Abstract
MicroRNAs (miRNAs) are associated with the formation and progression of many types of cancers. In the present study, the aim was to elucidate the involvement of miR-146a-5p in the regulation of human breast cancer (BC) cell growth and invasion, as well as the mechanisms underlying its effects. Reverse transcription-quantitative polymerase chain reaction results revealed that miR-146a-5p was markedly downregulated in BC tissues relative to those of adjacent normal tissues. miR-146a-5p expression was also markedly downregulated in BC cells. Overexpression of miR-146a-5p significantly suppressed the proliferation, invasion and migration of BC MDA-MB-453 and MCF7 cells. Furthermore, the results indicated that miR-146a-5p downregulated the expression of interleukin-1 receptor-associated kinase 1 (IRAK1) by directly binding to its 3′-untranslated region in BC cells. Furthermore, IRAK1 expression was observed to be markedly upregulated and inversely correlated with miR-146a-5p expression in BC tissues. Mechanical studies indicated that restoring IRAK1 expression reversed the miR-146a-5p-induced inhibitory effects on proliferation and invasion of BC cells. In conclusion, miR-146a-5p may act as a tumor suppressor in BC by directly targeting IRAK1. These results highlighted the potential of miR-146a-5p as a novel therapeutic target for the treatment of BC.
Keywords: breast cancer, microRNA-146a-5p, interleukin-1 receptor-associated kinase 1, growth, migration, invasion
Introduction
MicroRNAs (miRNAs) are a class of ~22-bp non-coding RNAs that are expressed in many organisms (1,2). Mature miRNAs arise from one arm of endogenous hairpin transcripts, the primary miRNAs (pri-miRNAs), by sequential processing in the nucleus and cytoplasm. Mature miRNAs regulate the expression of target genes post-transcriptionally. In particular, miRNAs inhibit translation through imperfect base-pairing interactions with the 3′-UTRs of their respective target genes or degrade their target mRNAs through perfect or near-perfect base paring (3,4). An individual miRNA is capable of regulating dozens of distinct mRNAs. More than 650 human miRNAs are believed to modulate about one-third of the mRNA species encoded in the genome.
MicroRNAs are associated with the development and progression of various types of cancers, including breast cancer (BC), brain cancer, chronic lymphocytic leukemia, colorectal neoplasia, hepatocellular carcinoma, and lung cancer (5–8). BC is one of the most common cancers affecting adult females, and studies have shown that BC is also associated with the deregulation of several miRNAs. Previous studies reported that various miRNAs, such as miR-139-5p (9), miR-17-5p (10), and miR-219a-5p (11), function as tumor suppressors to regulate BC development and progression. In the present study, our findings revealed that miR-146a-5p is downregulated in BC cells. Interleukin-1 receptor-associated kinase 1 (IRAK1) was predicted as a direct target of miR-146a-5p. Elevated expression of IRAK1 in BC tissues promotes the growth, migration, and invasion of BC cells.
Therefore, we aimed to explore the biological function of miR-146a-5p in BC and to verify the potential mechanisms involved. Results revealed that strong miR-146a-5p expression in BC patients significantly repressed cell proliferation, migration, and invasion. Furthermore, IRAK1 as a direct target of miR-146a-5p can reverse the tumor suppressive effects of miR-146a-5p.
Materials and methods
Patient and tissue samples
A total of 20 paired BC tissues were collected from patients who underwent surgical treatment between October 2015 and December 2017 at the Women's Hospital, Zhejiang University School of Medicine (Zhejiang, China). Tissue samples were snap frozen in liquid nitrogen immediately after surgical resection and stored at −80°C. Sample collection was performed after obtaining written informed consent from all patients. The present study was approved by the Institutional Review Board of Women's Hospital, Zhejiang University School of Medicine. Patients who received radiotherapy and/or immunotherapy before or after surgical treatment were not included in the present study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Women's Hospital, Zhejiang University School of Medicine. All patients enrolled in the present study provided written informed consents.
Cell lines and culture
BC cell lines, including the noncancerous breast cell line MCF10A and the BC cell lines MDA-MB-453 and MCF7 were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China). All cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen; Thermo Fisher Scientific, Inc.) and grown in humidified 5% CO2 at 37°C. miR-146a-5p mimics and controls were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). Transfection was performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as previously described.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BC and noncancerous breast cells using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. For microRNA analysis, RT-qPCR was performed using the TaqMan MicroRNA Reverse Transcription Kit, TaqMan Universal PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the target-specific primers. For mRNA analysis, RT-qPCR was performed using the TaqMan High-Capacity cDNA Reverse Transcription Kit, TaqMan Fast PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the corresponding primers. GAPDH was used as an internal control for normalizing IRAK1 expression levels. RT-qPCR was performed in triplicate on a RealPlex4 real-time PCR detection system from Eppendorf (Hamburg, Germany). The thermocycling conditions for RT-qPCR were as follows: Initial denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 10 sec. The relative expression levels were calculated using the 2−∆∆Cq method (12).
Immunohistochemistry (IHC)
Tissue specimens were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Afterwards, sections were deparaffinized and rehydrated. Sections were treated with hydrogen peroxide for 10 min to block endogenous peroxide activity. After antigen retrieval using a microwave, the sections were treated with 1% bovine serum albumin to block non-specific binding. Sections were then incubated with rabbit anti-IRAK1 (Abcam, Cambridge, MA, USA) in a humidified chamber overnight at 4°C. After washing thrice with phosphate-buffered saline (PBS) for 5 min each wash, tissue sections were treated with biotinylated anti-rabbit secondary antibody (Abcam), followed by further incubation with streptavidin-horseradish peroxidase complex. After rinsing, sections were treated with diaminobenzidine (DAB; Abcam) as chromogen, and counterstained with hematoxylin. Samples incubated with PBS instead of the primary antibody served as negative controls.
Cell proliferation assay
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (Amresco, LLC, Solon, OH, USA) assays were performed to evaluate cell proliferation. Briefly, 5×103 cells were separately seeded in 96-well microtiter plates (Corning Incorporated, Corning, NY, USA) and transfected with miR-146a-5p, control or miR-146a-5p mimics + IRAK1 plasmid, and then routinely cultured for 3 days. Medium supplemented with 0.5 mg/ml MTT was added to each well. The cells were incubated at 37°C for 4 h, after which 100 µl of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan. Absorption at the wavelength of 490 nm was measured using a SpectraMax M5 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Cell migration and invasion assay
Cell migration was evaluated by performing a wound healing assay. Briefly, transfected cells were cultured in 6-well plates (5×104 cells/well). Upon reaching 90–95% confluence, the cell monolayer was scratched using a sterile plastic micropipette tip, after which cells were cultured under standard conditions for 24 h. Following several washes, wound recovery was observed and photographed using an X71 inverted microscope (Olympus, Tokyo, Japan).
The Transwell invasion assay was performed to determine cell invasion. First, 1×105 transfected cells were seeded into the upper chamber of Matrigel-coated inserts containing serum-free medium. Medium containing 10% FBS was added to the lower chamber as chemoattractant. The cells were allowed to invade the chamber for 48 h at 37°C with 5% CO2. Cells that invaded the lower surface of the filter were fixed in 70% ethanol for 30 min and stained with 0.1% crystal violet for 10 min. The number of cells that migrated to the lower side was counted in five randomly selected fields under an X71 inverted microscope (Olympus).
Luciferase activity assay
The 3′-UTR of wild-type IRAK1 was amplified from a human cDNA library. Mutations in the miR-146a-5p binding site were introduced by site-directed mutagenesis using a fast mutation kit (NEB, Beverly, MA, USA). The PCR fragment was cloned into the psiCHECK-2 vector downstream of the firefly luciferase coding region within XhoI and NotI (Takara Bio, Inc., Otsu, Japan). The psiCHECK-2-control was used as internal control.
Western blot analysis
Total protein lysates were resolved by 10% SDS-PAGE and transferred onto polyvinyl difluoride membranes (EMD Millipore, Bedford, MA, USA). After blocking in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) with 5% non-fat dry milk for 30 min, membranes were washed four times in TBS-T and incubated with primary antibodies overnight at 4°C. All primary antibodies were obtained from Abcam and used at the following dilutions: IRAK1 (1/500), Twist (1/500), MMP-2 (1/500), MMP-9 (1/500), and anti-GAPDH (1/1,000). After thorough washing, membranes were incubated with horseradish peroxidase-linked goat polyclonal anti rabbit IgG secondary antibodies at a dilution of 1:2,000 for 1 h at room temperature. Immunoreactivity was detected by enhanced chemiluminescence (ECL Kit; Pierce; Thermo Fisher Scientific, Inc.) and exposure to radiography film. GAPDH served as the loading control.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). Two-tailed Student's t-test was performed to compare the differences between two groups. One-way analysis of variance followed by Dunnett's multiple comparison was employed to compare the differences among three independent treatment groups. Correlation between miR-146a-5p and IRAK1 expression levels in BC tissues was determined by conducting Pearson's correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-146a-5p expression in human BC tissues and cell lines
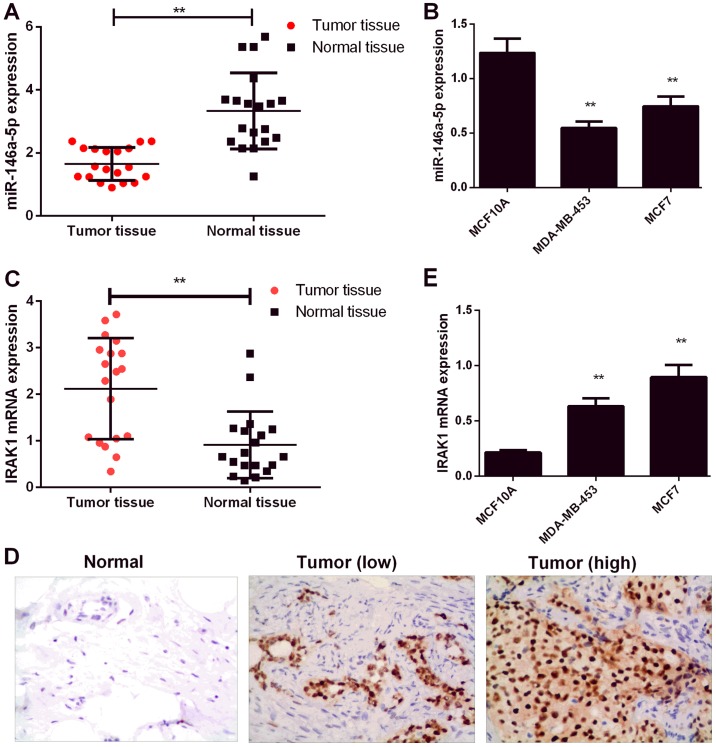
In the initial effort to determine the biological role of miR-146a-5p in BC, we examined miR-146a-5p expression levels in 20 BC patient tissue samples and normal tissues by RT-PCR. As shown in Fig. 1A, miR-146a-5p expression was significantly downregulated in BC tissues relative to that in normal tissues (P<0.01). Consistent with the above results, BC cell lines (MDA-MB-453 and MCF7) showed miR-146a-5p downregulation relative to those of normal human breast tissue MCF10A cells (Fig. 1B). IRAK1 expression is known to be significantly upregulated in BC (13). In addition, IRAK1 expression levels were determined by RT-PCR and IHC. As shown in Fig. 1C and D, IRAK1 levels were significantly upregulated in the BC tissues (P<0.01). IRAK1 levels were also upregulated in BC cell lines (MDA-MB-453 and MCF7) relative to those in MCF10A cells (P<0.01, Fig. 1E).
Figure 1.
miR-146-5p and IRAK1 expression levels in BC patients and BC cells. (A) miR-146-5p expression in BC and normal tissues as assessed by RT-qPCR (n=20). (B) miR-146-5p expression in normal breast cells (MCF10A) and BC cells (MDA-MB-453 and MCF7) as assessed by RT-qPCR. (C) Relative mRNA expression levels of IRAK1 in BC tissues and normal tissues as assessed by RT-qPCR (n=20). (D) Relative mRNA expression levels of IRAK1 in normal breast cells (MCF10A) and BC cells (MDA-MB-453 and MCF7) as assessed by RT-qPCR. (E) Protein expression of IRAK1 in normal breast tissue and BC tissue as evaluated by immunohistochemistry (magnification, ×200). Data are presented as mean ± standard deviation. **P<0.01 vs. MCF10A cells/as indicated. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; miR, microRNA; IRAK1, interleukin-1 receptor-associated kinase 1; BC, breast cancer.
miR-146a-5p overexpression inhibits the proliferation of BC cells
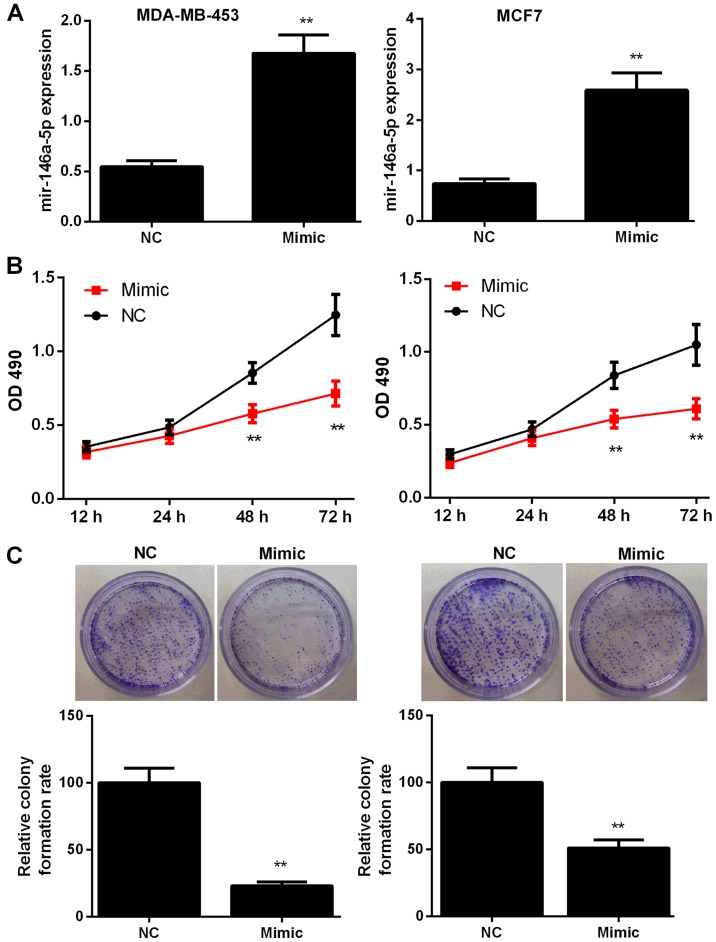
To explore the potential role of miR-146a-5p in BC, MDA-MB-453 and MCF7 cells were transfected with miR-control or miR-146a-5p mimic. The transfection efficiency was assessed by RT-qPCR (Fig. 2A). The effects of miR-146a-5p mimic on cell proliferation and colony formation were then evaluated. As shown in Fig. 2B, BC cells treated with the miR-146a-5p mimic showed significant inhibition of proliferation at 48 and 72 h compared with those in the NC groups (P<0.01). In addition, colony formation rate in the mimic-treated group was significantly lower at 48 h compared with that in the NC group (P<0.01, Fig. 2C).
Figure 2.
miR-146-5p represses the proliferation and colony formation of breast cancer cells. (A) miR-146-5p NC or mimic was transfected into MDA-MB-453 and MCF7 cells. Reverse transcription-quantitative polymerase chain reaction was performed to determine the corresponding transfection efficiencies. (B and C) Cell proliferation was evaluated in MDA-MB-453 and MCF7 cells transfected with NC or mimic by (B) MTT and (C) colony formation assays. Data are presented as the mean ± standard deviation. **P<0.01 vs. NC group. NC, negative control; miR, microRNA; OD, optical density.
miR-146a-5p represses BC cell migration and invasion
To determine whether miR-146a-5p influences the mobility of BC cells, we performed wound healing and Transwell assays to evaluate migration and invasion capabilities of MDA-MB-453 and MCF7 cells after transfection with miR-control or miR-146a-5p mimic. The results indicated that the migration and invasion capabilities of MDA-MB-453 and MCF7 cells were markedly reduced by transfection with miR-146a-5p mimic compared with those of the miR-control cells (P<0.01, Fig. 3A and B). In addition, protein expression levels of Twist, MMP-2, and MMP-9 were significantly downregulated in MDA-MB-453 and MCF7 cells after transfection miR-146a-5p mimic compared to those in cells transfected with miR-control (P<0.01, Fig. 3C). The above-mentioned findings demonstrated that miR-146a-5p exerted inhibitory effects on the migration and invasion capabilities of BC cells.
Figure 3.
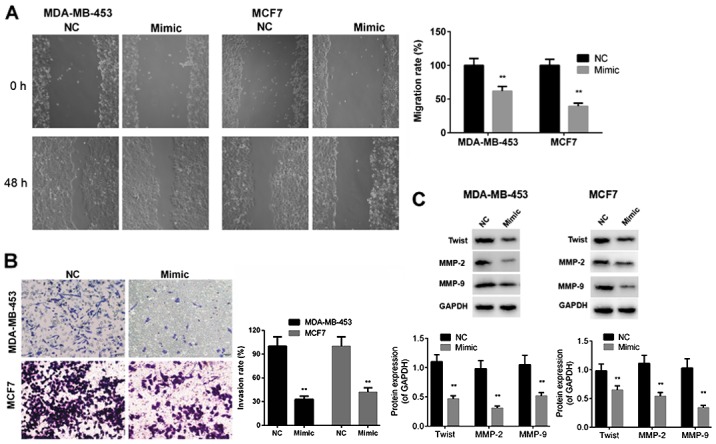
miR-146-5p suppresses the migration and invasion of breast cancer cells. (A) Migration of MDA-MB-453 and MCF7 cells transfected with NC or mimic was determined by performing wound healing assay. (B) Invasion ability was determined by performing Transwell invasion assay in MDA-MB-453 and MCF7 cells transfected with NC or mimic (magnification, ×200). (C) Expression levels of Twist, MMP-2 and MMP-9 were determined by western blot analysis. Data are presented as mean ± standard deviation. **P<0.01 vs. NC group. NC, negative control; miR, microRNA; MMP, matrix metalloproteinase.
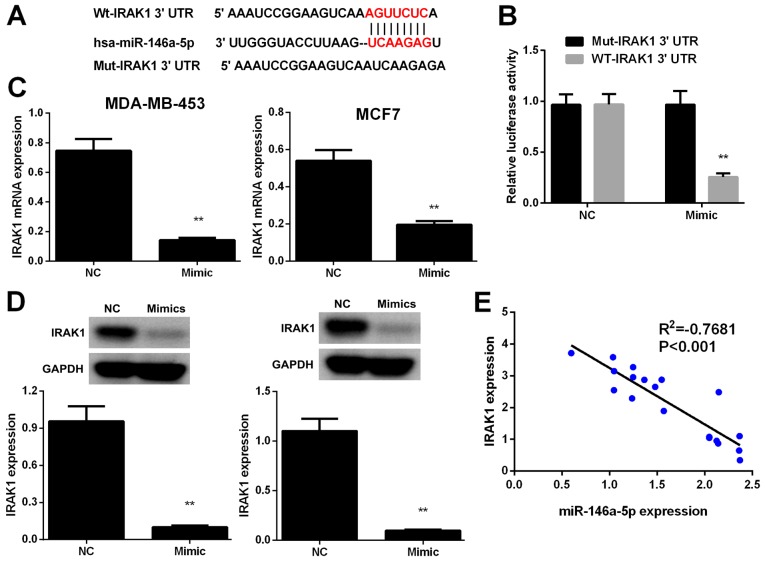
IRAK1 is a direct target of miR-146a-5p
To verify the potential mechanism by which miR-146a-5p suppresses BC cell proliferation, migration, and invasion, we used TargetScan and miRanda algorithms to identify putative miR-146a-5p targets. IRAK1, which is frequently reported to be involved in the occurrence, metastasis, and progression of human BC, was selected as a candidate miR-146a-5p target (Fig. 4A). Luciferase reporter assays were performed to confirm whether IRAK1 is regulated via direct binding of miR-146a-5p to its 3′-UTR. IRAK1 3′-UTR fragments harboring miR-146a-5p target sequences and its corresponding mutant fragments were subcloned into firefly luciferase vectors. Results demonstrated that luciferase activity was markedly lower in the cells co-transfected with miR-146a-5p mimic and wild-type IRAK1 3′-UTR compared to those in the NC cells, whereas co-transfection with miR-146a-5p mimic and the mutant IRAK1 3′-UTR failed to repress luciferase activity (Fig. 4B).
Figure 4.
IRAK1 is a target of miR-146-5p. (A) miR-146-5p-targeting sites in the IRAK1-3′-UTR and its mutant sequence abrogates the binding of IRAK1 to target mRNA. (B) Luciferase reporter assays were performed using MCF7 cells co-transfected with the miR-146-5p mimic and IRAK1-Wt-3′-UTR or IRAK1-Mut-3′-UTR reporter plasmid. (C) The mRNA and (D) protein levels of IRAK1 in MDA-MB-453 and MCF7 cells transfected with the miR-146-5p mimic or NC were determined by reverse transcription-quantitative polymerase chain reaction and western blot analysis, respectively. (E) Pearson's correlation analysis was performed to determine the correlations between the expression levels of IRAK1 and miR-146-5p in human breast cancer samples (n=20). Data are presented as mean ± standard deviation. **P<0.01 vs. NC group. IRAK1, interleukin-1 receptor-associated kinase 1; miR, microRNA; NC, negative control; UTR, untranslated region; Wt, wild-type; Mut, mutant.
IRAK1 expression levels in MDA-MB-453 and MCF7 cells were evaluated by RT-PCR and western blot analysis. Results showed that IRAK1 was downregulated in BC cells transfected with miR-146a-5p mimics relative to that in the NC cells (Fig. 4C and D). In addition, results of Pearson's correlation analysis revealed that miR-146a-5p expression was inversely correlated with IRAK1 expression (P<0.01, Fig. 4E). The above results indicated that miR-146a-5p directly binds to the IRAK1 3′-UTR to suppress its expression.
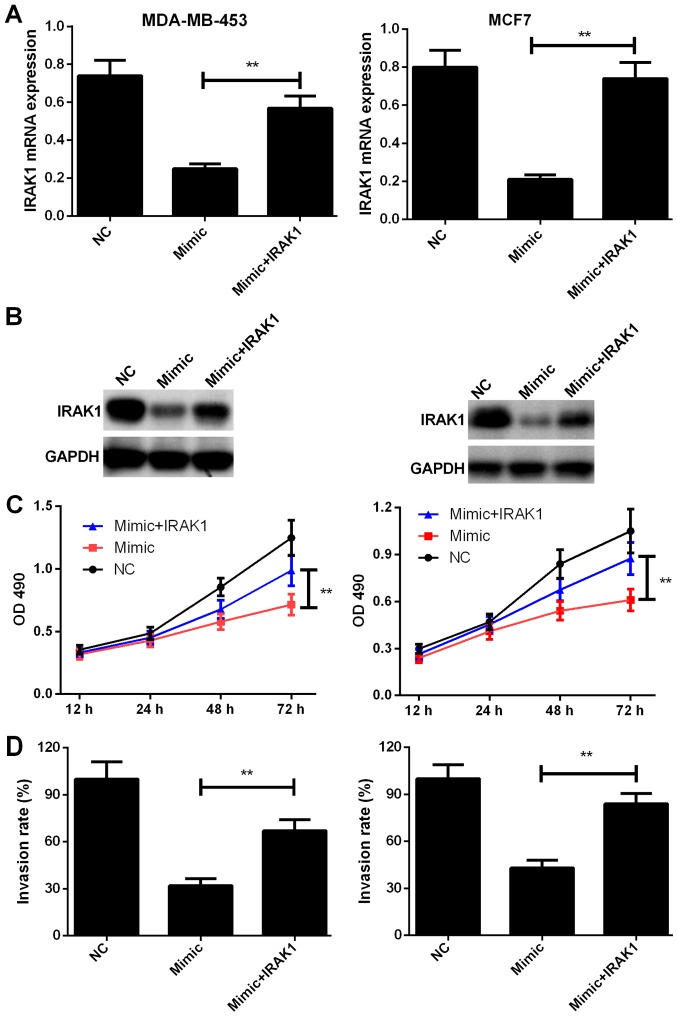
IRAK1 overexpression alleviates the inhibitory effects of miR-146a-5p on the proliferation, migration, and invasion capabilities of BC cells. We further evaluated the functional correlation between miR-146a-5p and IRAK1. IRAK1 expression was rescued in MDA-MB-453 and MCF7 cells transfected with the miR-146a-5p mimic. The transfection efficiencies were determined by RT-PCR and western blotting (P<0.01, Fig. 5A and B). As shown in Fig. 5C and D, IRAK1 overexpression in BC cells transfected with miR-146a-5p mimic group significantly enhanced cell proliferation and promoted the invasion capabilities (P<0.01), which suggested that restoring IRAK1 expression alleviates miR-146a-5p-induced suppression of proliferation and invasion of MDA-MB-453 and MCF7 cells. The above-mentioned results indicated that miR-146a-5p exerts repressive effects on the proliferation and invasion behavior of BC cells by targeting IRAK1.
Figure 5.
Restoration of IRAK1 expression reverses miR-146-5p mimic-induced inhibition of the proliferation and invasion of breast cancer cells. (A and B) IRAK1 expression in miR-146-5p mimic cells was validated by (A) reverse transcription-quantitative polymerase chain reaction and (B) western blot analysis. (C) Proliferation was evaluated using MDA-MB-453 and MCF7 cells transfected with NC, miR-146-5p mimic, or miR-146-5p mimic + IRAK1 by performing the MTT assay. (D) Invasion ability was evaluated in MDA-MB-453 and MCF7 cells transfected with NC, miR-146-5p mimic, or miR-146-5p mimic + IRAK1 by performing a Transwell invasion assay. Data are presented as mean ± standard deviation. **P<0.01 vs. miR-146-5p mimic group. IRAK1, interleukin-1 receptor-associated kinase 1; miR, microRNA; NC, negative control; OD, optical density.
Discussion
Increasing evidence has demonstrated that abnormal miRNA expression patterns play crucial roles in BC occurrence and metastasis. A better understanding of the biological functions of miRNAs is important for the development of effective therapeutic strategies for BC treatment and for identifying novel diagnostic markers for BC. Multiple studies have shown that miR-146a-5p downregulation is involved in tumorigenesis and metastasis of diverse human cancers. Xu et al (14) reported that miR-146a plays a critical role in the apoptosis of androgen-independent prostate cancer cells apoptosis by regulating ROCK/Caspase-3 signaling. Yuwen et al (15) suggested that the serum exosomal miR-146a-5p can be used as a novel putative biomarker for predicting the efficacy of cisplatin for the treatment of lung cancer and for real-time monitoring of drug resistance. The findings of Min et al (16) revealed that miR-146a-5p influences cell proliferation and apoptosis in a cellular context-dependent manner and selectively disarms the TRAF6-mediated branch of the TGF-β signaling in oral squamous cell carcinoma cell lines by sparing Smad4 involvement. Nevertheless, the biological role of miR-146a-5p in BC remains unknown.
In the present study, we first examined the expression of miR-146a-5p and interleukin-1 receptor-associated kinase 1 (IRAK1). Our results revealed that miR-146a-5p was markedly downregulated and IRAK1 was significantly upregulated in BC tissues and cell lines. We further conducted functional studies to elucidate the role of miR-146a-5p in BC. Results revealed that miR-146a-5p overexpression inhibited BC cell proliferation, colony formation, migration, and invasion. The above findings suggested the potential role of miR-216a as a tumor suppressor in BC.
To verify the potential molecular mechanisms by which miR-146a-5p exerts antitumor effects in BC, we performed bioinformatics analysis using TargetScan and miRanda algorithms. The analysis predicted IRAK1 as a direct target of miR-146a-5p. Subsequently, results of dual luciferase reporter assays supported the above findings and indicated that IRAK1 is a direct target of miR-146a-5p. IRAK1 is a downstream factor in the toll-like receptor (TLR) signaling, and IRAK1 expression is known to be significantly upregulated in many human cancers, including lung cancer (17), hepatocellular carcinoma (18), endometrial carcinoma (19), papillary thyroid carcinoma (20), and BC (21). Abnormal IRAK1 expression is known to be closely correlated with tumorigenesis and metastasis. A previous study demonstrated that IRAK1 is overexpressed in BC and was found to promote aggressive cell growth, metastasis, and development of resistance to paclitaxel treatment (21). Si et al (22) found that miR-146a-5p regulates proliferation and metastasis of triple-negative BC (TNBC) cells by regulating SOX5. First, our analysis identified IRAK1 as a novel miR-146a-5p target. Results of RT-PCR and western blot analysis revealed that IRAK1 expression was considerably downregulated in BC cells transfected with miR-146a-5p mimics when compared to the controls. In addition, IRAK1 expression was found to be markedly upregulated in BC tissues and inversely correlated with miR-146a-5p expression. In addition, we rescued IRAK1 expression in BC cells transfected with miR-146a-5p mimic. Restoring IRAK1 expression was demonstrated to alleviate the inhibitory effects of miR-146a-5p on the proliferation, migration, and invasion of BC cells. The above-mentioned results indicated that miR-146a-5p acts as a tumor suppressor partially by directly targeting IRAK1.
In summary, miR-146a-5p is downregulated in BC tissues and cell lines and plays a tumor suppressor role by directly targeting IRAK1. Our current findings provided new insights into the molecular mechanism underlying BC occurrence and progression. Therefore, miR-146a-5p can be considered as a novel therapeutic target for the treatment of BC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JPL and LFD designed and performed the experiments. FFC contributed to data analysis. YFF enrolled the patients and measured the RNA levels in the clinical samples. JPL conceived and initiated the present study, and wrote the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Women's Hospital, Zhejiang University School of Medicine (Zhejiang, China). All patients enrolled in the present study provided written informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liang Z, Xi Y. MicroRNAs mediate therapeutic and preventive effects of natural agents in breast cancer. Chin J Nat Med. 2016;14:881–887. doi: 10.1016/S1875-5364(17)30012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernyi VS, Tarasova PV, Kozlov VV, Saik OV, Kushlinskii NE, Gulyaeva LF. Search of MicroRNAs regulating the receptor status of breast cancer in silico and experimental confirmation of their expression in tumors. Bull Exp Biol Med. 2017;163:655–659. doi: 10.1007/s10517-017-3872-1. [DOI] [PubMed] [Google Scholar]
- 3.Lü L, Mao X, Shi P, He B, Xu K, Zhang S, Wang J. MicroRNAs in the prognosis of triple-negative breast cancer: A systematic review and meta-analysis. Medicine. 2017;96:e7085. doi: 10.1097/MD.0000000000007085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sempere LF, Keto J, Fabbri M. Exosomal MicroRNAs in breast cancer towards diagnostic and therapeutic applications. Cancers. 2017;9(pii):E71. doi: 10.3390/cancers9070071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li D, Wang H, Song H, Xu H, Zhao B, Wu C, Hu J, Wu T, Xie D, Zhao J, et al. The microRNAs miR-200b-3p and miR-429-5p target the LIMK1/CFL1 pathway to inhibit growth and motility of breast cancer cells. Oncotarget. 2017;8:85276–85289. doi: 10.18632/oncotarget.19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mjelle R, Sellæg K, Sætrom P, Thommesen L, Sjursen W, Hofsli E. Identification of metastasis-associated microRNAs in serum from rectal cancer patients. Oncotarget. 2017;8:90077–90089. doi: 10.18632/oncotarget.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awasthi R, Rathbone MJ, Hansbro PM, Bebawy M, Dua K. Therapeutic prospects of microRNAs in cancer treatment through nanotechnology. Drug Deliv Transl Res. 2018;8:97–110. doi: 10.1007/s13346-017-0440-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang YN, Chen ZH, Chen WC. Novel circulating microRNAs expression profile in colon cancer: A pilot study. Eur J Med Res. 2017;22:51. doi: 10.1186/s40001-017-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pajic M, Froio D, Daly S, Doculara L, Millar E, Graham PH, Drury A, Steinmann A, de Bock CE, Boulghourjian A, et al. miR-139-5p modulates radiotherapy resistance in breast cancer by repressing multiple gene networks of DNA repair and ROS defense. Cancer Res. 2018;78:501–515. doi: 10.1158/0008-5472.CAN-16-3105. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Lai Y, Ma J, Liu Y, Bi J, Zhang L, Chen L, Yao C, Lv W, Chang G, et al. miR-17-5p suppresses cell proliferation and invasion by targeting ETV1 in triple-negative breast cancer. BMC Cancer. 2017;17:745. doi: 10.1186/s12885-017-3674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuang C, Yuan Y, Song T, Wang H, Huang L, Luo X, He H, Huo L, Zhou H, Wang N, et al. miR-219a-5p inhibits breast cancer cell migration and epithelial-mesenchymal transition by targeting myocardin-related transcription factor A. Acta Biochim Biophys Sin. 2017;49:1112–1121. doi: 10.1093/abbs/gmx114. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Wee ZN, Yatim SM, Kohlabauer VK, Feng M, Goh JY, Bao Y, Lee PL, Zhang S, Wang PP, Lim E, et al. Corrigendum: IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat Commun. 2015;6:10054. doi: 10.1038/ncomms9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Huang Y, Niu X, Tao T, Jiang L, Tong N, Chen S, Liu N, Zhu W, Chen M. Hsa-miR-146a-5p modulates androgen-independent prostate cancer cells apoptosis by targeting ROCK1. Prostate. 2015;75:1896–1903. doi: 10.1002/pros.23068. [DOI] [PubMed] [Google Scholar]
- 15.Yuwen DL, Sheng BB, Liu J, Wenyu W, Shu YQ. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017;21:2650–2658. [PubMed] [Google Scholar]
- 16.Min SK, Jung SY, Kang HK, Park SA, Lee JH, Kim MJ, Min BM. Functional diversity of miR-146a-5p and TRAF6 in normal and oral cancer cells. Int J Oncol. 2017;51:1541–1552. doi: 10.3892/ijo.2017.4124. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Dang Y, Li P, Rong M, Chen G. Expression of IRAK1 in lung cancer tissues and its clinicopathological significance: A microarray study. Int J Clin Exp Pathol. 2014;7:8096–8104. [PMC free article] [PubMed] [Google Scholar]
- 18.Ye ZH, Gao L, Wen DY, He Y, Pang YY, Chen G. Diagnostic and prognostic roles of IRAK1 in hepatocellular carcinoma tissues: An analysis of immunohistochemistry and RNA-sequencing data from the cancer genome atlas. Onco Targets Ther. 2017;10:1711–1723. doi: 10.2147/OTT.S132120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Wang Y, Duan X, Wang Y, Zhang Z. Interleukin-1 receptor-associated kinase 1 correlates with metastasis and invasion in endometrial carcinoma. J Cell Biochem. 2018;119:2545–2555. doi: 10.1002/jcb.26416. [DOI] [PubMed] [Google Scholar]
- 20.Chou CK, Chi SY, Huang CH, Chou FF, Huang CC, Liu RT, Kang HY. IRAK1, a target of miR-146b, reduces cell aggressiveness of human papillary thyroid carcinoma. J Clin Endocrinol Metab. 2016;101:4357–4366. doi: 10.1210/jc.2016-2276. [DOI] [PubMed] [Google Scholar]
- 21.Wee ZN, Yatim SM, Kohlbauer VK, Feng M, Goh JY, Bao Y, Lee PL, Zhang S, Wang PP, Lim E, et al. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat Commun. 2015;6:8746. doi: 10.1038/ncomms9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Si C, Yu Q, Yao Y. Effect of miR-146a-5p on proliferation and metastasis of triple-negative breast cancer via regulation of SOX5. Exp Ther Med. 2018;15:4515–4521. doi: 10.3892/etm.2018.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.