Abstract
Background
Early and excessive alcohol use is a significant threat to healthy development. Evidence supports the effectiveness of electronic alcohol interventions for young drinkers. However, effects are typically small and studies targeting under 18-year-olds are scarce. This trial is the first to evaluate the effectiveness of a single-session, brief, motivational, web-based intervention (ProWISE) plus weekly text-message-initiated individualised prompts (TIPs) in reducing alcohol consumption and alcohol-related harm among children and adolescents aged ≥ 12 years. TIPs are designed to decrease risky alcohol use by reaching youth in the contexts of their everyday lives and by providing individualised feedback on drinking intentions, actual drinking and succession in achieving personal goals for low-risk drinking or abstinence.
Methods/Design
The trial is part of the multicentre consortium ProHEAD testing e-interventions for mental health problems in children and adolescents. Participants in grades 6–13 aged ≥ 12 years will be recruited in schools which participate in ProHEAD (target N = 15,000). Main criterion for inclusion in the ProWISE-TIP trial is a positive screening for at-risk alcohol use in the CRAFFT-d questionnaire (target n = 1076). In a multicentre, four-arm, randomised controlled design the following groups will be compared: (A) web-based intervention plus TIPs for 12 weeks; (B) web-based intervention plus text-message-initiated assessment of alcohol consumption for 12 weeks; (C) web-based intervention only; and (D) alcohol-related psychoeducation. TIPs will be delivered shortly before and after high-risk situations for excessive alcohol use and will be tailored to age, gender, drinking motives and alcohol consumption. Study participants will be followed up at three, six and nine months in the ProWISE-TIP trial and at one and two years in the ProHEAD consortium. Primary outcome is alcohol use in the past 30 days at nine months after enrolment. Secondary outcomes are alcohol-related problems, co-occurring substance use, health service utilisation, mental health problems and quality of life.
Discussion
Trial results will generate important evidence on how to enhance effectiveness of single-session, web-based alcohol interventions for youth. The ProWISE-TIP intervention, if effective, can be used as a stand-alone alcohol intervention or as an add-on to school-based or community-based alcohol prevention programs.
Trial registration
German Clinical Trials Register, DRKS00014606 Registered on 20 April 2018.
Electronic supplementary material
The online version of this article (10.1186/s13063-018-3160-z) contains supplementary material, which is available to authorized users.
Keywords: Adolescence, Binge drinking, Web-based intervention, Text-message intervention, Booster, ProHEAD
Background
Early and excessive alcohol use in adolescence is a significant threat to healthy development and a major public health concern. According to the Global Burden of Disease Study, alcohol use is among the top three risk factors contributing to the worldwide burden of disease [1]. While overall alcohol use by children and adolescents in Germany has decreased in the past 15 years [2], binge drinking, i.e. the consumption of five (four for girls) or more alcoholic beverages at one drinking occasion, is prevalent among 16.7% and 11.4% of male and female 12- to 17-year-olds, respectively, in the past 30 days [3]. Among 16- to 17-year-old boys, 37.1% report binge drinking in the past month (26.7% among girls) [3], indicating that a substantial proportion of the young population is at risk for experiencing short- and long-term negative consequences of risky alcohol use. Even heavier alcohol consumption patterns are common in German youth. In a state-wide representative sample, 5.6% of adolescents reported problematic alcohol use [4]; in a nationwide representative sample, the prevalence of problem drinking was 5.0% (girls: 5.1%, boys: 5.0%) in 12- to 17-year-olds [5]. Furthermore, in 2016 a total of 23,627 patients aged ≤ 19 years were admitted to German hospitals with an ICD-10 F10 diagnosis of Mental and Behavioural Disorders due to Use of Alcohol [6]. Among the 15- to 20-year-old inpatients, this diagnosis was the second most frequent reason for hospitalisation. Other short-term health risks associated with risky alcohol use for children and adolescents include aggressive and risky sexual behaviour, as well as elevated rates of injury and traffic accidents [7]. Moreover, heavy episodic drinking in adolescence is associated with a number of social and developmental problems, such as social conflicts, delinquency and problems of academic adjustment [8, 9], which also put children and adolescents at risk for chronification of problematic substance use patterns into adulthood [10]. Beyond these personal risks, alcohol-related problems also impose significant economic burden on public healthcare [11].
Thus, early recognition and indicated preventive interventions are needed to tackle risky alcohol use in childhood and adolescence. Acknowledging this, the American Academy of Pediatrics has published a policy statement with the recommendation to introduce substance use screening, brief intervention and referral to treatment (SBIRT) in all clinical settings serving paediatric populations [12]. In Germany, however, despite recommendation in the S3-Guideline for treatment of alcohol-related disorders in children and adolescents [13], alcohol SBIRT for children and adolescents is not part of standard primary care [2, 6]. Early detection of and intervention for risky alcohol use is especially challenging among youth, since members of this age group typically show the smallest rates of access to the help system [14–16].
Web-based interventions have been increasingly acknowledged in their capacity to lessen existing barriers for contacting the help system, particularly for at-risk populations [17, 18]. Evidence of such interventions to foster positive behaviour change, symptom reduction and improvement of health status is growing for a range of behavioural and mental health problems, including screening, prevention and early intervention for problematic substance use [19, 20]. Evidence indicates that fully automated brief motivational interventions are feasible and well accepted [21] and have the potential to reduce drinking and related harms for emerging adult at-risk drinkers up to 12 months after the intervention [22] with typically small but consistent effect sizes comparable to more conventional health professional-delivered interventions for substance use outcomes [23]. In a previous study, our research group developed a fully automated, single-session, web-based, brief motivational intervention targeting at-risk substance-using children and adolescents (aged 16–18 years), which was tested in four European countries [24, 25]. Compared to the assessment-only control group, the intervention was effective in decreasing past-month drinking as assessed by an AUDIT-C-based index score for drinking frequency, quantity and frequency of binge drinking at the three-month follow-up. However, results were limited by a high drop-out rate at the three-month follow-up (85.5%). In sum, aggregated evidence [17, 26] supports the utilisation of electronic alcohol interventions in reducing alcohol consumption and related harms in populations of young drinkers, but effect sizes tend to be small and different hypothesis have been tested as to how to increase effects of web-based alcohol interventions.
User engagement (i.e. the degree to which users find, access and actually use intervention content) is crucial for digital intervention programs, especially when they are designed as stand-alone interventions not supported by face-to-face interactions with a counsellor [27]. Designing user-accepted interventions requires tailoring of content, design and usability to the target groups preferences (user-centredness) [27]. User-centred interventions correspond with the habits and characteristics of the target population which should be reflected in the functional specifications of a behaviour change program. Failure in addressing user needs and characteristics adequately has been identified as a major barrier to uptake and impact of web-based interventions [28]. In addition to tailoring, self-monitoring, personalised feedback and reminders (‘prompts’) were found to be essential features enhancing user engagement as well as effectiveness of web-based alcohol interventions [17, 27].
In this trial, we will therefore test the effects of the introduction of highly individualised prompts (i.e. ‘messages, reminders, or brief feedback communicated to participants multiple times over the duration of an intervention’ [29]) to a web-based alcohol intervention. These prompts have the potential to reach participants in the context of their everyday lives as proposed by the ecological momentary intervention (EMI) approach [30]. Frequent contacts preceding high-risk situations seem especially appropriate for the target population of children and adolescents, because adolescence is a developmental period of increased proneness to risk-taking behaviour such as excessive alcohol use [31]. In this developmental phase, impulsive processes influence behaviour more strongly and self-regulatory processes have a smaller impact on behaviour than in adulthood. Additionally, self-regulatory processes presumably have a smaller impact on behaviour in high-risk situations, e.g. in situations with high peer pressure for drinking or under the influence of acute alcohol [32], so that interventions which aim at strengthening self-regulatory processes are presumed to have a stronger impact if delivered shortly before high-risk situations [30, 33].
A recent meta-analysis provided support for the effectiveness of prompts in increasing user engagement with digital interventions, especially when they were introduced shortly after completion of the intervention [34]. In particular, text-message-based prompts were found to increase user engagement and yield significant effects on an increased readiness to change drinking behaviour as well as reductions in heavy drinking [17]. Effectiveness of text-messaging interventions for adolescents’ and young adults’ substance use was additionally supported by a recent meta-analysis including 14 studies testing this approach [35]. Findings revealed that text-message-based interventions lead to substantial reductions in alcohol and tobacco use with a summary effect size of d = 0.25. Furthermore, a recent study with n = 765 at-risk alcohol consuming young adults (aged 18–25 years) found a weekly text-message-based assessment of drinking intentions and tailored feedback over a period of 12 weeks to be effective in reducing frequency of binge drinking, drinks per drinking day and prevalence of alcohol-related injury at nine-month follow-up [33]. In sum, evidence for the effectiveness of individualised prompts as an add-on to a web-based alcohol intervention is promising. However, the majority of evidence for young populations stems from studies with college students [31] and studies targeting under 18-year-olds are scarce [17, 19, 22, 29]. In the light of the early onset of alcohol misuse and addictive developmental trajectories [10], it is timely that the evidence base for this approach is broadened by testing it in the age group of under 18-year-olds.
Aims
In this trial we will further develop a previously evaluated youth-specific, web-based, single-session, brief motivational alcohol intervention [24] to increase positive effects on reductions in alcohol consumption and alcohol-related harm in children and adolescents aged ≥ 12 years identified as risky drinkers. Specifically, we will integrate text-message-initiated individualised prompts (TIPs) following the initial delivery of the web-based, single-session, brief motivational intervention (ProWISE intervention) and test this version of the intervention in a randomised controlled trial (RCT). Main goals of this trial are: (1) development of a user-centred, time-efficient and youth-specific text-message-initiated technique for delivering highly individualised prompts assessing drinking intentions and actual alcohol use and providing individualised feedback; (2) pilot testing of feasibility and acceptability of the new intervention components (i.e. TIPs) and their refinement according to results from the pilot testing and evaluation of focus group interviews; and (3) evaluation of effectiveness of the TIPs as boosters for the ProWISE intervention in a four-arm randomised controlled design.
Methods/Design
Design
The ProWISE-TIP trial is a sub-project (SP) of the multisite, prospective ProHEAD consortium. The aim of ProHEAD is the development, implementation and evaluation of Internet-based programs promoting mental health in healthy children and adolescents, preventing mental health problems (depression, eating disorders, risky alcohol use) in those who are at high risk and enhancing help-seeking of children and adolescents with mental health problems [36]. The ProHEAD consortium aims to improve access to prevention and care for young people aged ≥ 12 years by capitalising on novel E-health tools. For a detailed description of the ProHEAD consortium design, please refer to Kaess et al. [36]; for details on the other SPs, please refer to the respective study protocols in this issue [37–39].
Effectiveness of the ProWISE-TIP intervention will be tested in a four-armed, randomised controlled design with observer blinded group allocation with trial conditions: (A) ProWISE plus TIP for 12 weeks (ProWISE-TIP); (B) ProWISE plus text-message-initiated assessment (TA) for 12 weeks (ProWISE-TA); (C) ProWISE intervention only (ProWISE only); and (D) web-based psychoeducation on alcohol use in childhood and adolescence (control group). Participants will be followed up at three, six and nine months after enrolment in the SP and at one and two years after enrolment in the central project (ProHEAD consortium) (Fig. 1). A schedule of enrolment, assessment and intervention is provided in Fig. 3 and a populated Standard Protocol Items: Recommendations for Interventions Trials (SPIRIT) Checklist is provided in Additional file 1.
Fig. 1.
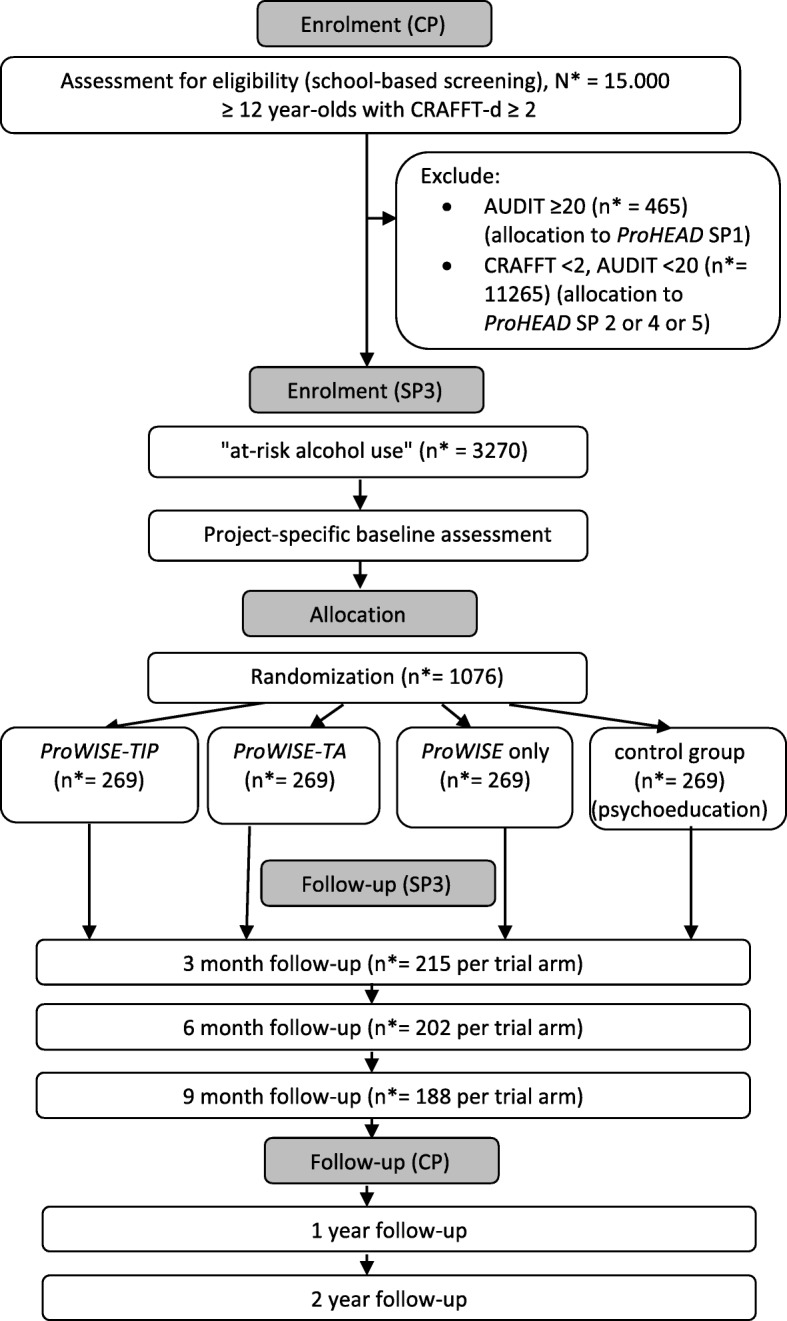
Trial flow diagram. Note: * refers to the proposed sample size, TIP text-message-based individualised prompt, TA text-message-based assessment, CP central project (ProHEAD project), SP3 sub-project 3 (ProWISE-TIP trial)
Fig. 3.

Schedule of enrolment, assessment and intervention. Note: CP central project, SP3 sub-project 3, ProWISE single-session web-based brief motivational alcohol intervention, PE psychoeducation, TA text-message-initiated assessment, TIP text-message-initiated individualised prompt, CRAFFT-d screen for risky alcohol use (Car, Relax, Alone, Forget, Friends, Trouble), AUDIT Alcohol Use Disorders Identification Test, Adapted AUDIT-C (30d) Alcohol Use Disorders Identification Test - Consumption adapted to assess alcohol use in the past 30 days, RAPI Rutgers Alcohol Problem Index, RTC Algorithm Readiness to change drinking algorithm, DMQ-R SF Drinking Motive Questionnaire-Revised Short Form, AHSQ Actual Help-Seeking Questionnaire, SDQ Strengths and Difficulties Questionnaire, KIDSCREEN-10 Quality of life measure for children and adolescents, CAST Cannabis Abuse Screening Test, SCL-9-K Short version of the Symptom Checklist, SCS-K-D German short form of the Self-Control Scale, AIC Adolescent Injury Checklist
Participants
The project will utilize a large-scale school-based sample of children and adolescents (N = 15,000 children and adolescents, grades 6–13, aged ≥ 12 years), recruited in five regions of Germany (Hamburg, Heidelberg, Leipzig, Marburg, Schwäbisch Gmünd). In the central project (ProHEAD consortium), online assessments of a broad variety of mental health problems and health-risk behaviours will be conducted at baseline and at two annual follow-ups. Following the baseline assessment, children and adolescents will be identified as either currently ‘healthy’, ‘high-risk’ (including subthreshold mental health problems and diverse health-risk behaviours) or having ‘mental health problems’ (which includes clinically relevant levels of psychopathology and/or indicators of threat to self and others). Based on these screening profiles, children and adolescents will be allocated to one of five programs according to their individual needs, i.e. increasing face-to-face mental help-seeking in children and adolescents with a screening result at baseline indicating mental health problems (SP1) [36], preventing eating disorder symptoms in children and adolescents with a screening result at baseline indicating a risk for the development of an eating disorder (SP2) [37], reducing alcohol misuse in children and adolescents with a screening result at baseline indicating risk for the development of alcohol misuse (SP3, ProWISE-TIP trial), preventing depressive symptoms in children and adolescents with a screening result at baseline indicating a risk for the development of depression (SP4) [38], promoting mental health and preventing mental health problems in children and adolescents with a screening result at baseline indicating no mental health problems (SP5) [39]. All programs provide online information as well as additional modules such as prompts or chat counselling with mental health professionals.
Inclusion and exclusion criteria
Children and adolescents (1) in grades 6–13 aged ≥ 12 years, (2) with a positive screening result for at-risk alcohol use based on the CRAFFT-d screening tool (≥ 2) [40], (3) and written informed consent from both participant and legal guardian are eligible for inclusion in the ProWISE-TIP trial. Children and adolescents with (1) current alcohol use disorder according to the Alcohol Use Disorders Identification Test (AUDIT) (≥ 20) [41] or (2) current psychiatric disorders, (3) illiteracy, (4) no possession of a mobile phone, no access to the Internet or no sufficient German language skills will be excluded from participation in the ProWISE-TIP trial (Fig. 1). Children and adolescents with a total score of ≥ 20 in the AUDIT [41] or a total score of 20–40 points on the Strengths and Difficulties Questionnaire (SDQ) [42] or a score above the defined thresholds for one of its sub-scales: emotional symptoms (scores: 7–10), conduct problems (scores: 5–10), hyperactivity/inattention (scores: 7–10), peer relationship problems (score > 5) will be excluded from the ProWISE-TIP trial and included in SP1 of the ProHEAD consortium. Children and adolescents with a negative screening result for risky alcohol use (CRAFFT-d score < 2) will be included in SPs 2, 4 or 5 of the ProHEAD consortium, depending on which inclusion criteria they meet. Study participants fulfilling inclusion criteria for more than one SP addressing children and adolescents at risk for developing an eating disorder, alcohol-related problems or depressive symptoms (SP2–4) will be allocated randomly to one SP (see CP study protocol [36] for details). There are no restrictions for study participants to take up concomitant care outside ProHEAD.
Recruitment and randomisation
Participants for the trial will be recruited through the ProHEAD infrastructure with five centres (Heidelberg, Hamburg, Leipzig, Marburg, Schwäbisch Gmünd) actively recruiting and drawing children and adolescents from a national school-based sample. For a detailed description of the recruitment process, please refer to Kaess et al. [36]. After enrolment in the central project, study participants will be allocated to one of the five SPs according to their screening result (Fig. 1). All participants receive an e-mail containing a link to an encrypted webpage where they can register to the respective SP. Participants with a positive screening for at-risk alcohol use are invited to take part in the ProWISE-TIP trial and referred to the SP-specific online baseline assessment (Fig. 3). After completion of the assessment, participants will be randomised to one of the four study arms (A) ProWISE plus TIP for 12 weeks (ProWISE-TIP); (B) ProWISE plus TA for 12 weeks (ProWISE-TA); (C) ProWISE intervention only (ProWISE only); (D) web-based psychoeducation on alcohol use in childhood and adolescence (control group). Randomisation is conducted automatically by the computer program on the basis of predefined lists and following a permutated block design. Randomisation will be stratified by age and gender. Once participants have completed the trial-specific online baseline assessment, they automatically receive a computer-generated e-mail containing a link to access the online content in their respective trial condition.
The ProWISE-TIP intervention
The ProWISE intervention
The intervention tested in this trial is based on a previously positively evaluated, single-session, web-based, motivational intervention for risky substance use in children and adolescents [24, 43]. For the current study, the intervention was refined in order to address children and adolescents aged ≥ 12 years (previously 16–18 years) and in order to address alcohol use only (previously alcohol and other substance use).
The ProWISE intervention is fully automated and relies on an interactive system to generate individually tailored content. All system-generated information directly refers to the participant’s statements assessed in the first place (e.g. alcohol use, gender, weight, perceptions of peer drinking). Navigation through the program is designed as a dialogue based on motivational interviewing (MI) techniques [44] between the user and the program. The intervention comprises the following six components: (1) feedback on individual drinking patterns with information on associated health and developmental risks; (2) normative feedback on descriptive drinking norms about gender- and age-matched peer drinking levels using graphed comparative information; (3) feedback on blood alcohol concentration (BAC) and associated health and other risks for the reported peak drinking episode; (4) importance and confidence rulers with a short summary and feedback to elicit and strengthen readiness to change and exploration of personal strengths, resources and strategies for goal attainment; (5) decisional balance for selection of personal costs and benefits of current alcohol use and a subsequent graphical display of comparative gains and losses of behaviour change in a balance sheet to illustrate ambivalence; and (6) identification and selection of personal high-risk situations for alcohol use and provision of behavioural strategies, e.g. to resist peer pressure. Completion of the ProWISE intervention takes approximately 20 min.
Text-message-initiated individualised prompts (TIPs)
The development of the TIPs will be realised in two phases. In phase 1 we will develop the content of the TIPs. Following the abovementioned EMI approach [30], children and adolescents will receive the prompts querying drinking intentions and providing individualised feedback shortly before high-risk situations for excessive alcohol use, i.e. before weekends, every Thursday at 18:00 h. On days following high-risk situations for drinking, i.e. every Sunday, participants will be prompted to report their actual alcohol consumption and will receive tailored feedback according to the relation of drinking intention and actual consumption.
Based on prior research findings on moderators of brief alcohol intervention efficacy, content of the Thursday TIPs will be individually tailored with respect to gender, age, drinking motives and willingness to set a goal for low-risk drinking or abstinence [45, 46]. Sunday TIPs will be tailored to age, gender, actual drinking [47] and to the degree of attainment of the drinking goal defined in the previous Thursday TIP. TIPs’ content will be informed by data which participants provide: (1) in the school-based online baseline assessment (age, gender, alcohol use); (2) in the SP-specific online assessment following enrolment in the SP (drinking motives); and (3) in the assessments of drinking intentions, willingness to set a drinking goal and actual alcohol use as part of the Thursday and Sunday TIPs.
Tailoring of the prompts to participants’ personal drinking motives and risk and consumption profiles is designed to yield high user-centredness, thereby promoting user engagement, a necessary prerequisite for effectiveness of any fully automated intervention [17, 27]. Timing and frequency of the prompts is chosen in order to reach adolescents in the context of their everyday lives, thereby strengthening self-regulatory processes. According to the framework for the prediction of risky behaviour in adolescents [32], risk behaviour is influenced by reflective control processes on the one hand and impulsive processes on the other hand, which, in turn, are influenced by boundary conditions, such as habitualness and motivational state, and characteristics of the situation. The weekly contact with participants before and after potential drinking occasions is supposed to strengthen self-control motivation (through motivational interviewing techniques for eliciting and strengthening motivation to change) and self-control ability (through provision of tailored harm-reduction and drink-less strategies), thereby influencing risky behaviour directly and indirectly (see Fig. 2).
Fig. 2.
Adapted framework for the prediction of risky behaviour in adolescents (adapted from Wiers et al., 2010; © 2010 Wiers, Ames, Hofmann, Krank and Stacey)
Like the ProWISE intervention, conversational style of the TIPs is designed in an MI congruent way. As central mechanisms of action, the TIPs are designed to increase self-monitoring and promote awareness of potential discrepancies between drinking intentions and actual consumption, thereby raising the awareness of ambivalence as an important mechanism in the development of a motivation to change according to MI theory [44]. According to a recent study, 40% of adolescents and young adults (aged 16–25 years) consume alcohol heavily despite they intent not to [48]. Individualised feedback on this discrepancy embedded in real-life context was found to be effective in reducing heavy drinking [33]. Furthermore, content of the TIPs draws on elements of MI theory by highlighting participants’ responsibility for change and freedom of choice, by providing information on harm-reduction and drink-less strategies as well as by providing feedback on alcohol use and by promoting self-efficacy for the achievement of low-risk alcohol use or abstinence.
Phase 2 of the TIP development will include pilot testing of feasibility and acceptability of the TIPs with N = 20 adolescents of the target population of ≥ 12- year-olds with risky alcohol consumption. Two focus groups (n = 5 each) will additionally provide feedback on content and conversational style of the TIPs, which will be adapted accordingly.
Control conditions
Participants in the control groups will receive either: (1) the ProWISE intervention plus weekly TA of alcohol use for 12 weeks every Sunday (ProWISE-TA); or (2) the ProWISE intervention only; or (3) web-based psychoeducation on alcohol use in childhood and adolescence (control). The psychoeducation control group is included in this study in order to test if findings from the previous evaluation of the ProWISE intervention can be replicated, since the intervention has been refined for the current study. Previously, the ProWISE intervention was tested as a self-help program (1) without school-based screening and referral to intervention, (2) in a different age group (16–18 years), and (3) in a slightly different population of at-risk drinkers (i.e. not excluding children and adolescents with AUDIT scores ≥ 20). The ProWISE-TA condition is included in the study design in order to differentiate effects of the TIPs from assessment reactivity [49].
Data collection
Participants will complete school-based online assessments at time of enrolment in the CP (t0) as well as at one and two years after enrolment (t5, t6) (Fig. 3). SP-specific assessments will be administered following enrolment in the SP (t1) and at the SP-specific follow-ups at three (t2), six (t3) and nine (t4) months after enrolment in SP3. All data will be collected online via central servers that are used for both the school-based assessments and the different interventions that are conducted via the ProHEAD online platform. Data will be handled in accordance with German legal regulations concerning data protection and data security (Data Protection Law of the Federal State of Baden-Wuerttemberg, Data Protection Law of the Free and Hanseatic City of Hamburg and German National Data Protection Laws) as well as EU General Data Protection Regulation. Data storage and transfer will be encrypted. All follow-up assessments will be conducted online via a password-secured website and will be recorded in the individual case report forms (CRF). An independent Data and Safety Monitoring Committee (DSMB) will oversee all aspects of data collection, handling and analysis. The DSMB will comprise independent researchers with expertise in research methodology, child and adolescent mental health, and technology-based alcohol interventions. Members of the DSMB will have their first meeting before inclusion of the first study participant.
Measures
Sociodemographic data [50] will be assessed as part of the school-based online assessment. Measures assessed for the CP and the other SPs are described elsewhere [36–39]. The CRAFFT-d screening test for risky alcohol use in adolescence in its validated German version [40] will be applied as part of the school-based screening to identify children and adolescents with at-risk alcohol use which will be included in the ProWISE-TIP trial. This six-item questionnaire assesses alcohol-related risk behaviours applying a binary yes/no response format (e.g. ‘Do you ever use alcohol to relax, feel better about yourself, or fit in?’; ‘Do you ever use alcohol while you are by yourself?’). Two or more positive answers indicate risky alcohol use. The CRAFFT-d is validated in the age group of adolescents aged 12–18 years [40].
Drinking motives required for individualisation of the TIPs will be assessed by the Drinking Motive Questionnaire Revised Short Form (DMQ-R SF) [46]. This youth-specific self-report questionnaire comprises 12 items assessing the frequency (never / rarely / sometimes / often / always) of different motives for drinking in the past 12 months (‘In the last 12 months, how often did you drink…’). Three items each represent the four drinking motives enhancement (e.g. ‘…because it’s fun?’), social (e.g. ‘…because it helps you enjoy a party?’), conformity (e.g. ‘…to fit in with a group you like?’) and coping (e.g. ‘...to forget about your problems?’). Scores for drinking motives are calculated by mean sum scores of the items representing the respective drinking motive with higher scores representing more predominant motives. The DMQ-R SF is validated in the age group 12–18 [46].
Primary outcome measure
For reasons of comparability, we chose to use the same primary outcome as in our previous study, testing the effectiveness of the original version of the web-based brief alcohol intervention without TIPs [24]. Therefore, the primary outcome is alcohol use in the past 30 days at nine-month follow-up as assessed by an index score for frequency and quantity of drinking and frequency of binge drinking calculated from the first three items of the Alcohol Use Disorders Identification Test (AUDIT-C) [41] adapted to assess drinking in the past 30 days. Frequency of alcohol consumption and frequency of having six drinks at one occasion (binge drinking) are assessed with response options ranging from 0 (never) to 4 (four or more times a week). The assessment of quantity of standard drinks consumed per typical drinking occasion (0 = one or two to 4 = ten or more) is supported by a graphical overview over units of alcoholic drinks defined as standard drinks. The AUDIT-C is validated in a sample of 14- to 18-year-old students in Germany [51].
Secondary outcome measures
As secondary outcomes, alcohol-related problems will be assessed by a brief version of the Rutgers Alcohol Problem Index (brief RAPI) [52], a youth-specific measure for alcohol-related problems at the SP-specific follow-ups. Participants are asked 16 questions about the frequency of experiencing different situations in the past three months while they were drinking alcohol or as a result of their alcohol use (e.g. ‘Not able to do your homework or study for a test’; ‘Got into fights with other people (friends, relatives, strangers)’; ‘Wanted to stop drinking but you couldn’t’). Response options range from ‘never’, ‘1–2 times’, ‘3–5 times’, ‘6–10 times’ to ‘more than 10 times’. Higher sum scores indicate more severe alcohol-related problems. The brief RAPI was validated in a sample of 12- to 18-year-olds [52]. Alcohol-related injuries in the past six months will be assessed by a modified version of the Adolescent Injury Checklist (AIC) [53]. Prevalence of experiencing nine different injuries while or shortly after consuming alcohol are assessed (e.g. ‘In the past 6 months, were you injured while or shortly after drinking alcohol … by being in a physical fight with someone? / ... by falling? / ... while riding in a car, truck or bus?’). Response options are ‘yes = 1 / no = 0’ with a higher sum score indicating higher prevalence of alcohol-related injuries in the past six months. Alcohol-related sexually risky behaviour will be recorded by assessing the frequency of experiencing six different risk situations while drinking or shortly after drinking alcohol (e.g. ‘I was sexually harassed’, ‘I had sex, which I couldn’t fully remember later on’). Co-occurring substance use will be assessed as a secondary outcome by the 30-day prevalence of cannabis and other illegal drug use. Additionally, readiness to change alcohol use will be assessed using the Brief Readiness to Change Drinking Algorithm as proposed by Epler et al. [54]. The algorithm comprises three items allowing to categorise risky drinkers’ motivation to change into the stages ‘pre-contemplation’, ‘contemplation’ and ‘action’ according to the Transtheoretical Model of Behaviour Change [55]. Response options for items vary (e.g. ‘Has the amount you drink changed in the past 3 months? Yes, I drink less / Yes, I drink more / No, I drink the same’) and allow allocation of the respondent to one of the three stages of change as described above. The Brief Readiness to Change Drinking Algorithm was validated in an adult population [54].
Additional secondary outcomes will be assessed in the school-based follow-ups at one and two years after enrolment. Help-seeking will be assessed using an adapted version of the Actual Help-Seeking Questionnaire (AHSQ) [56], which assesses actual help-seeking behaviour by listing potential help sources and measuring whether help has been sought from the respective sources within a specified time-period for a specified problem. The 13-item questionnaire comprises three subscales: whether informal help has been sought; whether formal help has been sought; and whether no help has been sought. Response options are 0 = ‘no’, 1 = ‘yes, in the past 12 months’ and 2 = ‘yes, but longer ago then 12 months’. The Actual Help-Seeking Questionnaire (AHSQ) is validated in a sample of 16- to 19-year-old students [56]. Mental health symptoms will be assessed using the Strengths and Difficulties Questionnaire (SDQ), a self-report screening questionnaire for children and adolescents aged 2–17 years [42]. The four subscales (emotional, conduct, hyperactivity and peer problems), each scored on a scale of 0–10, will be assessed. Higher scores indicate a higher level of psychopathology. Quality of life will be assessed using the KIDSCREEN-10 [57]. The KIDSCREEN is an international cross-culturally comparable quality-of-life assessment instrument validated for children and adolescents aged 8–18 years. The KIDSCREEN-10 index comprises 10 items, which provide a global measure of health-related quality of life. Items are answered on a 5-point response scale and are coded in a way that higher values indicate better quality of life. Furthermore, program usage patterns (frequency of replies to TIPs, duration, completion) based on log-file data will be analysed.
Further measures
To measure problematic cannabis use in our sample, we will use the Cannabis Abuse Screening Test (CAST) [58]. The CAST consists of one screening item (to evaluate whether cannabis was used in the last year) and six further questions concerning different aspects of cannabis consumption (e.g. if cannabis was already smoked in the morning). A higher sum value in the CAST indicates a more problematic cannabis use. The CAST is validated in a sample of 14- to 22-year-old youth [58]. Additionally, mental wellbeing in our SP sample will be assessed using a short version of the Symptom Checklist (SCL-K-9) [59], which comprises nine items providing a global severity index for general psychopathology. Respondents are asked to indicate how strongly they have suffered from different symptoms in the past seven days. Response options range from 0 = ‘not at all’ to 4 = ‘very strongly’ with higher scores indicating higher levels of psychopathology. The SCL-K-9 is validated in a sample of 14- to 92-year-olds. In order to control for potential moderators and mediators of intervention effectiveness, we will assess dispositional self-control capacity applying the German short form of the Self-Control Scale (SCS-K-D) [60]. The SCS-K-D consists of 13 items with a five-level response format. A higher total score in the SCS-K-D indicates a higher dispositional self-control capacity. The SCS-K-D is validated in a sample of school children attending tenth grade (mean age = 16.6 years). Furthermore, parental monitoring will be assessed by a validated seven-item questionnaire on adolescents’ perceptions of parents’ monitoring comprising the sub-scales parental knowledge (e.g. ‘my parents/guardian know where I am after school’), youth disclosure (e.g. ‘if I am going to be home late, I tell my parents/guardian’), parental solicitation (‘when I go out, my parents/guardian ask me where I’m going’) and parental control (‘when I go out, my parents/guardian tell me what time I’m going to return’) [61]. The parental monitoring scale was developed for and applied in child and adolescent samples [61, 62].
Health economic measures
Additionally, cost-effectiveness and cost-utility analyses will be conducted. Data on the cost of interventions will be compared to study outcomes to determine the incremental cost-effectiveness ratio (ICER) of interventions. The ICER is defined as the differential cost of a new treatment and treatment as usual, divided by the outcome differential of the two. Cost-utility analyses will provide information on cost per quality-adjusted life years (QALYs). QALYs are measures combining the additional life years gained by a certain healthcare intervention or program with the quality of life an individual attributes to this lifespan into one single parameter. Thus, QALYs are subjective and universally applicable outcome parameters for comparing health benefits across sectors, disorders, samples or populations. It can be assessed in both patients and healthy individuals. Health-related quality of life will be assessed using the KIDSCREEN-10 [57]. In addition, the health service utilisation of the participants will be assessed by the ‘Mannheimer Modul Ressourcenverbrauch’ (MRV) [63] and transformed into cost estimates for including intervention and treatment as usual costs into the cost-effectiveness and cost-utility analyses of the various study interventions. For transforming health utilisation data, a catalogue of so-called ‘unit costs’ will be compiled for all types of treatments, services or other healthcare measures that were used by study individuals and controls.
Sample size and power calculation
Two focus groups will be realised in study phase 2 with five participants each. N = 20 at-risk alcohol-consuming children and adolescents will be recruited for the pilot testing of the newly designed TIPs. For the RCT, N = 15,000 children and adolescents will be assessed for eligibility (ProHEAD school sample). The estimated number of eligible participants for the ProWISE-TIP trial based on current data on the prevalence of risky alcohol consumption is n = 3270 [51]. Criteria for the allocation of participants to the five individual ProHEAD trials are based on latest scientific evidence. However, this is the first time that the overall algorithm is applied on a consortium-wide basis simultaneously screening for various mental health problems. Therefore, an intermediate data analysis will be conducted following completion of 10% of the screening assessments (N = 1500) in order to determine the actual allocation ratio to the five ProHEAD trials and to adjust the screening algorithm if necessary. Based on prior research, we expect that the ProWISE-TIP intervention reaches a small effect size (f = 0.10) when compared to the ProWISE-only condition and a medium effect size (f = 0.25) when compared to the psychoeducation control group [33, 35]. Thus, a total sample size of N = 1076 (intention-to-treat) (n = 269 in each trial arm) are needed (power = 0.80; alpha = 0.05, two-tailed; f = 0.10) in order to show superiority of the ProWISE-TIP intervention.
Statistical analysis
Focus groups in study phase 1 will be evaluated by qualitative content analysis of data gained from the two focus groups. Details on study population will be provided by descriptive data analysis. In the RCT, intention to treat (ITT) analyses of primary data will be based on the available clinical data from all randomised participants after the nine-month follow-up. Missing data will be replaced by multiple imputations. For the primary endpoint, a mixed linear repeated measurement model (LMM) with the participant ID as random, group and time as fixed factor will be performed which is more robust against drop-outs than models without random factor [64] and uses the direct maximum likelihood as the statistical estimation procedure, which results in unbiased estimators under the missing-at-random-assumption [65]. We will use the closed test principle to evaluate differences between the means of groups. An additional analysis will be conducted on the per-protocol sample. The secondary endpoints alcohol-related problems, co-occurring substance use and help-seeking in those with transition to full-threshold alcohol use disorder, other mental health symptoms and quality of life will be examined in an exploratory manner with appropriate procedures, including subgroup analyses of gender of participants. Interim analyses will be performed after each wave of recruitment. Statistical analyses will be carried out with SPSS, Version 22 [66]. Associations between program usage patterns and reductions in alcohol use will be examined using latent class analysis (LCA) with Mplus, Version 5 [67].
Compliance / Rate of loss to follow-up
According to recent reports on follow-up rates in trials testing web-based alcohol interventions and text-message-based interventions, follow-up data for 80% of randomised individuals are expected at the three-month follow-up, 75% at the six-month follow-up and 70% at the nine-month follow-up (primary endpoint) in the four trial arms [19, 24, 33]. These follow-up rates seem feasible because of the recruitment setting (schools) and the use of incentives. Participants will receive online gift vouchers after completion of each program-specific assessment at the follow-ups at three (€10), six (€20) and nine (€25) months.
Methods against bias
Proactive recruitment of entire classes in schools will reduce potential selection bias. The Coordination Center for Clinical Trials (KKS) Heidelberg will monitor study-related procedures at the five recruiting centres. Specifically, the recruitment of schools within the target regions and the recruitment of students within these schools will be monitored in order to ensure adherence to the study manual and documentation guidelines as well as equivalent procedures at all sites.
Allocation concealment will be practiced with software support. Validated and standardised measures will be employed. Study participants will be informed about aim and rationale of the study as well as on the randomisation procedure. School-based data collection will be fully automated and web-based, preceded by an in-person introduction to the study and informed consent procedure. SP-specific data assessment will be fully automated and web-based (observer-blind). Weekly TIPs and TAs will be delivered fully automated. To reduce publication bias, the trial was registered in a clinical trials registry (DRKS00014606). Important protocol modifications will be communicated to the registry.
Dissemination of results
Results of the ProWISE-TIP trial will be published in international peer-reviewed journals and presented on national and international conferences. Additionally, the ProHEAD consortium will disseminate results via the ProHEAD website and the press campaigns accompanying the development of the project. Information regarding the availability of the ProWISE-TIP intervention after the study phase will be communicated to relevant stakeholders, such as schools, youth-specific counselling services and prevention programme providers.
Discussion
Early onset of alcohol use and excessive alcohol use is a major risk factor for a number of serious negative short-term consequences and for chronification of harmful consumption patterns into adulthood [10]. Therefore, prevention and early intervention addressing risky alcohol use is important to take place at a young age. However, adolescents are hard to reach with prevention measures and they typically show little access to the help system [14–16]. In this trial, we thus aim at developing a user-centred, youth-specific, fully automated, electronic alcohol intervention which has the potential to lower existing barriers for service utilisation and therefore reaches populations of at-risk alcohol-consuming children and adolescents who are often underserved. To our knowledge, this is the first trial to investigate effectiveness and cost-effectiveness of highly individualised, high-frequent booster messages as an add-on to a single-session, web-based, alcohol intervention in the target population of children and adolescents aged ≥ 12 years. The fully automated ProWISE-TIP intervention allows for a standardised delivery of highly tailored content which can potentially be disseminated cost-effectively at a large scale while providing a low-threshold opportunity for service-use, particularly for young non-treatment seeking at-risk populations.
The four-arm trial design will allow evaluating: (1) if the theory-based weekly TIPs following the ProWISE intervention decrease alcohol use among children and adolescents with at-risk drinking patterns significantly stronger compared to psychoeducation only; and (2) if the TIPs add effectiveness to the evidence-based single session ProWISE intervention without additional prompts. Furthermore, the inclusion of the ProWISE-TA condition in the four-arm design of the trial will allow to differentiate effects of the weekly TIP interventions from well documented effects caused by the weekly assessment itself [49].
The results of this study will contribute to the current evidence on user engagement and on effectiveness of web-based alcohol brief interventions with additional boosters for the very young target group of children and adolescents aged ≥ 12 years. The ProWISE-TIP intervention, if proven effective, can be used as a stand-alone youth-specific brief alcohol intervention or as an add-on to future school-based or community-based alcohol prevention programs. The intervention has the potential to be used for early detection and intervention in a variety of settings, such as youth work, schools, party scene, sports clubs or as part of routine paediatric medical care.
Trial status
The ProWISE-TIP intervention is currently being developed. Recruitment of participants is predicted to start between October 2018 and January 2019. School-based two-year follow-ups are predicted to be completed by March 2021.
Additional file
Standard Protocol Items: Recommendations for Interventions Trials (SPIRIT) Checklist. (DOC 123 kb)
Acknowledgements
This trial is funded by the German Federal Ministry of Education and Research (BMBF) Grant (01GL1744B). Michael Kaess is the coordinator and Stephanie Bauer the co-coordinator of the ProHEAD consortium. The consortium comprises six study sites in Germany. Site leaders are: Michael Kaess (University Hospital Heidelberg); Stephanie Bauer (University Hospital Heidelberg); Rainer Thomasius (University Medical Center Hamburg-Eppendorf); Christine Rummel-Kluge (University Leipzig), Heike Eschenbeck (University of Education Schwäbisch Gmünd); Hans-Joachim Salize (Medical Faculty Mannheim/Heidelberg University); and Katja Becker (Philipps-University of Marburg). Further members of the consortium are: Katja Bertsch, Sally Bilic, Romuald Brunner, Johannes Feldhege, Christina Gallinat, Sabine C. Herpertz, Julian Koenig, Sophia Lustig, Markus Moessner, Fikret Özer, Peter Parzer, Franz Resch, Sabrina Ritter, Jens Spinner (all University Hospital Heidelberg); Silke Diestelkamp, Kristina Wille (both University Medical Center Hamburg-Eppendorf); Sabrina Baldofski, Elisabeth Kohls, Lina-Jolien Peter (all University Leipzig); Vera Gillé, Hanna Hofmann, Laya Lehner (all University of Education Schwäbisch Gmünd); Elke Voss (Medical Faculty Mannheim/Heidelberg University); and Jens Pfeiffer and Alisa Samel (both Philipps-University of Marburg). We thank Christina Merkel and Boris Orth from the Federal Centre for Health Education (BzgA) for conducting additional analyses with data on adolescent alcohol use in a national representative sample of 12- to 17-year-old children and adolescents in order to facilitate provision of age-specific normative feedback on peer drinking patterns to participants in the ProWISE-TIP trial. We would like to thank Johanna Hahn for assisting in adapting the ProWISE intervention and Anna Segendorf for graphical support. We thank Peter-M. Sack for comments on a former draft of this paper.
Funding
The project is funded by the Federal Ministry of Education and Research (BMBF), Germany, grant number 01GL1744D. The BMBF has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. None of the project partners involved in the trial has a potential conflict of interest to declare.
Availability of data and materials
Not applicable.
Abbreviations
- Adapted AUDIT-C (30d)
Alcohol Use Disorders Identification Test - Consumption adapted to assess alcohol use in the past 30 days
- AHSQ
Actual Help-Seeking Questionnaire
- AIC
Adolescent Injury Checklist
- AUDIT
Alcohol Use Disorders Identification Test
- BAC
Blood alcohol concentration
- CAST
Cannabis Abuse Screening Test
- CP
Central project
- CRAFFT-d
Screen for risky alcohol use (Car, Relax, Alone, Forget, Friends, Trouble)
- CRF
Case report form
- DMQ-R SF
Drinking Motive Questionnaire-Revised Short Form
- DRKS
German Register for Clinical Trials
- EMI
Ecological momentary intervention
- ICER
Incremental cost-effectiveness ratio
- ITT
Intention to treat
- KIDSCREEN-10
Quality of life measure for children and adolescents
- LCA
Latent class analysis
- LLM
Linear mixed models
- MI
Motivational interviewing
- MRV
Mannheimer Modul zum Ressourcenverbrauch
- PE
Psychoeducation
- ProWISE
Prevention of risky alcohol use through Web-based Intervention and SMS-based REminders
- QALY
Cost per quality-adjusted life year
- RAPI
Rutgers Alcohol Problem Index
- RTC Algorithm
Readiness to change drinking algorithm
- SBIRT
Substance use screening, brief intervention and referral to treatment
- SCL-9-K
Short version of the Symptom Checklist
- SCS-K-D
German short form of the Self-Control Scale
- SDQ
Strengths and Difficulties Questionnaire
- SP
Sub-project
- SPIRIT
Standard Protocol Items: Recommendations for Interventions Trials
- TA
Text-message-initiated assessment
- TIP
Text-message-initiated individualised prompt
Authors’ contributions
SD developed the initial trial protocol and design, developed concept and content of the TIPs, adapted the ProWISE intervention and drafted the manuscript. LW provided methodological support and feedback on all stages of the development of the trial protocol and design and was, together with SD, responsible for selecting measures. MK is the principal investigator of the trial and the coordinator of the ProHEAD consortium, SB is the co-coordinator of the ProHEAD consortium. RT, MK, KB, HE and CRK are the site leaders of the five recruiting centres; SB and MM are responsible for technological support; HJS is responsible for health economic analysis. CB supported adaptation of the ProWISE intervention for this trial. NA contributed to designing the trial. All authors revised the manuscript for important intellectual content and approved the final version of the manuscript.
Ethics approval and consent to participate
The ProHEAD consortium received full ethical approval from the Ethics Committee I of the Medical Faculty of the University of Heidelberg (Ethics Committee No.: S-086/2018) on 1 March 2018. Secondary ethics votes for conducting ProHEAD in the respective federal states of the five recruiting centres within the ProHEAD consortium have been applied for. The trial has obtained a trial registration number from the German Clinical Trials Register: DRKS00014606, registered on 20 April 2018. Participants and their legal guardians provide written informed consent before participating in the first school-based assessment and they are free to withdraw consent at any time. The Ethics Committee of the Medical Faculty of the University of Heidelberg will be informed in case of severe adverse events and other unintended effects of trial participation. The study will be conducted in accordance with the ethical principles following the Declaration of Helsinki (DoH), 1996, and the Guidelines for Good Clinical Practice (ICH-GCP) in the latest revision. Data collection, storage and analysis will adhere to National and European Data Protection Laws.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Silke Diestelkamp, Email: s.diestelkamp@uke.de.
Lutz Wartberg, Email: lwartberg@uke.de.
Michael Kaess, Email: michael.kaess@med.uni-heidelberg.de, Email: michael.kaess@upd.ch.
Stephanie Bauer, Email: stephanie.bauer@med.uni-heidelberg.de.
Christine Rummel-Kluge, Email: christine.rummel-kluge@medizin.uni-leipzig.de.
Katja Becker, Email: Katja.Becker@med.uni-marburg.de.
Heike Eschenbeck, Email: heike.eschenbeck@ph-gmuend.de.
Hans-Joachim Salize, Email: hans-joachim.salize@zi-mannheim.de.
Markus Moessner, Email: markus.moessner@med.uni-heidelberg.de.
Christiane Baldus, Email: c.baldus@uke.de.
Nicolas Arnaud, Email: n.arnaud@uke.de.
Rainer Thomasius, Email: thomasius@uke.de.
the ProHEAD consortium:
Michael Kaess, Stephanie Bauer, Rainer Thomasius, Christine Rummel-Kluge, Heike Eschenbeck, Hans-Joachim Salize, Katja Becker, Katja Bertsch, Sally Bilic, Romuald Brunner, Johannes Feldhege, Christina Gallinat, Sabine C. Herpertz, Julian Koenig, Sophia Lustig, Markus Moessner, Fikret Özer, Peter Parzer, Franz Resch, Sabrina Ritter, Jens Spinner, Silke Diestelkamp, Kristina Wille, Sabrina Baldofski, Elisabeth Kohls, Lina-Jolien Peter, Vera Gillé, Hanna Hofmann, Laya Lehner, Elke Voss, Jens Pfeiffer, and Alisa Samel
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orth B. Drug affinity of adolescents in the Federal Republic of Germany 2015. Köln: Bundeszentrale fuer gesundheitliche Aufklaerung; 2016. [Google Scholar]
- 3.Orth B. Der Alkoholkonsum Jugendlicher und junger Erwachsener in Deutschland. Ergebnisse des Alkoholsurveys 2016 und Trends. Köln: Bundeszentrale für gesundheitliche Aufklärung; 2017. [Google Scholar]
- 4.Wartberg L, Brunner R, Kriston L, Durkee T, Parzer P, Fischer-Waldschmidt G, et al. Psychopathological factors associated with problematic alcohol and problematic Internet use in a sample of adolescents in Germany. Psychiatry Res. 2016;240:272–277. doi: 10.1016/j.psychres.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 5.Wartberg L, Kriston L, Thomasius R. Prevalence of problem drinking and associated factors in a representative German sample of adolescents and young adults. J Public Health (Oxf). 2018. 10.1093/pubmed/fdy163 [Epub ahead of print]. [DOI] [PubMed]
- 6.Gesundheitsberichterstattung des Bundes. Diagnosedaten der Krankenhäuser ab 2000. http://www.gbe-bund.de. Accessed 14 May 2018.
- 7.Sindelar HA, Barnett NP, Spirito A. Adolescent alcohol use and injury: a summary and critical review of the literature. Minerva Pediatr. 2004;56:291–309. [PubMed] [Google Scholar]
- 8.Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005;29:317–325. doi: 10.1097/01.ALC.0000156453.05028.F5. [DOI] [PubMed] [Google Scholar]
- 9.Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviours among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- 10.Viner RM, Taylor B. Adult outcomes of binge drinking in adolescence: findings from a UK national birth cohort. J Epidemiol Community Health. 2007;61:902–907. doi: 10.1136/jech.2005.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toumbourou JW, Stockwell T, Neighbors C, Marlatt GA, Sturge J, Rehm J. Interventions to reduce harm associated with adolescent substance use. Lancet. 2007;369:1391–1401. doi: 10.1016/S0140-6736(07)60369-9. [DOI] [PubMed] [Google Scholar]
- 12.Levy SJL, Williams JF, Committe on Substance Use and Prevention Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211. doi: 10.1542/peds.2016-1211. [DOI] [PubMed] [Google Scholar]
- 13.Thomasius R, Sack PM, Arnaud N, Hoch E. Treatment of alcohol-related disorders in children and adolescents: age-specific recommendations according to the new interdisciplinary S3-Guideline [Behandlung alkoholbezogener Störungen bei Kindern und Jugendlichen: Altersspezifische Empfehlungen der neuen interdisziplinären S3-Leitlinie] Z Kinder Jug-Psych. 2016;44:295–305. doi: 10.1024/1422-4917/a000435. [DOI] [PubMed] [Google Scholar]
- 14.Gibb SJ, Fergusson DM, Horwood LJ. Burden of psychiatric disorder in young adulthood and life outcomes at age 30. J Psychiatry. 2010;197:122–127. doi: 10.1192/bjp.bp.109.076570. [DOI] [PubMed] [Google Scholar]
- 15.Kaess M, Brunner R, Parzer P, Carli V, Apter A, Balazs JA, et al. Risk-behaviour screening for identifying adolescents with mental health problems in Europe. Eur Child Adolesc Psychiatry. 2014;23:611–620. doi: 10.1007/s00787-013-0490-y. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham JA, Breslin FC. Only one in three people with alcohol abuse or dependence ever seek treatment. Addict Behav. 2004;29:221–223. doi: 10.1016/S0306-4603(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 17.O'Rourke L, Humphris G, Baldacchino A. Electronic communication based interventions for hazardous young drinkers: a systematic review. Neurosci Biobehav Rev. 2016;68:880–890. doi: 10.1016/j.neubiorev.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Murray E. Web-based interventions for behaviour change and self-management: potential, pitfalls, and progresses. Med 2.0. 2012;1:e3. doi: 10.2196/med20.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riper H, Blankers M, Hadiwijaya H, Cunningham J, Clarke S, Wierset R, et al. Effectiveness of guided and unguided low-intensity internet interventions for adult alcohol misuse: a meta-analysis. PLoS One. 2014;9:e99912. doi: 10.1371/journal.pone.0099912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dedert E, McDuffie JR, Stein R, McNiel JM, Kosinski AS, Freiermuth CE, et al. Electronic interventions for alcohol misuse and Alcohol Use Disorders: a systematic review. Ann Inter Med. 2015;163:205–214. doi: 10.7326/M15-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shingleton RM, Palfai TP. Technology-delivered adaptations of motivational interviewing for health-related behaviours: a systematic review of the current research. Patient Educ Couns. 2016;99:17. doi: 10.1016/j.pec.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White A, Kavanagh D, Stallman H, Klein B, Kay-Lambkin F, Proudfoot J, et al. Online alcohol interventions: a systematic review. J Med Internet Res. 2010;12:e62. doi: 10.2196/jmir.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey KB, Scott-Sheldon LA, Elliott JC, Bolles JR, Carey MP. Computer-delivered interventions to reduce college student drinking: a meta-analysis. Addiction. 2009;104:1807–1819. doi: 10.1111/j.1360-0443.2009.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnaud N, Baldus C, Elgán TH, De Paepe N, Tønnesen H, Csémy L, et al. Effectiveness of a web-based screening and fully automated brief motivational intervention for adolescent substance use: a randomized controlled trial. J Med Inter Res. 2016;18:e103. doi: 10.2196/jmir.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnaud N, Bröning S, Drechsel M, Thomasius R, Baldus C. Web-based screening and brief intervention for poly-drug use among teenagers: study protocol of a multicentre two-arm randomized controlled trial. BMC Public Health. 2012;12:826. doi: 10.1186/1471-2458-12-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton R, Deluca P, Phillips T, Kaner E, Newbury-Birch D, Drummond C. Alcohol screening & brief intervention for adolescents: The how, what and where of reducing alcohol consumption and related harm among young people. Alcohol Alcohol. 2014;49:207–212. doi: 10.1093/alcalc/agt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short CE, Rebar AL, Plotnikoff RC, Vandelanotte C. Designing engaging online behaviour change interventions: A proposed model of user engagement. Eur Health Psychol. 2015;17:32–38. [Google Scholar]
- 28.Kelders SM, Kok RN, Ossebaard HC, Van Gemert-Pijnen JE. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J Med Internet Res. 2012;14:e152. doi: 10.2196/jmir.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behaviour interventions: systematic review. J Med Internet Res. 2009;11:e1638. doi: 10.2196/jmir.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckjord E, Shiffman S. Background for real-time monitoring and intervention related to alcohol use. Alcohol Res. 2014;36:9–18. [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg LA. Dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 32.Wiers RW, Ames SL, Hofmann W, Krank M, Stacey AW. Impulsivity, impulsive and reflective processes and the development of alcohol use and misuse in adolescents and young adults. Front Psychol. 2010;1:1–12. doi: 10.3389/fpsyg.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suffoletto B, Kristan J, Chung T, Jeong K, Fabio A, Monti P, et al. An interactive text message intervention to reduce binge drinking in young adults: a randomized controlled trial with 9-month outcomes. PLoS One. 2015;10:e0142877. doi: 10.1371/journal.pone.0142877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alkhaldi G, Hamilton FL, Lau R, Webster R, Michie S, Murray E. The effectiveness of prompts to promote engagement with digital interventions: a systematic review. J Med Internet Res. 2016;18:e6. doi: 10.2196/jmir.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason M, Ola B, Zaharakis N, Zhang J. Text messaging interventions for adolescent and young adult substance use: a meta-analysis. Prev Sci. 2015;16:181–188. doi: 10.1007/s11121-014-0498-7. [DOI] [PubMed] [Google Scholar]
- 36.Kaess M, Ritter S, Lustig S, Bauer S, Becker K, Eschenbeck H, et al. Promoting Help-seeking using E-technology for Adolescents with Mental Health Problems: Study Protocol for a Randomized Controlled Trial within the ProHEAD Consortium. (accepted). [DOI] [PMC free article] [PubMed]
- 37.Bauer S, Bilic S, Reetz C, Oezer F, Becker K, Eschenbeck H, et al. Efficacy and Cost-Effectiveness of Internet-based Selective Eating Disorder Prevention: Study Protocol for a Randomized Controlled Trial within the ProHEAD Consortium. (accepted). [DOI] [PMC free article] [PubMed]
- 38.Baldofski S, Kohls E, Bauer S, Becker K, Bilic S, Eschenbeck H, et al. Efficacy and Cost-Effectiveness of Two Online Interventions for Children and Adolescents at Risk for Depression (E.motion trial): Study Protocol for a Randomized Controlled Trial within the ProHEAD Consortium. (accepted). [DOI] [PMC free article] [PubMed]
- 39.Eschenbeck H, Lehner L, Hofmann H, Bauer S, Becker K, Diestelkamp S, et al. School-Based Mental Health Promotion in Children and Adolescents with StresSOS using Online or Face-to-Face Interventions: Study Protocol for a Randomized Controlled Trial within the ProHEAD Consortium. (accepted). [DOI] [PMC free article] [PubMed]
- 40.Tossmann P, Kasten L, Lang P, Strüber E. Bestimmung der konkurrenten Validität des CRAFFT-d – Ein Screeninginstrument für problematischen Alkoholkonsum bei Jugendlichen [Definition of the concurrent validity of the CRAFFT-d. A screening tool for problematic alcohol use in adolescents] Z Kinder Jugendpsychiatr Psychother. 2009;37:451–459. doi: 10.1024/1422-4917.37.5.451. [DOI] [PubMed] [Google Scholar]
- 41.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: guidelines for use in primary care. 2. Geneva: World Health Organization; 2001. [Google Scholar]
- 42.Goodman A, Goodman R. Strengths and difficulties questionnaire as a dimensional measure of child mental health. J Am Acad Child Adolesc Psychiatry. 2009;48:400–403. doi: 10.1097/CHI.0b013e3181985068. [DOI] [PubMed] [Google Scholar]
- 43.Arnaud N, Baldus C, Elgán TH, Tönnesen H, De Paepe N, Csemy L, et al. Moderators of outcome in a web-based substance use intervention for adolescents. Sucht. 2015;61:377–387. doi: 10.1024/0939-5911.a000397. [DOI] [Google Scholar]
- 44.Miller RW, Rollnick S. Motivational interviewing. Preparing people for change. New York: Guilford; 2013. [Google Scholar]
- 45.Wurdak M, Wolstein J, Kuntsche E. Effectiveness of a drinking-motive-tailored emergency-room intervention among adolescents admitted to hospital due to acute alcohol intoxication — a randomized controlled trial. Prev Med Rep. 2016;3:83–89. doi: 10.1016/j.pmedr.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuntsche E, Kuntsche S. Development and validation of the Drinking Motive Questionnaire Revised Short Form (DMQ-R SF) J Clin Child Adolesc Psychol. 2009;38:899–908. doi: 10.1080/15374410903258967. [DOI] [PubMed] [Google Scholar]
- 47.Carey KB, Scott-Sheldon LAJ, Carey MP, DeMartini KS. Individual-level interventions to reduce college student drinking: a meta analytic review. Addict Behav. 2007;32:2469–2495. doi: 10.1016/j.addbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labhart F, Kuntsche E. Presentation at the 42nd Annual Alcohol Epidemiology Symposium of the Kettil Bruun Society, Stockholm, Sweden. 2016. The spirit is willing, but the flesh is weak: why young people drink more than intended on weekend evenings - an event-level study. [Google Scholar]
- 49.McCambridge J, Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PLoS One. 2011;6:e23748. doi: 10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laucht M, Esser G, Schmidt MH, Ihle W, Löffler W, Stöhr RM, et al. Children at risk: the role of early biological and psychosocial risk factors in the development of infants and toddlers [“Risikokinder”: Zur Bedeutung biologischer und psychosozialer Risiken für die kindliche Entwicklung in den beiden ersten Lebensjahren] 1992. [PubMed] [Google Scholar]
- 51.Rumpf HJ, Wohlert T, Freyer-Adam J, Grothues J, Bischof G. Screening questionnaires for problem drinking in adolescents: performance of AUDIT, AUDIT-C, CRAFFT and POSIT. Eur Addict Res. 2013;19:121–127. doi: 10.1159/000342331. [DOI] [PubMed] [Google Scholar]
- 52.Earleywine M, LaBrie JW, Pedersen ER. A brief Rutgers Alcohol Problem Index with less potential for bias. Addict Behav. 2008;33:1249–1253. doi: 10.1016/j.addbeh.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jelalian E, Spirito A, Rasile D, Vinnick L, Rohrbeck C, Arrigan M. Risk-taking, reported injury, and perception of future injury among adolescents. J Pediatr Psychol. 1997;22:513–532. doi: 10.1093/jpepsy/22.4.513. [DOI] [PubMed] [Google Scholar]
- 54.Epler AJ, Kivlahan DR, Bush KR, Dobie DJ, Bradley KA. A brief readiness to change drinking algorithm: concurrent validity in female VA primary care patients. Addict Behav. 2005;30:389–395. doi: 10.1016/j.addbeh.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. doi: 10.1037/0022-006X.51.3.390. [DOI] [PubMed] [Google Scholar]
- 56.Rickwood DJ, Braithwaite VA. Social-psychological factors affecting help-seeking for emotional problems. Soc Sci Med. 1994;39:563–572. doi: 10.1016/0277-9536(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 57.Ravens-Sieberer U, Erhart M, Rajmil L, Herdman M, Auquier P, Bruil J, et al. Reliability, construct and criterion validity of the KIDSCREEN-10 score: a short measure for children and adolescents’ well-being and health-related quality of life. Qual Life Res. 2010;19:1487–1500. doi: 10.1007/s11136-010-9706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Legleye S, Karila L, Beck F, Reynaud M. Validation of the CAST, a general population Cannabis Abuse Screening Test. J Subst Use. 2007;12:233–242. doi: 10.1080/14659890701476532. [DOI] [Google Scholar]
- 59.Klaghofer R, Brähler E. Construction and test statistical evaluation of a short version of the SCL-90–R [Konstruktion und Teststatistische Prüfung einer Kurzform der SCL-90–R] Z Klin Psychol Psychiatr Psychother. 2001;49:115–124. [Google Scholar]
- 60.Bertrams A, Dickhäuser O. Messung dispositioneller Selbstkontroll-Kapazität – Eine deutsche Adaptation der Kurzform der Self-Control Scale (SCS-K-D) [Measuring dispositional self-control capacity. A German adaptation of the short form of the Self-Control Scale (SCS-K-D)] Diagnostica. 2009;55:2–10. doi: 10.1026/0012-1924.55.1.2. [DOI] [Google Scholar]
- 61.Wang B, Stanton B, Li X, Cottrell L, Deveaux L, Kaljee L. The influence of parental monitoring and parent-adolescent communication on Bahamian adolescent risk involvement: a three-year longitudinal examination. Soc Sci Med. 2013;97:161–169. doi: 10.1016/j.socscimed.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Feigelman S, Stanton B. Perceived parental monitoring and health risk behaviors among urban low-income African-American children and adolescents. J Adolsc Health. 2000;27:43–48. doi: 10.1016/S1054-139X(99)00077-4. [DOI] [PubMed] [Google Scholar]
- 63.Voß E, Salize HJ. Health care utilization and cost-effectiveness analyses in prevention studies in the mental health care field. Ment Health Prev. 2016;4:19–23. doi: 10.1016/j.mhp.2016.01.004. [DOI] [Google Scholar]
- 64.Heck RH, Thomas SL, Tabata LN. Multilevel and longitudinal modeling with IBM SPSS. London: Routledge; 2013. [Google Scholar]
- 65.Brown H, Prescott R. Applied Mixed Models in Medicine. 3. Chichester: Wiley; 2015. [Google Scholar]
- 66.IBM Corp . IBM SPSS Statistics for Windows, Version 22.0. Armonk: IBM Corp; 2013. [Google Scholar]
- 67.Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles: Muthén & Muthén; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standard Protocol Items: Recommendations for Interventions Trials (SPIRIT) Checklist. (DOC 123 kb)
Data Availability Statement
Not applicable.



