Abstract
Certain types of cancer exhibit downregulated expression of zonula occludens-1 (ZO-1), which serves an important function in tumor progression; however, the underlying molecular mechanisms that lead to this downregulation in cancer remain unclear. In the present study, the expression of ZO-1 in liver cancer (LC) tissues was investigated. Western blot and reverse transcription-quantitative polymerase chain reaction assays were used to detect the expression of ZO-1 protein and mRNA in LC tissues and paired adjacent non-tumorous tissues. The results indicated that, compared with non-tumorous tissues, the expression of ZO-1 was significantly downregulated at the protein (P<0.001) and mRNA (P=0.006) levels in LC tissue samples. In addition, various cellular and molecular methods were applied, including MTT, colony formation, flow cytometry and Transwell assays. The results indicated that overexpression of ZO-1 inhibited cell viability, proliferation and migration, and induced G0/G1 phase arrest in vitro.
Keywords: liver cancer, zonula occludens-1, tumor progression
Introduction
Liver cancer (LC) is the sixth most common type of cancer, ranking as high as third for cancer-associated mortality globally (1), and is particularly prevalent in Asia (2). It has been proposed that the incidence and mortality rates of LC has been increasing (3). Owing to the high prevalence of hepatitis B virus (HBV) infection in Chinese populations, HBV-associated liver cirrhosis or LC has become a major disease burden in China (4), accounting for between 75 and 90% of malignant tumors in adult livers (5). Early detection of LC allows for curative or palliative treatment with surgical treatments such as liver resection and liver transplantation (6). However, owing to a lack of detectable early symptoms, insidious onset and its high recurrence rate following surgery, there is a relatively low reported 5-year survival rate (7,8). It is therefore important to develop novel methods to prevent cancer recurrence and improve the prognosis for patients with LC. Although an increasing number of molecular biomarkers with high sensitivity and specificity for LC have been reported, none has so far justified its routine use in clinical practice (9). Furthermore, to the best of our knowledge, there has been no previous investigation of the potential function of zonula occludens-1 (ZO-1) in LC.
ZOs are members of the membrane-associated guanylate kinase (MAGUK) protein family, including ZO-1 (10), ZO-2 (11) and ZO-3 (12). ZO-1 is a 220-kDa scaffolding protein which contains various domains (an Src homology 3 domain, three PDZ domains, a proline-rich region and a guanylate kinase domain) that allow its interaction with specialized sites of plasma membrane as well as with other proteins (13,14). ZO-1 is associated directly with actin filaments, anchoring tight junction transmembrane proteins to the actin cytoskeleton (15,16). ZO-1 is a characteristic factor of tight junctions, which has also been demonstrated in epithelial (E-)cadherin junctions (17–19). In addition, it has a scaffolding function, serving an increasingly vital function in signal transduction by clustering critical membrane proteins (20). Deletions or mutations in the ZO-1 gene led to overgrowth, suggesting that ZO-1 may function as a tumor suppressor (13). For example, insulin-like growth factor I receptor (IGF-IR) induces E-cadherin-mediated cell-cell adhesion by upregulating ZO-1 in breast cancer cells. On the other hand, the expression of IGF-IR and ZO-1 increased growth, and survival of the primary tumor may decrease the occurrence of metastasis (21). Decreased ZO-1 expression has been identified to be associated with increased invasiveness in breast cancer (22), colorectal cancer (23) and gastrointestinal tumors (24). Furthermore, it is reported that ZO-1 is involved in tumor invasion associated with epithelial-mesenchymal transition processes (25).
In the present study, ZO-1 expression in LC tissue samples was investigated. In addition, the effect of expression of ZO-1 on LC cell viability, proliferation and migration were also investigated. Furthermore, the effects of ZO-1 on the LC cell cycle were also determined in vitro. Taken together, the results of the present study indicated that the ZO-1 gene may act as a tumor suppressor in LC, and serve an important function in LC development and progression.
Materials and methods
Cell culture and transfection
HepG2 cells (an LC cell line) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 100 mg/ml penicillin and 100 mg/ml streptomycin. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. For overexpression of ZO-1, the coding sequence of ZO-1 was amplified and subcloned into the pcDNA3.1(+) vector (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Cells were transfected with a negative control vector or a ZO-1-expressing plasmid using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Patients and tissue specimens
Fresh LC and surrounding non-tumor tissue samples were obtained from 30 randomly selected patients with LC, including 18 males and 12 females (age range, 40–60 years), all of whom had undergone surgical resection at Liaocheng People's Hospital (Liaocheng, China) between January 2014 and January 2015. The tumor tissues and their adjacent normal liver tissues, which were located >5 cm from the LC, were collected and maintained at −80°C for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis. None of the patients had received adjuvant therapies before surgery. All of the tissues were sampled and then verified by pathological examination. The histopathological type and stage of LC were determined according to the criteria of the World Health Organization classification (26). Tumor differentiation was assessed according to the Edmonson and Steiner grading system (27). All LC tissues were collected following approval by the Ethics Committee of Liaocheng People's Hospital.
Western blot analysis
Fresh LC tissues and the surrounding non-tumor liver tissues were treated with lysis buffer containing protease inhibitors (Promega Corporation, Madison, WI, USA). Following centrifugation at 20,000 × g at 4°C for 20 min, the supernatant was collected for determination of total protein concentration using the DC protein assay method (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to maintain equal loads (20 µg/lane). Then protein samples were electrophoretically separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes, which were then blocked at room temperature for 1 h with 5% non-fat dried milk in Tris-buffered saline containing Tween-20 (TBST; 50 mm Tris/HCl, 100 mm NaCl and 0.1% Tween-20, pH 7.4). Subsequently, membranes were incubated with a polyclonal goat anti-human ZO-1 antibody (1:500; catalog no. sc-33725; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. Following three washes with TBST for 5 min, the membranes were further incubated with IRDye800-conjugated anti-goat immunoglobulin G secondary antibody (1:5,000; catalog no. P/N 925–32210; Rockland Immunochemicals, Inc., Limerick, PA, USA) for 2 h at room temperature. Anti-β-actin antibody (1:2,000; catalog no. sc-70319; Santa Cruz Biotechnology, Inc.) was used as a loading control. Finally, membranes were scanned using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA) and analyzed using PDQuest software (version 7.2.0; Bio-Rad Laboratories, Inc.).
RT-qPCR
TRIzol® reagent (Thermo Fisher Scientific, Inc.) was used to extract RNA from paired LC samples. cDNA was synthesized from total RNA using an Omniscript RT kit (Qiagen, Inc., Valencia, CA, USA), according to the manufacturer's protocol. Subsequently, qPCR was used to determine the mRNA level of ZO-1, which was performed using a Mastercycler Ep Realplex instrument (Eppendorf, Hamburg, Germany). Reaction volumes of 25 µl included 2 µl cDNA, 12 µl 2×Fast EvaGreenTM qPCR Master mix (Biotium Inc., Freemont, CA, USA), 1 µl primers (10 mM) and 10 µl RNase/DNase-free water. Cycling parameters were as follows: Hot start at 95°C for 10 min; 40 cycles of amplification/quantification at 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec during which time fluorescence was determined. Melting curve analysis was performed using continuous fluorescence acquisition between 65 and 97°C. These cycling parameters generated single amplicons for the two primer sets used according to the presence of a single melt peak. The relative expression level for each target gene was normalized using the Cq value of GAPDH (internal reference) using the 2−ΔΔCq relative quantification method (28). Primer sequences were as follows: GAPDH forward, 5′-AACTTCCGTTGCTGCCAT-3′ and reverse, 5′-TTTCTTCCACAGGGCTTTG-3′; and ZO-1 forward, 5′-TATTATGGCACATCAGCACG-3′ and reverse, 5′-TGGGCAAACAGACCAAGC-3′.
Cell proliferation assay
An MTT assay was used to detect the effect of ZO-1 on cellular proliferation. In total, 5×103 cells were plated in each well of a 96-well plate. Following incubation for 24 h, 20 µl MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added, prior to incubation at 37°C for another 4 h in a 5% CO2 incubator. Following removal of the supernatants, the formazan crystals were dissolved in 100 µl/well dimethylsulfoxide. A multilabel plate reader (PerkinElmer, Inc., Waltham, MA, USA) to determine the absorbance of each sample at 490 nm. Three independent experiments were performed.
Colony formation assay
Cells were seeded in a 6-well plate at a density of 1×103 cells/well. Following culture for 2 weeks, cells were fixed with 4% paraformaldehyde for 20 min and then enumerated following staining with 1% crystal violet. Three independent experiments were performed.
Cell cycle analysis
Cell cycle distribution was analyzed using flow cytometry. A total of 48 h after transfection of cells with a negative control vector or a ZO-1 overexpressing plasmid, cells were trypsinized, rinsed with PBS, fixed with 70% ethanol at 4°C overnight, and treated with RNase A (0.02 mg/ml) in the dark at room temperature for 30 min. Cells were resuspended in 0.05 mg/ml propidium iodide and analyzed using flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA). DNA histograms were analyzed using ModFit LT (version 2.0; Verity Software House, Inc., Topsham, ME, USA). For each sample, >104 events were recorded.
Migration assays
A Transwell chamber assay (EMD Millipore, Billerica, MA, USA) was used to determine cell migration. Cells (1×105 cells/well) were suspended in 100 µl serum-free DMEM. Subsequently, the upper chamber of the inserts was added, and then DMEM containing 10% FBS was added to the lower chamber as the chemotactic factor. Following 24 h incubation at 37°C, the cells that migrated were fixed and stained at room temperature for 30 min with a dye solution which contained 0.2% crystal violet and 20% methanol. The number of migrated cells was determined under an inverted microscope (IX71; Olympus Corporation, Tokyo, Japan) at ×200 magnification in random fields in each well.
Statistical analysis
Statistical analyses were performed using SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA). One-way analysis of variance followed by a post hoc Dunnett's test was used to analyze the comparison of the means for three groups. Student's t-test was used to evaluate the differences between two groups. Results are presented as the mean ± standard error of the mean. P<0.05 was considered to indicate a statistically significant difference.
Results
ZO-1 expression is downregulated in LC tissues
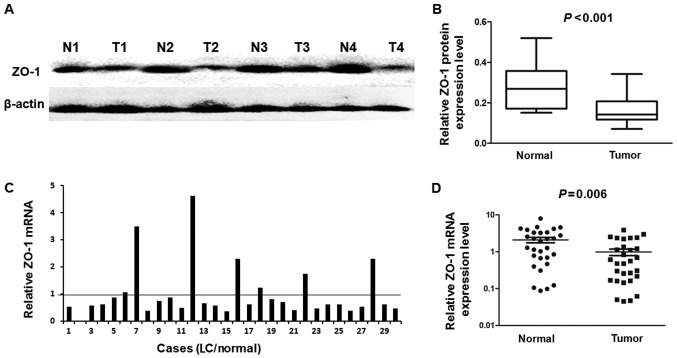
Western blotting was used to detect the protein levels of 18 randomly selected pairs of LC and their matched adjacent liver tissues. Fig. 1A presents four representative cases of the western blot result. The relative quantity of ZO-1 protein expression was normalized to the β-actin in the same samples. Compared with their adjacent normal liver tissues, the expression of ZO-1 protein was downregulated in the LC tissues (13/18), and the mean ZO-1 protein level in LC tissues was significantly decreased compared with in their adjacent normal liver tissues (P<0.001; Fig. 1B). These results were further confirmed by determining mRNA levels by RT-qPCR, which was used to determine the mRNA level of ZO-1 in 30 paired LC cancerous and matched adjacent normal liver tissues. The results indicated that the expression of the ZO-1 mRNA level was significantly lower in 23/30 (76.7%) LC tissues compared with the adjacent non-tumor tissues (Fig. 1C). The mean mRNA expression level of ZO-1 was significantly decreased in LC tissues compared with that in their corresponding normal liver tissues (P=0.006; Fig. 1D).
Figure 1.
Expression of ZO-1 in LC tissue samples. (A) Expression of ZO-1 protein in 18 paired tissues was analyzed by western blotting. Representative images of ZO-1 expression are presented. (B) Relative ZO-1 protein expression level was significantly decreased in 13/18 (72.2%) LC tissues compared with the corresponding adjacent liver tissues (P<0.001). (C) Relative mRNA levels of ZO-1 in 30 pairs of LC tissues and corresponding adjacent liver samples determined using the reverse transcription-quantitative polymerase chain reaction. (D) Mean relative mRNA expression level of ZO-1 in LC tissues compared with paired adjacent liver tissues (P=0.006). ZO-1, zonula occludens-1; LC, liver cancer; T, tumor; N, normal.
Overexpression of ZO-1 inhibits LC cell viability and proliferation in vitro
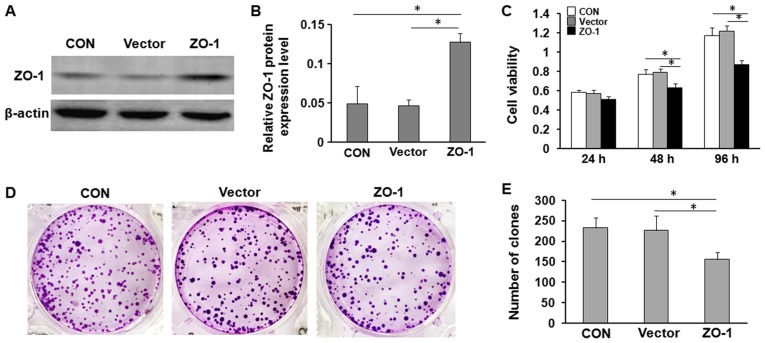
Since ZO-1 was significantly decreased in LC tissues, it was investigated whether overexpression of ZO-1 affected cell viability and proliferation of LC cells. The effects of ZO-1 on LC cell viability and proliferation were further evaluated using MTT and colony formation assays, respectively. The results indicated that overexpression of ZO-1 significantly inhibited the viability of HepG2 cells, and markedly decreased the number of colonies compared with the control and negative control vector cells (Fig. 2).
Figure 2.
Exogenous expression of ZO-1 inhibits the viability and proliferation of LC cells in vitro. (A) Relative ZO-1 expression was determined by western blotting. HepG2 cells without plasmid (CON), or transfected with empty plasmid (Vector) or ZO-1-overexpression plasmid (ZO-1) for 24 h. (B) Quantification of western blot analysis. (C) Overexpression of ZO-1 significantly inhibited the viability of LC cells. Cell viability was determined using an MTT assay at 1, 2 and 4 days. *P<0.05. (D) Colony formation assays of HepG2 cells with plasmid (CON) or transfected with ZO-1-overexpression plasmid (ZO-1) or empty plasmid (Vector). (E) Quantification of colony formation assays. Results are expressed as the mean ± standard deviation of three independent experiments. *P<0.05. ZO-1, zonula occludens-1; LC, liver cancer.
Upregulation of ZO-1 decreases LC cell cycle and migration in vitro
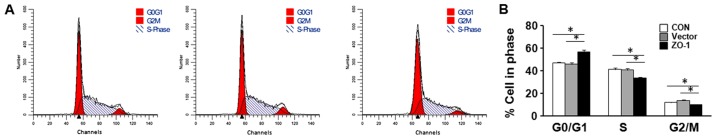
To investigate the potential mechanism responsible for the effects of ZO-1 on the proliferation of LC cells, the cell cycle was analyzed in HepG2 cells transfected with ZO-1-overexpressing plasmid or negative control plasmid using flow cytometry. In cell cycle analysis, a significant increase in the G0/G1 phase and decrease in the S-G2 phase was identified (Fig. 3).
Figure 3.
Effects of ZO-1 on cell cycle arrest of LC cells in vitro. (A) Following transfection with ZO-1-overexpression plasmid (ZO-1) or empty plasmid (vector) for 48 h, or untransfected (CON), the cell cycle distribution of HepG2 cells was determined by propidium iodide staining and flow cytometry. (B) ZO-1 overexpression caused a significant accumulation of cells in G0/G1 phase and a marked decrease in S-G2 phase compared with empty-vector-transfected cells. *P<0.05.
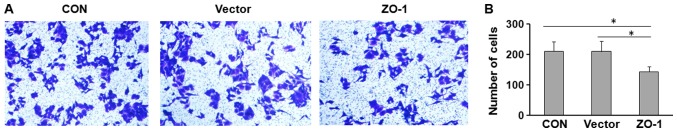
Furthermore, the potential effect of ZO-1 on cell migration was investigated using Transwell assays. HepG2 cells were transfected with ZO-1-overexpressing or control plasmid and seeded in the Transwell chamber. Overexpression of ZO-1 significantly decreased the migratory capacity of HepG2 cells (Fig. 4).
Figure 4.
Overexpression of ZO-1 suppresses cell migration in vitro. HepG2 cells were seeded into Transwell chambers. After 24 h, the migrated cells were (A) stained with 0.1% crystal violet (magnification, ×200) and (B) enumerated. Results are expressed as the mean ± standard deviation of three independent experiments. *P<0.05.
Discussion
LC is one of the most prevalent tumors globally and the third leading cause of cancer-associated mortality (29,30). Worldwide, ~750,000 new cases of LC are diagnosed each year. Population-based analysis indicated that the incidence rate continues to parallel the death rate, which indicates that the majority of individuals who develop LC succumb to this disease (31). Although tumor resection and liver transplantation are effective treatments for selected patients with LC, the prognosis of LC remains poor because the disease is often at a fairly advanced stage at the time of diagnosis (32). Surgical treatment is not applicable for patients at advanced tumor stages (33). LC is involved in multiple gene alterations including tumor suppressor inactivation, oncogene activation and apoptosis-associated gene dysregulation (34). Therefore, there is an urgent requirement to identify a sensitive and specific biomarker for the detection of liver cancer at the curative stage.
ZO-1 serves as a scaffolding protein that links the transmembrane tight junction proteins to cytoplasmic proteins and the actin cytoskeleton (15,35). As a member of the MAGUK family of putative signaling proteins, ZO-1 may be involved in signal transduction, and ZO-1 has been identified to bind a target of Ras: AF6 (36). Previous studies indicated that epidermal growth factor and vascular endothelial growth factor are able to increase ZO-1 tyrosine phosphorylation, modulate its subcellular localization, and consequently lead to increased permeability (37–39). ZO-1 serves an important function in maintaining tight junction integrity, which is disrupted in a number of invasive cancers and intestinal diseases (40). Consequently, studies have demonstrated that ZO-1 downregulation is involved in tumor development and progression (41,42).
To the best of our knowledge, the present study is the first to investigate the expression of ZO-1 and its function in LC progression. Using RT-qPCR and western blot analysis, it was identified that the expression of ZO-1 was decreased at the mRNA and protein levels in the majority of tumor tissues. Furthermore, it was also identified that overexpression of ZO-1 significantly inhibited cell viability and migration of LC cells in vitro. In addition, upregulation of ZO-1 induced cell cycle arrest. These results suggested that ZO-1 could also serve a tumor suppressor function in LC, and that abnormal ZO-1 expression may be associated with tumor progression and metastasis of LC. Further investigation into the potential molecular mechanism underlying the effects of ZO-1 are required. The mechanisms which contributed to ZO-1 downregulation in LC also require further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XZ, YQ and CN designed the study. XZ, YQ, LW, HZ and FT conducted the experiments, performed the data analysis and wrote the manuscript. CN and YQ analyzed the data and revised the manuscript. All authors discussed the results and reviewed the manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from each patient and the present study was approved by the Ethics Committee of Liaocheng People's Hospital (Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Parkin DM, Bray MF, Ferlay MJ, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Schütte K, Bornschein J, Malfertheiner P. Liver cancer-epidemiological trends and risk factors. Dig Dis. 2009;27:80–92. doi: 10.1159/000218339. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Cai MY, Tong ZT, Zheng F, Liao YJ, Wang Y, Rao HL, Chen YC, Wu QL, Liu YH, Guan XY, et al. EZH2 protein: A promising immunomarker for the detection of liver cancers in liver needle biopsies. Gut. 2011;60:967–976. doi: 10.1136/gut.2010.231993. [DOI] [PubMed] [Google Scholar]
- 5.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 6.Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of liver cancer increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 8.Chong CC, Wong GL, Lai PB. Impact of antiviral therapy on post-hepatectomy outcome for hepatitis B-related liver cancer. World J Gastroenterol. 2014;20:6006–6012. doi: 10.3748/wjg.v20.i20.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen SK, Brown RS, Siegel AB. Liver cancer: Review of current treatment with a focus on targeted molecular therapies. Therap Adv Gastroenterol. 2010;3:55–66. doi: 10.1177/1756283X09346669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukita S, Furuse M, Itoh M. Molecular architecture of tight junctions: Occludin and ZO-1. Soc Gen Physiol Ser. 1997;52:69–76. [PubMed] [Google Scholar]
- 15.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 16.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179–35185. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 17.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in Cadherin-based cell adhesion through its direct binding to α catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provost E, Rimm DL. Controversies at the cytoplasmic face of the cadherin-based adhesion complex. Curr Opin Cell Biol. 1999;11:567–572. doi: 10.1016/S0955-0674(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 20.Fanning AS, Anderson JM. PDZ domains: Fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999;103:767–772. doi: 10.1172/JCI6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauro L, Bartucci M, Morelli C, Andò S, Surmacz E. IGF-I receptor-induced cell-cell adhesion of MCF-7 breast cancer cells requires the expression of junction protein ZO-1. J Biol Chem. 2001;276:39892–39897. doi: 10.1074/jbc.M106673200. [DOI] [PubMed] [Google Scholar]
- 22.Hoover KB, Liao SY, Bryant PJ. Loss of the Tight Junction MAGUK ZO-1 in breast cancer: Relationship to glandular differentiation and Loss of heterozygosity. Am J Pathol. 1998;153:1767–1773. doi: 10.1016/S0002-9440(10)65691-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaihara T, Kusaka T, Nishi M, Kawamata H, Imura J, Kitajima K, Itoh-Minami R, Aoyama N, Kasuga M, Oda Y, et al. Dedifferentiation and decreased expression of adhesion molecules, E-cadherin and ZO-1, in colorectal cancer are closely related to liver metastasis. J Exp Clin Cancer Res. 2003;22:117–123. [PubMed] [Google Scholar]
- 24.Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S, et al. Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol. 1997;151:45–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM, Birembaut P, Gilles C. Beta-catenin and ZO-1: Shuttle molecules Involved in tumor Invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185:61–65. doi: 10.1159/000101304. [DOI] [PubMed] [Google Scholar]
- 26.Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, Kondo R, Nomura Y, Koura K, Ueda K, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37:496–505. doi: 10.1097/PAS.0b013e31827332b0. [DOI] [PubMed] [Google Scholar]
- 27.Pirisi M, Leutner M, Pinato DJ, Avellini C, Carsana L, Toniutto P, Fabris C, Boldorini R. Reliability and reproducibility of the edmondson grading of hepatocellular carcinoma using paired core biopsy and surgical resection specimens. Arch Pathol Lab Med. 2010;134:1818–1822. doi: 10.5858/2009-0551-OAR1.1. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Liu H, Luo L. Analysis of relative gene expression using different real-time quantitative PCR. Acta Agronomica Sinica. 2007;33:1214–1218. (In Chinese) [Google Scholar]
- 29.Yang Y, Liu YM, Wei MY, Wu YF, Gao JH, Liu L, Zhou WP, Wang HY, Wu MC. The liver tissue bank and clinical database in China. Front Med China. 2010;4:443–447. doi: 10.1007/s11684-010-0190-7. [DOI] [PubMed] [Google Scholar]
- 30.Fares N, Péron JM. Epidemiology, natural history, and risk factors of liver cancer. Rev Prat. 2013;63(216-217):220–222. (In French) [PubMed] [Google Scholar]
- 31.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 32.Ng KK, Lo CM, Chan SC, Chok KS, Cheung TT, Fan ST. Liver transplantation for liver cancer: The Hong Kong experience. J Hepatobiliary Pancreat Sci. 2010;17:548–554. doi: 10.1007/s00534-009-0165-8. [DOI] [PubMed] [Google Scholar]
- 33.Amram ML, Benamran DA, Roth AD. Targeted therapies in digestive oncology. Rev Med Suisse. 2011;7:1131-1132–1134-1136. (In French) [PubMed] [Google Scholar]
- 34.Jain S, Singhal S, Lee P, Xu R. Molecular genetics of hepatocellular neoplasia. Am J Transl Res. 2010;2:105–118. [PMC free article] [PubMed] [Google Scholar]
- 35.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Itallie CM, Balda MS, Anderson JM. Epidermal growth factor induces tyrosine phosphorylation and reorganization of the tight junction protein ZO-1 in A431 cells. J Cell Sci. 1995;108:1735–1742. doi: 10.1242/jcs.108.4.1735. [DOI] [PubMed] [Google Scholar]
- 38.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 39.Merwin JR, Anderson JM, Kocher O, Van Itallie CM, Madri JA. Transforming growth factor beta 1 modulates extracellular matrix organization and cell-cell junctional complex formation during in vitro angiogenesis. J Cell Physiol. 1990;142:117–128. doi: 10.1002/jcp.1041420115. [DOI] [PubMed] [Google Scholar]
- 40.Giri S, Poindexter KM, Sundar SN, Firestone GL. Arecoline induced disruption of expression and localization of the tight junctional protein ZO-1 is dependent on the HER 2 expression in human endometrial Ishikawa cells. BMC Cell Biol. 2010;11:53. doi: 10.1186/1471-2121-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Németh Z, Szász AM, Somorácz Á, Tátrai P, Németh J, Gyorffy H, Szíjártó A, Kupcsulik P, Kiss A, Schaff Z. Zonula occludens-1, occludin, and E-cadherin protein expression in biliary tract cancers. Pathol Oncol Res. 2009;15:533–539. doi: 10.1007/s12253-009-9150-4. [DOI] [PubMed] [Google Scholar]
- 42.Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004;40:2717–2725. doi: 10.1016/j.ejca.2004.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.