Abstract
Cervical cancer is one of the most prevalent female cancer types in developing countries. ThinPrep cytological test (TCT) and human papillomavirus (HPV) detection are canonical screening methods for cervical cancer currently. However, there are limitations to these techniques. The aim of the present study was to identify efficient and practical methods for the screening of cervical intraepithelial neoplasia (CIN) and carcinoma. Residual PreservCyt specimens were obtained from 1,000 women who were admitted between August 2013 and December 2015. TCT, human telomerase RNA component (h-TERC) fluorescent in situ hybridization (FISH), MYC-specific FISH and surface plasmon resonance (SPR)-HPV genotyping were performed, followed by histopathology for those patients with positive results in any of the four tests. As a result, 106, 64, 56 and 112 patients were positive in the TCT, h-TERC, c-MYC and SPR-HPV tests, respectively, resulting in 213 being scheduled for histopathology; inflammation was identified in 159 patients, CIN I in 31, CIN II in 14, CIN III in seven and invasive cervical cancer in two patients. Using histopathology as the gold standard, TCT exhibited the highest sensitivity (87.04%), while h-TERC analysis had the highest specificity (81.76%). Parallel tests demonstrated that the Youden's index of TCT + h-TERC was the highest (0.49), while the serial analysis reported that TCT + HPV had the highest Youden's index (0.53) compared with any of the biomarkers alone (TCT, 0.50; HPV, 0.29; h-TERC, 0.47). In conclusion, dual positive TCT and HPV may be an efficient approach for basic screening of cervical lesions. h-TERC amplification may serve as an auxiliary test to improve the specificity.
Keywords: cervical intraepithelial neoplasia, cervical carcinoma, human papillomavirus, diagnosis, screening
Introduction
Cervical cancer is one of the most prevalent female cancer types in developing countries, second only to breast cancer (1). In China, it is estimated that ~61,691 women are newly diagnosed with invasive cervical cancer (ICC) annually and 29,526 of these succumbed to this disease in 2012 (2). Shanxi province has been identified as a high-incidence area, with an incidence rate of 23.04 per 100,000 (3,4). Cervical cancer develops from the associated precursor lesion, cervical intraepithelial neoplasia (CIN). Thus, it is necessary to develop effective strategies for mass screening of CIN, in order to reduce the incidence of cervical cancer.
The ThinPrep cytological test (TCT) and human papillomavirus (HPV) detection are canonical screening methods for cervical cancer currently (5–7). However, there are limitations to these techniques. For example, TCT is only capable of identifying patients with abnormal cell morphology, and cannot determine if these patients are at high-risk of lesion progression. Additionally, the results of TCT may be influenced by sampling and slide quality, based on the skill and experience of the practitioner (8). It has also been reported that HPV infection is reversible in the reproductive system and only 1–2% of persistent HPV infections progress to neoplasia (9,10). Thus, it is beneficial to investigate novel biomarkers for the diagnosis of CIN and evaluation of the likelihood of lesion progression.
Recent studies have demonstrated that ICC invariably possesses extra copies of the chromosome arm 3q, which contains the human telomerase RNA component (h-TERC) gene in the 3q26 region (11,12). h-TERC encodes the template for telomerase RNA, corresponding to the repeat sequence which is added in tandem to the ends of chromosomes, in order to maintain the telomere length (13). Thus, abnormal amplification of h-TERC may lead to the cells having increased telomeres and enhanced proliferation ability, resulting in the formation of cervical tumors (12,13). Detection of h-TERC amplification may be beneficial for the diagnosis of CIN and ICC (14). c-MYC, located in chromosomal region 8q24, is reported to be the most common integration site in the HPV genome (15). The c-MYC gene may be overexpressed simultaneous to the amplification of HPV, which is associated with the acquisition of a malignant phenotype in cervical cells (16). Therefore, c-MYC may also be an important oncogene involved in tumor progression, and hence may be a potential biomarker for cervical cancer (17). Previously, there have been studies to evaluate the diagnostic value of h-TERC (18) or c-MYC (19) alone in TCT and HPV, or a combination of h-TERC and c-MYC (11) in CIN and cancer. However, limited studies have been performed in order to determine which is the most efficient and practical method based on the four tests (TCT, HPV DNA, h-TERC and c-MYC) (8). Additionally, the majority of studies are aimed at demonstrating the sensitivity and specificity of the aforementioned biomarkers in distinguishing high-grade cervical lesions (>CIN2+ or ≥CIN2) and invasive cancer types from low-grade lesions (11,18,19); consequently, studies rarely focus on the difference between normal and all precursor lesions as well as ICC. Furthermore, the conclusions remain inconsistent between different studies. For example, Zheng et al (18) observed that among three methods, h-TERC testing displayed the highest specificity and positive predictive value, and HPV testing displayed the highest sensitivity, while TCT was not optimal for any of the diagnostic parameters. Jiang et al (20) demonstrated that for the detection of advanced cervical lesions, cytological evaluations were optimal (93.3%) in terms of specificity, followed by h-TERC amplification (83.8%) and HPV DNA analyses (39.3%). Zhao et al (19) reported that the specificity of fluorescence in situ hybridization (FISH) analysis with a c-MYC-specific probe seemed to be higher compared with TCT and HPV DNA testing; however, this was not consistent with the results from Gao et al (8) or Li et al (11). The diagnostic performance of c-MYC [area under the curve (AUC)=0.865] was slightly superior to that of h-TERC (AUC=0.843) in the study performed by Gao et al (8), although contrasting results were obtained from Li et al (11) (c-MYC AUC=0.799 vs. h-TERC AUC=0.838). Therefore, further studies are required in order to confirm the diagnostic abilities of h-TERC and c-MYC compared with TCT and HPV DNA testing.
The aim of the present study was to determine the optimal programs for screening precancerous lesions and cervical carcinoma among women in Shanghai, one of the largest cities in China (21), by TCT, h-TERC- and MYC-specific FISH and surface plasmon resonance (SPR)-HPV genotyping.
Materials and methods
Patients and specimens
Residual liquid-based cytology PreservCyt specimens were obtained from 1,000 women (aged 20–68 years) who were admitted to the gynecological clinic of Ren Ji Hospital of Shanghai Jiao Tong University (Shanghai, China) for routine screening between August 2013 and December 2015. None of the patients had received cytological tests within 6 months prior to cervical therapy, including radical surgery (hysterectomy and bilateral pelvic lymphadenectomy) with or without adjuvant radiotherapy or chemotherapy. Furthermore, none of the patients had additional neoplastic diseases. All specimens underwent HPV DNA, h-TERC, c-MYC and TCT testing. Histopathological examination was performed for patients who received positive results for any of the aforementioned tests. The Ethics Committee of Ren Ji Hospital of Shanghai Jiao Tong University approved this study. Written informed consent was obtained from all patients when the biopsy was performed.
Cytological examination
The liquid-based specimens were automatically processed with the Cytyc T2000 ThinPrep® system (Cytic Corp., Marlborough, MA, USA) and stained with the Papanicolaou stain method [95% ethyl alcohol fixation for 10 min; Harris hematoxylin (Papanicolaou solution A; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 3 min; running water, 2–3 times; 0.05% hydrochloric acid, 30 sec; 95% ethyl alcohol rinsing 2–3 times; orange G (Papanicolaou solution B, Sigma-Aldrich; Merck KGaA; 1 drop for 10 sec); 95% ethyl alcohol for 30 sec; 100% ethyl alcohol for 30 sec; polychromatic solution EA50 for 3 min; 95% ethyl alcohol for 2 min; 100% ethyl alcohol for 2 min; xylene for 2 min; all at room temperature; and mounted in a mounting medium (Permount; Thermo Fisher Scientific, Inc., Waltham, MA, USA)]. Cytological diagnoses were classified independently by two cytopathology physicians, under double-blinded conditions according to the 2001 Bethesda System (22). Diagnoses were as follows: i) Negative for intraepithelial lesion or malignancy; ii) epithelial cell abnormality e.g. atypical squamous cells of undetermined significance (ASCUS); iii) atypical squamous cells in which high-grade squamous intraepithelial lesion cannot be excluded (ASCH); iv) low-grade squamous intraepithelial lesion (LSIL); v) high-grade squamous intraepithelial lesion (HSIL); vi) squamous cell carcinoma; and vii) atypical glandular cells.
h-TERC and c-MYC gene detection
FISH was used in order to detect the amplification of h-TERC and c-MYC genes as previously described (8), according to the following procedures.
Preparation of slides
ThinPrep samples were enzymatically digested in 0.1% collagenase (type B; 20–30 min), centrifuged (2,000 × g at 37°C for 10 min), re-suspended in deionized water (20 min) and fixed twice with a methanol + acetic acid mixture (volume ratio, 3:1; 10 min) to prepare one-layer slides.
Pretreatment
Following rinsing twice with 2X sodium chloride plus sodium citrate (SSC; 5 min), the slides were placed in 0.1 M HCl for 5 min and incubated with a pepsin solution (0.05% pepsin/0.01 M HCl; (Sigma-Aldrich; Merck KGaA) for 10 min. Thereafter, the samples were subjected to dehydration through a graded ethanol series (70, 85 and 100%).
FISH
The prepared samples and probe mixture (consisting of probe, 2 ml; hybridization buffer, 7 ml; and deionized water, 1 ml) were denatured in 70% formamide/2X SSC for 5 min and dehydrated with the above graded ethanol series for 3 min each. Dual-color fluorescence probes were used for the detection of h-TERC [red, gene locus-specific probe (GLP) h-TERC probe; green, GLP chromosome 3 centromere-specific (CSP3) control probe], while the single probe was performed for measurement of c-MYC (red) (GP Medical Technologies, Beijing, China). The denatured probes were added onto the denatured slides and hybridized overnight at 42°C. Following removal of the cover slips, the slides were rinsed with 0.3% NP-40/0.4X SSC for 2 min at 67°C, followed by washing with 0.1% NP-40/2X SSC and 70% ethanol for 30 sec and 3 min, respectively. The slides were mounted with DAPI at 37°C for 10–20 min to counterstain.
FISH signal interpretation
Images of the slides were obtained on a fluorescence microscope (DM 2500; Leica Microsystems GmbH, Wetzlar, Germany) and analyzed using VideoTest-FISH software (version 2.0; VideoTest, St. Petersburg, Russia). The cell areas were determined through a DAPI filter (×100 objective). A nucleus with >2h-TERC/CSP3 signals (Fig. 1) or >2c-MYC signals (Fig. 2) was scored positive, while all remaining cells were considered normal.
Figure 1.
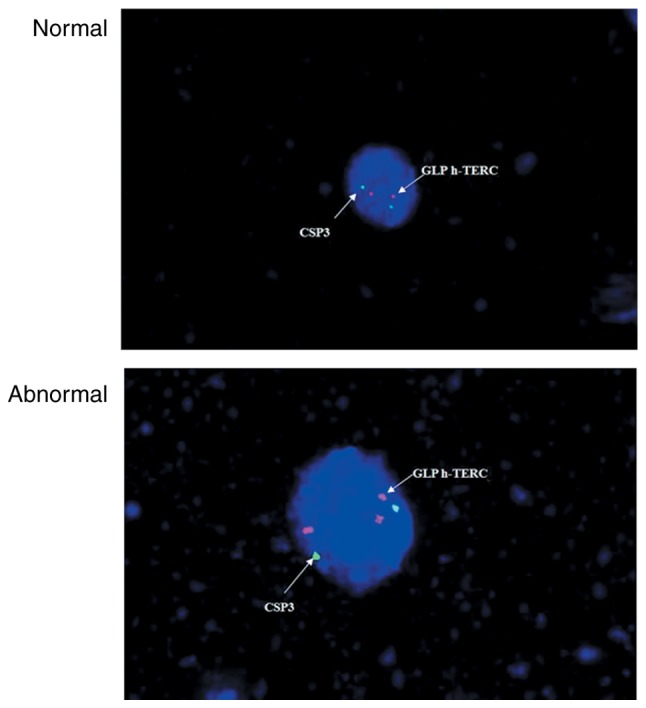
Normal vs. abnormal h-TERC signal from fluorescence in situ hybridization. CSP, chromosome 3 centromere-specific; h-TERC, human telomerase RNA component; GLP, gene locus-specific probe.
Figure 2.
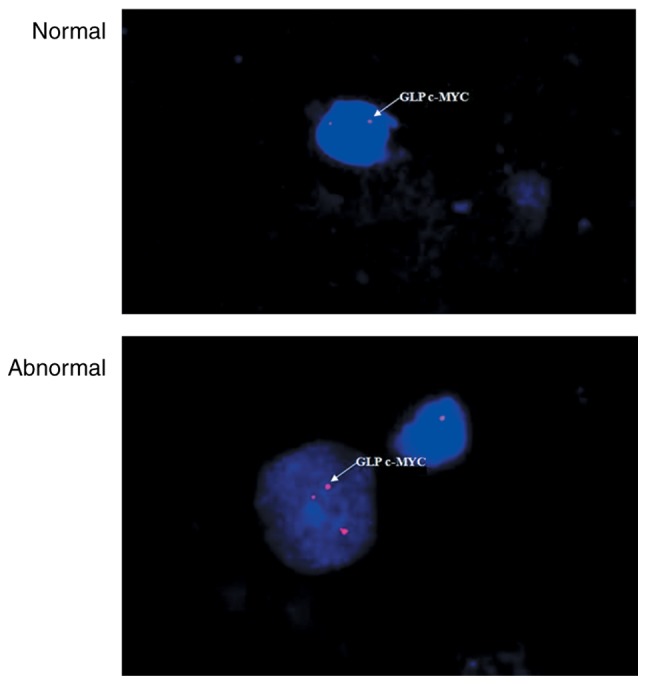
Normal vs. abnormal c-MYC signal from fluorescence in situ hybridization. CSP, chromosome 3 centromere-specific; GLP, gene locus-specific probe.
HPV DNA detection and genotyping
SPR-based tests conducted at the Beijing Jinpujia Medical Technology Co., Ltd. (Beijing, China) were used for HPV DNA detection and genotyping (23,24). Briefly, genomic DNA in ThinPrep cell suspension samples was isolated using Fast Extract Solution and amplified on an Eppendorf Mastercycler polymerase chain reaction (PCR) machine (model no. 5333; Eppendorf, Hamburg, Germany) with Taq polymerase (cat. no. R001A; Takara, Inc., Otsu, Japan) using the following procedure: 2 min at 50°C, 4 min at 94°C; 28 cycles of 30 sec at 94°C, 45 sec at 48°C, and 20 sec at 72°C; followed by 25 cycles of 30 sec at 94°C, 45 sec at 65°C and 20 sec at 72°C. The HPV genotyping of the amplified DNA product was performed using an SPR biosensor chip reading instrument (W2600; Beijing Jinpujia Medical Technology Co., Ltd., Beijing, China) which is able to detect 16 high-risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68 and 81) and eight low-risk (6, 11, 40, 42, 43, 44, 54 and 70) HPV subtypes simultaneously. The primers were provided by the Beijing Jinpujia Medical Technology Co., Ltd. and not shown.
Histopathological evaluation
A cell block was prepared from the ThinPrep samples by centrifugation at 1,500 × g for 10 min at 4°C and fixed in 10% formaldehyde overnight at 4°C. Subsequently, the specimen were transferred to graded ethanol for dehydration (70, 80, 90, 95 and 100%; each for 3 min), embedded in 5-µm-thick paraffin and stained with hematoxylin (5 min) and eosin (5 min) at room temperature for the pathological examination. The diagnosis was divided into four categories: i) Normal/inflammation; ii) CIN I (mild atypical hyperplasia and cellular atypism); iii) CIN II (moderate atypical hyperplasia and distinct cellular atypism); and iv) CIN III (severe atypical hyperplasia and significant cellular atypism) and ICC.
Statistical analysis
All the statistical analyses were performed with SPSS software (version 18.0 for Windows; SPSS Inc., Chicago, IL, USA). The enumerative results were expressed as n (%) and compared using a χ2 test (or Fisher's exact test). Using the histopathological diagnosis as the gold standard, the sensitivity [a/(a+c) ×100%], specificity [d/(b+d) ×100%], positive predictive value [a/(a+b) ×100%], negative predictive value [d/(c+d) ×100%], misdiagnosis rate [b/(b+d) ×100%], omission diagnosis rate [c/(a+c) ×100%] and Youden's index (Y=sensitivity + specificity-1.0) of the four screening programs for identification of CIN and ICC were calculated, where ‘a’ referred to true positive, ‘b’ was the false positive, ‘c’ was the false negative, and ‘d’ was true negative. Combined diagnosis was evaluated in parallel (if any of the methods were positive) or series (if all the methods were positive). P<0.05 was considered to indicate a statistically significant difference.
Results
Correlation between histopathological findings and TCT, h-TERC, c-MYC and HPV testing
Of the 1,000 cytological cases, 106 were reported to be positive in the TCT test, including ASCUS in 57 patients, ASCH in 13 patients, LSIL in 30 patients and HSIL in six patients. FISH analysis revealed that 64 patients (6.40%) were h-TERC positive and 56 (5.60%) were c-MYC positive. SPR-HPV genotyping demonstrated that 112 patients (11.20%) were HPV positive, of which 75 were infected with high-risk types, 28 were infected with low-risk types and nine were combined high- and low-risk types. In addition, infection with a single HPV subtype was observed in 66 patients (58.93%) and infection with multiple HPV subtypes was reported in 46 patients (41.07%).
Following screening, 213 patients were scheduled for histopathological examination due to a positive result in any of the aforementioned tests. The results reported inflammation in 159 patients, CIN I in 31 patients, CIN II in 14 patients, CIN III in 7 patients and ICC in two patients.
According to the histopathological findings, the rate of h-TERC, c-MYC expression and high-risk HPV infection [with the dominant genotypes of HPV16 (75.0%, 21/28), HPV18 (72.7%, 8/11), HPV58 (57.1%, 8/14), HPV52 (25.0%, 2/8) and HPV33 (20.0%, 1/5) (data not shown)], increased gradually with the increase in the severity of lesions (P<0.05; Table I). However, no significant differences in ASCH and HSIL of cytological examination were observed among different histopathological diagnoses. The LSIL ratio in the cytological examination was significantly elevated, but ASCUS ratio was significantly declined with the increase in the severity of lesions (P<0.05; Table I).
Table I.
Cytological, positive h-TERC, c-MYC expression and HPV infection findings according to histopathological diagnosis.
| TCT, n (%) | HPV positive, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histopathological classification | No. patients | ASCUS | ASCH | LSIL | HSIL | h-TERC positive, n (%) | c-MYC positive, n (%) | High risk | Low risk | Combined |
| Normal | 159 | 46 (28.93) | 1 (0.63) | 12 (7.55) | 0 (0.00) | 29 (18.24) | 32 (20.13) | 41 (25.79) | 25 (15.72) | 6 (3.77) |
| CIN I | 31 | 10 (32.26) | 7 (22.58) | 8 (25.81) | 0 (0.00) | 18 (58.06) | 11 (35.48) | 16 (51.61) | 2 (6.45) | 3 (9.68) |
| CIN II | 14 | 1 (7.14) | 5 (35.71) | 6 (42.86) | 1 (7.14) | 9 (64.29) | 7 (50.00) | 10 (71.43) | 1 (7.14) | 0 (0.00) |
| CIN III | 7 | 0 (0.00) | 0 (0.00) | 3 (42.86) | 4 (57.14) | 6 (85.71) | 4 (57.14) | 6 (85.71) | 0 (0.0) | 0 (0.00) |
| ICC | 2 | 0 (0.00) | 0 (0.00) | 1 (50.00) | 1 (50.00) | 2 (100.00) | 2 (100.00) | 2 (100.00) | 0 (0.00) | 0 (0.00) |
| P-value | − | <0.001 | 0.076 | 0.003 | 0.342 | 0.01 | <0.001 | 0.02 | 0.52 | 0.552 |
CIN, cervical intraepithelial neoplasia; ICC, invasive cervical cancer; ASCUS, atypical squamous cell of undetermined significance; ASCH, atypical squamous cells in which high-grade squamous intraepithelial lesion cannot be excluded; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; TCT, ThinPrep cytological test; HPV, human papillomavirus; h-TERC, human telomerase RNA component.
Diagnostic value of each method for cervical lesions
Using the pathological result of ‘normal’ as the criterion, the sensitivity, specificity, positive predictive value, negative predictive value, misdiagnosis rate, omission diagnosis rate and Youden's index of TCT, HPV, h-TERC and c-MYC for the detection of cervical lesions, including CIN and ICC, were determined. The results demonstrated that TCT exhibited the highest sensitivity (87.04%) and lowest omission diagnosis rate (12.96%), while h-TERC gene analysis had the highest specificity (81.76%) and the lowest misdiagnosis rate (18.24%), suggesting these two methods may be important when screening cervical lesions (Table II). These results were confirmed with the Youden's index, with a score of 0.5 and 0.47 for TCT and h-TERC, respectively. Although no significant difference in the sensitivity was observed between TCT and HPV, as well as between h-TERC and c-MYC (P>0.05), Youden's indices of HPV and c-MYC were approximately 0.2, suggesting their poor utility for case detection (Table II).
Table II.
Diagnostic value of TCT, h-TERC, c-MYC and HPV for cervical lesions.
| Test | Results | CIN and ICC | Normal | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Misdiagnosis rate (%) | Omission diagnosis rate (%) | Youden's index |
|---|---|---|---|---|---|---|---|---|---|---|
| TCT | + | 47 | 59 | 87.04 | 62.89 | 44.34 | 93.46 | 37.11 | 12.96 | 0.50 |
| − | 7 | 100 | − | − | − | − | − | − | − | |
| h-TERC | + | 35 | 29 | 64.81a | 81.76a | 54.69 | 87.25 | 18.24a | 35.19a | 0.47 |
| − | 19 | 130 | − | − | − | − | − | − | − | |
| c-MYC | + | 24 | 32 | 44.44a | 79.87a | 42.86 | 80.89a | 20.13a | 55.56a | 0.24 |
| − | 30 | 127 | − | − | − | − | − | − | − | |
| HPV | + | 40 | 72 | 74.07c | 54.72b,c | 35.71b | 86.14 | 45.28b,c | 25.93a,c | 0.29 |
| − | 14 | 87 | − | − | − | − | − | − | − |
P<0.05 vs. TCT
P<0.05 vs. h-TERC
P<0.05 vs. c-MYC. TCT, ThinPrep cytological test; HPV, human papillomavirus; h-TERC, human telomerase RNA component; CIN, cervical intraepithelial neoplasia; ICC, invasive cervical cancer.
Furthermore, the combined diagnostic values of the above tests were analyzed. The results indicated that the sensitivity increased from 87.04 to >90% when the TCT was combined with the h-TERC, c-MYC and/or HPV test (any positive; Table III), however the specificity decreased from 62.89 to <50%, with the highest specificity observed in combined h-TERC and c-MYC testing (62.89%). Nevertheless, the Youden's index of TCT and h-TERC remained relatively high (0.49), further implying their importance.
Table III.
Parallel and serial tests of combined TCT, h-TERC, c-MYC and HPV testing for diagnosis of cervical lesions.
| A, Parallel | |||||||
|---|---|---|---|---|---|---|---|
| Method | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Misdiagnosis rate (%) | Omission diagnosis rate (%) | Youden's index |
| TCT + h-TERC | 100.00 | 49.06 | 40.00 | 100.00 | 50.94 | 0.00 | 0.49 |
| TCT + c-MYC | 94.44 | 46.54 | 37.50 | 96.10 | 53.46 | 5.56 | 0.41 |
| TCT + HPV | 98.15 | 27.67 | 31.55 | 97.78 | 72.33 | 1.85 | 0.26 |
| h-TERC + c-MYC | 75.93 | 62.89 | 41.00 | 88.50 | 37.11 | 24.07 | 0.39 |
| h-TERC + HPV | 88.89 | 38.99 | 33.10 | 91.18 | 61.01 | 11.11 | 0.28 |
| c-MYC + HPV | 85.19 | 37.11 | 31.51 | 88.06 | 62.89 | 14.81 | 0.22 |
| TCT + h-TERC + c-MYC | 100.00 | 33.33 | 33.75 | 100.00 | 66.67 | 0.00 | 0.33 |
| TCT + h-TERC + HPV | 100.00 | 14.47 | 28.42 | 100.00 | 85.53 | 0.00 | 0.14 |
| TCT + c-MYC + HPV | 98.15 | 14.47 | 28.04 | 95.83 | 85.53 | 1.85 | 0.13 |
| h-TERC + c-MYC + HPV | 90.74 | 21.38 | 28.16 | 87.18 | 78.62 | 9.26 | 0.12 |
| B, Serial | |||||||
| Method | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Misdiagnosis rate (%) | Omission diagnosis rate (%) | Youden's index |
| TCT + h-TERC | 51.85 | 95.60 | 80.00 | 85.39 | 4.40 | 48.15 | 0.47 |
| TCT + c-MYC | 37.04 | 96.23 | 76.92 | 81.82 | 3.77 | 62.96 | 0.33 |
| TCT + HPV | 62.96 | 89.94 | 68.00 | 87.73 | 10.06 | 37.04 | 0.53 |
| h-TERC + c-MYC | 33.33 | 98.74 | 90.00 | 81.35 | 1.26 | 66.67 | 0.32 |
| h-TERC + HPV | 50.00 | 97.48 | 87.10 | 85.16 | 2.52 | 50.00 | 0.47 |
| c-MYC + HPV | 33.33 | 97.48 | 81.82 | 81.15 | 2.52 | 66.67 | 0.31 |
| TCT + h-TERC + c-MYC | 25.93 | 99.37 | 93.33 | 79.80 | 0.63 | 74.07 | 0.25 |
| TCT + h-TERC + HPV | 38.89 | 98.11 | 87.50 | 82.54 | 1.89 | 61.11 | 0.37 |
| TCT + c-MYC + HPV | 27.78 | 99.37 | 93.75 | 80.20 | 0.63 | 72.22 | 0.27 |
| h-TERC + c-MYC + HPV | 24.07 | 98.74 | 86.67 | 79.29 | 1.26 | 75.93 | 0.23 |
TCT, ThinPrep cytological test; HPV, human papillomavirus; h-TERC, human telomerase RNA component.
In addition, serial tests (both or all positive) were also performed. As a result, the specificity of any combination was notably higher compared with the parallel test, yet the sensitivity was decreased, with the highest sensitivity being 62.96%. According to the Youden's index, TCT + HPV (0.53) may be the most effective for diagnosis of cervical lesions, followed by TCT + h-TERC (0.47) and h-TERC + HPV (0.47) (Table III). Comprehensively, dual positive TCT and HPV were suggested to be an efficient approach for the basic screening of cervical lesions. h-TERC amplification may serve as an auxiliary test for TCT and HPV testing, to improve the specificity. This conclusion may be credible due to the highest Youden's index in TCT + HPV + h-TERC (0.37) among the three combination groups (TCT + h-TERC + c-MYC, 0.25; TCT + c-MYC + HPV, 0.27; h-TERC + c-MYC + HPV, 0.23).
Discussion
In the present study, for the first time to the best of our knowledge, the optimal programs for screening CIN and cervical carcinoma among women in Shanghai were demonstrated (21), through simultaneous detection of TCT, h-TERC, MYC and HPV. The results demonstrated that although TCT (87.04%) and HPV testing (74.07%) alone had higher sensitivity for screening CIN and cervical carcinoma, their specificities (62.89 and 54.72%, respectively) were significantly lower compared with the alternative tests analyzed, suggesting that there may be limitations when using these tests alone to screen precancerous cervical lesions. This trend seemed to be in accordance with previous studies (19,25); however, the quantitative values were slightly different, which may be attributed to the differences in the sample size (25), regional location of the population (21) and detection methods (18,19). In particular, it has been reported that there are discordant results between SPR and hybrid capture II (HC2) tests or direct DNA sequencing assays (23,24). Nevertheless, the SPR test has the incomparable advantages of a low-cost, rapid detection and an easy-to-use method with the potential for automation (23,24). In addition, the SPR method is able to test 24 HPV subtypes, which is higher compared with the 13 subtypes which are currently able to be tested using the HC2 test (21), or the 21 subtypes which are able to be detected via the CAPE HPV genotyping assay kit (19). The results from the present study suggested that the SPR test may be a superior approach for HPV genotyping in a clinical setting. When the TCT and HPV tests were combined and one or both of the markers amplified to be positive, the sensitivity and the specificity were increased to 98.15 and 89.94%, respectively, displaying the highest Youden's index values (0.53) compared with all alternative combinations. These results implied that the combination of the TCT and HPV tests may improve the sensitivity of cervical disease screening and reduce the rate of misdiagnosis, leading to the timely detection of this disease in high-risk populations. These results further demonstrated the necessity of detection of TCT and HPV in the clinic as previously reported (25,26).
Although combined TCT and HPV tests may improve the sensitivity and the specificity to 98.15 and 89.94% respectively, a number of patients may still be misdiagnosed and missed. Therefore, alternative biomarkers are required to serve as supplementary detection methods. The present study reported that h-TERC gene analysis had the highest specificity (81.76%) and the lowest misdiagnosis rate (18.24%). Additionally, the combination of TCT, HPV and h-TERC displayed 100% sensitivity and 98.11% specificity, suggesting h-TERC may be the principal gene in cervical carcinogenesis, which is in line with results from previous studies (19,27). It has been reported that the h-TERC gene is significantly upregulated in numerous cancer types (28,29). Inhibition of h-TERC blocks tumor growth and promotes cell apoptosis, including cervical cancer (30). This phenomenon was also observed in the present study, with the h-TERC-positive rate significantly increased from the histological diagnoses of normal (18.24%), CIN I (58.06%), CIN II (64.29%), CIN III (85.71%) to ICC (100%). These results indicate that h-TERC amplification may be a useful genetic testing marker that might assist in the clinical histopathological analysis for the differential diagnosis of normal or CIN cases, and may even distinguish between low-grade (<CIN I) and high-grade (>CIN II) cervical lesions.
Furthermore, extensive studies have demonstrated that the transcription factor c-MYC may serve an important role in carcinogenesis, due to its effect on basic cell processes including cell proliferation, the cell cycle and apoptosis (31–33). Thus, c-MYC amplification may also be a potential diagnostic indicator for cervical cancer (8,11,19). In the present study, it was observed that the c-MYC-positive rates increased with increasing severity of histological diagnosis. However, the fact that Youden's index of c-MYC itself was lower and the addition of c-MYC did not increase the Youden's index for the alternative methods, suggests that c-MYC may be not the most ideal biomarker in the screening of cervical lesions.
There are a number of limitations to the present study. First, the study was a single center study and the sample size was relatively small, which may have led to the underestimation or overestimation of the diagnostic values of the biomarkers. Second, the aim of the study was to demonstrate the diagnostic performance of the biomarkers for differentiating CIN and ICC from healthy controls; therefore, further investigations are required in order to validate the utility of the biomarkers for screening different cervical lesion severities (11). Third, beyond h-TERC and c-MYC genes, numerous studies have reported that the epigenetically-regulated genes, including paired box 1 (34) and zinc finger protein 582 (35), may serve as genetic biomarkers for detecting high-grade CIN lesions and cervical cancer. Therefore, it may be beneficial for further studies to also consider these epigenetic factors.
In conclusion, the results of this preliminary study revealed that a combination of TCT and HPV testing may be the most efficient approach for the basic screening of cervical lesions in Shanghai. h-TERC amplification may serve as an auxiliary test to improve the specificity. However, further investigation with larger samples collected from a multicenter study is required in order to confirm these results.
Acknowledgements
Not applicable.
Funding
This study was funded by The Shanghai Municipal Health and Family Planning Commission Fund (grant no. 15GWZK0701).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author's contributions
WJ and WD participated in the design of this study. WL and ZH were involved in the sample collection. WJ and LQ served important roles in statistical analyses. WJ, WL and WD contributed to the acquisition and interpretation of data. WJ and WD drafted and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committee of Ren Ji Hospital of Shanghai Jiao Tong University approved this study. Written informed consent was obtained from all patients when the biopsy was performed.
Patient consent for publication
All patients agreed with their data used for publication purposes.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Di J, Rutherford S, Chu C. Review of the cervical cancer burden and population-based cervical cancer screening in China. Asian Pac J Cancer Prev. 2015;16:7401–7407. doi: 10.7314/APJCP.2015.16.17.7401. [DOI] [PubMed] [Google Scholar]
- 3.Wang XZ, Liu KR, Yuan FM, Hou JZ, Zhang JJ, Zhang YZ. An analysis of both high incidence of esophageal and cervical cancer in Yangcheng County, Shanxi Province. China Cancer. 2011;4:259–261. [Google Scholar]
- 4.Wang Z, Wang J, Fan J, Zhao W, Yang X, Wu L, Li D, Ding L, Wang W, Xu J, et al. Risk factors for cervical intraepithelial neoplasia and cervical cancer in Chinese women: Large study in Jiexiu, Shanxi Province, China. J Cancer. 2017;8:924–932. doi: 10.7150/jca.17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Zhang L, Zhao G, Che L, Zhang H, Fang J. The clinical research of Thinprep Cytology Test (TCT) combined with HPV-DNA detection in screening cervical cancer. Cell Mol Biol (Noisy-le-grand) 2017;63:92–95. doi: 10.14715/cmb/2017.63.2.14. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Wang Y, Liu L, Guo C, Liu Z, Nie S. Prevalence of human papillomavirus infection and genotyping for population-based cervical screening in developed regions in China. Oncotarget. 2016;7:62411–62424. doi: 10.18632/oncotarget.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JL, Yang YZ, Dong WW, Sun J, Tao HT, Li RX, Hu Y. Application of human papillomavirus in screening for cervical cancer and precancerous lesions. Asian Pac J Cancer Prev. 2013;14:2979–2982. doi: 10.7314/APJCP.2013.14.5.2979. [DOI] [PubMed] [Google Scholar]
- 8.Gao K, Eurasian M, Zhang J, Wei Y, Zheng Q, Ye H, Li L. Can genomic amplification of human telomerase gene and C-MYC in liquid-based cytological specimens be used as a method for opportunistic cervical cancer screening? Gynecol Obstet Invest. 2015;80:153–163. doi: 10.1159/000371760. [DOI] [PubMed] [Google Scholar]
- 9.Szarewski A. HPV vaccination and cervical cancer. Curr Oncol Rep. 2012;14:559–567. doi: 10.1007/s11912-012-0259-3. [DOI] [PubMed] [Google Scholar]
- 10.Chandra R. Relevance of persistent infection with high-risk HPV genotypes in cervical cancer progression. MLO Med Lab Obs. 2013;45 40, 42, 44. [PubMed] [Google Scholar]
- 11.Li T, Tang L, Bian D, Jia Y, Huang X, Zhang X. Detection of hTERC and c-MYC genes in cervical epithelial exfoliated cells for cervical cancer screening. Int J Mol Med. 2014;33:1289–1297. doi: 10.3892/ijmm.2014.1699. [DOI] [PubMed] [Google Scholar]
- 12.Kuglik P, Kasikova K, Smetana J, Vallova V, Lastuvkova A, Moukova L, Cvanova M, Brozova L. Molecular cytogenetic analyses of hTERC (3q26) and MYC (8q24) genes amplifications in correlation with oncogenic human papillomavirus infection in Czech patients with cervical intraepithelial neoplasia and cervical carcinomas. Neoplasma. 2015;62:130–139. doi: 10.4149/neo_2015_017. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Bryan TM, Reddel RR. Increased copy number of the TERT and TERC telomerase subunit genes in cancer cells. Cancer Sci. 2008;99:1092–1099. doi: 10.1111/j.1349-7006.2008.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bin H, Ruifang W, Ruizhen L, Yiheng L, Zhihong L, Juan L, Chun W, Yanqiu Z, Leiming W. Detention of HPV L1 capsid protein and hTERC gene in screening of cervical cancer. Iran J Basic Med Sci. 2013;16:797–802. [PMC free article] [PubMed] [Google Scholar]
- 15.Ferber MJ, Thorland EC, Brink AA, Rapp AK, Phillips LA, Mcgovern R, Gostout BS, Cheung TH, Chung TK, Fu WY, Smith DI. Preferential integration of human papillomavirus type 18 near the c-myc locus in cervical carcinoma. Oncogene. 2003;22:7233–7242. doi: 10.1038/sj.onc.1207006. [DOI] [PubMed] [Google Scholar]
- 16.Abba MC, Laguens RM, Dulout FN, Golijow CD. The c-myc activation in cervical carcinomas and HPV 16 infections. Mutat Res. 2004;557:151–158. doi: 10.1016/j.mrgentox.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Kübler K, Heinenberg S, Rudlowski C, Keyver-Paik MD, Abramian A, Merkelbach-Bruse S, Büttner R, Kuhn W, Schildhaus HU. c-myc copy number gain is a powerful prognosticator of disease outcome in cervical dysplasia. Oncotarget. 2015;6:825–835. doi: 10.18632/oncotarget.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Liang P, Zheng Y, Yi P, Liu Q, Han J, Huang Y, Zhou Y, Guo J, Li L. Clinical significance of hTERC gene detection in exfoliated cervical epithelial cells for cervical lesions. Int J Gynecol Cancer. 2013;23:785–790. doi: 10.1097/IGC.0b013e31828f39a0. [DOI] [PubMed] [Google Scholar]
- 19.Zhao WH, Hao M, Cheng XT, Yang X, Wang ZL, Cheng KY, Liu FL, Bai YX. c-myc gene copy number variation in cervical exfoliated cells detected on fluorescence in situ hybridization for cervical cancer screening. Gynecol Obstet Invest. 2016;81:416–423. doi: 10.1159/000442286. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J, Wei LH, Li YL, Wu RF, Xie X, Feng YJ, Zhang G, Zhao C, Zhao Y, Chen Z. Detection of TERC amplification in cervical epithelial cells for the diagnosis of high-grade cervical lesions and invasive cancer: A multicenter study in China. J Mol Diagn. 2010;12:808–817. doi: 10.2353/jmoldx.2010.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Y, Ma C, Zou J, Zhu Y, Yang R, Xu Y, Zhang Y. Prevalence characteristics of high-risk human papillomaviruses in women living in Shanghai with cervical precancerous lesions and cancer. Oncotarget. 2016;7:24656–24663. doi: 10.18632/oncotarget.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, Jr, et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 23.He X, Yang P, Wang H, Wang Y, Liu S. Human papillomavirus genotyping by surface plasmon resonance-based test. Clin Lab. 2016;62:2079–2084. doi: 10.7754/Clin.Lab.2016.151233. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Yang H, Zhang H, Yang F, Zhou M, Jia C, Lan Y, Ma Y, Zhou L, Tian S, et al. A surface plasmon resonance--based system to genotype human papillomavirus. Cancer Genet Cytogenet. 2010;200:100–105. doi: 10.1016/j.cancergencyto.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Pan QJ, Hu SY, Guo HQ, Zhang WH, Zhang X, Chen W, Cao J, Jiang Y, Zhao FH, Qiao YL. Liquid-based cytology and human papillomavirus testing: A pooled analysis using the data from 13 population-based cervical cancer screening studies from China. Gynecol Oncol. 2014;133:172–179. doi: 10.1016/j.ygyno.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Castle PE, Aslam S, Behrens C. Cervical precancer and cancer risk by human papillomavirus status and cytologic interpretation: Implications for risk-based management. Cancer Epidemiol Biomarkers Prev. 2016;25:1595–1599. doi: 10.1158/1055-9965.EPI-16-0330. [DOI] [PubMed] [Google Scholar]
- 27.Zappacosta R, Ianieri MM, Buca D, Repetti E, Ricciardulli A, Liberati M. Clinical role of the detection of human telomerase RNA component gene amplification by fluorescence in situ hybridization on liquid-based cervical samples: Comparison with human papillomavirus-DNA testing and histopathology. Acta Cytol. 2015;59:345–354. doi: 10.1159/000438719. [DOI] [PubMed] [Google Scholar]
- 28.Baena-Del Valle JA, Zheng Q, Esopi DM, Rubenstein M, Hubbard GK, Moncaliano MC, Hruszkewycz A, Vaghasia A, Yegnasubramanian S, Wheelan SJ, et al. MYC drives overexpression of telomerase RNA (hTR/TERC) in prostate cancer. J Pathol. 2018;244:11–24. doi: 10.1002/path.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penzo M, Ludovini V, Treré D, Siggillino A, Vannucci J, Bellezza G, Crinò L, Montanaro L. Dyskerin and TERC expression may condition survival in lung cancer patients. Oncotarget. 2015;6:21755–21760. doi: 10.18632/oncotarget.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Li H, Yao G, Li W, Wang F, Jiang Z, Li M. Inhibition of telomerase RNA (hTR) in cervical cancer by adenovirus-delivered siRNA. Cancer Gene Ther. 2007;14:748–755. doi: 10.1038/sj.cgt.7701056. [DOI] [PubMed] [Google Scholar]
- 31.Yuan Y, Zhang J, Cai L, Ding C, Wang X, Chen H, Wang X, Yan J, Lu J. Leptin induces cell proliferation and reduces cell apoptosis by activating c-myc in cervical cancer. Oncol Rep. 2013;29:2291–2296. doi: 10.3892/or.2013.2390. [DOI] [PubMed] [Google Scholar]
- 32.Liao LM, Sun XY, Liu AW, Wu JB, Cheng XL, Lin JX, Zheng M, Huang L. Low expression of long noncoding XLOC_010588 indicates a poor prognosis and promotes proliferation through upregulation of c-Myc in cervical cancer. Gynecol Oncol. 2014;133:616–623. doi: 10.1016/j.ygyno.2014.03.555. [DOI] [PubMed] [Google Scholar]
- 33.Cui F, Hou J, Huang C, Sun X, Zeng Y, Cheng H, Wang H, Li C. C-Myc regulates radiation-induced G2/M cell cycle arrest and cell death in human cervical cancer cells. J Obstet Gynaecol Res. 2017;43:729–735. doi: 10.1111/jog.13261. [DOI] [PubMed] [Google Scholar]
- 34.Luan T, Hua Q, Liu X, Xu P, Gu Y, Qian H, Yan L, Xu X, Geng R, Zeng X, Zeng X. PAX1 methylation as a potential biomarker to predict the progression of cervical intraepithelial neoplasia: A meta-analysis of related studies. Int J Gynecol Cancer. 2017;27:1480–1488. doi: 10.1097/IGC.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 35.Liou YL, Zhang Y, Liu Y, Cao L, Qin CZ, Zhang TL, Chang CF, Wang HJ, Lin SY, Chu TY, et al. Comparison of HPV genotyping and methylated ZNF582 as triage for women with equivocal liquid-based cytology results. Clin Epigenetics. 2015;7:50. doi: 10.1186/s13148-015-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


