Abstract
Changes in expression levels of serum interleukin-4 (IL-4), IL-10 and adiponectin (APN) in patients with postoperative infection of colorectal cancer were studied. The clinical data of 159 patients receiving radical surgery for colorectal cancer in Xiangyang No. 1 People's Hospital, Hubei University of Medicine from January 2014 to December 2017, were retrospectively analyzed. A total of 67 patients with postoperative infection were enrolled into the infection group, while the remaining 92 patients without infection were enrolled into the non-infection group. The expression levels of serum IL-4, IL-10 and APN of patients were detected via enzyme-linked immunosorbent assay. The correlation of IL-4, IL-10 and APN levels with stage of colorectal cancer were explored by the Spearmans correlation analysis. The expression levels of IL-4 and IL-10 in the infection group were significantly higher than those in the non-infection group at day 3 after surgery (P<0.05). The expression level of APN in the infection group was lower than that in the non-infection group at day 3 after surgery (P<0.05). The serum IL-4 and IL-10 levels in pulmonary infection was higher and the serum IL-10 level in pulmonary infection was higher than those in incision infection and abdominal infection (P<0.05). The IL-4 and IL-10 levels in patients with colorectal cancer in the infection group at day 3 after surgery had a significant positive correlation with the stage of colorectal cancer (r=0.9357, P<0.001; r=0.9717, P<0.001), and the APN level in patients with colorectal cancer in the infection group at day 3 after surgery had a significant negative correlation with the stage of colorectal cancer (r=−0.9736, P<0.001). The serum IL-4 and IL-10 levels in patients with postoperative infection of colorectal cancer are positively correlated with the stage of cancer, while the serum APN level was negatively correlated with the stage of cancer.
Keywords: IL-4, IL-10, APN, postoperative infection of colorectal cancer, stage of colorectal cancer, correlation
Introduction
Colorectal cancer is a malignant tumor of the digestive tract derived from mucous epithelium, including colon cancer and rectal cancer (1). With the changes in dietary structure and living habits, the incidence rate of colorectal cancer ranks 3rd following lung cancer and breast cancer, and its mortality rate shows a gradual increasing trend (2). The 5-year survival rate of early colorectal cancer can be up to 90%, so the early diagnosis and treatment is extremely important (3). Radical surgery dominates the treatment of early colorectal cancer, supplemented by chemotherapy. Patients who can tolerate surgery with middle-advanced cancer and surgical indications should undergo radical surgery. Good efficacy has been obtained in most patients who have received radical surgery for colorectal cancer (4). However, radical surgery for colorectal cancer is difficult, and the patient's immunity is low, so postoperative infection occurs easily (5). The risk of infection is increased due to the decline in the patient's defense after surgery, and incision, pulmonary and abdominal infections often occur after exogenous or endogenous surgical site infection (6).
Interleukin-4 (IL-4) and IL-10 can activate and regulate immune cells, and mediate the activation, proliferation and differentiation of T and B cells, which play an important role in inflammatory and immune responses (7). Adiponectin (APN) is an insulin-sensitizing hormone, its level can predict the development of inflammation and it has displayed anti-inflammatory potential in clinical tests (8). Studies have demonstrated that inflammation is involved in the occurrence and development of postoperative infection, and there are significant changes in the levels of serum IL-4, IL-10 and APN, which can evaluate the postoperative infection and serve as indexes for the clinical auxiliary diagnosis of postoperative infection of malignant tumor (9). Currently, the correlation of changes in serum IL-4, IL-10 and APN levels with postoperative infection of colorectal cancer are rarely reported. In this experiment, the data of patients with infection after radical surgery for colorectal cancer were retrospectively analyzed, the levels of serum IL-4, IL-10 and APN were compared in patients with postoperative infection of colorectal cancer and pulmonary infection, and their correlation with the stage of colorectal cancer in patients with postoperative infection were analyzed and explored, so as to provide references for the clinical nursing and treatment after radical surgery for colorectal cancer.
Patients and methods
Data of patients
The clinical data of 159 patients receiving radical surgery for colorectal cancer in Xiangyang No. 1 People's Hospital, Hubei University of Medicine (Xiangyang, China) from January 2014 to December 2017 were retrospectively analyzed. A total of 67 patients with postoperative infection were enrolled into the infection group, including 39 males and 28 females aged 22–76 years with an average age of 42.38±6.94 years, while the remaining 92 patients without infection were enrolled into the non-infection group, including 54 males and 38 females aged 19–71 years with an average age of 39.24±5.31 years. To ensure the accuracy and reliability of experimental results, clinical data of patients were compared between the two groups, and there were no significant differences (P>0.05), proving that they were comparable between the two groups. Basic data of patients are shown in Table I.
Table I.
Basic data of 159 patients receiving radical surgery for colorectal cancer [n (%)].
| Parameters | Infection group (n=67) | Non-infection group (n=92) | χ2 | P-value |
|---|---|---|---|---|
| Sex | 0.004 | 0.951 | ||
| Male | 39 (58.21) | 54 (58.70) | ||
| Female | 28 (41.79) | 38 (41.30) | ||
| Age (years) | 0.070 | 0.791 | ||
| <30 | 27 (40.30) | 39 (42.39) | ||
| ≥30 | 40 (59.70) | 53 (57.61) | ||
| Smoking | 0.222 | 0.637 | ||
| Yes | 42 (62.69) | 61 (66.30) | ||
| No | 25 (37.31) | 31 (33.70) | ||
| Dietary habit | 2.390 | 0.122 | ||
| Low fiber | 19 (28.36) | 37 (40.22) | ||
| High fiber | 48 (71.64) | 55 (59.78) | ||
| Intestinal obstruction | 0.164 | 0.686 | ||
| Yes | 50 (74.63) | 66 (71.74) | ||
| No | 17 (25.37) | 26 (28.26) | ||
| Surgical mode | 0.258 | 0.611 | ||
| Laparoscopic surgery | 42 (62.69) | 54 (58.70) | ||
| Traditional laparotomy | 25 (37.31) | 38 (41.30) | ||
| Type of tumor | 0.421 | 0.810 | ||
| Right hemicolon cancer | 15 (22.39) | 21 (22.83) | ||
| Left hemicolon cancer | 19 (28.36) | 22 (23.91) | ||
| Rectal cancer | 33(49.25) | 49 (53.26) | ||
| Pathological stage | 0.272 | 0.965 | ||
| Stage I | 17 (25.37) | 23 (25.00) | ||
| Stage II | 19 (28.36) | 26 (28.26) | ||
| Stage III | 24 (35.82) | 31 (33.70) | ||
| Stage IV | 7 (10.45) | 12 (13.04) | ||
| Type of infection | − | − | ||
| Incision infection | 39 (58.21) | − | ||
| Abdominal infection | 15 (22.39) | − | ||
| Pulmonary infection | 13 (19.40) | − |
Exclusion and inclusion criteria
Inclusion criteria: patients whose examination results of pathological section were in line with the manifestations of colorectal cancer, patients aged above 18 years, patients who were not treated in other hospitals, and patients who underwent radical surgery for colorectal cancer in Xiangyang No. 1 People's Hospital Affiliated to Hubei University of Medicine. Exclusion criteria: patients who did not cooperate in related diagnosis and treatment, pregnant or lactating patients, patients with other diseases that may be related to such cytokine expression and changes, patients with genetic diseases, patients with tumors other than colorectal cancer, or patients with communication disorders or cognitive disorders. The subjects or their families signed the informed consent and cooperated with medical workers in related diagnosis and treatment.
This study was approved by the Ethics Committee of Xiangyang No. 1 Peoples Hospital, Hubei University of Medicine (Xiangyang, China).
Methods
After 4 ml fasting peripheral venous blood was drawn from all patients in the early morning before surgery and at day 3 after surgery, the serum was isolated via centrifugation at 2,000 × g for 15 min at 4°C (Beckman Coulter, Inc., Brea, CA, USA) and stored in a cryogenic refrigerator (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at −20°C. The levels of serum IL-4, IL-10 and APN in both groups were detected via enzyme-linked immunosorbent assay (ELISA) in strict accordance with instructions of the human IL-4 and IL-10 ELISA kits (Shanghai Kanglang Biological Technology Co., Ltd., Shanghai, China) and human APN ELISA kit (Shanghai MLbio Co., Ltd., Shanghai, China). The standard, blank and sample wells were set. Then, 50 µl standard samples were accurately loaded, and the sample well was added with 40 µl of sample diluent and then 10 µl of sample to be detected and mixed evenly. The plate was sealed with sealing membrane, followed by incubation at 37°C for 1 h. The sealing membrane was uncovered. The solution was discarded and the plate was dried. Each well was filled with 100 µl washing solution. The sealing membrane was covered and the washing solution was discarded after 30 sec. The above procedure was repeated 5 times, and the plate was dried. The standard well and sample well were added with 100 µl ELISA reagent. Color developing agents A (60 µl) and B (60 µl) were added into each well and mixed evenly, and the sealing membrane was covered, followed by color development in the dark at 37°C for 30 min. Then 50 µl stop buffer was added into each well to terminate the reaction. The optical density (OD) of each well was immediately detected at a wavelength of 450 nm by using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA), and the concentrations of serum IL-4, IL-10 and APN were calculated.
Statistical analysis
SPSS 17.4 [AsiaAnalytics (formerly SPSS China)] software system was used for statistical analysis. The basic enumeration data of patients were expressed as percentage [n (%)], and Chi-square test was performed. IL-4, IL-10 and APN levels were expressed as mean ± standard deviation. t-test was adopted for the comparison of differences between the two groups at different time-points, and F analysis was used for the expression difference among different types of infection. The correlation of IL-4, IL-10 and APN levels with the stage of colorectal cancer in patients with postoperative infection of colorectal cancer was analyzed via Spearmans correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Changes in expression levels of serum IL-4, IL-10 and APN in both groups
There were no differences in the expression levels of IL-4, IL-10 and APN between the two groups before surgery (P>0.05). The expression levels of IL-4 and IL-10 in the infection group were significantly higher than those in the non-infection group at day 3 after surgery (P<0.05), and they were higher in both groups after surgery than those before surgery (P<0.05), but the changes in the expression levels in the infection group were more significant than those in the non-infection group. There was no difference in the expression level of APN between the two groups before surgery (P>0.05). The expression level of APN in the infection group was lower than that in the non-infection group at day 3 after surgery (P<0.05), and it was lower in both groups after surgery than that before surgery (P<0.05), but the change in the expression level was more significant in the infection group than that in the non-infection group (Table II).
Table II.
Changes in expression levels of serum IL-4, IL-10 and APN.
| IL-4 (pg/l) | IL-10 (ng/l) | APN (pg/l) | ||||
|---|---|---|---|---|---|---|
| Groups | Before surgery | 3 d after surgery | Before surgery | 3 d after surgery | Before surgery | 3 d after surgery |
| Infection group (n=67) | 162.78±13.76 | 357.64±16.48a | 153.67±29.78 | 219.41±52.97a | 146.50±37.53 | 107.26±26.61a |
| Non-infection group (n=92) | 159.43±12.84 | 216.73±13.51a | 154.91±28.56 | 174.43±37.65a | 145.92±36.26 | 129.12±29.68a |
| t | 0.117 | 59.16 | 0.791 | 6.261 | 0.922 | 4.787 |
| P-value | 1.576 | <0.001 | 0.266 | <0.001 | 0.098 | <0.001 |
Statistically significant difference compared with that before surgery (P<0.05). IL, interleukin; APN, adiponectin.
Expression levels of serum IL-4, IL-10 and APN in different types of infection
The serum IL-4 level in pulmonary infection was higher than that in incision infection and abdominal infection (P<0.05), and it was also higher in abdominal infection than that in incision infection, displaying no statistically significant difference (P>0.05). The serum IL-10 level in pulmonary infection was higher than that in incision infection and abdominal infection, showing statistically significant differences (P<0.05), and it was also higher in abdominal infection than that in incision infection without a statistically significant difference (P>0.05). The serum APN level in pulmonary infection was lower than that in incision infection and abdominal infection, showing statistically significant differences (P<0.05), and it was also lower in abdominal infection than that in incision infection without a statistically significant difference (P>0.05; Table III).
Table III.
Changes in expression levels of serum IL-4, IL-10 and APN in different types of infection.
| Items | Incision infection (n=39) | Abdominal infection (n=15) | Pulmonary infection (n=13) | F | P-value |
|---|---|---|---|---|---|
| IL-4 (pg/l) | 327.84±24.51 | 344.52±21.46 | 355.75±28.54a | 7.122 | 0.002 |
| IL-10 (ng/l) | 197.55±48.72 | 209.24±52.63 | 225.61±49.25 | 1.605 | 0.209 |
| APN (pg/l) | 135.61±27.64 | 126.91±23.97 | 108.24±24.39a | 5.315 | 0.007 |
Statistically significant difference compared with that in incision infection (P<0.05). IL, interleukin; APN, adiponectin.
Correlation of serum IL-4, IL-10 and APN with stage of colorectal cancer in patients in infection group
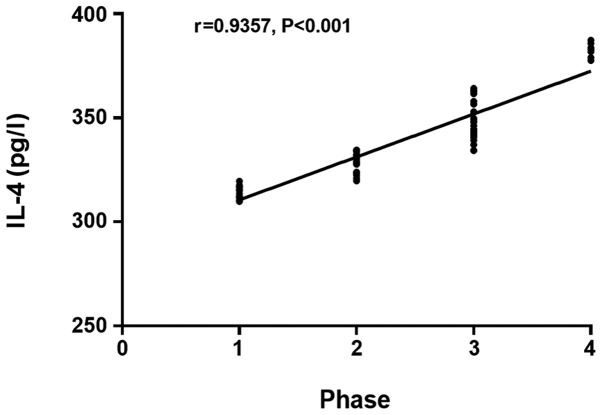
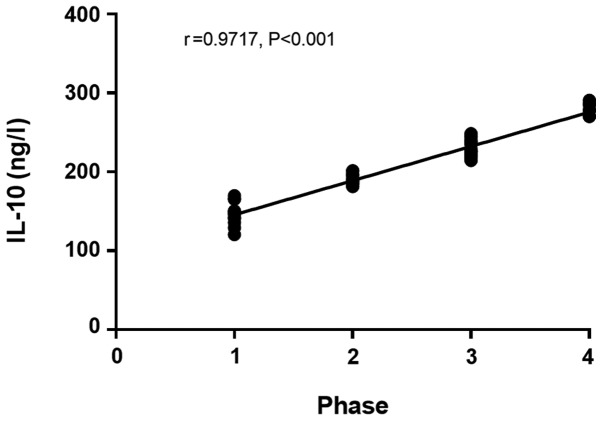
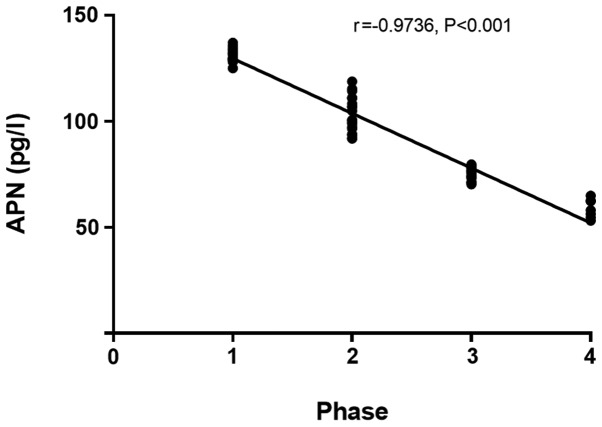
The IL-4 and IL-10 levels in patients with colorectal cancer in the infection group at day 3 after surgery had a significant positive correlation with the stage of colorectal cancer (r=0.9357, P<0.001) (r=0.9717, P<0.001; Figs. 1 and 2), and the APN level in patients with colorectal cancer in the infection group at day 3 after surgery had a significant negative correlation with the stage of colorectal cancer (r=−0.9736, P<0.001; Fig. 3).
Figure 1.
Correlation between IL-4 level at day 3 after surgery and stage of colorectal cancer in the infection group. Spearmans correlation analysis shows that the IL-4 level in patients with colorectal cancer at day 3 after surgery in the infection group has a significant positive correlation with the stage of colorectal cancer (r=0.9357, P<0.001). IL-4, interleukin-4.
Figure 2.
Correlation between IL-10 level at day 3 after surgery and stage of colorectal cancer in the infection group. Spearmans correlation analysis shows that the IL-10 level in patients with colorectal cancer at day 3 after surgery in the infection group has a significant positive correlation with the stage of colorectal cancer (r=0.9717, P<0.001). IL-10, interleukin-10.
Figure 3.
Correlation between APN level at day 3 after surgery and stage of colorectal cancer in the infection group. Spearmans correlation analysis reveals that the APN level in patients with colorectal cancer at day 3 after surgery in the infection group has a significant negative correlation with the stage of colorectal cancer (r=−0.9736, P<0.001). APN, adiponectin.
Discussion
The large intestine functions to absorb the liquid in the food debris and to make the food debris feces, which is an important component of the digestive system and the lower part of the digestive tract (10). At the same time, the large intestine also secretes mucin, so that the feces can be excreted easily and the intestinal wall can be protected from the mechanical damage (11). Colorectal cancer is divided into colon cancer and rectal cancer. There are no obvious symptoms in the early stage of colorectal cancer, and it has developed into advanced colorectal cancer if the lesion metastasizes to the stomach, liver and extra-territorial lymph nodes (12,13). The position of colorectal cancer is low, so it can be diagnosed via digital rectal examination and colonoscopy. However, the large intestine is located deep in the pelvic cavity, so surgery is difficult and incomplete, and the postoperative recurrence rate and infection rate are extremely high (14). After radical surgery for colorectal cancer, pulmonary infection can occur in patients due to malnutrition, hypoproteinemia and anemia, abdominal infection can also be caused by increased abdominal pressure formed due to special functions of the large intestine, and there are various abdominal fats and the incision is prone to effusion, thus leading to incision infection (15–17).
After infection, the tissues in the patient's body will be stimulated by pain, trauma and stress, thereby releasing a large number of inflammatory factors. Both IL-4 and IL-10 are anti-inflammatory factors, which can exert an immunomodulatory effect in the large intestine through stimulating B cells, mastocytes, macrophages and T cells (18). IL-4 and IL-10 can inhibit the secretion of TNF, IL-1 and IL-6 and promote the body's immune response through downregulating inflammatory mediators (19). In the post-operative infection of patients with colorectal cancer, the activation process of Th1 and Th2 cells can be delayed under the combined action of IL-4 and IL-10, thereby enhancing the Th-type response and improving the body repair (20). APN, anti-inflammatory factor, is consumed due to the aggravation of inflammation in patients with postoperative infection of colorectal cancer, leading to significant decline in APN in the body. At the same time, the low-level APN can accelerate the occurrence of inflammatory response (21).
This study revealed that there were no differences in the expression levels of IL-4, IL-10 and APN between the two groups before surgery (P>0.05). The expression levels of IL-4 and IL-10 in the infection group were significantly higher than those in the non-infection group at day 3 after surgery (P<0.05), and they were higher in both groups after surgery than those before surgery (P<0.05), but the changes in expression levels in the infection group were more significant than those in the non-infection group. The expression level of APN in the infection group was lower than that in the non-infection group at day 3 after surgery (P<0.05), and it was lower in both groups after surgery than that before surgery (P<0.05), but the change in the expression level was more significant in the infection group than that in the non-infection group. The levels of anti-inflammatory factors IL-4 and IL-10 in the infection group were higher than those in the non-infection group, indicating that in the progression of postoperative infection of colorectal cancer, the anti-inflammatory effect of the patient's body is enhanced, and the decline in APN can strengthen the anti-inflammatory effect. The serum IL-4 level in pulmonary infection was higher than that in incision infection and abdominal infection (P<0.05), and it was also higher in abdominal infection than that in incision infection (P>0.05). The serum IL-10 level in pulmonary infection was higher than that in incision infection and abdominal infection (P<0.05), and it was also higher in abdominal infection than that in incision infection (P>0.05). The serum APN level in pulmonary infection was lower than that in incision infection and abdominal infection (P<0.05), and it was also lower in abdominal infection than that in incision infection (P>0.05). The expression levels of anti-inflammatory factors were different among different types of infections. Moreover, the IL-4 and IL-10 levels in patients with colorectal cancer in the infection group at day 3 after surgery had a significant positive correlation with the stage of colorectal cancer, and the APN level in patients with colorectal cancer in the infection group at day 3 after surgery had a significant negative correlation with the stage of colorectal cancer. It is reported (22) that the body immunity of patients with advanced colorectal cancer is poorer than that of patients with early colorectal cancer, which are consistent with the results in this study, and the expression levels of IL-4 and IL-10 in patients with advanced colorectal cancer were higher than those in patients with early colorectal cancer, while the expression level of APN was lower than that in patients with early colorectal cancer.
In this study, the sample size was small due to the limited medical resources in Xiangyang No. 1 People's Hospital Affiliated to Hubei University of Medicine, so there might be a certain contingency in the results. In the future, subjects in this study will be followed up for survey for a longer time, to obtain optimal results.
In conclusion, the serum IL-4, IL-10 and APN levels have a certain correlation with the presence or absence of postoperative infection of colorectal cancer, the type of infection and the stage of colorectal cancer, which are worthy of clinical promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JH and ZW performed ELISA. SZ analyzed the general data of patients and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Xiangyang No. 1 People's Hospital, Hubei University of Medicine (Xiangyang, China). Patients who participated in this study, signed the informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, et al. US Preventive Services Task Force: Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315:2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 4.Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O'Connor E, Smith N, Whitlock EP. Screening for colorectal cancer: updated evidence report and systematic review for the US preventive services task force. JAMA. 2016;315:2576–2594. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 5.Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 6.McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23:2832–2840. doi: 10.1245/s10434-016-5204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tei M, Wakasugi M, Kishi K, Tanemura M, Akamatsu H. Incidence and risk factors of postoperative delirium in elderly patients who underwent laparoscopic surgery for colorectal cancer. Int J Colorectal Dis. 2016;31:67–73. doi: 10.1007/s00384-015-2335-2. [DOI] [PubMed] [Google Scholar]
- 8.Sakellariou S, Fragkou P, Levidou G, Gargalionis AN, Piperi C, Dalagiorgou G, Adamopoulos C, Saetta A, Agrogiannis G, Theohari I, et al. Clinical significance of AGE-RAGE axis in colorectal cancer: associations with glyoxalase-I, adiponectin receptor expression and prognosis. BMC Cancer. 2016;16:174. doi: 10.1186/s12885-016-2213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230–240. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- 10.Kojima M, Ikeda K, Saito N, Sakuyama N, Koushi K, Kawano S, Watanabe T, Sugihara K, Ito M, Ochiai A. Neuroendocrine tumors of the large intestine: clinicopathological features and predictive factors of lymph node metastasis. Front Oncol. 2016;6:173. doi: 10.3389/fonc.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bettington M, Walker N, Rosty C, Brown I, Clouston A, McKeone D, Pearson SA, Klein K, Leggett B, Whitehall V. Serrated tubulovillous adenoma of the large intestine. Histopathology. 2016;68:578–587. doi: 10.1111/his.12788. [DOI] [PubMed] [Google Scholar]
- 12.Sinicrope FA, Okamoto K, Kasi PM, Kawakami H. Molecular biomarkers in the personalized treatment of colorectal cancer. Clin Gastroenterol Hepatol. 2016;14:651–658. doi: 10.1016/j.cgh.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18:197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med. 2016;13:120–135. doi: 10.20892/j.issn.2095-3941.2015.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koelzer VH, Zlobec I, Lugli A. Tumor budding in colorectal cancer-ready for diagnostic practice? Hum Pathol. 2016;47:4–19. doi: 10.1016/j.humpath.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K, Kumamoto T, Nojiri K, Matsuyama R, Takeda K, Endo I. Impact of postoperative morbidity on long-term survival after resection for colorectal liver metastases. Ann Surg Oncol. 2016;23(Suppl 5):929–937. doi: 10.1245/s10434-010-1352-1. [DOI] [PubMed] [Google Scholar]
- 17.Mokutani Y, Mizushima T, Yamasaki M, Rakugi H, Doki Y, Mori M. Prediction of postoperative complications following elective surgery in elderly patients with colorectal cancer using the comprehensive geriatric assessment. Dig Surg. 2016;33:470–477. doi: 10.1159/000446709. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg. 2016;263:326–336. doi: 10.1097/SLA.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 19.Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, Ciclitira PJ, Al-Hassi HO. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22:3117–3126. doi: 10.3748/wjg.v22.i11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passardi A, Scarpi E, Cavanna L, Dall'Agata M, Tassinari D, Leo S, Bernardini I, Gelsomino F, Tamberi S, Brandes AA, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7:33210–33219. doi: 10.18632/oncotarget.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriarity A, O'Sullivan J, Kennedy J, Mehigan B, McCormick P. Current targeted therapies in the treatment of advanced colorectal cancer: A review. Ther Adv Med Oncol. 2016;8:276–293. doi: 10.1177/1758834016646734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.