Abstract
Effect of miR-21 and miR-138 on the proliferation of colon cancer cells and their association with prognosis were investigated. Expression levels of miR-21 and miR-138 in colorectal cancer and normal adjacent tissues were compared. Differential expression of miR-21 and miR-138 in colon cancer tissues with different clinicopathological features were analyzed. miR-21 and miR-138 expression vectors were established and transfected into cells of colon cancer cell line SW480. Methyl thiazolyl tetrazolium (MTT) assay was used to detect the proliferation of SW480 cells. Kaplan-Meier method and log-rank test were used to study the relationship between miR-21 and miR-138 expression and prognosis. Cox proportional hazards model was used to analyze the factors related to prognosis of colon cancer. Expression level of miR-21 in colon cancer tissues was significantly higher than that in adjacent tissues, and expression level of miR-138 was lower in cancer tissues than in adjacent tissues (P<0.001). Expression of miR-21 and miR-138 was associated with the degree of differentiation, lymph node metastasis, distant metastasis, and TNM stage (P<0.05). miR-21 promotes proliferation of colon cancer cell line SW480, and miR-138 inhibits cell proliferation. Survival analysis showed that the survival time of patients with high expression of miR-21 was significantly shorter than that of patients with low expression of miR-21, while survival time of patients with high expression of miR-138 was significantly longer than that of patients with low expression of miR-138 (log-rank, P<0.05). miR-21 is highly expressed in colon cancer tissues and is positively associated with the degree of malignancy of patients but negatively associated with survival. miR-138 expression is low in colon cancer tissues and is negatively associated with the degree of malignancy of patients but positively associated with survival. miR-21 and miR-138 may be involved in the regulation of colon cancer cell proliferation.
Keywords: miR-21, miR-138, colon cancer, cell proliferation, prognosis
Introduction
Colon cancer is a common malignancy (1,2). In the United States, morbidity and mortality of colon cancer ranks third among all malignancies (3,4). Colon cancer is also the third most common malignancy in developing countries. It has been reported that >600,000 patients die of this cancer every year. Incidence of colon cancer is higher in females than in males (5). Colon cancer affects >1,2 million new cases (6). The average age of onset of colon cancer is ~40 years, and the highest incidence is observed in the population age group of 50–60 years. The onset age in developing countries is 10 years earlier than that in developed countries (7). Studies have confirmed that the pathological process of tumors is related to the expression of serum miRNAs. The expression of miRNAs plays an important role in the development and progression of colon cancer, as well as in the diagnosis and prognosis (8). miRNAs are a group of non-coding RNAs of ~19–25 nucleotides in length that regulate the expression of multiple genes at the post-transcriptional level, thereby playing an important role in cell proliferation, differentiation, apoptosis and tumor development (9).
It has been reported that the increased expression of miR-21 is closely related to various malignant tumors such as breast, lung and prostate cancer, and it has been shown to promote cell proliferation and inhibit cell apoptosis of various tumor cell lines (10–13). miR-138 is ~23–24 nt in size, and its gene is located on human chromosomes 3p21 and 16q13. miR-138 plays a role as tumor suppressor gene in tumor regulation (14–16). miR-138 is lowly expressed in various types of tumors and negatively regulates the proliferation and metastasis of gastric cancer cells and other tumor cells (17). Therefore, miR-21 and miR-138 are closely associated with tumor development. Our study aimed to analyze the expression of miR-21 and miR-138 in patients with colon cancer and its effect on proliferation of SW480 cells and the prognosis of patients, so as to investigate the role of miR-21 and miR-138 in the development of colon cancer and provide references for the diagnosis and prognosis of this disease.
Materials and methods
Specimen collection
Tumor tissues and adjacent healthy tissues within 50 mm around the tumors were collected from 128 patients who underwent surgical resection from January 2010 to January 2013 in Nanfang Hospital (Guangzhou, China). The patients included 68 males and 60 females, and their age ranged from 30–78 years. Tissues were stored at −80°C before using. All cancer specimens were confirmed by pathological examination, and no cancer cells and obvious inflammatory cells were observed in cancer tissues. Colon cancer tissues were diagnosed and staged according to the TNM staging criteria of the American Joint Committee on Cancer (7th edition). No history of a tumor, major organ dysfunction, such as liver or kidney, and abnormal hemorrhage or abnormal coagulation function were observed. All patients had complete medical record and follow-up data. No preoperative radiotherapy, chemotherapy or other anticancer treatments were performed. All patients and their families were informed and signed a consent. The study was approved by the Ethics Committee of Nanfang Hospital.
Reagents and instruments
Human colon cancer cell line SW480 was purchased from Shanghai Cell Bank of the Chinese Academy of Sciences (cat. no. TCHu172; Shanghai, China). Leibovitz's L-15 medium was purchased from Changzhou Beiyuanxin Biotechnology Co., Ltd. (Changzhou, China), and RNA extraction kit (TRIzol) was purchased from Shanghai Pufei Biotechnology Co., Ltd. (Shanghai, China). TaqMan microRNA assay kit was purchased from (ABI; Thermo Fisher Scientific, Inc., Waltham, MA, USA). NanoDrop 2000 UV spectrophotometer was purchased from Thermo Fisher Scientific, Inc. Countess II FL Automated Cell Counter was purchased from ABI (Thermo Fisher Scientific, Inc.). MTT test kit was purchased from Shanghai Lianmai Biological Engineering Co., Ltd. (Shanghai, China).
Methods
Total RNA extraction
TRIzol reagent was used to extract total RNA from colon cancer and adjacent tissues in accordance with the manufacturer's instructions. Concentration and purity of extracted RNA were measured using NanoDrop 2000 UV spectrophotometer, and RNA integrity was detected by agarose gel electrophoresis.
Reverse transcription
Reverse transcription was performed on the extracted RNA. The primer sequences were designed and synthesized by Takara Biotechnology Co., Ltd. (Beijing, China). U6 was used as an endogenous control. Reverse transcription system (15 µl): 0.5 µl of reverse transcript primer, 0.5 µl of reverse transcriptase, 2.0 µl of buffer, 2 µl of RNA, and RNase Free dH2O was added to make a final volume of 15 µl. Reaction conditions: 37°C for 10 min and 95°C for 5 min. Synthesized cDNA was stored at 4°C.
RT-qPCR
Quantitative PCR reaction system: 1 µl of TaqMan microRNA assay (20X), 1 µl of cDNA (1:15 dilution), 1.33 µl of TaqMan 2X Universal PCR Master Mix II, and 10 µl of TaqMan 2X Universal PCR Master Mix II, RNase Free dH2O were added to make a final volume of 20 µl. Countess II FL Automated Cell Counter quantitative PCR instrument was used for PCR amplification. Reaction conditions were: 95°C for 5 min, followed by 45 cycles of 95°C for 20 sec and 60°C for 45 sec. U6 was used as the endogenous control. Each reaction was repeated 3 times. Expression level of miR-21 and miR-138 was analyzed by 2−ΔΔCq method. Primer sequences are listed in Table I (18).
Table I.
Primer sequences of of miR-21, miR-138 and U6.
| Items | Forward | Reverse |
|---|---|---|
| miR-21 | 5′-GCGGTAGCTTATCAGACTGA-3′ | 5′-TGCGTGTCGTGGAGTC-3′ |
| miR-138 | 5′-GGTGTCGTGGAGTCGGCAA-3′ | 5′-AACTTCACAACACCAGCTTA-3′ |
| U6 | 5′-CTCGCTTCGGCAGCACA-3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
Detection of human colon cancer cell proliferation by MTT assay
Construction of miR-21 and miR-138 expression vectors was performed by GenePharma Co., Ltd. (Shanghai, China). miR-21 expression vector (positive group A) and blank vector (negative group A), miR-138 expression vector (positive group B) and blank vector (negative group B) and trypsin-digested SW480 cells were cultured in Leibovitz's L-15 medium at 37°C with pH 6.8–7.4 and 5% CO2, according to the instructions of Lipofectamine 2000.
After transfection, the cells of human colon cancer cell line SW480 were used to prepare single cell suspension and were cultured in 96-well plates. Some cells were collected 6 h later and 20 µl MTT (5 mg/ml) was added, followed by cell culture for another 4 h at 37°C. Supernatant was removed and DMSO was added. After shaking on a shaker for 15 min, OD values at a wavelength of 570 nm were measured at 6, 24, 48, and 72 h using an enzyme-linked immunosorbent assay detector and a growth curve was generated. MTT test kit was purchased from Shanghai Lianmai Biological Engineering Co., Ltd.
Follow-up
Patients were followed up by telephone and outpatient visit. The relationship between the expression levels of miR-21 and miR-138 and clinicopathological features of colon cancer was observed. The endpoint of follow-up was the patient's death or until April 2017, and associations between miR-21 and miR-138 expression and survival of patients were analyzed.
Statistical analysis
SPSS 21.0 statistical software package (Shanghai Kabei Information Technology Co., Ltd., Shanghai, China) was used for statistical analysis. Measurement data are expressed as mean ± standard deviation. Measurement data with normal distribution were analyzed by t-test. Kaplan-Meier was used for survival analysis. Log-rank test was used to compare survival rates. Cox proportional hazards model was used to analyze factors related to prognosis of colon cancer patients. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-21 and miR-138 in colon cancer and adjacent tissues
The expression levels of miR-21 and miR-138 were detected by RT-qPCR. Results showed that the expression level of miR-21 in colon cancer tissues was significantly higher than that in adjacent healthy tissues, while the expression level of miR-138 was lower in colon cancer tissues than in adjacent healthy tissues (P<0.001) (Table II; Figs. 1 and 2).
Table II.
Expression of miR-21 and miR-138 in colon cancer and adjacent tissues.
| Groups | n | miR-21 | t value | P-value | miR-138 | t value | P-value |
|---|---|---|---|---|---|---|---|
| Colon cancer tissues | 128 | 5.86±0.34 | 49.120 | <0.001 | 0.46±0.24 | 4.707 | <0.001 |
| Adjacent healthy tissues | 128 | 3.48±0.43 | 0.58±0.16 |
Figure 1.
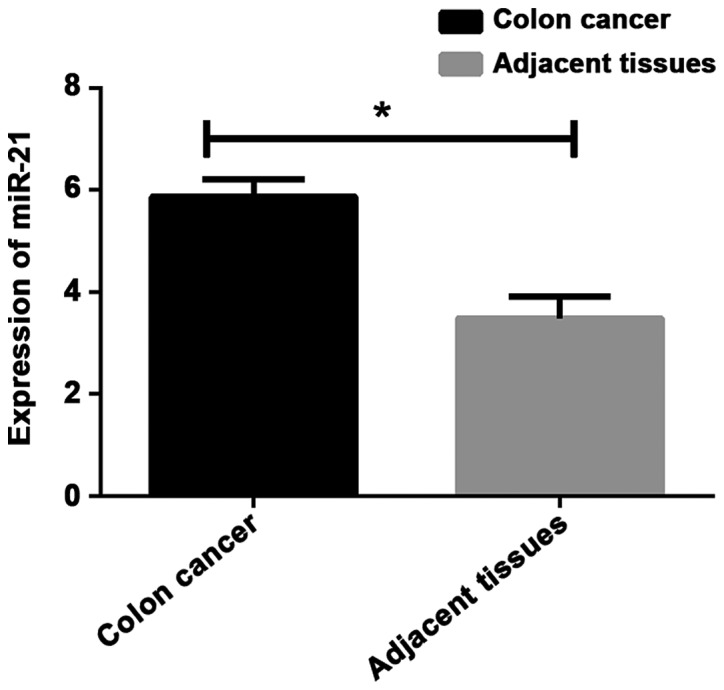
Expression of miR-21 in colon cancer and adjacent tissues. Results of RT-qPCR showed that the expression level of miR-21 in colon cancer tissues was significantly higher than that in adjacent tissues. *P<0.001, compared with adjacent healthy tissues.
Figure 2.
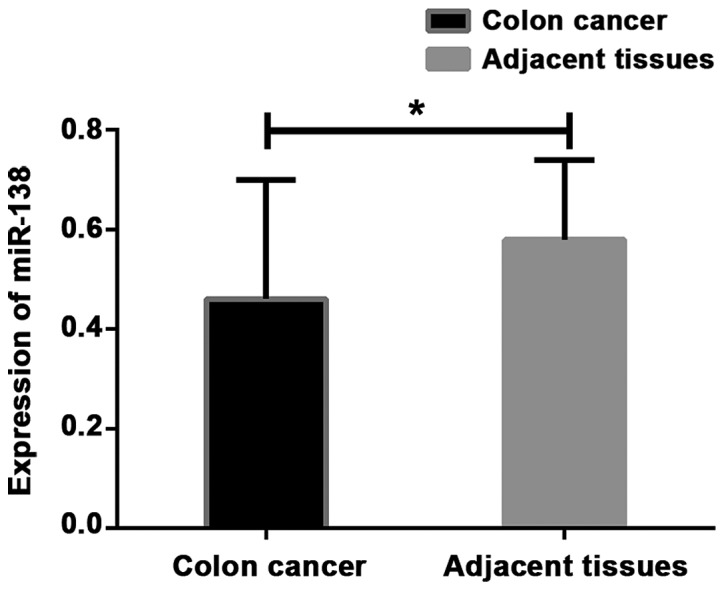
Expression of miR-138 in colon cancer and adjacent tissues. Results of RT-qPCR showed that the expression level of miR-138 in colon cancer tissues was significantly lower than that in adjacent tissues. *P<0.001, compared with adjacent healthy tissues.
Association between the expression of miR-21 and miR-138 and clinicopathological features
There was no association between the expression levels of miR-21 and miR-138 and age and sex (P>0.05). The expression levels of miR-21 and miR-138 were associated with differentiation, lymph node metastasis, distant metastasis, and TNM staging (P<0.05) (Table III).
Table III.
Association between the expressions of miR-21 and miR-138 and clinicopathological features.
| Variables | n (%) | miR-21 | t value | P-value | miR-138 | t value | P-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 0.438 | 0.662 | 0.622 | 0.535 | |||
| ≥45 | 76 (59.4) | 5.47±2.06 | 0.48±0.12 | ||||
| <45 | 52 (40.6) | 5.31±1.98 | 0.46±0.24 | ||||
| Sex | 0.408 | 0.684 | 1.220 | 0.225 | |||
| Male | 68 (53.1) | 5.78±2.47 | 0.43±0.16 | ||||
| Female | 60 (46.9) | 5.64±1.05 | 0.47±0.21 | ||||
| Differentiation | 2.000 | 0.048 | 3.541 | 0.001 | |||
| Low-moderate | 89 (69.5) | 5.08±2.13 | 0.48±0.15 | ||||
| High | 39 (30.5) | 5.89±2.06 | 0.38±0.14 | ||||
| Lymph node metastasis | 3.237 | 0.002 | 2.094 | 0.038 | |||
| Yes | 62 (48.4) | 5.86±1.64 | 0.42±0.23 | ||||
| No | 66 (51.6) | 5.07±1.08 | 0.49±0.14 | ||||
| Distant metastasis | 3.786 | <0.001 | 4.051 | <0.001 | |||
| Yes | 48 (37.5) | 6.18±2.13 | 0.42±0.17 | ||||
| No | 80 (62.5) | 5.04±1.28 | 0.57±0.22 | ||||
| TNM stage | 3.681 | <0.001 | 3.818 | <0.001 | |||
| I+II | 84 (65.6) | 5.01±1.47 | 0.52±0.14 | ||||
| III+IV | 44 (34.4) | 6.14±1.95 | 0.41±0.18 |
Effects of miR-21 and miR-138 on proliferation of colon cancer cells
Effects of miR-21 and miR-138 on proliferation of colon cancer SW480 cells were detected by MTT assay. Results showed that miR-21 could promote cell proliferation. OD values at 6, 24, 48, and 72 h after miR-21 expression vector transfection (positive group A) were higher than those of cells with blank vector transfection (negative group B). miR-138 inhibited cell proliferation, and OD values at 6, 24, 48, and 72 h after miR-1338 expression vector transfection (positive group A) were lower than those of cells with blank vector transfection (negative group B) (P<0.05) (Figs. 3 and 4).
Figure 3.
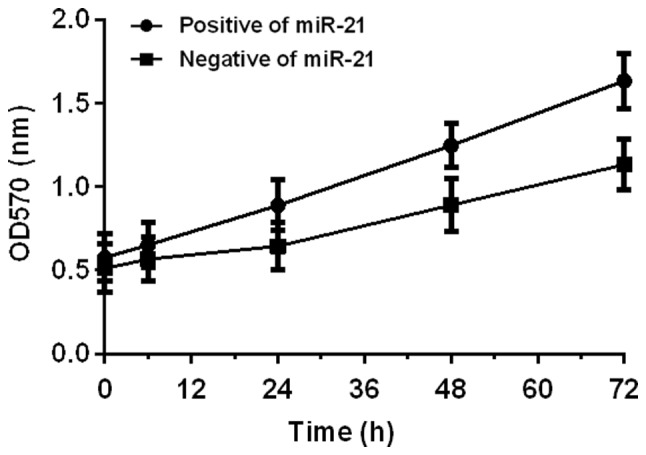
Effect of miR-21 on colon cancer SW480 cell proliferation. Proliferation of SW480 cells was determined by MTT assay. The OD values of the two groups of cells increased with time, and the OD values at 6, 24, 48, and 72 h after the miR-21 expression vector transfection (positive group A) were higher than those of cells with blank vector transfection (negative group B) (P<0.05). MTT, methyl thiazolyl tetrazolium.
Figure 4.
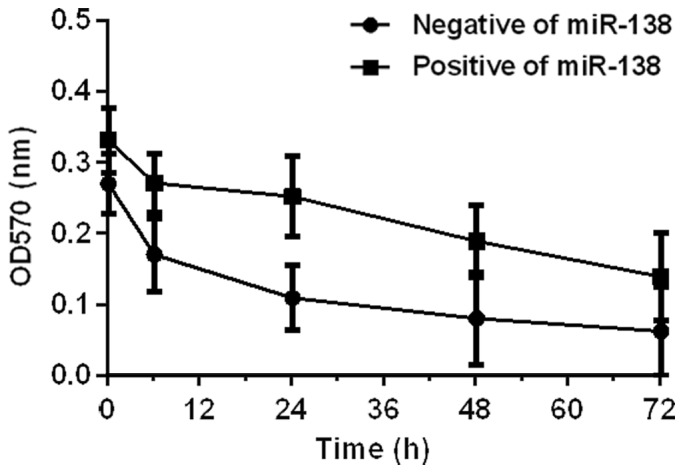
Effect of miR-138 on colon cancer SW480 cell proliferation. Proliferation of SW480 cells was determined by MTT assay. The OD values of the two groups of cells decreased with time, and the OD values at 6, 24, 48, and 72 h after miR-138 expression vector transfection (positive group A) were lower than those of cells with blank vector transfection (negative group B) (P<0.05). MTT, methyl thiazolyl tetrazolium.
Survival analysis
The median expression level of miR-21 in colon cancer tissues was 5.595. According to the median expression level of miR-21, patients were divided into high expression group (n=64) and low expression group (n=64). The median expression level of miR-138 in colon cancer tissues was 0.483. According to the median expression level of miR-138, patients were divided into high expression group (n=64) and low expression group (n=64). Mean survival time of the miR-21 low expression group was 35.46±1.08 months, and the mean survival time of the miR-21 high expression group was 30.82±2.84 months. Mean survival time of the miR-138 low expression group was 34.16±1.88 months, and the mean survival time of the miR-138 high expression group was 36.86±2.02 months. Survival analysis showed that the survival time of patients with high expression of miR-21 was significantly shorter than that of patients with low expression of miR-21. The survival time of miR-138 high expression group was longer than that of miR-138 low expression group (log-rank, P<0.05). Patient's survival was negatively associated with the level of miR-21 expression. Univariate prognostic analysis showed that the factors affecting the prognosis of patients with colon cancer include miR-21 and miR-138 expression, tissue differentiation, TNM staging, and lymph node metastasis. Cox multivariate regression analysis showed that miR-21, miR-138 expression, and TNM staging were independent risk factors for poor prognosis of colon cancer (Figs. 5 and 6; Tables IV and V).
Figure 5.
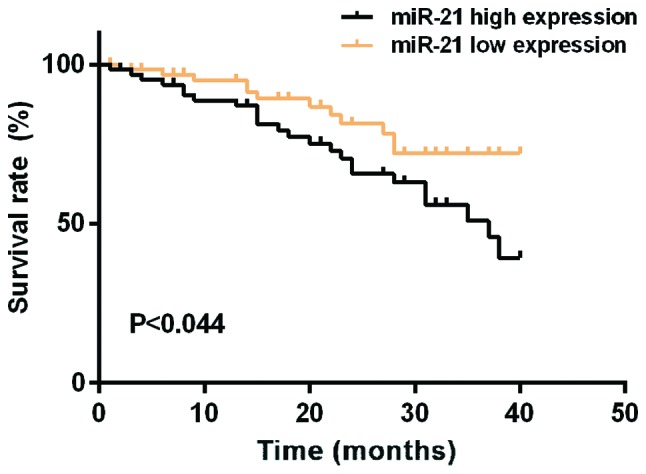
Relationship between miR-21 and prognosis of colon cancer patients. The survival curves showed that the survival time of patients with high expression of miR-21 was significantly shorter than that of patients with low expression of miR-21. The survival time of patients with miR-21 was negatively associated with the expression of miR-21 (P<0.044).
Figure 6.
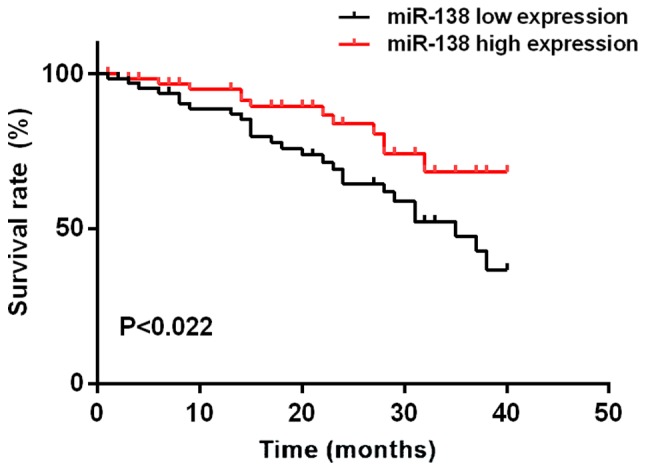
Relationship between miR-138 and prognosis of colon cancer patients. The survival curves showed that the survival time of patients with high expression of miR-138 was significantly longer than that of patients with low expression of miR-138 (P<0.022).
Table IV.
Univariate analysis of factors affecting colon cancer.
| miR-21 | miR-138 | |||
|---|---|---|---|---|
| Variables | P-value | HR (95% CI) | P-value | HR (95% CI) |
| Expression level (low vs. high) | <0.001 | 38.842 (15.203–96.215) | <0.001 | 32.562 (13.524–87.347) |
| Sex (male vs. female) | 0.559 | 1.012 (0.797–1.620) | 0.845 | 1.062 (0.523–1.946) |
| Age (<45 years vs. ≥45 years) | 0.114 | 1.132 (1.028–1.715) | 0.173 | 0.986 (0.946–1.042) |
| TNM stage (I and II vs. III and IV) | 0.013 | 2.821 (1.346–2.857) | 0.013 | 4.182 (2.152–7.864) |
| Lymph node metastasis (yes vs. no) | 0.011 | 3.053 (1.282–7.323) | 0.004 | 2.452 (1.265–4.124) |
| Differentiation (low vs. high) | 0.025 | 0.921 (0.831–1.525) | 0.032 | 0.042 (0.152–0.945) |
Table V.
Multivariate analysis of association between miR-21 and miR-138 and colon cancer.
| miR-21 | miR-138 | |||
|---|---|---|---|---|
| Variables | P-value | HR (95% CI) | P-value | HR (95% CI) |
| Expression level (low vs. high) | 0.034 | 36.842 (15.203–96.215) | 0.016 | 33.542 (13.524–87.347) |
| TNM stage (I and II vs. III and IV) | 0.028 | 2.867 (1.348–2.857) | 0.021 | 4.325 (2.248–7.856) |
Discussion
With the improvement of people's living standards and changes in eating habits, the incidence of colon cancer has shown an increasing trend (19). Multiple factors, multiple stages, and multiple genetic mutations lead to the development and progression of colon cancer, and multiple oncogenes and tumor suppressor genes are involved. Treatment of colon cancer is currently dominated by comprehensive treatment, and the most effective one is surgical treatment combined with targeted chemotherapy before and after surgery. With the development of molecular biology and cell biology, it has been found that the expression of oncogenes and tumor suppressor genes can affect the proliferation and differentiation of tumor cells, and has a correlation with the occurrence and development of tumors and prognosis (20–22). At present, it has been confirmed that at least 400 miRNAs in the human genome are closely related to tumors (23).
The results of this study showed that the expression level of miR-21 in colon cancer tissues was significantly higher than that in adjacent healthy tissues, and the expression level of miR-138 was lower in colon cancer tissues than in adjacent tissues (P<0.001). The expression levels of miR-21 and miR-138 were associated with the degree of differentiation, lymph node metastasis, distant metastasis, and TNM stage (P<0.05). MTT results showed that miR-21 could promote cell proliferation. OD values at 6, 24, 48, and 72 h after miR-21 expression vector transfection (positive group A) were higher than those of cells with blank vector transfection (negative group B). miR-138 inhibited cell proliferation, and OD values at 6, 24, 48, and 72 h after miR-1338 expression vector transfection (positive group A) were lower than those of cells with blank vector transfection (negative group B) (P<0.05). Studies on different tumor tissues and cell lines have shown that miR-21 can promote cell proliferation (24,25). Capraro et al (26) have shown that miR-138 expression is low in glioma cells and can significantly inhibit the proliferation and migration of glioma cells, and thus functions as a tumor suppressor gene, which is consistent with the findings of the present study. Therefore, miR-21 may be a potential marker for the diagnosis and prognosis of colon cancer. miR-138 can inhibit the proliferation of cancer cells. Survival analysis showed that survival time of patients with high expression of miR-21 was significantly shorter than that of patients with low expression of miR-21. Survival time of miR-138 high expression group was longer than that of miR-138 low expression group (log-rank, P<0.05). Cox multivariate regression analysis showed that miR-21 and miR-138 expression, and TNM staging were independent risk factors for poor prognosis of colon cancer (Table V). Thus, miR-21 can predict the recurrence of colon cancer, and it is also one of the risk factors for the progression of colon cancer. Expression of miR-21 was positively associated with the degree of malignancy and the risk of disease progression, and was negatively associated with the survival of patients, while miR-138 is the opposite. Expression of miR-21 and miR-138 in the pathological specimens of patients with colon cancer is an index that can determine the prognosis of colon cancer. Combination of routine postoperative immunohistochemical pathological examination and detection of miR-21 and miR-138 expression may improve the diagnosis of colon cancer and is worthy of clinical application.
Our study has also some limitations. The sample size was small, and the patients were from a single hospital, which may affect our conclusions. Our study only investigated the effects of the two miRNAs on colon cancer cell proliferation, while the involvement of other genes and interactions with other factors were not studied. Further studies are needed to explore the mechanism and the roles of those two miRNAs.
In conclusion, miR-21 is highly expressed in colon cancer tissues, and is positively associated with the degree of malignancy of patients and negatively associated with survival. miR-138 expression is low in colon cancer tissues, and negatively associates with the degree of malignancy of patients and positively associated with survival. miR-21 and miR-138 may be involved in the regulation of colon cancer cell proliferation. Our study provides references for clinical diagnosis, treatment and prognosis of colon cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and Technology Project of Guangdong Province (no. 2017B090901067) and the Foundation of Social Development Project of the Science and Technology Department of Jiangsu Province (BE2015719).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
CY wrote the manuscript and analyzed the follow-up data. QX and LJ extracted total RNA. QX and BS assisted with reverse transcription. XJ performed PCR and XH was responsible for MTT assay. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Nanfang Hospital (Guangzhou, China). Signed informed consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Sung JJ, Lau JY, Goh KL, Leung WK. Asia Pacific Working Group on Colorectal Cancer: Increasing incidence of colorectal cancer in Asia: Implications for screening. Lancet Oncol. 2005;6:871–876. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 3.Bae JM, Cho NY, Kim TY, Kang GH. Clinicopathologic and molecular characteristics of synchronous colorectal cancers: Heterogeneity of clinical outcome depending on microsatellite instability status of individual tumors. Dis Colon Rectum. 2012;55:181–190. doi: 10.1097/DCR.0b013e31823c46ce. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, Chang DT, Nikiforova MN, Kuan SF, Pai RK. Clinicopathologic features of synchronous colorectal carcinoma: A distinct subset arising from multiple sessile serrated adenomas and associated with high levels of microsatellite instability and favorable prognosis. Am J Surg Pathol. 2013;37:1660–1670. doi: 10.1097/PAS.0b013e31829623b8. [DOI] [PubMed] [Google Scholar]
- 5.Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen J, Su F, Yao H, Song E. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem. 2011;286:19127–19137. doi: 10.1074/jbc.M110.216887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Simoglou C, Gymnopoulou E, Simoglou L, Gymnopoulou M, Nikolaou K, Gymnopoulos D. Surgery for colorectal cancer in the small town of Komotini. J Multidiscip Healthc. 2012;5:273–276. doi: 10.2147/JMDH.S30554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62:283–298. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 9.Garalde DR, Snell EA, Jachimowicz D, Sipos B, Lloyd JH, Bruce M, Pantic N, Admassu T, James P, Warland A, et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat Methods. 2018;15:201–206. doi: 10.1038/nmeth.4577. [DOI] [PubMed] [Google Scholar]
- 10.Hollis M, Nair K, Vyas A, Chaturvedi LS, Gambhir S, Vyas D. MicroRNAs potential utility in colon cancer: Early detection, prognosis, and chemosensitivity. World J Gastroenterol. 2015;21:8284–8292. doi: 10.3748/wjg.v21.i27.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas J, Lupold SE. The transcriptional regulation of miR-21, its multiple transcripts, and their implication in prostate cancer. Cell Cycle. 2010;9:923–929. doi: 10.4161/cc.9.5.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, Cui L, Sun W, Zhou H, Yuan X, Huo M, Chen J, Lou Y, Guo J. MicroRNA-21 is a new marker of circulating tumor cells in gastric cancer patients. Cancer Biomark. 2011-2012;10:71–77. doi: 10.3233/CBM-2011-0231. [DOI] [PubMed] [Google Scholar]
- 13.Reis ST, Pontes-Junior J, Antunes AA, Dall'Oglio MF, Dip N, Passerotti CC, Rossini GA, Morais DR, Nesrallah AJ, Piantino C, et al. miR-21 may acts as an oncomir by targeting RECK, a matrix metalloproteinase regulator, in prostate cancer. BMC Urol. 2012;12:14. doi: 10.1186/1471-2490-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/S0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths-Jones S. The microRNA registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Na YJ, Sung JH, Lee SC, Lee YJ, Choi YJ, Park WY, Shin HS, Kim JH. Comprehensive analysis of microRNA-mRNA co-expression in circadian rhythm. Exp Mol Med. 2009;41:638–647. doi: 10.3858/emm.2009.41.9.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu ST, Chen L, Wang HJ, Tang XD, Fang DC, Yang SM. hTERT promotes the invasion of telomerase-negative tumor cells in vitro. Int J Oncol. 2009;35:329–336. [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Ji BC, Yu CC, Yang ST, Hsia TC, Yang JS, Lai KC, Ko YC, Lin JJ, Lai TY, Chung JG. Induction of DNA damage by deguelin is mediated through reducing DNA repair genes in human non-small cell lung cancer NCI-H460 cells. Oncol Rep. 2012;27:959–964. doi: 10.3892/or.2012.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 23.Pillai RS. MicroRNA function: Multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 25.Buscaglia LE, Li Y. Apoptosis and the target genes of microRNA-21. Chin J Cancer. 2011;30:371–380. doi: 10.5732/cjc.30.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capraro V, Zane L, Poncet D, Perol D, Galia P, Preudhomme C, Bonnefoy-Berard N, Gilson E, Thomas X, El-Hamri M. Telomere deregulations possess cytogenetic, phenotype, and prognostic specificities in acute leukemias. Exp Hematol. 2011;39:195–202.e2. doi: 10.1016/j.exphem.2010.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


