Abstract
We previously reported that the dissected pancreatic tissue margin (DPM) and the preoperative serum level of carbohydrate antigen 19-9 (preCA19-9) were independent prognostic factors in pancreatic ductal adenocarcinoma (PDAC). In the current study, the prognostic relevance of these factors, including their molecular associations, were validated. A total of 161 patients with PDAC underwent a pancreatectomy between 1986 and 2013, and a multivariate Cox proportional hazards model and a propensity score-based model validated the prognostic importance of DPM. The prognostic factors were compared with the mutation profiles of the K-ras and TP53 genes. Univariate prognostic analysis of disease-specific survival (DSS) demonstrated that DPM (P<0.0001), preCA19-9 (P<0.0001) and Union for International Cancer Control (UICC) stage (P<0.0001), were all significantly associated with poor outcome in PDAC. A multivariate Cox proportional hazards model confirmed that preCA19-9 (P=0.0002) and DPM (P=0.0002) remained as prognostic factors independent of UICC stage (P=0.0015). The combination of preCA19-9 and DPM to predict prognosis could accurately identify the long-term survivors of PDAC (70% 5-year DSS), and a multivariate logistic regression model identified that DPM was the most effective predictor of mortality. The prognostic relevance of DPM was also confirmed (P=0.0008) through propensity score-based background adjustment of patient bias. K-ras gene mutation was significantly associated with DPM (P=0.0002), and DPM-positive patients demonstrated recurrence of distant metastasis in 67% of cases. Therefore, DPM is a critical prognostic indicator in PDAC. In combination with preCA19-9, DPM may be useful to identify long-term survivors of PDAC. Furthermore, to the best of our knowledge, the current study was the first to discover that DPM can represent a poor prognosis based putatively on its association with the K-ras gene mutation.
Keywords: carbohydrate antigen 19-9, dissected peripancreatic tissue margin, prognostic factor, K-ras
Introduction
Pancreatic cancer is the twelfth most common malignancy, with 337,872 cases reported in 2012, and the seventh leading cause of cancer-associated mortality, with 330,391 mortalities reported in 2012, worldwide (1). Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies. PDAC is the fourth leading cause of cancer-associated mortality in the USA (2); without any substantial improvement in curative therapies, it is anticipated to be the second leading cause of cancer-associated mortality by 2030 (3). Surgical resection is currently the only option to potentially achieve long-term survival (4). However, the 5-year survival rate is only ~10% following surgical therapy (5); therefore, postoperative adjuvant chemotherapy is being developed to improve prognosis. In the USA in 1985, the Gastrointestinal Tumor Study Group reported the use of postoperative adjuvant therapy for the first time (6). Later, in 2007, the CONKO-001 trial evaluated the use of gemcitabine (7). The effectiveness of postoperative S-1 adjuvant chemotherapy was revealed in Japan in 2016 (8). Nevertheless, the 5-year survival rate following surgery and adjuvant chemotherapy remains at only ~20% (5,9). Due to postoperative quality of life degradation and other factors, there are a number of problems that result in insufficient adjuvant chemotherapy. Therefore, the importance of neoadjuvant therapy has recently been the focus of numerous studies (10). Treatment options for PDAC will diversify in the future and it will be necessary to make the optimal choice of therapy.
PDAC is postulated to lead to systemic metastasis, which is accompanied by an accumulation of various gene mutations (11,12). However, which gene mutations are associated with clinicopathological prognostic factors is not clear. By identifying gene mutations that are associated with prognostic factors, there is a possibility that the range of treatment options will be increased. We previously reported that the preoperative carbohydrate antigen 19-9 (preCA19-9) level and the dissected peripancreatic tissue margin (DPM) were independent prognostic factors in PDAC (13). To date, it has repeatedly been reported that a positive margin and CA19-9 are prognostic factors in a univariate manner (14–16). However, each previous study included different clinicopathological factors and multivariate prognostic analysis revealed different sets of independent prognostic factors. To the best of our knowledge, our previous study was the first to report that the two factors can be simultaneously evaluated as independent prognostic factors (13). Analysis that combines these important prognostic factors may be useful in clinical management. However, our previous study had limitations, including a small sample size and follow-up period.
The aims of the current study were to validate the prognostic relevance of preCA19-9 and DPM as independent prognostic factors in primary PDAC and to clarify the associations between predictive factors and gene mutation status.
Materials and methods
Registration of patients
From January 1986 to December 2013, 161 patients (median, 65; range 38–88), including 85 male and 76 female patients, with histologically confirmed PDAC underwent a pancreatectomy with D0-D3 lymph node dissection at the Department of Surgery, Kitasato University Hospital (Kanagawa, Japan). Patients with histological variants, including mucinous cystic adenocarcinoma, intraductal papillary adenocarcinoma, acinar cell carcinoma and endocrine carcinoma were excluded from the study. Preoperative workup included an ultrasonography, computed tomography, endoscopic retrograde pancreatography, endoscopic ultrasonography and magnetic resonance cholangiopancreatography to evaluate primary and metastatic tumor sites.
The preoperative serum CA19-9 levels were investigated immediately prior to surgery, avoiding the effects of obstructive jaundice and/or inflammation of the biliary duct. The recommended upper limit of a normal CA19-9 level is 37 U/ml, when CA19-9 levels are determined and defined from the standard deviations of healthy individuals. CA19-9 levels were determined using a chemiluminescent enzyme immunoassay kit (LUMIPLSE G; cat. no., CA19-9-N) obtained from Fujirebio US, Inc. (Malvern, PA, USA).
The surgical resection tissues were obtained from pancreaticoduodenectomy (n=39), pylorus-preserving pancreaticoduodenectomy (n=74), distal pancreatectomy (n=43) and total pancreatectomy (n=5). Partial resection of the portal vein was performed if the surgeon observed tumor invasion of the portal vein. Intraoperative pathologic assessment of the proximal or distal pancreatic margins was performed using frozen-tissue sections. If the pancreatic margin was positive for cancerous cells, further resection of the pancreas was performed. The present study was approved by the Institutional Review Board for Observation and Epidemiological Study, Kitasato University Medical Ethics Organization (approval no. B18-017; Kanagawa, Japan). All patients agreed to the use of their samples in scientific research and written informed consent was obtained from all patients.
Pathological investigation
All histological and other clinicopathological factors were judged independently and blindly by histopathologists, and all histopathologic factors from the Japanese classification system (JCS) version 6 (17) were obtained from medical records. In the current study, stage was determined according to the 6th edition of the International Union Against Cancer (UICC) tumor-node-metastasis classification (18). Assessment of cancer infiltration at the surgical margin was evaluated according to the JCS, as DPM, pancreatic cut end margin or bile duct cut end margin. According to the National Comprehensive Cancer Network guideline (19), the surgical margin was defined as a superior mesenteric artery (SMA) margin, posterior margin, portal vein groove margin, portal vein margin, pancreatic margin or pancreatic surface and bile duct margin. Details of the DPM included the SMA margin, posterior margin and portal vein groove margin. Surgical margin-positive was defined as tumor cells present in the dissection surface, as judged by histopathologists. When requested by a pathologist, the gene mutation was investigated using non-radioisotopic single-strand conformation polymorphism (SSCP) from each DNA sample (n=97).
DNA examination and search for the mutated K-ras gene using SSCP
For simultaneous DNA analysis, the previously described protocols were conducted (20,21). Briefly, this consisted of five steps. Firstly, to prevent cross-contamination, small sections of fresh solid tissue were sampled by scraping with disposable bamboo combs, which are 3×3×120 mm rods made of bamboo with a spatula like ends. Secondly, one-step DNA extraction with lysis buffer containing proteinase K, Nonidet P-40 and Tween 20 was performed. Subsequently. polymerase chain reaction (PCR) was performed with each gene primer presented in Table I and the conditions presented in Table II. Next, SSCP analysis with polyacrylamide gels was performed, and detection was achieved by silver staining. In this analysis, mutated bands were evident at 1:64 dilutions of the mutated alleles.
Table I.
Primer sequences used for polymerase chain reaction.
| Gene | Exon | Forward sequence | Reverse sequence | Amplified fragment length, bp | Codons |
|---|---|---|---|---|---|
| K-ras | 1 | 5′-GACTGAATATAACTTGTGG-3′ | 5′-GCTATTGTTGGATCAATATTC-3′ | 108 | 2–37 |
| 2 | 5′-GATTCCTACAGGAAGCAAGT-3′ | 5′-TAATGGTGAATATCTTC-3′ | 185 | 38–97 | |
| TP53 | 5 | 5′-TTCCTCTTCCTGCAGTACTC-3′ | 5′-GCCCCAGCTGCTCACCATCGCTA-39 | 214 | 125–186 |
| 6 | 5′-GCCTCTGATTCCTCACTGATTG-3′ | 5′-AGTTGCAAACCAGACCTCAG-3′ | 157 | 187–224 | |
| 7 | 5′-CCTCATCTTGGGCCTGTGTTATC-3′ | 5′-CAAGTGGCTCCTGACCTGGAGTC-3′ | 154 | 225–261 | |
| 8 | 5′-CCTATCCTGAGTAGTGGTAA-3′ | 5′-GTCCTGCTTGCTTACCTCGC-3′ | 166 | 262–306 | |
| 9 | 5′-GCCTCTTTCCTAGCACTGCC-3′ | 5′-CCAAGACTTAGTACCTGAAG-3′ | 101 | 307–331 |
Table II.
Polymerase chain reaction cycle conditions.
| Fresh samples | |
|---|---|
| Conditions | No. of cycles |
| 94°C 3 min | 1 |
| 52°C for 1 min, 72°C for 1 min, 94°C for 30 sec | 2 |
| 52°C for 45 sec, 72°C for 30 sec, 94°C for 20 sec | 35 |
| 52°C for 45 sec, 72°C for 3 min | 1 |
Follow-up and postoperative therapy
A total of 108 patients (67%) received empirical adjuvant therapy; this consisted of 105 patients who received chemotherapy and 3 patients who received radiotherapy. Another 23 patients (14%) received therapy for remnant tumor; specifically 22 patients who received chemotherapy and 1 patient who received radiotherapy. A number of chemotherapy regimens consisted of 5-fluorouracil (5-FU)-based chemotherapy, including 5-FU only (n=3), 5-FU/cisplatin (n=9) or 5-FU/Adriamycin/mitomycin-C/nimustine hydrochloride (n=2) by venous, portal or arterial infusion. Other chemotherapy regimens included tegafur/uracil (n=11), 5′-deoxy-5-fluorouridine (n=3) or TS-1 (n=3) as oral therapy, or mitomycin-C (n=7). In addition to gemcitabine (n=83) or gemcitabine+TS-1 (n=6) by venous or arterial infusion. Radiotherapy consisted of 1.8 Gy per day to a total dose of 50 Gy. Routine follow-up consisted of physical examination, laboratory studies and computed tomography imaging at 3- to 4-month intervals for the first 2 years, at 6-month intervals for years 3 to 5, and followed by annual follow-ups from thereon. Only the first sites of recurrence were recorded.
Statistical analysis
Continuous variables were compared using the Student's t-test and nominal variables were compared using Pearson's chi-square test. The Kaplan-Meier method was used for disease-specific survival (DSS) analysis and the difference in survival rate was assessed by log-rank test (22). DSS was measured from the date of surgery to the date of mortality or last follow-up. Mortality associated with causes other than PDAC were not counted in this measurement. The variables that demonstrated prognostic potential suggested by univariate analysis (P<0.05) were subjected to multivariate analysis with a Cox proportional hazards model. Propensity scores were matched using a caliper width of 0.2 multiplied by the standard deviation of values that was calculated by a logistic regression analysis. A receiver operating characteristic (ROC) curve was generated using JMP software v.11.0 (SAS institute, Cary, NC, USA) and the area under the curve (AUC) was used to optimize the best cut-off value. The associations between K-ras gene mutation and prognostic factors were examined with the Student's t-test. P<0.05 was considered to indicate a statistically significant difference. All statistical analysis was performed using JMP software v.11.0.
Results
Patients' characteristics and univariate prognostic analysis in PDAC
The first aim of the current study was to validate the prognostic relevance of preCA19-9 and DPM, which have previously been identified as independent prognostic factors in PDAC (12). The characteristics of 161 patients with PDAC and the univariate prognostic factors are summarized in Table III. Significant prognostic factors included lymphatic permeation factor (ly; P=0.036), vascular permeation factor (v; P=0.0087), tumor differentiation (P=0.0037), intrapancreatic nerve invasion factor (ne; P=0.00025), retropancreatic tissue invasion factor (RP; P=0.0053), portal venous system invasion factor (PV; P=0.0133), extrapancreatic nerve plexus invasion factor (PL; P=0.0004), arterial system invasion factor (A; P=0.0019), preCA19-9 level (P<0.0001), DPM (P<0.0001) and UICC 6th stage (P<0.0001). Arterial invasion was composed of pT4 (celiac artery or superior mesenteric artery; n=2) and non-pT4 (splenic artery; n=3). By increasing the number of patients from the previous pilot study (13), several pathological factors, which are not UICC staging factors, including ly, v, ne, RP, PV and A, were newly identified as univariate prognostic factors in PDAC.
Table III.
Univariate and multivariate prognostic analysis for 161 patients with PDAC who underwent pancreatectomy.
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variable | No. | RR | 95% CI | P-valuea | RR | 95% CI | P-valuea |
| Age, years | |||||||
| <65/>65 | 79/82 | 0.8 | 0.6–1.2 | 0.3150 | |||
| Sex | |||||||
| Male/female | 85/76 | 1.2 | 0.8–1.8 | 0.2628 | |||
| Lymphatic invasion | |||||||
| Absence/presence | 22/139 | 1.8 | 1.1–3.5 | 0.0360 | 1.5 | 0.81–2.95 | 0.2109 |
| Venous invasion | |||||||
| Absence/presence | 13/148 | 4 | 1.5–16.4 | 0.0087 | 1.6 | 0.56–6.91 | 0.4082 |
| Tumor differentiation | |||||||
| Well/other (moderate, poorly) | 137/24 | 2.1 | 1.2–3.4 | 0.0037 | 1.8 | 0.32–1.01 | 0.0563 |
| Intrapancreatic nerve invasionth | |||||||
| Absence/presence | 17/144 | 3.6 | 1.6–10.2 | 0.0025 | 2 | 0.87–6.1 | 0.1083 |
| Retropancreatic tissue invasion | |||||||
| Absence/presence | 62/99 | 1.8 | 1.2–2.7 | 0.0053 | 1.2 | 0.70–1.94 | 0.5635 |
| Portal venous system invasion | |||||||
| Absence/presence | 131/30 | 1.8 | 1.1–2.9 | 0.0133 | 2 | 1.15–3.22 | 0.0143 |
| Extrapancreatic nerve plexus invasion | |||||||
| Absence/presence | 126/35 | 2.1 | 1.4–3.2 | 0.0004 | 1.5 | 1.15–3.22 | 0.1671 |
| Arterial system invasion | |||||||
| Absence/presence | 156/5 | 3.8 | 1.3–8.6 | 0.0019 | 2.4 | 0.78–6.32 | 0.1173 |
| PreCA19-9 level, U/ml | |||||||
| <37/>37 | 39/122 | 3.5 | 2.1–6.4 | <0.0001 | 2.8 | 1.58–5.46 | 0.0003 |
| Dissected pancreatic tissue margin | |||||||
| Negative/positive | 101/60 | 2.9 | 2.0–4.3 | <0.0001 | 2.5 | 1.58–3.99 | <0.0001 |
| Stageb | |||||||
| 0–I | 9 | Reference | <0.0001 | Reference | 0.0032 | ||
| II | 122 | 9.6 | 2.1–169 | 4.5 | 0.92–80.4 | ||
| III | 7 | 56.7 | 9.9–1067.6 | 13.5 | 2.0–268 | ||
| IV | 23 | 30.5 | 6.3–547.8 | 9.4 | 1.81–173 | ||
| PreCA19-9 and DPM combination | |||||||
| A; Over 37 U/ml and Positive | 51 | Reference | <0.0001 | ||||
| B; Over 37 U/ml and Negative | 71 | 2.6 | 1.7–3.9 | ||||
| C; <37 U/ml and Positive | 9 | 2.6 | 1.2–6.3 | ||||
| D; <37 U/ml and Negative | 30 | 10.2 | 5.0–23.8 | ||||
According to log-rank test.
According to the 6th edition of the International Union Against Cancer staging system. RR, relative risk; CI, confidence interval; preCA19-9, preoperative carbohydrate antigen 19-9; DPM, dissected peripancreatic tissue margin.
Multivariate Cox proportional hazards model in PDAC
The ten variables that exhibited prognostic potential identified by univariate prognostic analysis, including ly, v, tumor differentiation, ne, RP, PV, PL, A, preCA19-9, DPM and UICC 6th stage, were subjected to multivariate analysis. This analysis revealed that preCA19-9 [P=0.0002, relative risk (RR)=2.8], DPM positive (P=0.0002, RR=2.4), PV (P=0.01, RR=2.0) and A (P=0.035, RR=3.3) were the remaining prognostic factors independent of UICC stage (P=0.0015; Table III).
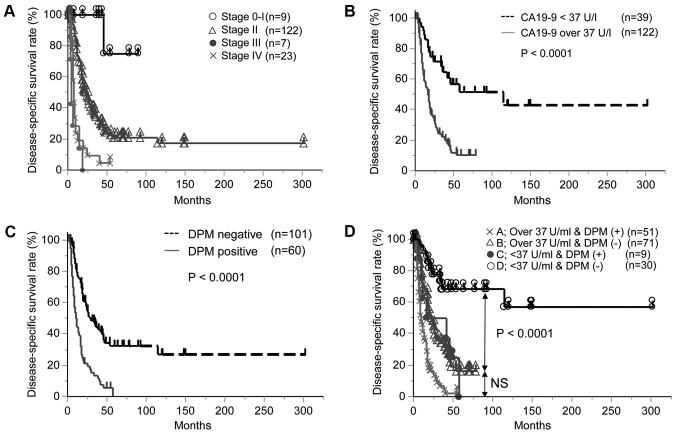
Similar to previous studies (23,24), preCA19-9 and DPM were identified as independent prognostic factors. The Kaplan-Meier curves of each factor are presented in Fig. 1A for UICC stage, Fig. 1B for PreCA19-9 and Fig. 1C for DPM. Notably, all DPM-positive patients succumbed to the disease within 5 years, while a number of patients with preCA19-9 levels >37 U/ml were alive with no recurrence after five years.
Figure 1.
Disease-specific survival rates of pancreatic ductal adenocarcinoma. (A) Kaplan-Meier curve of Union for International Cancer Control stage. (B) Kaplan-Meier curve of preCA19-9 level. (C) Kaplan-Meier curve of DPM status. (D) Kaplan-Meier curve according to combinations of preCA19-9 level and DPM status. Group A, preCA19 >37 U/ml and DPM-positive; group B, preCA19-9 >37 U/ml and DPM-negative; group C, preCA19-9 <37 U/ml and DPM-positive; and group D, preCA19-9 <37 U/ml and DPM-negative. preCA19-9, preoperative carbohydrate antigen 19-9; DPM, dissected peripancreatic tissue margin.
In addition, survival outcomes for the combination of preCA19-9 and DPM are presented in Table III and Fig. 1D. The prognosis of patients with PDAC was sub-classified into four groups (A, B, C and D) according to preCA19-9 and DPM (group A, preCA19-9 >37 U/ml and DPM-positive; group B, preCA19-9 >37 U/ml and DPM-negative; group C, preCA19-9 <37 U/ml and DPM-positive; and group D, preCA19-9 <37 U/ml and DPM-negative). Of note, group D demonstrated the best clinical outcome and included long-term survivors (10/30) of PDAC (Fig. 1D).
Prognostic relevance of DPM-positive status in PDAC following propensity score matching
A DPM-positive status may be the best indicator of highly aggressive tumor characteristics in PDAC; therefore, a risk model for DPM prediction was generated by logistic regression analysis. The clinicopathological factors analyzed were as follows: age, gender, ly, v, ne, RP, PV, A, PL, UICC stage and preCA19-9. Using this prediction model, an ROC curve was generated and presented in Fig. 2A (AUC=0.80) and the propensity score (PS) to predict DPM was calculated. The density distribution of PS was analyzed between DPM-positive and DPM-negative patients, and a notable difference in the PS distribution was identified between the groups (Fig. 2B). The matching of PS was performed by logit exchange. The logit exchange scores of the matched cases between both DPM-positive and DPM-negative cases were presented linearly in a scatter plot (Fig. 2C). The logit exchange scores of the matched cases between DPM-positive and DPM-negative were distributed in Kernel density estimation differently from the unmatched cases (Fig. 2D, left panel), as compared with the differential ranges prior to matching (Fig. 2D, right panel). Following matching, no significant differences were identified for factors potentially affecting DPM between DPM-positive and DPM-negative cases (Table IV). Notably, the factors identified to be associated with DPM positive were RP and RL, and these factors were corrected by score matching. As a result, DPM was demonstrated as a robust prognostic factor in PDAC (P=0.0008; Fig. 2E).
Figure 2.
Propensity score matching method. (A) Receiver operating characteristic curve generated by multiplex logistic analysis demonstrated an AUC of 0.80. (B) Density distribution of propensity score. (C) Logit exchange scores of both DPM-positive and DPM-negative cases were aligned linearly in scatter plot. (D) Logit exchange scores of matched DPM-positive and DPM-negative cases were distributed in the same range in Kernel density estimation. (E) Kaplan-Meier curve of DPM following propensity score matching. DPM, dissected peripancreatic tissue margin; AUC, area under the curve.
Table IV.
Distribution of potential factors associated with DPM between DPM positive and negative cases prior to and following propensity score matching.
| Prior to matching | Following matching | |||||
|---|---|---|---|---|---|---|
| Variable | DPM positive (n=59) | DPM negative (n=101) | P-value | DPM positive (n=43) | DPM negative (n=43) | P-value |
| Agea | 63.4±9.4 | 64.4±10.3 | 0.5172c | 64.1±9.1 | 64.7±10.4 | 0.81c |
| Sex | ||||||
| Male/female | 30/29 | 55/46 | 0.6590d | 22/21 | 22/21 | 1.00d |
| PreCA19-9 level, U/ml | ||||||
| <37/>37 | 9/50 | 30/71 | 0.0400d | 8/35 | 10/33 | 0.59d |
| Stageb | ||||||
| 0–I/II/III/IV | 0/42/5/12 | 9/79/2/11 | 0.0097d | 0/33/2/8 | 0/35/2/6 | 0.84d |
| Lymphatic invasion | ||||||
| Absence/presence | 53/6 | 85/16 | 0.3056d | 38/5 | 37/6 | 0.75d |
| Venous invasion | ||||||
| Absence/presence | 56/3 | 91/10 | 0.2664d | 41/2 | 39/4 | 0.39d |
| Intrapancreatic nerve invasion | ||||||
| Absence/presence | 56/3 | 87/14 | 0.00675d | 40/3 | 40/3 | 1.00d |
| Retropancreatic tissue invasion | ||||||
| Absence/presence | 51/8 | 48/53 | <0.0010d | 35/8 | 32/11 | 0.44d |
| Portal venous system invasion | ||||||
| Absence/presence | 12/47 | 18/83 | 0.6939d | 11/32 | 7/36 | 0.28d |
| Extrapancreatic nerve plexus invasion | ||||||
| Absence/presence | 22/37 | 13/88 | 0.0003d | 9/34 | 11/32 | 0.61d |
| Arterial system invasion | ||||||
| Absence/presence | 3/56 | 2/99 | 0.2762d | 2/42 | 1/41 | 0.56d |
Data are presented as the mean ± standard deviation.
According to the 6th edition of the International Union Against Cancer staging system.
According to Student's t-test.
Accroding to Pearson's chi-square test. DPM, dissected pancreatic tissue margin; preCA19-9, preorperative carbohydrate antigen 19-9.
Subsequently, initial recurrent sites were investigated in DPM-positive patients with PDAC. Among the 60 DPM-positive patients, 52 recurrences were identified. Liver metastases were the most prevalent in 21 patients (35%), followed by lymph node metastases in 13 patients (22%), peritoneal dissemination in 5 patients (8%), lung metastasis in 1 patient (35%), nerve plexus of the SMA in 9 patients (15%) and remnant pancreas in 3 patients (5%).
Molecular association of DPM with mutational status of K-ras and p53 genes in PDAC
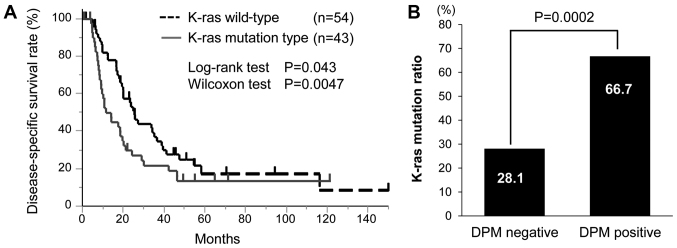
Subsequently, the associations of DPM with the mutational status of K-ras and TP53 genes in PDAC were investigated. For K-ras gene mutation, 96 patients with PDAC were investigated. K-ras gene mutation was identified in 42 patients (44%), among whom 34 patients possessed mutations in exon1 (codon 12 or 13). The Kaplan-Meier curve was generated according to K-ras gene mutation status (P=0.043 by the log-rank test and P=0.0047 by the Wilcoxon test; Fig. 3A). Since DPM has been cited as a poor prognostic factor in the present results, univariate analysis was performed for factors associated with DPM status (Table V). RP (P<0.001), PL (P=0.0003) and K-ras gene mutation status (P=0.0002) were significantly associated with DPM-positive status. Multivariate analysis confirmed that RP (P=0.037), PL (P=0.026) and K-ras gene mutation status (P=0.0004) remained as independent factors of DPM-positive statue. A significant association was identified between DPM and K-ras gene mutation status (Fig. 3B). DPM-positive patients had a K-ras gene mutation in 66.7% (26/39) of cases, while DPM-negative patients exhibited a K-ras gene mutation in 28.1% (16/57) of cases. For the TP53 gene mutation, 67 patients with PDAC were examined and a TP53 gene mutation was identified in 14 (20.9%). Of these, 6 patients were DPM-positive, and no significant difference was identified with regard to DPM status (P=0.9275).
Figure 3.
Associations between K-ras mutation and prognostic factors. (A) Comparison of disease-specific survival between K-ras gene wild-type and mutation. A significant difference in survival was identified using the Wilcoxon test (P=0.0047). (B) The association between DPM status and K-ras gene mutation was evaluated by the Student's t-test (P=0.0002). DPM, dissected peripancreatic tissue margin.
Table V.
Univariate and multivariate prognostic analysis for DPM.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | DPM positive (n=59) | DPM negative (n=101) | P-valuea | Odds ratio | 95% CI | P-valueb |
| Lymphatic invasion | ||||||
| Absence/presence | 53/6 | 85/16 | 0.3148 | |||
| Venous invasion | ||||||
| Absence/presence | 56/3 | 91/10 | 0.2820 | |||
| Intrapancreatic nerve invasion | ||||||
| Absence/presence | 56/3 | 87/14 | 0.0822 | |||
| Retropancreatic tissue invasion | ||||||
| Absence/presence | 51/8 | 48/53 | <0.0010 | 3.1 | 1.1–10.6 | 0.0373 |
| Portal venous system invasion | ||||||
| Absence/presence | 12/47 | 18/83 | 0.6939 | |||
| Extrapancreatic nerve plexus invasion | ||||||
| Absence/presence | 22/37 | 13/88 | 0.0003 | 3.9 | 1.2–14.5 | 0.0264 |
| Arterial system invasion | ||||||
| Absence/presence | 3/56 | 2/99 | 0.2762 | |||
| K-ras mutation | ||||||
| Negative/positive | 13/26 | 41/16 | 0.0002 | 5.2 | 2.07–14.2 | 0.0004 |
According to Pearson's chi-square test.
According to log-rank test. DPM, dissected pancreatic margin; CI, confidence interval.
Discussion
PDAC is one of the most fatal forms of human malignancy and exhibits a poor 5-year survival rate even following curative resection and postoperative adjuvant chemotherapy (5). The present study indicated that long-term survivors following pancreatectomy can be predicted by a combination of clinicopathological factors, including preCA19-9 and DPM, which were prognostic factors independent of the cancer stage. These factors have been repeatedly reported in previous studies, therefore, they have been highly validated. However, to the best of our knowledge, no studies have analyzed a combination of these factors. In the current study, patients negative for both preCA19-9 and DPM (n=30) demonstrated a 5-year DSS of 68.5% following surgery, with 25 of the patients undergoing postoperative adjuvant chemotherapy. This finding recapitulated and validated our previous study (13) and such prognostic information may be useful in the development of optimal treatment strategies for PDAC.
The most established biomarker for PDAC diagnosis and prognosis is preCA19-9 (14,23–28). Notably, preCA19-9 level could provide preoperative information and serve as a preoperative prognostic factor potentially able to affect the choice of treatment strategy, including the administration of neoadjuvant therapy. The current study determined that a pancreatectomy should be considered for patients with a low preCA19-9 level, while those with a high level of preCA19-9 tend to exhibit a poorer prognosis and may require neoadjuvant therapy prior to surgery to improve their survival rate. Previously, preoperative chemoradiation has been demonstrated to be a promising strategy to treat aggressive pancreatic cancer, including borderline resectable pancreatic cancer (10). The present study supports this novel promising therapeutic strategy for patients with PDAC and a high level preCA19-9.
By contrast, since DPM is a pathological factor, it cannot be preoperatively informative and preoperative prediction of future recurrence by DPM status is impossible. However, the present study demonstrated that DPM could accurately predict long-term survivors of PDAC following surgery, in combination with preCA19-9. Notably, all cases of DPM-positive PDAC inevitably succumbed to the disease; therefore, DPM status may indicate the tumor aggressiveness of PDAC and reflect specific molecular features.
To the best of our knowledge, the current study was the first to demonstrate that K-ras gene mutation is significantly associated with DPM-positive status, compared with DPM-negative status. K-ras gene mutation in the resected margin of PDAC has been reported to be a marker representing a poor prognosis (29,30). In the present study, K-ras gene mutation was an independent predictor for DPM-positive status in multivariate analysis. Supporting these results, PDAC clones with mutated K-ras gene have previously been demonstrated to be persistently present in the retroperitoneal margin. K-ras gene mutation has been identified in pancreatic intraepithelial neoplasia, which is a precursor lesion (31). In addition, a previous study that used high-resolution analysis reported >90% of cases have K-ras gene mutation in PDAC tumors (32,33). By contrast, but similar to our studies, conventional methods to investigate K-ras gene mutation have been widely reported. In a recent meta-analysis (34), the frequencies of K-ras gene mutations were reported to range from 47 to 88%. In the present study, the low rate of K-ras gene mutation is considered to be influenced by component rates of a small number of tumor cells mixed with a large number of stromal cells. Using conventional methods, it may be difficult to detect a small number of K-ras gene mutations in tumors, and the frequency of K-ras gene mutation therefore appears lower compared with that observed using high resolution analysis (35). An inducible KrasG12D model elucidated that a precursor lesion with an activated K-ras signal transforms to carcinoma but a precursor lesion with an inactivated K-ras signal undergoes apoptosis (36). These findings may support the hypothesis that mutation of the K-ras gene is associated with tumor aggressiveness and micrometastases, which have the potential to disseminate systemically in DPM-positive patients, as observed in the current study.
The detection rate of K-ras gene mutation in the present study was low. However, previous studies have identified the frequencies of K-ras gene mutation in other cancer types using conventional methods and have repeatedly demonstrated standard values (20,37–40). Therefore, it can be suggested that the present result reflects the conventional mutation rate of PDAC, which may represent dominant clones in the tumor tissues. With regard to positive association of DPM with K-ras gene mutation, validation was required for different patient sets, however the experiments performed in the current study could not be repeated due to technical reasons.
From 2014 to 2017, 96 patients with PDAC underwent a pancreatectomy at Kitasato University Hospital and 6 cases were examined for the K-ras gene mutation. Among the 6 patients with PDAC, K-ras gene mutation was identified in 2 (33%); patients with DPM-positive PDAC demonstrated a K-ras gene mutation in 50% (1/2) of cases, while DPM-negative patients exhibited a K-ras gene mutation in 25% (1/4) of cases. This result was consistent with the aforementioned results of the current study despite the small patient number. In the future, learning sets should be co-analyzed. Finally, as the recruitment period of the current study was long, numerous operators were involved in the study. Therefore, a number of factors could cause bias and affect the determination of DPM status. Periodical validation is required to confirm the results of the current study.
In conclusion, the present study validated and reiterated the prognostic relevance of DPM and preCA19-9 in patients with primary PDAC. This finding may be useful in the development of a novel treatment strategy for patients with PDAC and a poor prognosis. In addition, the current study revealed an association of DPM status with K-ras gene mutation in PDAC. This finding may facilitate the molecular understanding of PDAC and assist the development of molecular targeted therapy. Similar to other cancer types associated with a poor prognosis, multidisciplinary treatment, including preoperative treatment and aggressive surgery, and postoperative treatment, including molecular targeted therapy, should be adopted to further improve the prognosis of patients with PDAC. Thus, the present findings are important.
Acknowledgements
The authors would like to thank Miss Tomomi Miyake (Kitasato University School of Medicine) for her technical assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
NN, YK and KYa designed the study and wrote the initial draft of the manuscript. NN, YK and KYa analysed and interpreted the data and assisted in the preparation of the manuscript. HK, HU, KYo, TT, SI, KI, HT, TK, TY, MS, YK and MW contributed to the collection and interpretation of the data and critically reviewed the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board for Observation and Epidemiological Study, Kitasato University Medical Ethics Organization (approval no. B18-017). All patients agreed to the use of their samples in scientific research. And, written informed consents were obtained from all patients. This study only used these samples. All the data/samples were used anonymously.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.International Agency for Research on Cancer WHO. GLOBOCAN2012: Estimated Cancer Incidence, Mortality and Prevalence. 2012 [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2014. American Cancer Society. 2014 [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA. 2010;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 6.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 7.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.23.2582-a. [DOI] [PubMed] [Google Scholar]
- 8.Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01) Lancet. 2016;388:248–257. doi: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/S0140-6736(01)06651-X. [DOI] [PubMed] [Google Scholar]
- 10.Kim EJ, Ben-Josef E, Herman JM, Bekaii-Saab T, Dawson LA, Griffith KA, Francis IR, Greenson JK, Simeone DM, Lawrence TS, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer. 2013;119:2692–2700. doi: 10.1002/cncr.28117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 12.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waraya M, Yamashita K, Katagiri H, Ishii K, Takahashi Y, Furuta K, Watanabe M. Preoperative serum CA19-9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Ann Surg Oncol. 2009;16:1231–1240. doi: 10.1245/s10434-009-0415-7. [DOI] [PubMed] [Google Scholar]
- 14.Mirkin KA, Hollenbeak CS, Wong J. Prognostic impact of carbohydrate antigen 19-9 level at diagnosis in resected stage I–III pancreatic adenocarcinoma: A U.S. population study. J Gastrointest Oncol. 2017;8:778–788. doi: 10.21037/jgo.2017.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allema JH, Reinders ME, van Gulik TM, Koelemay MJ, Van Leeuwen DJ, de Wit LT, Gouma DJ, Obertop H. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer. 1995;75:2069–2076. doi: 10.1002/1097-0142(19950415)75:8<2069::AID-CNCR2820750807>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Wenger FA, Peter F, Zieren J, Steiert A, Jacobi CA, Müller JM. Prognosis factors in carcinoma of the head of the pancreas. Dig Surg. 2000;17:29–35. doi: 10.1159/000018797. [DOI] [PubMed] [Google Scholar]
- 17.Classification of pancreatic carcinoma. Tokyo: Kanahara; 2011. Japan Pancreas Society. [Google Scholar]
- 18.Sobin LH, Wittekind C, editors. 6th. New York: Wiley and Liss; 2002. International Union Againt Cancer (UICC): TNM classification of malignant tumors. [Google Scholar]
- 19.National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Pancreatic Adenocarcinoma. 2016 Version 2. [Google Scholar]
- 20.Yamashita K, Tatebayashi T, Shinoda H, Okayasu I. Simplified rapid non-radioactive PCR-SSCP method applied to K-ras mutation analysis. Pathol Int. 1996;46:801–804. doi: 10.1111/j.1440-1827.1996.tb03553.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita K, Yoshida T, Shinoda H, Okayasu I. Novel method for simultaneous analysis of p53 and K-ras mutations and p53 protein expression in single histologic sections. Arch Pathol Lab Med. 2001;125:347–352. doi: 10.5858/2001-125-0347-NMFSAO. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 23.Piagnerelli R, Marrelli D, Roviello G, Ferrara F, Di Mare G, Voglino C, Petrioli R, Marini M, Macchiarelli R, Roviello F. Clinical value and impact on prognosis of peri-operative CA 19-9 serum levels in stage I and II adenocarcinoma of the pancreas. Tumour Biol. 2016;37:1959–1966. doi: 10.1007/s13277-015-3986-x. [DOI] [PubMed] [Google Scholar]
- 24.Chan A, Prassas I, Dimitromanolakis A, Brand RE, Serra S, Diamandis EP, Blasutig IM. Validation of biomarkers that complement CA19.9 in detecting early pancreatic cancer. Clin Cancer Res. 2014;20:5787–5795. doi: 10.1158/1078-0432.CCR-14-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Nakashima A, Sakabe R, Shigemoto N, Kato Y, et al. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2321–2329. doi: 10.1245/s10434-010-1033-0. [DOI] [PubMed] [Google Scholar]
- 26.Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, Balakrishnan L, Dwivedi SB, Telikicherla D, Selvan LD, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger AC, Garcia M, Jr, Hoffman JP, Regine WF, Abrams RA, Safran H, Konski A, Benson AB III, MacDonald J, Willett CG. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: A prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918–5922. doi: 10.1200/JCO.2008.18.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, Bajetta E, Saletti P, Figer A, Scheithauer W, Herrmann R. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132–138. doi: 10.1016/S1470-2045(08)70001-9. [DOI] [PubMed] [Google Scholar]
- 29.Ohigashi H, Ishikawa O, Sasaki Y, Yamada T, Furukawa H, Imaoka S, Kasugai T, Ishiguro S, Ueda K, Miyoshi Y, Nakamura Y. K-ras point mutation in the nerve plexuses around the superior mesenteric artery in resectable adenocarcinoma of the pancreatic head: Distribution pattern and related factors. Arch Surg. 2000;135:1450–1455. doi: 10.1001/archsurg.135.12.1450. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Reber HA, Dry SM, Elashoff D, Chen SL, Umetani N, Kitago M, Hines OJ, Kazanjian KK, Hiramatsu S, et al. Unfavourable prognosis associated with K-ras gene mutation in pancreatic cancer surgical margins. Gut. 2006;55:1598–1605. doi: 10.1136/gut.2005.083063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, Moses HL. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao LY, Zhang LF, Xiu DR, Yuan CH, Ma ZL, Jiang B. Prognostic significance of K-ras mutations in pancreatic cancer: A meta-analysis. World J Surg Oncol. 2016;14:146. doi: 10.1186/s12957-016-0888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knijn N, Mekenkamp LJ, Klomp M, Vink-Börger ME, Tol J, Teerenstra S, Meijer JW, Tebar M, Riemersma S, van Krieken JH, et al. KRAS mutation analysis: A comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104:1020–1026. doi: 10.1038/bjc.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onozato W, Yamashita K, Yamashita K, Kuba T, Katoh H, Nakamura T, Sato T, Ihara A, Okayasu I, Watanabe M. Genetic alterations of K-ras may reflect prognosis in stage III colon cancer patients below 60 years of age. J Surg Oncol. 2011;103:25–33. doi: 10.1002/jso.21710. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita K, Kuba T, Shinoda H, Takahashi E, Okayasu I. Detection of K-ras point mutations in the supernatants of peritoneal and pleural effusions for diagnosis complementary to cytologic examination. Am J Clin Pathol. 1998;109:704–711. doi: 10.1093/ajcp/109.6.704. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita K, Kida Y, Shinoda H, Kida M, Okayasu I. K-ras point mutations in the supernatants of pancreatic juice and bile are reliable for diagnosis of pancreas and biliary tract carcinomas complementary to cytologic examination. Jpn J Cancer Res. 1999;90:240–248. doi: 10.1111/j.1349-7006.1999.tb00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamata H, Yamashita K, Kojo K, Ushiku H, Ooki A, Watanabe M. Discrepancies between the K-ras mutational status of primary colorectal cancers and corresponding liver metastases are found in codon 13. Genomics. 2015;106:71–75. doi: 10.1016/j.ygeno.2015.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.