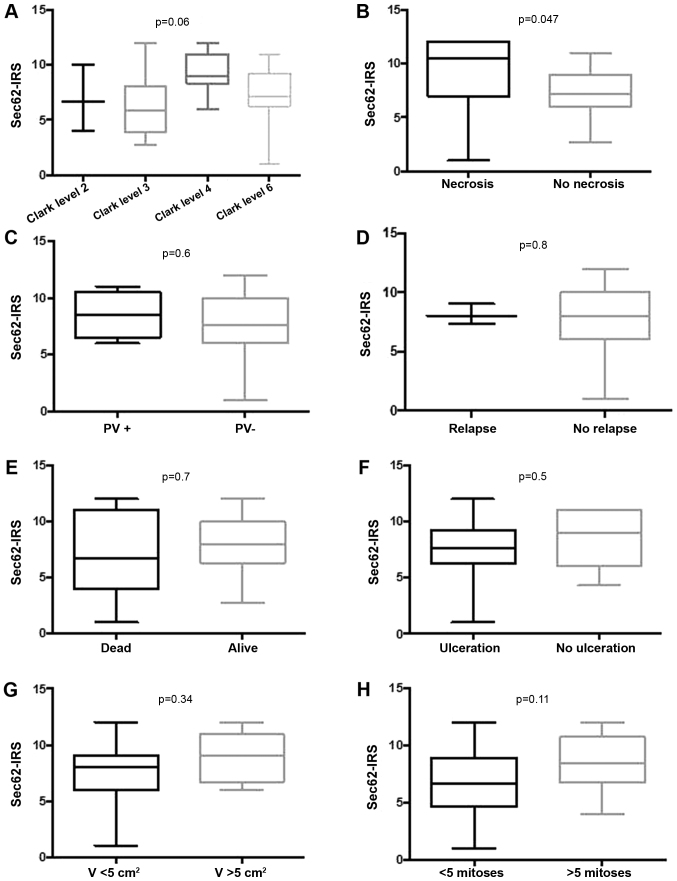
Immunoscore of 3q oncogene SEC62 could be investigated in 34 cases of all AFXs (Fig. 1). In 100% (34/34) of the samples cytoplasmatic expression of 3q oncogene SEC62 within the mesenchymal tumor cells was seen. Mean score was 7.7 with a minimum of 1 and a maximum score of 12. Exemplary cases of distinct Sec62-IRS-scores are shown in Fig. 1. Intriguingly, we found significantly higher expression of SEC62 in cases of AFX with tumor necrosis (Fig. 2G), while there was no statistically relevant dependency on invasion depth, ulceration, relapse of tumor, distant metastases or death due to tumor (Fig. 2). Hence, tendency of higher SEC62-IRS-scores were found for tumors with higher Clark levels and a tumor size greater 5 cm2, although reaching no significance level (Fig. 2G).
Figure 1.
Differential cytoplasmatic expression of 3q oncogene SEC62 in AFX. Left column see hematoxylin and eosin-stain, original magnification, ×200. Right column see Sec62-stain. Original magnification, ×200. (A) AFX, patient no. 21: Mixed type with high nuclear pleomorphism and partly storiform tumor growth of mainly spindle-shaped cells. (B) Patient no. 21: Sec62-IRS 3,6. (C) AFX, patient no. 20: Pleomorphic type tumor with bizarre tumor cells and highly atypical mitotic figures. Please note areas with epithelioid differentiation. (D) Patient no. 20: Sec62-IRS 6,6. (E) AFX, patient no. 15: Storiform type of tumor with predominantly spindle-shaped cells in a regular growth. (F) Patient no. 15: Sec62-IRS 8. AFX, atypical fibroxanthoma.
Figure 2.
Correlation of Sec62-IRS with clinical and histopathological features. (A) Correlation of Sec62-IRS with tumor stage according to the Clark level. (B) Correlation of Sec62-IRS with the presence of intratumoral necrotic areas. (C) Correlation of Sec62-IRS with the presence of polyomavirus DNA in lesional tissue. (D) Correlation of Sec62-IRS with a relapse of the disease. (E) Correlation of Sec62-IRS with the non-disease-specific survival of the patients. (F) Correlation of Sec62 with an ulcerous growth pattern of the tumor. (G) Correlation of Sec62-IRS with the volume of the tumor. (H) Correlation of Sec62-IRS with the frequency of mitoses. Sec62-IRS values are shown using box and whisker blots. Each box represents the range from the first quartile to the third quartile. The median is indicated by a line. The whiskers outside the boxes represent the ranges from the minimum to the maximum value of each group. IRS, immunoreactive score; PV, Polyomavirus.
Analysis of MCPyV DNA
MCPyV DNA positive cases did not show any statistically significant differences in 3q oncogene SEC62 expression, probably due to low number of positive cases (Fig. 2C). There was also no dependency between invasion depth of the tumor in correlation with virus-positive cases, Clark level, necroses, ulceration, relapse or death due to disease (data not shown).
Discussion
AFX is a mesenchymal neoplasm, rarely seen in daily routine, even in dermatology and dermatopathology units. Relationship of AFX and pleomorphic dermal sarcoma (PDS) is still not clearly identified (43). As AFX is a diagnosis of exclusion, it is mandatory to apply strict diagnostic criteria that include diligent immunohistochemical workup (8–12).
There is growing evidence that viruses play an important role in tumorigenesis, mainly in immunosuppressed patients and an estimated 20% of global cancer burden is related to viral infections (44). Merkel Cell Polyomavirus (MCPyV) was recently detected in Merkel cell carcinoma (MCC) and approximately 91.2% of MCC are MCPyV-positive (41,45). Intriguingly, 85% of all healthy adults and 58% of children younger than 10 years are MCPyV-positive displaying a high seroprevalence of MCPyV (46). In 2010 MCPyV was initially detected within samples of AFX with an incidence of 17% in this study (13). The authors concluded that MCPyV may act as a cofactor in the tumorigenesis of a subset of AFXs (13). In our cohort we were able to detect MCPyV-DNA in 9.7% only. Comparable to Andres et al, virus-positive AFXs were detected predominantly in older males with ulcerated tumors (13), but with no correlation to invasion depth or tumor size in our study. At the moment, the role of MCPyV in AFXs remains unclear. Due to high seroprevalence of MCPyV and possibility of viral persistence in several compartments of the body (body fluids, tissue biopsies, several organ specimens) its role in tumorigenesis of AFX remains to be determined (46). MCPyV is frequently detected on healthy human skin (46,47). Therefore, it cannot be excluded that MCPyV detection may only be a bystander phenomenon without clinical implication (46). Intriguingly, in 2016 Liu and colleagues managed to identify human dermal fibroblasts as the primary skin cell type supporting MCPyV gene expression and productive replication after infectious entry (48). Hence, they showed MCPyV infection is promoted via induction of matrix metalloproteinase (MMP) genes by the WNT/β-catenin signaling pathway. It is already known that UV radiation stimulates the WNT signaling and MMP expression (48,49). Taken together molecular pathways with connection to chronic UV exposure and host cell of MCPyV, our findings indicate towards a pathogenetic relationship between infection and AFX. Though, number of MCPyV cases actually is too low to draw clear conclusions.
Prognostic or predictive biomarkers do not exist for AFXs, neither do any histomorphologic parameters exist, that predict clinical outcome and metastatic potential. Of greater importance concerning prognosis than histomorphologic parameters is surgical margin status, as clear margins are associated with improved clinical outcome (50).
In contrast, histomorphologic parameters of prognostic relevance are well defined for malignant melanoma: Histogenetic subtype, Breslow thickness, Clark level, mitotic figures, ulceration, regression and others (51). Results of multivariate analysis of these factors in large melanoma cohorts are reflected in the AJCC (American Joint Committee on Cancer) for melanoma staging and classification from 2009 (52). Hence, in this study we were not able to identify clinical, viral or histomorphological parameters (displaying worse prognosis for instance in malignant melanoma) that correlated with clinical outcome of the patients. Discussion concerning differentiation of AFX from pleomorphic sarcoma is still ongoing.
For the 3q encoded SEC62 gene, an overexpression was found in a variety of human cancers (21,25,31,32,36,37,39) and in non-small cell lung cancer, cervical cancer as well as head and neck cancer, high SEC62 expression correlated with a significantly shorter overall survival. These findings indicate a general role of SEC62 as an oncogene in the pathogenesis of human cancer and emphasize the role of SEC62 expression level as a prognostic factor in various cancer entities (53). In our study, we found markedly increased SEC62 expression levels in the lesional tissue compared with the adjacent healthy squamous epithelium in the vast majority of cases pointing towards an oncogenic function of SEC62 in AFX, as well. While significantly higher SEC62 expression levels were found in AFX samples that showed intratumoral necrotic areas, which is known to be an adverse prognostic factor in this entity (1), we found no significant correlation of SEC62 expression with other clinical and histopathological features including the patients' survival. However, given the comparably low number of cases and the only marginal portion of cases of death, these data do not exclude a potential prognostic relevance of Sec62 in AFX which is further indicated by the association of high SEC62 expression levels with advanced Clark levels and tumor size (see Fig. 2A and G). As it is recommended that tumors displaying prognostically unfavorable features (extension to subcutaneous fat, corresponding to Clark level V, perineural invasion or necrosis), should be diagnosed as PDS. Hence, high expression levels of SEC62 could serve as a diagnostic parameter for distinction between AFX and PDS. This has to be further investigated with higher number of cases.
Regarding the functional role of Sec62 in tumor cell biology, it was shown that high Sec62 levels can stimulate the migration of tumor cells (21,31,39) as well as their resistance to ER stress (37). If comparable effects can be seen when SEC62 is overexpressed in AFX cells is a remaining question, which will be ambitious to answer keeping in mind the extremely rare metastasis rate of AFX (1,54) and the fact that there are no established AFX cell lines available.
Acknowledgments
The authors would like to thank Mrs. Anne Kerber, Mrs. Alexandra Stark, Mrs Monika Hoffmann and Mrs Ulrike Bechtel of Saarland University Medical Center (Homburg, Germany) for their technical support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CSLM and ML were major contributors in writing the manuscript, made substantial contributions to the conception and design of the study, and gave final approval of the version to be published. LK and FB performed the immunohistochemical staining. TP and SS performed the virological analyzes. SG conducted the statistical tests. TV and BS critically read the manuscript and were major contributors in the study design. All authors read and approve the final manuscript.
Ethics approval and consent to participate
Investigations were performed after approval by a local Human Investigations Committee (approval no. 281/10; Ethikkomission der Ärztekammer des Saarlandes). All participants gave their informed consent to participate in the study.
Patient consent for publication
Informed consent for publication of the data was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Koch M, Freundl AJ, Agaimy A, Kiesewetter F, Künzel J, Cicha I, Alexiou C. Atypical fibroxanthoma-histological diagnosis, immunohistochemical markers and concepts of therapy. Anticancer Res. 2015;35:5717–5735. [PubMed] [Google Scholar]
- 2.Kim YS, Lee HM, Kim JP, Lim CR. Unusual presentation of a type 1 Monteggia equivalent lesion: Simultaneous medial humeral condyle fracture with ipsilateral anterior dislocation of the radial head and acute plastic bowing of the ulna. J Pediatr Orthop B. 2014;23:383–388. doi: 10.1097/BPB.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 3.Tchernev G, Tronnier M, Ananiev J, Taneva T, Patterson JW, Gulubova M, Trafeli JP, Gegova A, Harrell M, Guarneri C, et al. Atypical fibroxanthoma-a diagnosis of exclusion! Wien Med Wochenschr. 2013;163:380–386. doi: 10.1007/s10354-012-0173-1. [DOI] [PubMed] [Google Scholar]
- 4.Ziemer M. Atypical fibroxanthoma. J Dtsch Dermatol Ges. 2012;10:537–550. doi: 10.1111/j.1610-0387.2012.07980_suppl.x. [DOI] [PubMed] [Google Scholar]
- 5.Helbig D, Ihle MA, Pütz K, Tantcheva-Poor I, Mauch C, Büttner R, Quaas A. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget. 2016;7:21763–21774. doi: 10.18632/oncotarget.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss A, Vanchinathan V, Kwon EJ. Aberrant tyrosinase expression in an atypical fibroxanthoma: A case report. J Cutan Pathol. 2017;44:467–469. doi: 10.1111/cup.12900. [DOI] [PubMed] [Google Scholar]
- 7.Lopez L, Velez R. Atypical fibroxanthoma. Arch Pathol Lab Med. 2016;140:376–379. doi: 10.5858/arpa.2014-0495-RS. [DOI] [PubMed] [Google Scholar]
- 8.Toll A, Gimeno J, Baró T, Hernández-Munoz MI, Pujol RM. Study of epithelial to mesenchymal transition in atypical fibroxanthoma and undifferentiated pleomorphic sarcoma to discern an epithelial origin. Am J Dermatopathol. 2016;38:270–277. doi: 10.1097/DAD.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 9.Mahalingam S, Shah A, Stewart A. Atypical Fibroxanthoma: A case series and review of literature. Auris Nasus Larynx. 2015;42:469–471. doi: 10.1016/j.anl.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 10.de Feraudy S, Mar N, McCalmont TH. Evaluation of CD10 and procollagen 1 expression in atypical fibroxanthoma and dermatofibroma. Am J Surg Pathol. 2008;32:1111–1122. doi: 10.1097/PAS.0b013e31816b8fce. [DOI] [PubMed] [Google Scholar]
- 11.Gleason BC, Calder KB, Cibull TL, Thomas AB, Billings SD, Morgan MB, Hiatt KM, Smoller BR. Utility of p63 in the differential diagnosis of atypical fibroxanthoma and spindle cell squamous cell carcinoma. J Cutan Pathol. 2009;36:543–547. doi: 10.1111/j.1600-0560.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 12.Tallon B, Beer TW. MITF positivity in atypical fibroxanthoma. Am J Dermatopathol. 2016;38:165–166. doi: 10.1097/DAD.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 13.Andres C, Puchta U, Flaig MJ. Detection of Merkel cell polyomavirus DNA in atypical fibroxanthoma in correlation to clinical features. Am J Dermatopathol. 2010;32:799–803. doi: 10.1097/DAD.0b013e3181dfcdff. [DOI] [PubMed] [Google Scholar]
- 14.Conti BJ, Devaraneni PK, Yang Z, David LL, Skach WR. Cotranslational stabilization of Sec62/63 within the ER Sec61 translocon is controlled by distinct substrate-driven translocation events. Mol Cell. 2015;58:269–283. doi: 10.1016/j.molcel.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer HA, Grau H, Kraft R, Kostka S, Prehn S, Kalies KU, Hartmann E. Mammalian Sec61 is associated with Sec62 and Sec63. J Biol Chem. 2000;275:14550–14557. doi: 10.1074/jbc.275.19.14550. [DOI] [PubMed] [Google Scholar]
- 16.Lakkaraju AK, Thankappan R, Mary C, Garrison JL, Taunton J, Strub K. Efficient secretion of small proteins in mammalian cells relies on Sec62-dependent posttranslational translocation. Mol Biol Cell. 2012;23:2712–2722. doi: 10.1091/mbc.e12-03-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang S, Benedix J, Fedeles SV, Schorr S, Schirra C, Schäuble N, Jalal C, Greiner M, Hassdenetufel S, Tatzelt J, et al. Different effects of Sec61α, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J Cell Sci. 2012;125:1958–1969. doi: 10.1242/jcs.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller L, de Escauriaza MD, Lajoie P, Theis M, Jung M, Müller A, Burgard C, Greiner M, Snapp EL, Dudek J, Zimmermann R. Evolutionary gain of function for the ER membrane protein Sec62 from yeast to humans. Mol Biol Cell. 2010;21:691–703. doi: 10.1091/mbc.e09-08-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdmann F, Schäuble N, Lang S, Jung M, Honigmann A, Ahmad M, Dudek J, Benedix J, Harsmann A, Kopp A, et al. Interaction of calmodulin with Sec61α limits Ca2+ leakage from the endoplasmic reticulum. EMBO J. 2011;30:17–31. doi: 10.1038/emboj.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang S, Erdmann F, Jung M, Wagner R, Cavalie A, Zimmermann R. Sec61 complexes form ubiquitous ER Ca2+ leak channels. Channels (Austin) 2011;5:228–235. doi: 10.4161/chan.5.3.15314. [DOI] [PubMed] [Google Scholar]
- 21.Linxweiler M, Schorr S, Schäuble N, Jung M, Linxweiler J, Langer F, Schäfers HJ, Cavalié A, Zimmermann R, Greiner M. Targeting cell migration and the endoplasmic reticulum stress response with calmodulin antagonists: A clinically tested small molecule phenocopy of SEC62 gene silencing in human tumor cells. BMC Cancer. 2013;13:574. doi: 10.1186/1471-2407-13-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schäuble N, Lang S, Jung M, Cappel S, Schorr S, Ulucan Ö, Linxweiler J, Dudek J, Blum R, Helms V, et al. BiP-mediated closing of the Sec61 channel limits Ca2+ leakage from the ER. EMBO J. 2012;31:3282–3296. doi: 10.1038/emboj.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bockmühl U, Schwendel A, Dietel M, Petersen I. Distinct patterns of chromosomal alterations in high- and low-grade head and neck squamous cell carcinomas. Cancer Res. 1996;56:5325–5329. [PubMed] [Google Scholar]
- 24.Sheu JJ, Lee CH, Ko JY, Tsao GS, Wu CC, Fang CY, Tsai FJ, Hua CH, Chen CL, Chen JY. Chromosome 3p12.3-p14.2 and 3q26.2-q26.32 are genomic markers for prognosis of advanced nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2709–2716. doi: 10.1158/1055-9965.EPI-09-0349. [DOI] [PubMed] [Google Scholar]
- 25.Jung V, Kindich R, Kamradt J, Jung M, Müller M, Schulz WA, Engers R, Unteregger G, Stöckle M, Zimmermann R, Wullich B. Genomic and expression analysis of the 3q25-q26 amplification unit reveals TLOC1/SEC62 as a probable target gene in prostate cancer. Mol Cancer Res. 2006;4:169–176. doi: 10.1158/1541-7786.MCR-05-0165. [DOI] [PubMed] [Google Scholar]
- 26.Chang YC, Yeh KT, Liu TC, Chang JG. Molecular cytogenetic characterization of esophageal cancer detected by comparative genomic hybridization. J Clin Lab Anal. 2010;24:167–174. doi: 10.1002/jcla.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen DG, White DJ, Hutchins AM, Scurry JP, Tabrizi SN, Garland SM, Armes JE. Progressive genetic aberrations detected by comparative genomic hybridization in squamous cell cervical cancer. Br J Cancer. 2000;83:1659–1663. doi: 10.1054/bjoc.2000.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heselmeyer K, Macville M, Schröck E, Blegen H, Hellström AC, Shah K, Auer G, Ried T. Advanced-stage cervical carcinomas are defined by a recurrent pattern of chromosomal aberrations revealing high genetic instability and a consistent gain of chromosome arm 3q. Genes Chromosomes Cancer. 1997;19:233–240. doi: 10.1002/(SICI)1098-2264(199708)19:4<233::AID-GCC5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Haverty PM, Hon LS, Kaminker JS, Chant J, Zhang Z. High-resolution analysis of copy number alterations and associated expression changes in ovarian tumors. BMC Med Genomics. 2009;2:21. doi: 10.1186/1755-8794-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dehan E, Ben-Dor A, Liao W, Lipson D, Frimer H, Rienstein S, Simansky D, Krupsky M, Yaron P, Friedman E, et al. Chromosomal aberrations and gene expression profiles in non-small cell lung cancer. Lung Cancer. 2007;56:175–184. doi: 10.1016/j.lungcan.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Bochen F, Adisurya H, Wemmert S, Lerner C, Greiner M, Zimmermann R, Hasenfus A, Wagner M, Smola S, Pfuhl T, et al. Effect of 3q oncogenes SEC62 and SOX2 on lymphatic metastasis and clinical outcome of head and neck squamous cell carcinomas. Oncotarget. 2017;8:4922–4934. doi: 10.18632/oncotarget.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linxweiler M, Linxweiler J, Barth M, Benedix J, Jung V, Kim YJ, Bohle RM, Zimmermann R, Greiner M. Sec62 bridges the gap from 3q amplification to molecular cell biology in non-small cell lung cancer. Am J Pathol. 2012;180:473–483. doi: 10.1016/j.ajpath.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 33.Wemmert S, Lindner Y, Linxweiler J, Wagenpfeil S, Bohle R, Niewald M, Schick B. Initial evidence for Sec62 as a prognostic marker in advanced head and neck squamous cell carcinoma. Oncol Lett. 2016;11:1661–1670. doi: 10.3892/ol.2016.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng L, Du J, Zhou Q, Cheng B, Li J, Zhang D, Ling C. Identification of cyclin B1 and Sec62 as biomarkers for recurrence in patients with HBV-related hepatocellular carcinoma after surgical resection. Mol Cancer. 2012;11:39. doi: 10.1186/1476-4598-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fumagalli F, Noack J, Bergmann TJ, Cebollero E, Pisoni GB, Fasana E, Fregno I, Galli C, Loi M, Soldà T, et al. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat Cell Biol. 2016;18:1173–1184. doi: 10.1038/ncb3423. [DOI] [PubMed] [Google Scholar]
- 36.Greiner M, Kreutzer B, Jung V, Grobholz R, Hasenfus A, Stöhr RF, Tornillo L., Dudek J, Stöckle M, Unteregger G, et al. Silencing of the SEC62 gene inhibits migratory and invasive potential of various tumor cells. Int J Cancer. 2011;128:2284–2295. doi: 10.1002/ijc.25580. [DOI] [PubMed] [Google Scholar]
- 37.Greiner M, Kreutzer B, Lang S, Jung V, Cavalié A, Unteregger G, Zimmermann R, Wullich B. Sec62 protein level is crucial for the ER stress tolerance of prostate cancer. Prostate. 2011;71:1074–1083. doi: 10.1002/pros.21324. [DOI] [PubMed] [Google Scholar]
- 38.Hagerstrand D, Tong A, Schumacher SE, Ilic N, Shen RR, Cheung HW, Vazquez F, Shresta Y, Kim SY, Giacomelli AO, et al. Systematic interrogation of 3q26 identifies TLOC1 and SKIL as cancer drivers. Cancer Discov. 2013;3:1044–1057. doi: 10.1158/2159-8290.CD-12-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linxweiler M, Bochen F, Schick B, Wemmert S, Al Kadah B, Greiner M, Hasenfus A, Bohle RM, Juhasz-Böss I, Solomayer EF, Takacs ZF. Identification of SEC62 as a potential marker for 3q amplification and cellular migration in dysplastic cervical lesions. BMC Cancer. 2016;16:676. doi: 10.1186/s12885-016-2739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 41.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling T, Oldak M, Walch-Rückheim B, Wickenhauser C, Doorbar J, Pfister H, Malejczyk M, Majewski S, Keates AC, Smola S. Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012;8:e1002833. doi: 10.1371/journal.ppat.1002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mentzel T, Requena L, Brenn T. Atypical Fibroxanthoma revisited. Surg Pathol Clin. 2017;10:319–335. doi: 10.1016/j.path.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Luo GG, Ou JH. Oncogenic viruses and cancer. Virol Sin. 2015;30:83–84. doi: 10.1007/s12250-015-3599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Álvarez-Argüelles ME, Melón S, Rojo S, Fernandez-Blázquez A, Boga JA, Palacio A, Vivanco B, de Oña M. Detection and quantification of Merkel cell polyomavirus. Analysis of Merkel cell carcinoma cases from 1977 to 2015. J Med Virol. 2017;89:2224–2229. doi: 10.1002/jmv.24896. [DOI] [PubMed] [Google Scholar]
- 46.Mancuso G, Antona J, Sirini C, Salvo M, Giacometti L, Olivero C, Trisolini E, Indellicato R, Boldorini R. Frequent detection of Merkel cell polyomavirus DNA in tissues from 10 consecutive autopsies. J Gen Virol. 2017;98:1372–1376. doi: 10.1099/jgv.0.000778. [DOI] [PubMed] [Google Scholar]
- 47.Foulongne V, Sauvage V, Hebert C, Dereure O, Cheval J, Gouilh MA, Pariente K, Segondy M, Burguière A, Manuguerra JC, et al. Human skin microbiota: High diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS One. 2012;7:e38499. doi: 10.1371/journal.pone.0038499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, Yang R, Payne AS, Schowalter RM, Spurgeon ME, Lambert PF, Xu X, Buck CB, You J. Identifying the target cells and mechanisms of merkel cell polyomavirus infection. Cell Host Microbe. 2016;19:775–787. doi: 10.1016/j.chom.2016.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, MacDonald M, You J. Merkel cell polyomavirus infection and Merkel cell carcinoma. Curr Opin Virol. 2016;20:20–27. doi: 10.1016/j.coviro.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nonaka D, Bishop PW. Sarcoma-like tumor of head and neck skin. Am J Surg Pathol. 2014;38:956–965. doi: 10.1097/PAS.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 51.Ivan D, Prieto VG. An update on reporting histopathologic prognostic factors in melanoma. Arch Pathol Lab Med. 2011;135:825–829. doi: 10.5858/2010-0229-RAR.1. [DOI] [PubMed] [Google Scholar]
- 52.Balch CM, Gershenwald JE, Soong SJ, Thimpson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–1206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linxweiler M, Schick B, Zimmermann R. Let's talk about Sec62: Sec61, Sec62 and Sec63 in signal transduction, oncology and personalized medicine. Signal Transduct Target Ther. 2017;2:17002. doi: 10.1038/sigtrans.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helwig EB, May D. Atypical fibroxanthoma of the skin with metastasis. Cancer. 1986;57:368–376. doi: 10.1002/1097-0142(19860115)57:2<368::AID-CNCR2820570230>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.