Abstract
The present study investigated the expression of p53 in ground-glass nodule (GGN) of lung cancer and non-lung cancer patients, and explored the correlation with prognosis. A total of 120 GGN patients admitted to the Department of Respiratory Medicine in the Second Affiliated Hospital of Zhejiang University School of Medicine during the period from March 2010 to March 2014 were selected. These patients included 60 lung cancer patients and 60 non-tumor patients. Biopsy or surgical specimens were collected. Fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) were used to detect p53 gene and protein expression in the two groups of GGN tissues. All patients were followed up for 3 years and the relationship between p53 protein expression and the overall survival (OS) of the two groups of patients was analyzed. In GGN cells of non-cancer patients, p53 absence was observed in 6 cases and the absence rate was 10.0%. In GGN cells of cancer patients, the absence rate was significantly higher than that of non-cancer GGN group (p<0.05). The positive rate of p53-positive cases in non-tumor patients GGN group was lower than that of in GGN tissues of lung cancer patients (p<0.05). There were no deaths in the GGN non-cancer group (n=60) within 3 years, while 43 deaths occurred in GGN lung cancer group. The median survival time and the 3-year survival rate of patients with p53 positive was lower than that of p53-negative patients (p<0.05). p53 was overexpressed in GGN of lung cancer patients, and p53 overexpression is significantly correlated with poor prognosis of lung cancer patients. p53 plays an important role in transformation from GGN to lung cancer. Detection of p53 expression in GGN tissue may provide guidance for the diagnosis and prognosis of lung cancer.
Keywords: ground-glass nodule, lung cancer, p53, prognosis
Introduction
Lung cancer is one of the most common malignancies in the world. With the aggregated environmental pollution, incidence of lung cancer gradually increases. Clinical data show that in all stages of lung cancer, the 5-year survival rate of patients is only 15%, and this situation has not improved in the past 30 years (1–3). Detection of most lung cancers depends on the discovery of ground-glass opacity (GGO) and ground-glass nodules (GGNs) (4). CT image of GGO appears as a lightly-enhanced cloud-like shadow circular nodule in the style of a frosted glass (5). Pathological basis of GGO is alveolar wall thickening, alveolar cavity collapse, alveolar cavity gas content reduction. Besides that, pulmonary inflammation, fibrosis, intrapulmonary lymph nodes, and inflammatory pseudo-tumors may also appear (6).
GGN in some cases can be diagnosed as cancer. GGN is divided into components (whether or not they contain solid components): simple/complete GGN (pGGN) and mixed or part-solid GGN (mGGN) (7). mGGN due to the occurrence of atypical hyperplasia in solid hyperplasia has a significantly higher risk of cancer than pGGN. Based on histopathological findings, these tissues are mainly divided into atypical adenomatoid hyperplasia (AAH), adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) (8,9). GGN cannot be diagnosed solely based on CT. Blood test is not reliable in some cases. Therefore, most patients were diagnosed at advanced stages (10). Therefore, identification of novel molecular targets for the treatment of GGN is urgently needed.
Inactivation of p53 is closely related to the development of many human tumors. p53 inhibits the replication of damaged DNA in normal cells and promotes the death (apoptosis) of these cells (11). Inactivated or altered p53 can cause abnormal cells with damaged DNA to survive and divide and transmit mutations to daughter cells, resulting in the occurrence of cancer. p53 is defective in most human cancers (12). However, expression of p53 gene and protein in lung GGN nodules has not been reported. Therefore, 60 cases of GGN lung cancer and 60 cases of GGN non-cancer patients were included in this study. Fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) were used to detect the expression of p53 protein and mRNA, respectively, and the relationship between the abnormal expression of p53 and the survival time of lung cancer patients was further analyzed.
Materials and methods
Clinical data
From March 2010 to March 2014, 120 patients with GGN admitted to the Department of Respiratory Medicine in The Second Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China) were selected including 60 patients with lung cancer and 60 non-cancer patients. Those patients included 64 males and 56 females, with an average age of 57±20.6 years. Simple pGGN was observed in 36 cases (12 cases in the upper right lobe, 13 cases in the left upper lobe, 10 cases in the left lower lobe and right lower lobe, and 1 case in the right middle lobe). Mixed mGGN was observed in 66 cases (19 cases in the upper right lobe, 16 cases in the left upper lobe, 14 cases in the left lower lobe, 14 cases in the right lower lobe and 3 cases in the right middle lobe). There were 75 cases with single GGN and 35 cases with multiple GGN. There was no significant difference in age, sex, location of lesions, and general GGN types between the two groups. CT image data and biopsy/surgical specimens were collected. All patients were followed up for 3 years after biopsy/surgery. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine, and each participant signed an informed consent.
H&E histopathology and IHC methods
H&E staining
Tissues of the biopsy or surgical specimens were fixed overnight in an appropriate amount of 4% paraformaldehyde (Google-BIO, Wuhan, China). After dehydration and embedding, paraffin sections were performed at a thickness of 0.4 µm. Sections were kept in an oven at 65°C for 4 h. After dehydration by passing a graded series of ethanol concentrations, sections were subjected to H&E staining, and rehydration by passing a graded series of ethanol concentrations. Sections were finally sealed with neutral gum. Tissue morphology was observed and photographed under a microscope (DM-5000B; Leica Microsystems GmbH, Wetzlar, Germany).
IHC
Streptomycin-biotin-peroxidase (SP) staining method was used. Tissue sections were routinely dewaxed, rehydrated, blocked with H2O2, digested with trypsin, and incubated with normal sheep serum at room temperature. Sections were incubated with mouse anti-human p53 primary monoclonal antibody (dilution, 1:200; cat. no. ab1101; Abcam, Cambridge, MA, USA) diluted in PBST overnight at 4°C, followed by incubation with biotinylated goat anti-mouse secondary polyclonal antibody (dilution, 1:900; cat. no. ab6788; Abcam) at room temperature for 30 min. After incubation with SP complex at room temperature for 20 min (dilution, 1:200), color development with DAB was performed. Hematoxylin staining was performed and sections were sealed. Brown nucleus indicated positive signals.
Analysis of the results
Sections were analyzed by two experienced pathologists using a double-blind method. Ten visual fields were selected under a 400-fold microscope (Olympus Corporation, Tokyo, Japan), and 100 cells of each field were counted. Cells with brown nucleus were counted to calculate the percentage of tumor cells. Percentage >6% was positive and ≤5% was negative.
Detection of p53 gene expression in GGN tissue by FISH
FISH method
Probe preparation: GLP p53 DNA (PathVysion™ p53 DNA Probe kit) was purchased from Vysis, Inc. (cat. no. FG0011; Vysis, Inc.: Abbott Laboratories, Downers Grove, IL, USA). This probe (green fluorescent marker) targets p53 on chromosome 17p13.1. Probe mixture (7 µl of hybridization buffer, 2 µl of probe, and 1 µl of deionized water) was mixed. The mixture was vortexed and stored in the dark (2). Hybridization: probe mixture was added to the surface of specimen, and the specimen was covered with a cover glass. After denaturing at 83°C in the dark for 5 min, hybridization was performed at 42°C for 16 h in the dark. The next day, sections were washed with 2X SSC solution (pH 7.2) at 46°C for 5 min, followed by incubation in 70% ethanol for 3 min. After air dry in the dark, 15 µl of DAPI counterstain was added to the surface of each slide. After incubation in the dark for 20 min, the results were observed under a fluorescence microscope.
Analysis of results
Olympus BX51 fluorescence microscope (Olympus Corporation) (DAPI/TRITC/FITC) was used to observe the results. Appearance of 0 or 1 green fluorescence (FITC) dot in the nucleus indicated p53 absence. Appearance of ≥2 green fluorescence (FITC) dots in nucleus was not counted. a total of 200 interphase cells were selected from each slide and the percentage of p53-deficient cells was calculated. Absence of >20% was defined as deletion.
Statistical analysis
Statistical data were analyzed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Enumeration data were expressed as percentage (%), and comparison between the two groups was performed using the Chi-square test. The Kaplan-Meier method was used to plot the survival curve, and survival curves were compared using the log-rank test. P<0.05 was considered to be statistically significant.
Results
CT signs and pathological data of patients with GGN
There was no significant difference between the two groups in terms of age, sex, site of lesions, and general GGN data. CT image of GGN patients showed an increase in local density of lungs, a focal cloudiness shade, and the shadow veins and bronchial texture were clearly discernible. Pathological studies showed that ground-glass changes in lungs were caused by filling of alveolar space with exudate, a small amount of lymphocytes, neutrophils, macrophages or amorphous substances, accompanied by alveolar wall thickening and other causes (Figs. 1 and 2).
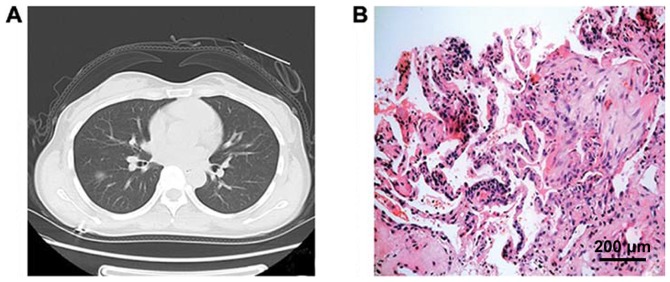
Figure 1.
CT signs and pathological data of GGN. (A) CT shows a GGN on the left lung with a round shape with clear and uniform boundary and ~15 mm in diameter. (B) Pathological examination suggests interstitial pneumonia, alveolar septa edema, and accumulation of macrophages in the alveolar space and hyaline membrane formation. GGN, ground-glass nodule.
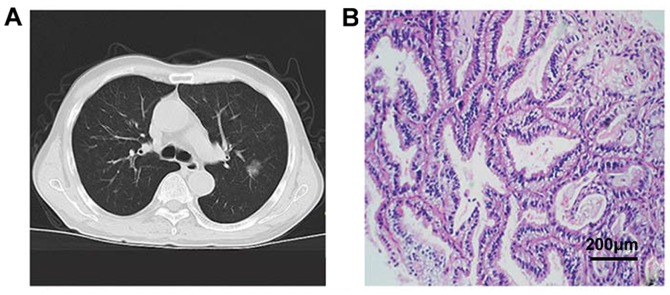
Figure 2.
CT signs and pathological data of GGN. (A) CT shows a GGN on the right upper lung with a diameter of ~8 mm and contains solid components. (B) H&E staining suggests epithelial adenocarcinoma, presence of alveolar structures, alveolar wall thickening, and cancer cells growing along the alveolar wall. GGN, ground-glass nodule.
Detection of p53 protein expression and gene absence in GGN tissues by IHC and FISH
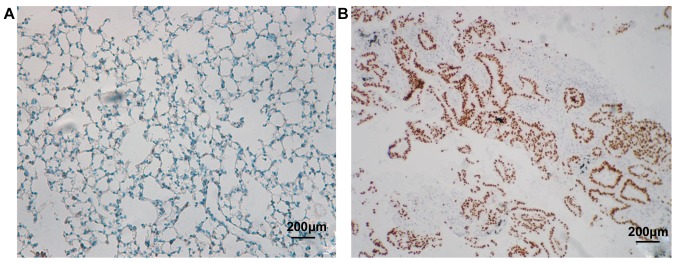
p53 is located in the nucleus and positive cells are brown. As shown in Fig. 3 and Table I, 8 out of 70 cases of non-cancer GGN patients enrolled in this study were p53 positive, and 52 cases were negative, and the positive rate was 13.33%. However, in 60 patients with lung cancer, GGN tissues of 39 patients showed p53 positive and 21 patients were negative, and the positive rate was 65.0%. There was a statistically significant difference between the two groups (P<0.05).
Figure 3.
p53 protein expression and gene deletion in GGN. (A) Negative expression of p53 protein in non-cancer GGN tissues. (B) Positive expression of p53 protein in lung cancer GGN tissues (×200-fold). GGN, ground-glass nodule.
Table I.
Relationship of p53 gene deletion and abnormal protein expression with the occurrence of lung cancer.
| p53 protein abnormal expression | p53 gene deletion | ||||
|---|---|---|---|---|---|
| Groups | Cases | p53 protein expression | Expression percentage (%) | p53 absence | Absence rate (%) |
| Non-cancer | 60 | 8 | 13.33 | 6 | 10 |
| Cancer | 60 | 39 | 65.0a | 34 | 56.67b |
P<0.01
P<0.001, compared with the non-cancer group.
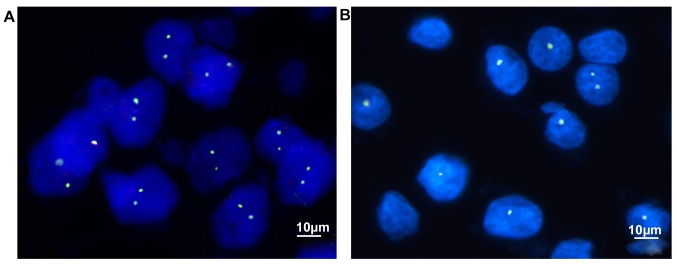
The standard for deletion of p53 absence by FISH was cells with 0 or 1 green fluorescence (FITC) dot appeared in intact nuclei. As shown in Fig. 4 and Table I, the number of p53 absence in 70 non-cancer GGN patients was 6 and the absence rate was 10.0%. The number of p53 deletions in 70 patients with non-cancer GGN was 34 and the absence rate was 56.67%. There was a statistically significant difference between the two groups (P<0.05, Fig. 5).
Figure 4.
p53 protein expression and gene deletion in GGN. (A) The application of fluorescence-labeled p53 DNA probe detected a 10% deletion rate of p53 gene in non-cancer GGN tissues. (B) In lung cancer GGN tissues, p53 gene deletion rate detected by fluorescence-labeled p53 DNA probe was 56.67% (FISH ×1,200). GGN, ground-glass nodule; FISH, Fluorescence in situ hybridization.
Figure 5.
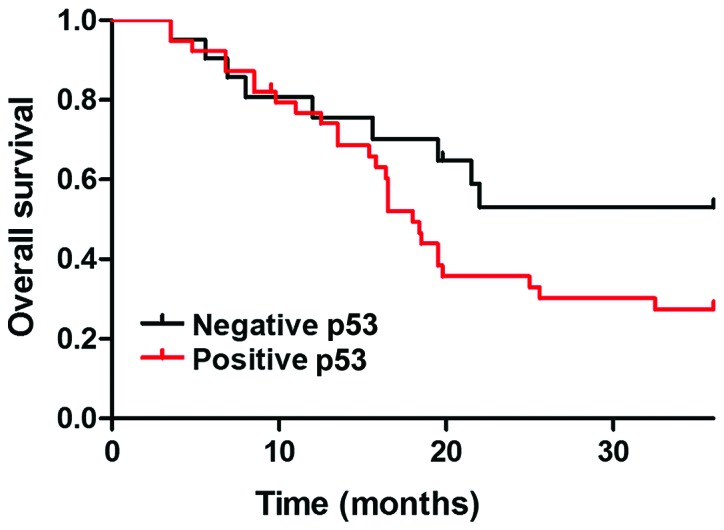
Relationship between p53 protein expression and survival in patients with lung cancer. The median survival time of patients with p53-positive expression was 16.8 months, and the 3-year survival rate was 31.5%. The median survival time of p53-negative patients was 19.8 months, and the 3-year survival rate was 49.6%. There was a statistically significant difference between the two groups (P<0.05).
Follow-up results
Median follow-up time for all patients was 961 days (25–1,121 days). No deaths occurred in the GGN non-cancer group (n=60), and 43 cases died in the GGN lung cancer group. The median survival time of patients with p53-positive expression was 16.8 months, and the 3-year survival rate was 31.5%. The median survival time of p53-negative patients was 19.8 months, and the 3-year survival rate was 49.6%. There was a statistically significant difference between the two groups (P<0.05).
Discussion
GGN cannot be diagnosed solely based on CT. Blood test is also not reliable in some cases. Therefore, most patients were diagnosed at advanced stages (13). According to the 2016 edition of the ‘Guidelines for the Classification, Diagnosis and Treatment of Lung Nodules in China,’ solid nodules >1 cm in diameter are defined as high-risk nodules and require further examination (thin nodule three-dimensional reconstruction CT scan, thin-layer contrast-enhanced CT scan, needle biopsy) to confirm the nature of lesion. CT scan should be performed 3 months later. If the nodule does not shrink or grow after 3 months, the possibility of malignancy should be considered. If the nodule shrinks, CT should be performed 6, 12, and 24 months later. If no change is observed, a long-term annual CT review is recommended and follow-up period should be ≥3 years (14).
From focal GGN to early stage lung cancer, the growth of GGN is inert, and this progress is relatively slow. Therefore, the follow-up time for GGN is usually at least 3 years. At initial stage (<8 mm), GGN in CT images was often characterized as pure and round lesions with low density and clear boundaries. At this stage, most GGNs were pGGN, and after surgery, they were mostly confirmed as AAH (precancerous lesion) or AIS (not invading the surrounding vascular interstitial, non-metastatic, 5-year survival rate of 100%). In extreme cases, it may also be MIA (which invades surrounding vascular stroma <5 mm, does not metastasize, and has a 5-year survival rate of 100% after resection) (15). In general, AAH has no obvious clinical symptoms and signs, and many AAHs are found in surgically resected lung specimens. In 2004 edition of World Health Organization's histological classification of lung cancer, AAH was considered to be a precancerous lesion of bronchioloalveolar carcinoma (BAC). The incidence of AAH in surgically resected specimens was 9.3–21.4%. The incidence of AAH in resected lung specimens for other reasons was 4.4–9.6%. However, 20% of pure GGN lesions grow or become mixed GGN during follow-up, whereas 40% of mixed GGN grow during follow-up (16). As the GGN gradually grows, the percentage of solid components of pure GGN increases and mGGNs is formed. There are even malignant changes such as lobed leaves, burrs, vacuoles, pleural depressions, and vascular intensiveness, which in turn lead to the occurrence of invasive adenocarcinomas that can invade blood vessels, intrapulmonary or systemic metastases (3). Therefore, to explore the molecular mechanism of GGN carcinogenesis is an important issue to be solved in the diagnosis of lung cancer.
Studies have shown that the activation of oncogenes and inactivation of tumor suppressor genes are major events in tumorigenesis. p53 is a ubiquitous tumor suppressor gene located on human chromosome 17q13.1 encoding p53 protein, which regulates cell cycle (17). When the intracellular DNA is destroyed, wild-type p53 is activated to induce cell cycle arrest in G1-M phase, hereby inhibiting cell proliferation. Half-life of the mutant p53 gene is significantly prolonged and T1/2 often reaches 20–40 h, and this abnormal protein expression can be detected in a variety of human tumors (18). With the advantages of high sensitivity, specificity, and intuitiveness, FISH can be used to observe chromosome structure and abnormalities in biological specimens. With tumor suppressor gene DNA as probe, FISH technology can be used to perform interphase nucleus analysis to observe the loss of gene expression in tumor cells, so as to provide molecular genetic basis for studies on the progression of different stages of tumors.
In this study, we used the FISH technique to hybridize the GLP p53 DNA probe to the short arm of chromosome 17 (17p13.1), and we also used IHC techniques to detect deletions and abnormal expression of tumor suppressor p53 gene in the GGN of non-lung cancer and lung cancer patients. In the past, p53 gene inactivation was reported to be induced by point mutations, which can only explain a small part of lung cancer cases in which p53 gene is inactivated (19). In the current experiment, the standard for FISH detection of p53 gene deletion was the appearance of 0 or 1 green fluorescence (FITC) dot in intact nuclei. In the GGN cells of non-cancer patients, p53 absence was observed in 6 cases and the absence rate was 10.0% (6/60). In the GGN cells of cancer patients, 53 absence was observed in 34 cases and the absence rate was 56.67% (34/60), which was significantly higher than that of the non-cancer GGN group (P<0.05). IHC results suggest that the number of p53-positive cases in the non-tumor patients GGN group is 8, and the positive rate is 13.33% (8/60). The number of p53-positive cases in the GGN tissues of lung cancer patients was 39, and the positive rate was 65.0% (39/60). The difference between the two groups was statistically significant (P<0.05). However, we detected p53 protein expression in lung cancer tissues both with and without p53 gene deletion.
According to the findings reported by Olivier et al (20), we believe that p53 abnormalities are common in lung cancer tissues and tumor cells contain loss of specific sites of p53 gene in the short arm of chromosome 17 and are accompanied by genetic mutations in the residual p53 allele. Deletion of the p53 gene may also happen, but no gene mutations have yet occurred. Furthermore, our 3-year follow-up data showed that no deaths occurred in the GGN non-cancer group (n=60), whereas 43 cases died in the GGN lung cancer group. The median survival time of patients with p53-positive group was 16.8 months, and the 3-year survival rate was 31.5%. The median survival time of p53-negative patients was 19.8 months, and the 3-year survival rate was 49.6%.
In summary, FISH and IHC were used to investigate the correlation between p53 gene abnormalities and survival of lung cancer patients. We found that p53 gene deletion and protein overexpression in lung cancer GGN tissues were significantly associated with poor prognosis. It is suggested that p53 plays an important role in the process of transformation of GGN to lung cancer. Therefore, detecting the deletion of p53 gene as well as the expression of p53 protein in GGN tissue will benefit the diagnosis and prognosis of lung cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZT, WC, DY and ZZ were devoted to interpreting the general data. ZT, WC and DY were responsible for IHC. ZT, WC, DY, LZ and FW interpreted the FISH results. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China). Signed informed consents were obtained from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. National Lung Screening Trial Research Team: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood DE. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Thorac Surg Clin. 2015;25:185–197. doi: 10.1016/j.thorsurg.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Asamura H, Kusumoto M, Kondo H, Tsuchiya R. ‘Early’ peripheral lung cancer: Prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg. 2002;74:1635–1639. doi: 10.1016/S0003-4975(02)03895-X. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Sone S, Abe H, Macmahon H, Doi K. Malignant versus benign nodules at CT screening for lung cancer: Comparison of thin-section CT findings. Radiology. 2004;233:793–798. doi: 10.1148/radiol.2333031018. [DOI] [PubMed] [Google Scholar]
- 6.Aoki T, Tomoda Y, Watanabe H, Nakata H, Kasai T, Hashimoto H, Kodate M, Osaki T, Yasumoto K. Peripheral lung adenocarcinoma: Correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology. 2001;220:803–809. doi: 10.1148/radiol.2203001701. [DOI] [PubMed] [Google Scholar]
- 7.Hattori A, Suzuki K, Matsunaga T, Fukui M, Tsushima Y, Takamochi K, Oh S. Tumour standardized uptake value on positron emission tomography is a novel predictor of adenocarcinoma in situ for c-stage IA lung cancer patients with a part-solid nodule on thin-section computed tomography scan. Interact Cardiovasc Thorac Surg. 2014;18:329–334. doi: 10.1093/icvts/ivt500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detterbeck FC, Marom EM, Arenberg DA, Franklin WA, Nicholson AG, Travis WD, Girard N, Mazzone PJ, Donington JS, Tanoue LT, et al. The IASLC Lung Cancer Staging Project: Background data and proposals for the application of TNM staging rules to lung cancer presenting as multiple nodules with ground glass or lepidic features or a pneumonic type of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11:666–680. doi: 10.1016/j.jtho.2015.12.114. [DOI] [PubMed] [Google Scholar]
- 9.Lee HY, Choi YL, Lee KS, Han J, Zo JI, Shim YM, Moon JW. Pure ground-glass opacity neoplastic lung nodules: Histopathology, imaging, and management. AJR Am J Roentgenol. 2014;202:W224–33. doi: 10.2214/AJR.13.11819. [DOI] [PubMed] [Google Scholar]
- 10.Goo JM, Park CM, Lee HJ. Ground-glass nodules on chest CT as imaging biomarkers in the management of lung adenocarcinoma. AJR Am J Roentgenol. 2011;196:533–543. doi: 10.2214/AJR.10.5813. [DOI] [PubMed] [Google Scholar]
- 11.Harris CC. p53 tumor suppressor gene: At the crossroads of molecular carcinogenesis, molecular epidemiology, and cancer risk assessment. Environ Health Perspect. 1996;104(Suppl 3):435–439. doi: 10.1289/ehp.96104s3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakeji Y, Korenaga D, Tsujitani S, Baba H, Anai H, Maehara Y, Sugimachi K. Gastric cancer with p53 overexpression has high potential for metastasising to lymph nodes. Br J Cancer. 1993;67:589–593. doi: 10.1038/bjc.1993.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao Y, Lu L, Dewan M, Chen AY, Corso J, Xuan J, Salganicoff M, Krishnan A. Multi-level ground glass nodule detection and segmentation in CT lung images. Med Image Comput Comput Assist Interv. 2009;12:715–723. doi: 10.1007/978-3-642-04271-3_87. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Fan Y, Wang Y, Qiao Y, Wang G, Huang Y, Wang X, Wu N, Zhang G, Zheng X, et al. China national guideline of classification, diagnosis and treatment for lung nodules (2016 version) Zhongguo Fei Ai Za Zhi. 2016;19:793–798. doi: 10.3779/j.issn.1009-3419.2016.12.12. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Zhao C, Yang Y, He Y, Hou L, Li X, Gao G, Shi J, Ren S, Chu H, et al. High discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground-glass nodules. J Thorac Oncol. 2015;10:778–783. doi: 10.1097/JTO.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Qiang JW, Ye JD, Ye XD, Zhang J. High resolution CT in differentiating minimally invasive component in early lung adenocarcinoma. Lung Cancer. 2014;84:236–241. doi: 10.1016/j.lungcan.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Muller PA, Vousden KH. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane DP, Cheok CF, Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol. 2010;2:a001222. doi: 10.1101/cshperspect.a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolly KW, Malkin D, Douglass EC, Brown TF, Sinclair AE, Look AT. Splice-site mutation of the p53 gene in a family with hereditary breast-ovarian cancer. Oncogene. 1994;9:97–102. [PubMed] [Google Scholar]
- 20.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.