Abstract
The expression of matrix metalloproteinase-2 (MMP-2) in brain glioma and its correlation with patients' clinicopathological characteristics and magnetic resonance imaging (MRI) features were investigated. A total of 104 patients with brain glioma admitted and treated in the First Affiliated Hospital of Anhui Medical University from June 2010 to September 2014 were randomly enrolled. MRI examination was performed before operation. Immunohistochemistry (IHC) was used to detect the expression levels of MMP-2 in brain glioma tissues and paired normal brain tissues after operation and to analyze the associations of MMP-2 expression with the clinicopathological characteristics of brain glioma and survival time of patients. The relationship between MMP-2 expression and preoperative MRI features of glioma was analyzed. The positive rate of MMP-2 expression in brain glioma was 73.08% (76/104), while that in paired normal brain tissues was only 12.5% (13/104), obviously lower than that in brain glioma tissues (P<0.05). The MMP-2 expression in the body of glioma was not related to the patients' sex, age, tumor location and pathological type (P>0.05), but there was a significant correlation with the tumor diameter and pathological grade of the patients (P<0.05). Analysis by Cox model suggested that tumor diameter, pathological grade and MMP-2 were independent prognostic factors for glioma (P<0.05). The overall survival (OS) of patients in the positive MMP-2 expression group was 16.4 months, while the OS in the negative MMP-2 expression group was 20.16 months, and the difference between the two groups was statistically significant (P<0.05). The positive expression of MMP-2 in glioma was closely related to the uniformity of MRI signal for tumor, tumor diameter, severity of peritumoral edema, degree of enhancement and pathological grade of tumor (P<0.05). MMP-2 is highly expressed in brain glioma, and it is a negative factor for prognosis. Therefore, the MRI manifestations of glioma can reflect to some extent the intensity of MMP-2 expression.
Keywords: glioma, MRI, MMP-2, prognosis
Introduction
Glioma is the most common kind of malignant primary brain tumor, whose major subtypes include astrocytoma, glioblastoma, oligodendroglioma and ependymoma. Among them, glioblastoma is the commonest histologic type with the highest malignancy (1,2). In spite of substantial progress in neurosurgery, radiotherapy and chemotherapy at present, the prognosis of glioma patients is still poor (3). Extensively infiltrative growth is shown in glioblastoma and anaplastic astrocytoma, and the 1-year survival rate of the patients is 40% (4). The reason for the fatality of the disease is that the extensive invasion of glioma cells into normal brain tissues becomes a main challenge for therapeutic intervention (5).
Matrix metalloproteinases (MMPs) are a category of zinc-dependent proteolytic endopeptidases, which is involved in the occurrence and development of multiple tumors. Latest studies on the mechanism of tumor invasion have shown that MMPs play a crucial role in the process (6). MMPs can enhance the invasive ability of tumor cells by degrading extracellular matrix proteins (such as collagen, fibronectin and proteoglycan) as well as growth factor binding protein, growth factor precursor, receptor tyrosine kinase, cell adhesion molecule and other proteases (7,8). A great many studies have proven that the upregulation of MMP expression is the leading cause of excessive proliferation of tumor cells, disseminated tumor growth, anti-apoptosis, neovascularization and inhibition of antitumor immune surveillance (9). Among the MMPs, the elevated expression level of MMP-2 (72 kDa type IV collagenase) is closely related to the increase and progression of glioma malignancy, thus attracting extensive attention.
Magnetic resonance imaging (MRI) is the most effective means in diagnosing various central nervous system diseases. Conventional MRI provides important anatomical and diagnostic information for understanding of tumors, and it has great value in many aspects, such as identification of tumor types, diagnosis and staging as well as assessment of parameters of tumor vessels (10).
In this study, MRI was applied to explore the preoperative imaging features of glioma patients, and immunohistochemistry (IHC) was utilized to detect the expression levels of MMP-2 protein in 104 cases of glioma tissues and surrounding normal brain tissues, respectively. The associations of MMP-2 protein expression in glioma tissues with the clinicopathological characteristics of the patients as well as preoperative MRI features were analyzed. It is conducive to understanding the MRI features of glioma, further increases the diagnostic accuracy of pathological grade before operation, is helpful in selecting treatment protocols and measuring therapeutic effects and provides an objective basis for formulation of clinical treatment plan and assessment of the patients' prognosis, which is of great significance for improving the patients' quality of life.
Patients and methods
Clinical data
A total of 104 patients with brain glioma admitted and treated in the First Affiliated Hospital of Anhui Medical University (Hefei, China) from June 2010 to September 2014 were enrolled randomly, including 58 males and 46 females aged 28–72 years, with an average age of 40.7 years. All the patients received treatment with surgical procedure, and they underwent MRI plain scan and contrast-enhanced scan before operation. After resection of the tumor, puncture biopsy was performed from the ‘non-functional area’ of the brain located outside the margin of the tumor under a microscope (BX-42; Olympus, Tokyo, Japan). These patients did not receive any treatment before operation. All the specimens obtained after operation were fixed in 10% formalin and embedded in paraffin. Then the specimens were sliced to sections, which were examined by histopathology, stained with hematoxylin and eosin (H&E) and checked by neuropathologists. The patients were divided into 4 groups according to the World Health Organization (WHO) classification: Grade I (n=16, including 13 cases of astrocytoma and 3 cases of ependymoma), grade II (n=12, including 6 cases of astrocytoma and 6 cases of oligodendroglioma), grade III (n=48, including 25 cases of anaplastic astrocytoma, 10 cases of anaplastic oligodendroglioma and 13 cases of anaplastic ependymoma) and grade IV (n=33, glioblastoma multiforme).
After operation, every patient was followed up to record the survival time. The duration of follow-up was 1–36 months, with an average duration of 19.8±6.1 months. Deaths caused by reasons other than this disease were excluded. The informed consent was signed by all the patients or their guardians. This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University.
Detection of MMP-2 protein level via IHC
The tumor specimens and normal brain tissues were embedded in paraffin and then sliced to 4-µm-thick sections. Paraformaldehyde (4%) (Beyotime, Shanghai, China) was used for the fixative for 10 mins at 4 °C The sections were baked at 60°C, followed by deparaffinization in xylene for 15 min, rehydration in a gradient of ethanol and washing with distilled water. Next, the sections were treated with 3% peroxidase for 30 min to block endogenous peroxidase. After being rinsed with phosphate-buffered saline (PBS) 3 times, the sections were incubated with 2% aliquot of normal serum for 5 min, so as to block non-specific binding. Primary mouse anti-human monoclonal antibody MMP-2 (diluted at 1:100; cat. no. sc-13594, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was added and incubated overnight in a wet box. After that, the sections were washed with PBS 3 times and then incubated in biotinylated secondary goat anti-mouse (HRP) IgG antibody (dilution, 1:2,000; cat. no. ab6789; Abcam, Cambridge, MA, USA) with specific immunoglobulin as the carrier at room temperature for 1 h, followed by washing with PBS 3 times. Avidin-biotin-peroxidase complex was added and incubated at room temperature for 1 h, and immunoperoxidase staining was conducted with 3,3′-diaminobenzidine tetrahydrochloride as the substrate, followed by adding of hematoxylin for temporary staining and rinsing with PBS 3 times. Then the sections were placed under an inverted microscope (Leica DM-5000B; Leica, Wetzlar, Germany) for photographing, 3 fields of vision were selected in each section and 3 photos were taken for each field of vision.
The cells with cytoplasm stained yellowish-brown or sepia were recorded as positive cells. Semiquantitative scoring was conducted from two aspects: i) extent of staining [0 point (no staining), 1 point (light staining), 2 points (moderate staining) and 3 points (deep staining)] and ii) percentage of stained cells [0 point (no stained cells), 1 point (stained cells <25%), 2 points (stained cells at 25–50%) and 3 points (stained cells >50%)]. The total scores (0–6 points) from both aspects were calculated. The score of 0–2 points represented negative, and that of 3–6 points positive.
MRI examination
A Signa 1.5T superconductive MRI scanner (GE Healthcare, Little Chalfont, Buckinghamshire, UK) with a head coil was used as the MRI scanning device, and scans using conventional spin echo (SE) sequence in the axial and sagittal planes were performed first. T1-weighted image (T1WI): Repetition time (TR): 2,500 msec and echo time (TE): 24 msec. T2-weighted image (T2WI): TR: 5,000 msec and TE: 98 msec. The slice thickness was 5 mm, with an interval of 1.2 mm, and data acquisition matrix was 256×256. T1 contrast-enhanced scans were performed in the axial, sagittal and coronal planes after intravenous injection of gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA).
Statistical analysis
The experimental results were analyzed using GraphPad Prism software (version 5.01; GraphPad Software, La Jolla, CA, USA). Chi-square test was applied to analyze the correlations of MMP-2 protein levels with the clinical indicators of glioma patients and MRI parameters. The patients' survival curves were constructed using Kaplan-Meier method and checked via log-rank test. P<0.05 indicates that the difference was statistically significant.
Results
Detection of MMP-2 expression in glioma tissues and paired normal brain tissues via IHC
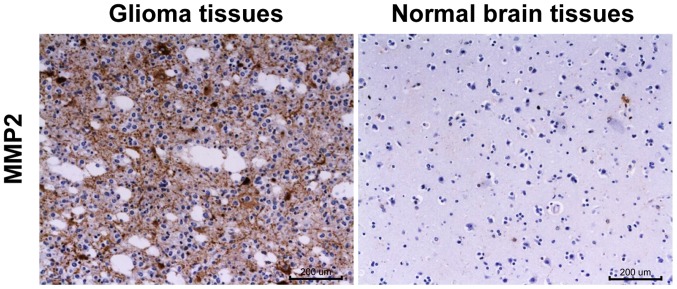
The MMP-2 was located in the cytoplasm, and the cytoplasm of the MMP-2 positive cells was stained in different shades of brown (Fig. 1). The positive expression rate of MMP-2 protein in 104 cases of brain glioma was 73.08% (76/104), while that in paired normal brain tissues was only 12.5% (13/104), obviously lower than that in brain glioma tissues (P<0.001) (Table I).
Figure 1.
Detection of MMP-2 expression in glioma tissues and paired normal brain tissues via IHC (magnification, ×200). MMP-2, matrix metalloproteinase-2; IHC, immunohistochemistry.
Table I.
Difference in MMP-2 protein expression in glioma tissues and paired normal brain tissues.
| MMP-2 | |||
|---|---|---|---|
| Groups | n | Positive n (%) | Negative n (%) |
| Glioma tissue | 104 | 76 (73.08) | 28 (26.92) |
| Surrounding normal brain tissue | 104 | 13 (12.5) | 91 (87.5) |
| χ2 | 12.81 | ||
| P-value | <0.001 | ||
MMP-2, matrix metalloproteinase-2.
Correlation of MMP-2 expression with glioma
The MMP-2 expression in the body of glioma was not related to the patients' sex, age, tumor location and pathological type (P>0.05), but there was a significant correlation with the tumor diameter and pathological grade of the patients (P<0.05) (Table II).
Table II.
Comparison of MMP-2 expression and clinicopathological characteristics of glioma.
| MMP-2 expression | ||||||
|---|---|---|---|---|---|---|
| Item | Groups | n (104) | Positive (n=76) | Negative (n=28) | χ2 | P-value |
| Sex | 0.84 | 0.115 | ||||
| Male | 58 | 42 | 16 | |||
| Female | 46 | 34 | 12 | |||
| Age (years) | 1.14 | 0.097 | ||||
| <30 years | 15 | 11 | 4 | |||
| 30–50 years | 35 | 26 | 9 | |||
| >50 years | 54 | 39 | 15 | |||
| Tumor diameter | 5.72 | 0.017 | ||||
| <3 cm | 31 | 16 | 15 | |||
| >3 cm | 73 | 60 | 13 | |||
| Tumor location | 1.61 | 0.063 | ||||
| Frontal lobe | 18 | 13 | 5 | |||
| Temporal lobe | 43 | 32 | 11 | |||
| Parietal lobe | 35 | 25 | 10 | |||
| Others | 8 | 6 | 2 | |||
| Pathological type | 1.53 | 0.058 | ||||
| Astrocytoma | 54 | 38 | 16 | |||
| Oligodendroglioma | 44 | 33 | 11 | |||
| Others | 6 | 5 | 1 | |||
| Pathological grade | 6.28 | 0.023 | ||||
| I–II | 23 | 13 | 10 | |||
| III | 48 | 34 | 14 | |||
| IV | 33 | 29 | 4 | |||
MMP-2, matrix metalloproteinase-2.
Analysis of the significance of MMP-2 expression and clinicopathological characteristics for judging prognosis via Cox model
Univariate analyses of sex, age, tumor diameter, tumor location, pathological type, pathological grade and MMP-2 expression were performed using the Cox model (ENTER method). The results showed that tumor diameter, pathological grade and MMP-2 were independent prognostic factors for glioma (P<0.05) (Table III). The survival analysis indicated that the overall survival (OS) of patients in the positive MMP-2 expression group was 16.4 months, while that in the negative MMP-2 expression group was 20.16 months, and the difference between the two groups was statistically significant (P<0.05). It suggested that MMP-2 is a negative prognostic factor (Fig. 2).
Table III.
Analyses of independent prognostic factors for glioma patients using Coxs proportional hazards regression model.
| Indexes | Regression coefficient (B) | Standard error (SE) | Wald test | Degree of freedom | P-value | Relative risk (RR) | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| Sex | 1.216 | 0.412 | 1.262 | 1 | 0.364 | 0.715 | 0.463–1.504 |
| Age | 0.930 | 0.341 | 0.724 | 2 | 0.157 | 1.019 | 0.635–1.358 |
| Tumor diameter | 0.342 | 0.525 | 9.583 | 1 | 0.024 | 1.662 | 1.242–1.852 |
| Tumor location | 1.262 | 0.526 | 2.931 | 2 | 0.457 | 0.992 | 0.793–1.274 |
| Pathological type | 0.672 | 0.327 | 8.342 | 2 | 0.134 | 1.213 | 1.141–1.955 |
| Pathological grade | 0.825 | 0.282 | 9.461 | 2 | 0.017 | 3.524 | 2.626–4.033 |
| MMP-2 level | 1.521 | 0.728 | 11.573 | 1 | 0.008 | 3.674 | 2.582–3.936 |
MMP-2, matrix metalloproteinase-2.
Figure 2.
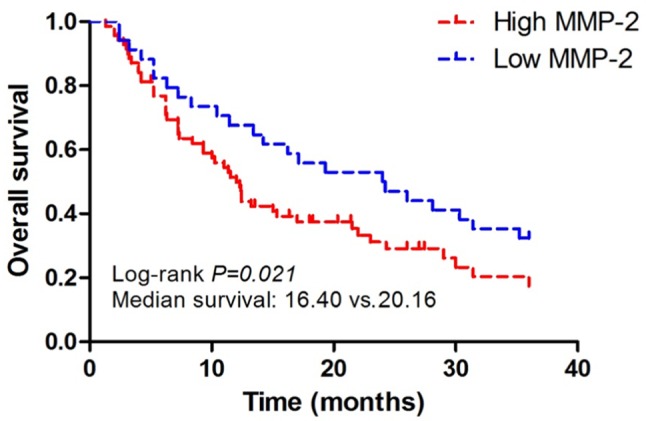
Survival curves of positive and negative MMP-2 expression groups. MMP-2, matrix metalloproteinase-2.
MRI data of glioma
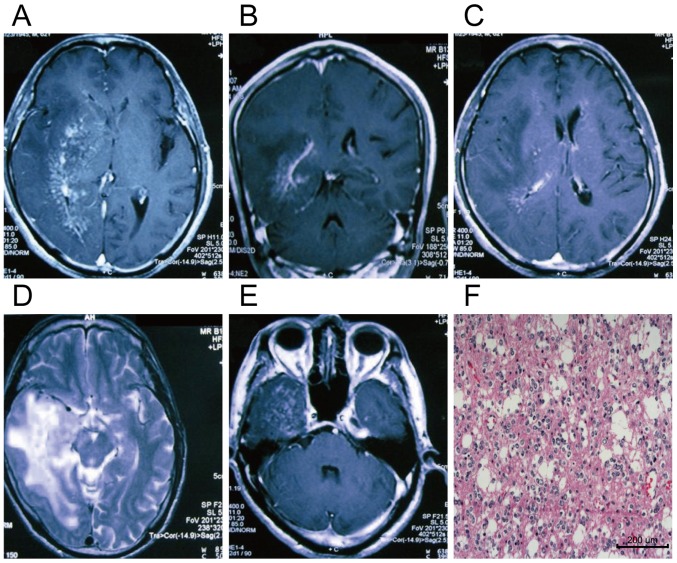
For most of the patients, the tumor presented areas of mixed isointense and hypointense signals on the T1WI as well as mixed isointense and hyperintense signals on the T2WI. On the T1WI, the parenchyma of tumor was enhanced remarkably after injection of enhancer Gd-DTPA, while the central necrotic and cystic areas were not enhanced. The MRI data of the patients with brain stem glioma are shown in Fig. 3.
Figure 3.
MRI and pathological data of a glioma patient. Male patient aged 62 years. (A-E) The MRI scanning images in different levels, and (F) shows the postoperative pathological image of glioma in the same patient (magnification, ×200). (C) The image on T2WI sequence, and (A, B and D) T1WIs after enhancement. Large areas of irregular abnormal signals are visible in the right temporal, parietal and occipital lobes, basal ganglia and centrum semioval. There are mixed isointense and hypointense signals on the T1WI as well as mixed isointense and hyperintense signals on the T2WI, and the surrounding edemas are not enhanced. MRI, magnetic resonance imaging; T1WI, T1-weighted image.
Relationship between MRI features of glioma and MMP-2 expression
The pathological data of MRI diagnosis before operation and expression levels of MMP-2 protein in glioma tissues were analyzed. It was found that the positive MMP-2 expression in glioma was closely associated with the uniformity of MRI signal for tumor, tumor diameter, severity of peritumoral edema, degree of enhancement and pathological grade of tumor, and there were statistically significant differences (P<0.05) (Table IV).
Table IV.
Relationship between MMP-2 expression and MRI features of the patients.
| MMP-2 expression | ||||||
|---|---|---|---|---|---|---|
| Items | Indexes | n (104) | Negative (n=28) | Positive (n=76) | χ2 | P-value |
| Tumor signal | 4.76 | 0.026 | ||||
| Uniform | 36 | 10 | 26 | |||
| Non-uniform | 68 | 18 | 50 | |||
| Tumor diameter | 5.25 | 0.017 | ||||
| ≤3 cm | 37 | 9 | 28 | |||
| >3 cm | 67 | 19 | 48 | |||
| Peritumoral edema | 8.35 | 0.023 | ||||
| Mild | 44 | 13 | 31 | |||
| Moderate to severe | 60 | 15 | 45 | |||
| Degree of enhancement | 5.04 | 0.008 | ||||
| Mild | 38 | 12 | 26 | |||
| Moderate to severe | 66 | 16 | 50 | |||
| Pathological grade | 11.42 | 0.015 | ||||
| I–II | 26 | 4 | 22 | |||
| III | 46 | 16 | 30 | |||
| IV | 32 | 8 | 24 | |||
MMP-2, matrix metalloproteinase-2.
Discussion
Glioma is a neuroectodermal tumor which is highly invasive, and degradation of extracellular matrix is the prerequisite of its invasive phenotype (11). Currently, substantial evidence has shown that MMP-2, a member of MMPs, plays an important role in such a process. MMP-2 is regarded as a crucial impact factor for a variety of pathological conditions, such as tissue remodeling and morphogenesis (12). In particular, invasive cell necrosis often occurs in the center of glioma which grows rapidly, while the invasiveness and angiogenesis occur in the margin of glioma mass (13).
Among the 104 cases in this experiment, the positive expression rate of MMP-2 was 73.08% (76/104), which was notably higher than that in normal brain tissues [12.5% (13/104)]. In addition, the higher positive expression rate of MMP-2 in glioma was associated with the clinicopathological characteristics that reflected poor biological behaviors of glioma. It was manifested that MMP-2 was expressed at low level in gliomas with small diameter and low malignancy, but it was highly expressed in malignant gliomas with larger diameter. These results are similar to the findings of other scholars (14). It can be seen that MMP-2 exerts its pathological effects by means of its abnormal expression in brain glioma. On the one hand, it can stimulate normal cells to convert into tumor cells and form more tumor lesions through many paths, including gene level and signal transduction. On the other hand, it can promote the distant migration of tumor cells by degrading the extracellular matrix, resulting in generation of new tumor lesions, fast growth of tumors, increase in volume and apparent peripheral space occupying effects (severe brain edema and other lesions). Furthermore, the MMP-2 expression was measured in glioma patients of different sex and age groups, and it was shown that there were no significant differences between males and females and among various age groups. It may imply that androgen, estrogen and age have no impact on the secretion of MMP-2 in glioma patients. It was evident in the survival analysis that MMP-2 influences the patient prognosis, it is a negative prognostic factor and that its positive expression indicates poor prognosis. These findings are consistent with the results of other studies.
Principal findings about the MMP-2 in glioma in promoting invasion, metastasis and other malignant biological processes of the tumor are as follows: i) MMP-2 can also activate potent MMP-9 and stimulate specific cascades to produce plenty of MMP-9 proteins (15). ii) The balance between MMP-2 and its inhibitor is crucial for proteolysis control. It was reported that tissue inhibitor of metalloproteinases-1 (TIMP-1) and TIMP-2, inhibitors of MMPs, are expressed in normal brain tissues. In tumor tissues, however, the expression is decreased remarkably in highly invasive glioma (16). Therefore, more TIMP may be produced in low-grade glioma than in high-grade one, and the MMP-2 level may be correspondingly lower, so different degrees of invasiveness can be clarified (3). In addition to invasion of glioma, MMP-2 is also involved in degradation of blood-brain barrier, neurodegeneration and angiogenesis (17). MMP-2 upregulates vascular endothelial growth factor (VEGF) protein and induces tumor angiogenesis through multiple signal transduction pathways. Moreover, it causes hematogenous metastases of cancer cells to form new cancer foci that cannot be distinguished by naked eye (18), thus affecting the prognosis of patients.
As a type of non-invasive imaging technology, MRI can be utilized to monitor the treatment of glioma and dynamic changes of subgroup tumors. The uniformity of plain scan signals of glioma is associated with the fact whether there is cystic degeneration, necrosis or hemorrhage in the tumor or not. Generally, there are only one or two kinds of pathological changes in the gliomas with homogeneous signals, while multiple pathological changes can be detected in the gliomas with heterogeneous signals (19). The tumor with high MMP-2 expression is often complicated with cystic degeneration, necrosis, hemorrhage and other pathological changes because of its strong invasiveness, and MRI is characterized by heterogeneous signals. However, there is less cystic degeneration, necrosis and hemorrhage occurring in the tumor with low MMP-2 expression, so the MRI is characterized by homogeneous signals. It can be seen that the uniformity of MRI signal for glioma can indicate the level of MMP-2 expression.
The degrees of enhancement and edema of glioma have correlations with the neovascular formation and degree of damage to blood-brain barrier in the tumor (20). T2WI is applied to assess the severity of peritumoral diseases. The tumor with high MMP-2 expression has a high pathological grade and strong invasiveness, and it can easily destroy the vascular basement membrane and increase its permeability, leading to tumor interstitial edema formed by aggregation of liquid and plasma proteins in the tumor stroma. On the other hand, the stronger the proliferation activity of the tumor is, the richer the tumor neovessels will be, and the more obvious the damage to blood-brain barrier will be. Therefore, the enhancement degree is more significant (21). It is evident that the severity of peritumoral edema and enhancement degree shown on glioma MRI can to some extent be reflected.
In conclusion, it was found in this study that MMP-2 is positively expressed in glioma, and it has a close correlation with the uniformity of MRI signal for tumor, severity of peritumoral edema, degree of enhancement and pathological grade of tumor.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Science Foundation Project (no. 81571308).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HZ and YY designed the study and performed the experiments. HZ, YM and HW collected the data. YM and LX analyzed the data. HZ and YY prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Hefei, China). Signed informed consents were obtained from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2018;62:753–766. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 2.Park DM, Rich JN. Biology of glioma cancer stem cells. Mol Cells. 2009;28:7–12. doi: 10.1007/s10059-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 3.Raymond E, Brandes AA, Dittrich C, Fumoleau P, Coudert B, Clement PM, Frenay M, Rampling R, Stupp R, Kros JM, et al. European Organisation for Research and Treatment of Cancer Brain Tumor Group Study: Phase II study of imatinib in patients with recurrent gliomas of various histologies: A European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26:4659–4665. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huse JT, Holland EC. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Wu Z, Wu T, Huang Y, Cheng Z, Li X, Sun T, Xie X, Zhou Y, Du Z. Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by downregulation of MMP2 and inactivation of ERK/MAPK signaling. Cell Death Dis. 2016;7:e2123. doi: 10.1038/cddis.2015.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahme GJ, Israel MA. Id4 suppresses MMP2-mediated invasion of glioblastoma-derived cells by direct inactivation of Twist1 function. Oncogene. 2015;34:53–62. doi: 10.1038/onc.2013.531. [DOI] [PubMed] [Google Scholar]
- 8.Liu LY, Man XX, Yao HX, Tan YY. Effects of pheophorbide a- mediated photodynamic therapy on proliferation and metastasis of human prostate cancer cells. Eur Rev Med Pharmacol Sci. 2017;21:5571–5579. doi: 10.26355/eurrev_201712_13994. [DOI] [PubMed] [Google Scholar]
- 9.Tabouret E, Boudouresque F, Farina P, Barrié M, Bequet C, Sanson M, Chinot O. MMP2 and MMP9 as candidate biomarkers to monitor bevacizumab therapy in high-grade glioma. Neuro Oncol. 2015;17:1174–1176. doi: 10.1093/neuonc/nov094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu LS, Kelm Z, Korfiatis P, Dueck AC, Elrod C, Ellingson BM, Kaufmann TJ, Eschbacher JM, Karis JP, Smith K, et al. Impact of software modeling on the accuracy of perfusion MRI in glioma. AJNR Am J Neuroradiol. 2015;36:2242–2249. doi: 10.3174/ajnr.A4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langlois B, Saupe F, Rupp T, Arnold C, van der Heyden M, Orend G, Hussenet T. AngioMatrix, a signature of the tumor angiogenic switch-specific matrisome, correlates with poor prognosis for glioma and colorectal cancer patients. Oncotarget. 2014;5:10529–10545. doi: 10.18632/oncotarget.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piromkraipak P, Sangpairoj K, Tirakotai W, Chaithirayanon K, Unchern S, Supavilai P, Power C, Vivithanaporn P. Cysteinyl leukotriene receptor antagonists inhibit migration, invasion, and expression of MMP-2/9 in human glioblastoma. Cell Mol Neurobiol. 2018;38:559–573. doi: 10.1007/s10571-017-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aroui S, Najlaoui F, Chtourou Y, Meunier AC, Laajimi A, Kenani A, Fetoui H. Naringin inhibits the invasion and migration of human glioblastoma cell via downregulation of MMP-2 and MMP-9 expression and inactivation of p38 signaling pathway. Tumour Biol. 2016;37:3831–3839. doi: 10.1007/s13277-015-4230-4. [DOI] [PubMed] [Google Scholar]
- 14.Yi GZ, Feng WY, Zhou Q, Liu YW, Qi ST. The impact of MMP-2 and its specific inhibitor TIMP-2 expression on the WHO grade and prognosis of gliomas in Chinese population: A Meta-Analysis. Mol Neurobiol. 2017;54:22–30. doi: 10.1007/s12035-015-9539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farina P, Tabouret E, Lehmann P, Barrie M, Petrirena G, Campello C, Boucard C, Graillon T, Girard N, Chinot O. Relationship between magnetic resonance imaging characteristics and plasmatic levels of MMP2 and MMP9 in patients with recurrent high-grade gliomas treated by Bevacizumab and Irinotecan. J Neurooncol. 2017;132:433–437. doi: 10.1007/s11060-017-2385-0. [DOI] [PubMed] [Google Scholar]
- 16.Cho HJ, Park JH, Nam JH, Chang YC, Park B, Hoe HS. Ascochlorin suppresses MMP-2-mediated migration and invasion by targeting FAK and JAK-STAT signaling cascades. J Cell Biochem. 2018;119:300–313. doi: 10.1002/jcb.26179. [DOI] [PubMed] [Google Scholar]
- 17.Roomi MW, Kalinovsky T, Rath M, Niedzwiecki A. Modulation of MMP-2 and MMP-9 secretion by cytokines, inducers and inhibitors in human glioblastoma T-98G cells. Oncol Rep. 2017;37:1907–1913. doi: 10.3892/or.2017.5368. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran RK, Sørensen MD, Aaberg-Jessen C, Hermansen SK, Kristensen BW. Expression and prognostic impact of matrix metalloproteinase-2 (MMP-2) in astrocytomas. PLoS One. 2017;12:e0172234. doi: 10.1371/journal.pone.0172234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schepkin VD. Sodium MRI of glioma in animal models at ultrahigh magnetic fields. NMR Biomed. 2016;29:175–186. doi: 10.1002/nbm.3347. [DOI] [PubMed] [Google Scholar]
- 20.Krivosheya D, Prabhu SS. Combining functional studies with intraoperative MRI in glioma surgery. Neurosurg Clin N Am. 2017;28:487–497. doi: 10.1016/j.nec.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Jain KK, Sahoo P, Tyagi R, Mehta A, Patir R, Vaishya S, Prakash N, Vasudev N, Gupta RK. Prospective glioma grading using single-dose dynamic contrast-enhanced perfusion MRI. Clin Radiol. 2015;70:1128–1135. doi: 10.1016/j.crad.2015.06.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.