Abstract
Background
Gastric Helicobacter pylori (H. pylori) is linked with chronic gastritis, peptic ulcer disease, and gastric malignancy. This study aims to investigate the association of gastric H. pylori with colorectal adenomatous polyps (CAP) in the Chinese population.
Methods
One thousand three hundred seventy five workers of China Petroleum and Chemical Corporation Sinopec Zhenhai Refining & Chemical Branch were recruited. Carbon-13 urea breathes test, and colorectal biopsies were utilized to detect H. pylori and CAP. The correlation between the number and distribution of CAP with H. pylori infection (HPI) was determined. Logistic regression models were applied to calculate the effect of H. pylori on the risk of CAP and pathway studio was used to attribute the cellular processes linking HPI and adenomatous polyps.
Results
One hundred Eighty participants were diagnosed as CAP, and 1195 participants were classified as healthy control. The prevalence of HPI in the CAP group was significantly higher than that in the healthy control group (57.8% verse 40.1%) (p<0.001). It was the number not the distribution of CAP corrected with H. pylori status. An increased risk of CAP was found to be associated with H. pylori (OR = 3.237; 95.0% CI 2.184–4.798, p = 0.00) even after multiple parameters adjustment. Pathway studio analysis demonstrated that HPI connected with CAP at multi-level.
Conclusions
HPI is associated with an increased risk of CAP in the Chinese population.
Electronic supplementary material
The online version of this article (10.1186/s12876-018-0918-4) contains supplementary material, which is available to authorized users.
Keywords: Helicobacter pylori, Colorectal adenomatous polyps, Colorectal carcinoma, Logistic regression, China
Background
Although advanced treatment strategy has significantly declined the mortality, colorectal carcinoma (CRC) is estimated to be the 3rd malignancy and the 4th primary cancer-related deaths [1–3]. 20% of patients without any symptom at the time of diagnosis may present with contiguous invasion, transperitoneal spread, and lymphatic or hematogenous dissemination [4–6]. In the matter of geographic pattern, the prevalence has increased in Canada, Australia, and the United States, while India and China show a relatively low risk [4, 7, 8].
Colorectal adenomatous polyp (CAP) can be considered as precancerous lesions, for hyper-proliferative epithelial cells can transform from adenomatous stage to CRC stage [9, 10]. The early diagnosing and early treatment of CAP depends on colonoscopy, which could significantly decrease the incidence of CRC. It is even demonstrated that even one-time colonoscopic screen at the age of 55-year could reduce 30–50% mortality [11–13]. Nowadays, more strategies are sought to promote eligible individuals to take part in this CRC screening programs.
Helicobacter pylori (H. pylori) affects about 50% of the world population which can lead to chronic gastritis, peptic ulcer disease, and even gastric malignancy [14–19]. However, there is still a lack of evidence to confirm the existence and the extent of association between H. pylori and CAP. This research aims to investigate whether HPI could be used as an alternative to colonoscopy screen to associate with CAP and to quantify such risk in Chinese population.
Methods and materials
Participants’ selection
One thousand three hundred seventy five workers and staff members of China Petroleum and Chemical Corporation Sinopec Zhenhai Refining & Chemical Branch who underwent regular physical check-up and colonoscopy from March 2013 to October 2014 in Zhenhai Lianhua Hospital were recruited. This study protocol was approved by the Ethics Committee of Zhenhai Lianhua Hospital, and the written consents from participants were obtained. The number and distribution of CAP were determined. Participants with inflammatory bowel disease, CRC, suboptimal bowel preparations, uncompleted colonoscopies, and short of H. pylori information were excluded.
Specimens
Encountered colorectal polyps were harvested and resected, which were further classified by the distribution as right-sided colon phenotype (cecum, ascending, and transverse colon), left-sided colon phenotype (descending colon, sigmoid, and rectum), and whole colon phenotype. All specimens were paraffin embedded.
Carbon-13 urea breath test for HPI
A non-invasive carbon-13 urea breath test (13C UBT) kit ordered from Shenzhen Headway Bio-Sci & Tech Co., Ltd. (Shenzhen, China) was used to detect H. pylori according to the manufacturer’s instruction. In brief, participants drunk 50 ml commercial orange juice deliquated with 13C-urea (75 mg). Expelled air samples were collected into Tedlar gas bags before and 30-min after the tracer ingestion, which was further detected with mass spectrometer systems. The cutoff value of delta over baseline (DOB) was set up as 4.0 per thousand to diagnose as H. pylori positive.
Pathway analysis
To find the association between HPI and CAP, the shortest path algorithm in pathway studio [20] was employed with default parameters, which built connections with the cell, cell processes, compounds, organs, and functional classes. Each edge was annotated with text mining result which was supported by one or more references, and the detailed information could be identified in Additional file 1, which is also online available at http://gousinfo.com/database/Data_Genetic/HPI_AP_Suplementary.xlsx, including the titles and sentences where a relationship has been recognized.
Statistical analysis
The distributions of continuous and categorical variables were analyzed with Student’s t-test and Chi-square test. A multivariable logistic regression analysis was adopted to estimate the predictive effect of H. pylori on the risk of CAP. P < 0.05 was considered as statistical significance.
Results
Population and clinicopathological characteristics
Characteristics of the participants were outlined in Table 1. The population was composed of 1375 subjects, among which 180 subjects were diagnosed as CAP by colorectal biopsies, and the other 1195 subjects were classified as healthy control. The average age of participants was 53.7 ± 10.5 for healthy control subjects and 58.6 ± 11.3 for subjects with CAP. The ratio of male gender among H. pylori positive participants (147/880, 16.7%) was significantly higher than that in female (33/495, 6.7%) (χ2 = 28.057,p<0.001). Participants with CAP showed a considerably increased age, waist circumference (WC), body mass index (BMI), systolic blood pressure (SBP), fasting blood glucose (FBG), uric acid (UA), and 13C DOB compared with healthy control.
Table 1.
Distribution of clinical variables
| Healthy control(n = 1195) | CAP group (n = 180) | t | p | |
|---|---|---|---|---|
| AGE (year) | 53.7 ± 10.5 | 58.6 ± 11.3 | 5.811 | < 0.001 |
| WC (cm) | 82.6 ± 8.8 | 85.5 ± 8.6 | 5.553 | < 0.001 |
| BMI(kg/m2) | 23.6 ± 2.8 | 24.3 ± 2.9 | 3.077 | =0.002 |
| SBP(mmHg) | 123(113,134) | 126 (118,138) | 3.270* | =0.001 |
| DBP(mmHg) | 78 (72,85) | 79 (72,86) | 0.763* | =0.445 |
| TC(mmol/L) | 4.87(4.34,5.49) | 5.00(4.35,5.63) | 1.123* | =0.261 |
| TG(mmol/L) | 1.20(0.82,1.72) | 1.32(0.92,1.81) | 1.703* | =0.089 |
| HDL-C(mmol/L) | 1.53(1.31,1.77) | 1.45(1.28,1.74) | 1.531* | =0.126 |
| LDL-C(mmol/L) | 2.64(2.17,3.16) | 2.81(2.33,3.25) | 1.904* | =0.057 |
| FBG(mmol/L) | 5.07(4.73,5.49) | 5.14 (4.85,5.67) | 2.713* | =0.007 |
| UA(μmol/L) | 321(265,380) | 328(275,397) | 1.707* | =0.088 |
| 13C(DOB) | 1.80(0.60,11.7) | 5.95(1.00,15.7) | 3.601* | < 0.001 |
note:* = Z value
The prevalence of HPI in CAP
The prevalence of HPI in the CAP group (104/180, 57.8%) was significantly higher than that in the healthy control group (479/1195, 40.1%) (χ2 = 20.1,p<0.001). CAP was mainly distributed in the left colon (58.3%). After Chi-square test, H. pylori prevalence in multiple polyp patients was higher than solitary polyp patients (66.2% vs. 51.5%) (χ2 = 3.944,p = 0.047), while patients with different polyp location phenotypes showed no significant difference of H. pylori prevalence (χ2 = 0.597,p = 0.742, Table 2).
Table 2.
The relation of CAP distribution and location with HPI
| H.Pylori positive (n = 104) | H. pylori negative (n = 76) | p | ||
|---|---|---|---|---|
| Number | solitary | 53 | 50 | 0.047 |
| multiple | 51 | 26 | ||
| Location | left-sided colon | 59 | 46 | 0.742 |
| right-sided colon | 27 | 20 | ||
| whole colon | 18 | 10 |
HPI associated with a higher odds ratio of CAP
Multiple logistic regression models for HPI concerning colorectal adenomas polyps were performed. Without parameters adjusted (model 1), HPI had a higher odds ratio (OR) of CAP (OR = 2.863;95.0% CI 1.975–4.150,p = 0.000). After adjustment of age and gender (model 2), subjects with H. pylori showed more prevalence of CAD (OR = 3.104;95.0% CI 2.104–4.775,p = 0.000). After additional adjustment for WC, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglyceride (TG), LDL, and HDL (model 3), the results did not materially change (OR = 3.237;95.0% CI 2.184–4.798,p = 0.000) (Table 3). All of these data indicated that HPI could be used as an independent factor in the risk analysis of CAP.
Table 3.
Multiple logistic regression models for H.pylori concerning colorectal adenomas polyps
| H. pylori | Partial regression coefficient | Standard error | Waldχ2 | p | OR (95.0% CI) |
|---|---|---|---|---|---|
| Model 1 | 1.052 | 0.189 | 30.821 | 0.000 | 2.863 (1.975,4.150) |
| Model 2 | 1.122 | 0.198 | 32.651 | 0.000 | 3.104 (2.104,4.577) |
| Model 3 | 1.175 | 0.201 | 34.213 | 0.000 | 3.237 (2.184,4.798) |
Note: no parameter was adjusted in Model 1; Model 2 adjusted for age, gender; Model 3 adjusted for age, gender, WC, BMI, systolic and diastolic blood pressure, total cholesterol, triglyceride, LDL, HDL
Potential functional pathways connecting CAP and HPI
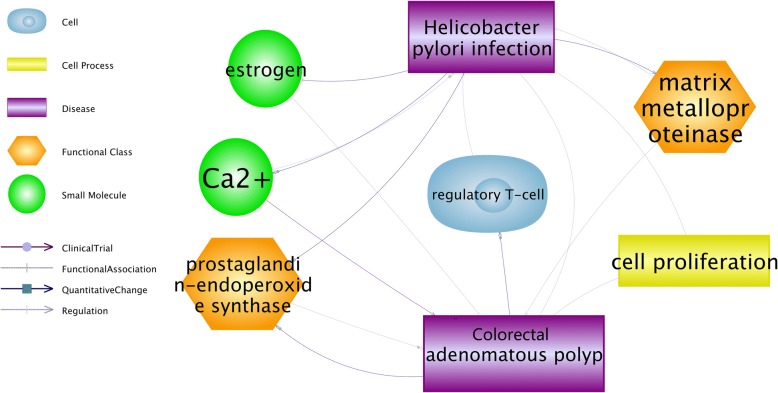
HPI demonstrated possible multi-level connections with CAP as shown in Fig. 1. The most common cellular processes were matrix metalloproteinase, prostaglandin-endoperoxide synthase, and cell proliferation. All of these might be the transformation processes involved in Helicobacter pylori infection and further development of adenomatous polyp. To note, for each relationship (edge) presented in Fig. 1, there were one or more supporting references, which have been provided in the Additional file 1 available at http://gousinfo.com/database/Data_Genetic/HPI_AP_Suplementary.xlsx.
Fig. 1.
Pathway analysis showed multiple cellular processes related to H. pylori infection and colorectal adenomatous polyps. A biological process annotation was created using the Pathway Studio 7.0 program to identify cell process and functional class associated with H. pylori infection and colorectal adenomatous polyps and the most common cellular processes were matrix metalloproteinase, prostaglandin-endoperoxide synthase, and mucin
Discussion
HPI shows clearly an association with the development of gastric carcinoma, while both positive [21–23] and negative [24, 25] associations reported with colorectal neoplasia. This investigation finds that H. pylori prevalence in CAP group is significantly increased than that in the healthy control group. H. pylori prevalence in multiple CAP group is higher than that in single CAP group. Moreover, pathway analysis revealed multiple pathways through which HPI could promote the development of CAP (Fig. 1). All of these indicate that HPI is associated with an increased risk of CAP and bioinformatics analysis suggests that functional processes of matrix metalloproteinase, prostaglandin-endoperoxide synthase, and mucin may link the pathogenesis of HPI and CAP.
The literature-based functional pathway analysis revealed five forward pathways (HPI➔CAP) and one backward pathway (CAP➔HPI), as shown in Fig. 1. These pathways could help to understand the potential mechanisms that HPI may influence the pathology of CAP. Specifically, HPI could up-regulate the expression of matrix metalloproteinases [26], which may take part in not only colorectal carcinogenesis from adenomatous polyps but also colorectal tumor invasion and initiation of metastatic cascade [27]. HPI has been known to induce the expression of pro-inflammatory cyclooxygenase enzyme (COX-2) [28], and cyclooxygenase has been suggested as a promoter for CAP in familial adenomatous polyposis [29]. Additionally, HPI has been shown to decrease the level of estrogen [30] that protect against the development of colorectal cancers and adenomatous polyps [31]. HPI could also increase Ca2+ concentration [32], and Ca2+ is a critical chemopreventive agent in adenomatous polyps after polypectomy and after colorectal surgery for colorectal cancer. [33] On the other, pathway analysis also revealed a potential CAP➔HPI pathway. The underlying literature information showed that human adenomatous polyps are accompanied with accumulated Treg, [34] and Tregs contribute to the persistence of HPI [35]. These forward and backward pathways suggested a possible vicious circle where HPI associates with CAP in a promoting manner.
HPI is detected using a non-invasive carbon-13 urea breath test, and CAP is diagnosed by colonoscopy, all of these suggest the reliable of the results. Confounding elements such as age, gender, and metabolism related parameters cannot be neglected. The participants involved in this study are age and gender-matched, and multiple logistic regression models adjusted for multiple parameters shows H. pylori could be a risk factor for CAP. Patients with two or more colorectal adenomatous polyps are a high-risk group for HPI and the development of advanced adenomas cancer [36].
Based on previous researches, HPI probably occurs in childhood or adolescence and is associated with of increased gastrin release, which could promote the development of potential adenocarcinoma. Patients who have undergone colonoscopic surveillance and have colorectal adenomatous polyps removed show low chance of CRC development in the future. For most colon cancers are considered to have a premalignant adenomatous polyp phase [37, 38], it is important to perform colonoscopic surveillance, while in some cases such an operation is not practicable owing to the concern of complication and the lack of advanced medical intervention.
A positive relation between H. pylori and colorectal polyps is observed, which is in accordance with the CRC mouse models [39] and other population investigation [23, 40–42]. Moreover, for the first time, our data show that HPI could be used as an independent factor in the risk analysis of CAP in China. The pathogenic mechanisms responsible for this association remain uncertain. H. pylori have been detected in colorectal malignant tissues; however, the possibility that H. pylori are a direct activator of colonic carcinogenesis remains purely hypothetical. On the other hand, experimental data have suggested some potential oncogenic interactions between H. pylori and colorectal mucosae, such as inflammatory responses induction and perpetuation, gut microflora alteration, and gastrin release [43]. Because our study is based on a regular physical check-up instead of an epidemiology-based investigation, it may have some limitations to interpret the results. More prospective, long-term epidemiology-based investigations are urgent to confirm the link between H. pylori and CAP, which can be used as an alternative to colonoscopy.
Conclusion
This study supports a possible connection between HPI and the risk of CAP which may require further investigation.
Additional file
Supporting information for the pathway analysis. Additional file 1 presents the detailed information of each and all relationships identified from the pathway analysis (Fig. 1), including relationship type and the supporting references for each relationship (title, publication year, author and related sentences).(XLSX 22 kb)
Acknowledgments
Not applicable.
Funding
This study is partially supported by the Social Development Program of Ningbo, China (2013C50044); and the Social Development and Science & Technology Program of Zhenhai, Ningbo, China (2016S009). Both grants supported the data collection used in this study. Social Development Project of Ningbo, China (No. 2016C51007).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI
Body mass index
- CAP
Colorectal adenomatous polyps
- DBP
Diastolic blood pressure
- FBG
Fasting blood glucose
- H. pylori
Helicobacter pylori
- OR
Odd Ratio
- SBP
Systolic blood pressure
- TC
Total cholesterol
- TG
Triglyceride
- UA
Uric acid
- WC
Waist circumference
Authors’ contributions
CC and ZZ contributed to the study design, data collection and analyzed, and manuscript development. YM, JD, YX and HC contributed to the data collection and analysis, and manuscript development. All authors have read and approved the manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Hospital of Zhenhai Refine-Chemical Company, Ningbo, China. Informed written consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
ChangxiChen, Email: ccx06@163.com.
Yushan Mao, Email: zhys007@sina.com.
Juan Du, Email: 1693844635@qq.com.
Yimin Xu, Email: xxyymm1123@126.com.
Zhongwei Zhu, Phone: +86-13867899022, Email: zhzhwei08@163.com.
Hongbao Cao, Email: caohon2010@gmail.com.
References
- 1.Nguyen H, Duong HQ. The molecular characteristics of colorectal cancer: implications for diagnosis and therapy. Oncol Lett. 2018;16(1):9–18. doi: 10.3892/ol.2018.8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mármol I, Sánchez-De-Diego C, Pradilla DA, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: a general overview and future perspectives in colorectal Cancer. Int J Mol Sci. 2017;18(1):197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S, Sun HJ. Surgical treatment of colorectal Cancer. 2018. Epidemiology of colorectal Cancer in Asia-Pacific region; pp. 3–10. [Google Scholar]
- 4.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clinics in Colon & Rectal Surgery. 2009;22(04):191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han J, Hur H, Min BS, Lee KY, Kim NK. Predictive factors for lymph node metastasis in submucosal invasive colorectal carcinoma: a new proposal of depth of invasion for radical surgery. World J Surg. 2018;1:1–7. doi: 10.1007/s00268-018-4482-4. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Hu X, Meng Y, Zhao H, Cao Q, Jin M. The prognosis significance and application value of peritoneal elastic lamina invasion in colon cancer. PLoS One. 2018;13(4):e0194804. doi: 10.1371/journal.pone.0194804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolatkhah R, Somi MH, Kermani IA, Ghojazadeh M, Jafarabadi MA, Farassati F, Dastgiri S. Increased colorectal cancer incidence in Iran: a systematic review and meta-analysis. BMC Public Health. 2015;15(1):997. doi: 10.1186/s12889-015-2342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford-Williams F, March S, Ireland MJ, Rowe A, Goodwin B, Hyde MK, Chambers SK, Aitken JF, Dunn J. Geographical variations in the clinical Management of Colorectal Cancer in Australia: a systematic review. Front Oncol. 2018;8:116. doi: 10.3389/fonc.2018.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggioprice L, Treuting P, Zeng W, Tsang M, Bielefeldtohmann H, Iritani BM. Helicobacter infection is required for inflammation and Colon Cancer in Smad3-deficient mice. Cancer Res. 2006;66(2):828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soylu A, Ozkara S, Alıs H, Dolay K, Kalaycı M, Yasar N, Kumbasar AB. Immunohistochemical testing for helicobacter pylori existence in neoplasms of the colon. BMC Gastroenterol. 2008;8(1):1–6. doi: 10.1186/1471-230X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Enezi SA, Alsurayei SA, Ismail AE, Aly NYA, Ismail WA, Abou-Bakr AA. Adenomatous colorectal polyps in patients referred for colonoscopy in a regional Hospital in Kuwait. Saudi Journal of Gastroenterology. 2010;16(3):188. doi: 10.4103/1319-3767.65194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner H, Zwink N, Ludwig L, Hoffmeister M. Should screening colonoscopy be offered from age 50? Deutsches Arzteblatt International. 2017;114(6):94. doi: 10.3238/arztebl.2017.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plattner D. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. Eur Surg. 2004;36(2):121–122. doi: 10.1007/s10353-004-0078-3. [DOI] [Google Scholar]
- 14.Moss S, Calam J. Helicobacter pylori and peptic ulcers: the present position. Gut. 1992;33(3):289–292. doi: 10.1136/gut.33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mommersteeg MC, Yu J, Peppelenbosch MP, Fuhler GM. Genetic host factors in helicobacter pylori-induced carcinogenesis: emerging new paradigms. Biochim Biophys Acta. 2018;1869(1):42–52. doi: 10.1016/j.bbcan.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Kountouras J, Kapetanakis N, Polyzos SA, Katsinelos P, Gavalas E, Tzivras D, Zeglinas C, Kountouras C, Vardaka E, Stefanidis E. Active helicobacter pylori infection is a risk factor for colorectal mucosa: early and advanced colonic neoplasm sequence. Gut & Liver. 2017;11(5):733–734. doi: 10.5009/gnl16389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kountouras J, Kapetanakis N: CD44 and helicobacter pylori-related colon oncogenesis. Saudi Medical Journal 2015, 36(10):1249–1249. [DOI] [PMC free article] [PubMed]
- 18.Shmuely H, Melzer E, Braverman M, Domniz N, Yahav J. Helicobacter pylori infection is associated with advanced colorectal neoplasia. Scand J Gastroenterol. 2014, 49(1):35–42. [DOI] [PubMed]
- 19.Kountouras J, Kapetanakis N, Zavos C, Romiopoulos I, Polyzos SA, Tsiaousi E, Michael S, Vardaka E, Nikolaidou C, Venizelos I. Impact of helicobacter pylori infection on colon oncogenesis. Am J Gastroenterol. 2013;108(4):625–626. doi: 10.1038/ajg.2013.17. [DOI] [PubMed] [Google Scholar]
- 20.Nikitin A, Egorov S, Daraselia N, Mazo I. Pathway studio--the analysis and navigation of molecular networks. Bioinformatics. 2003;19(16):2155–2157. doi: 10.1093/bioinformatics/btg290. [DOI] [PubMed] [Google Scholar]
- 21.Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, Goebell H. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group Digestion. 1999;60(3):210–215. doi: 10.1159/000007661. [DOI] [PubMed] [Google Scholar]
- 22.Hartwich A, Konturek S, Pierzchalski P, Zuchowicz M, Labza H, Konturek P, Karczewska E, Bielanski W, Marlicz K, Starzynska T. Helicobacter pylori infection, gastrin, cyclooxygenase-2, and apoptosis in colorectal cancer. Int J Color Dis. 2001;16(4):202–210. doi: 10.1007/s003840100288. [DOI] [PubMed] [Google Scholar]
- 23.Kapetanakis NKJ, Zavos C, Polyzos SA, Venizelos I, Nikolaidou C. Association of Helicobacter pylori infection with colorectal cancer. Immunogastroenterology. 2013;2:47–56. doi: 10.7178/ig.24. [DOI] [Google Scholar]
- 24.Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115(2):275–280. doi: 10.1016/S0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- 25.Moss SF, Neugut AI, Garbowski GC, Wang S, Treat MR, Forde KA. Helicobacter pylori seroprevalence and colorectal neoplasia: evidence against an association. J Natl Cancer Inst. 1995;87(10):762–763. doi: 10.1093/jnci/87.10.762. [DOI] [PubMed] [Google Scholar]
- 26.Yeh YC, Sheu BS, Cheng HC, Wang YL, Yang HB, Wu JJ. Elevated serum matrix Metalloproteinase-3 and -7 in H. pylori -related gastric Cancer can be biomarkers correlating with a poor survival. Digestive Diseases & Sciences. 2010;55(6):1649–1657. doi: 10.1007/s10620-009-0926-x. [DOI] [PubMed] [Google Scholar]
- 27.Groblewska M, Mroczko B, Gryko M, Kędra B, Szmitkowski M. Matrix metalloproteinase 2 and tissue inhibitor of matrix metalloproteinases 2 in the diagnosis of colorectal adenoma and cancer patients. Folia Histochem Cytobiol. 2010;48(4):564. doi: 10.2478/v10042-010-0076-1. [DOI] [PubMed] [Google Scholar]
- 28.Kang W, Tong JH, Chan AW, Lung RW, Chau SL, Wong QW, Wong N, Yu J, Cheng AS, To KF Stathmin1 plays oncogenic role and is a target of microRNA-223 in gastric cancer. PLoS One. 2012;7(3):e33919. doi: 10.1371/journal.pone.0033919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delker DA, Wood AC, Snow AK, Samadder NJ, Samowitz WS, Affolter KE, Boucher KM, Pappas LM, Stijleman IJ, Kanth P. Chemoprevention with cyclooxygenase and epidermal growth factor receptor inhibitors in familial adenomatous polyposis patients: mRNA signatures of duodenal neoplasia. Cancer Prev Res. 2017;11(1). [DOI] [PMC free article] [PubMed]
- 30.Xu ZH, Zhang J, Yang D, Zhang JH. Progress of research between helicobacter pylori infection and osteoporosis. Zhongguo Gu Shang. 2011;24(11):966–968. [PubMed] [Google Scholar]
- 31.Woodson K, Lanza E, Tangrea JA, Albert PS, Slattery M, Pinsky J, Caan B, Paskett E, Iber F, Kikendall JW, Lance P, Shike M, Weissfeld J, Schatzkin A. Hormone replacement therapy and colorectal adenoma recurrence among women in the polyp prevention trial. J Natl Cancer Inst. 2001;93(23):1799–1805. doi: 10.1093/jnci/93.23.1799. [DOI] [PubMed] [Google Scholar]
- 32.Kameoka S, Kameyama T, Hayashi T, Sato S, Ohnishi N, Hayashi T, Murata-Kamiya N, Higashi H, Hatakeyama M, Takaoka A. Helicobacter pylori induces IL-1β protein through the inflammasome activation in differentiated macrophagic cells. Biomed Res. 2016;37(1):21–27. doi: 10.2220/biomedres.37.21. [DOI] [PubMed] [Google Scholar]
- 33.Duris I, Hruby D, Pekarkova B, Huorka M, Cernakova E, Bezayova T, Ondrejka P. Calcium chemoprevention in colorectal cancer. Hepato-Gastroenterology. 1996;43(7):152–154. [PubMed] [Google Scholar]
- 34.Akeus P, Langenes V, von Mentzer A, Yrlid U, Sjöling Å, Saksena P, Raghavan S, Quiding-Järbrink M. Altered chemokine production and accumulation of regulatory T cells in intestinal adenomas of APC(Min/+) mice. Cancer Immunol Immunother. 2014;63(8):807–819. doi: 10.1007/s00262-014-1555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang TJ. The number of Foxp3-positive regulatory T cells is increased in helicobacter pylori gastritis and gastric cancer. Pathol Res Pract. 2010;206(1):34–38. doi: 10.1016/j.prp.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Atkin WS, Saunders BP: Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 2002, 51 Suppl 5(Supplement 5):V6. [DOI] [PMC free article] [PubMed]
- 37.Hyman NH, Anderson P, Blasyk H. Hyperplastic polyposis and the risk of colorectal Cancer. Dis Colon Rectum. 2004;47(12):2101–2104. doi: 10.1007/s10350-004-0709-6. [DOI] [PubMed] [Google Scholar]
- 38.Cottet V, Jooste V, Fournel I, Bouvier AM, Faivre J, Bonithon-Kopp C. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012;61(8):1180–1186. doi: 10.1136/gutjnl-2011-300295. [DOI] [PubMed] [Google Scholar]
- 39.Doetschman T. GI GEMs: genetically engineered mouse models of gastrointestinal disease. Gastroenterology. 2011;140(2):380–385. doi: 10.1053/j.gastro.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brim H, Zahaf M, Laiyemo AO, Nouraie M, Pérezpérez GI, Smoot DT, Lee E, Razjouyan H, Ashktorab H: Gastric helicobacter pylori infection associates with an increased risk of colorectal polyps in African Americans. BMC Cancer,14,1(2014-04-28) 2014, 14(1):1–7. [DOI] [PMC free article] [PubMed]
- 41.Mizuno S, Morita Y, Inui T, Asakawa A, Ueno N, Ando T, Kato H, Uchida M, Yoshikawa T, Inui A. Helicobacter pylori infection is associated with colon adenomatous polyps detected by high-resolution colonoscopy. Int J Cancer. 2005;117(6):1058–1059. doi: 10.1002/ijc.21280. [DOI] [PubMed] [Google Scholar]
- 42.Hu KC, Wu MS, Chu CH, Wang HY, Lin SC, Po HL, Bair MJ, Liu CC, Su TH, Chen CL. Hyperglycemia combined helicobacter pylori infection increases risk of synchronous colorectal adenoma and carotid artery plaque. Oncotarget. 2017;8(65):108655–108664. doi: 10.18632/oncotarget.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel S, Lipka S, Shen H, Barnowsky A, Silpe J, Mosdale J, Pan Q, Fridlyand S, Bhavsar A, Abraham A. The association of H. pylori and colorectal adenoma: does it exist in the US Hispanic population? Journal of Gastrointestinal Oncology. 2014;5(6):463. doi: 10.3978/j.issn.2078-6891.2014.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information for the pathway analysis. Additional file 1 presents the detailed information of each and all relationships identified from the pathway analysis (Fig. 1), including relationship type and the supporting references for each relationship (title, publication year, author and related sentences).(XLSX 22 kb)
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.