Abstract
The biological features of pancreatic cancer and the associated hypoxic environment around the cancer cells often lead to resistance to radiotherapy and chemotherapy. The present study was performed in order to explore the effect pancreatic stellate cells (PSCs) have on the proliferation of pancreatic cancer cells. In the present study, PSCs from human pancreatic cancer tissues were isolated, and the PSCs markers α-smooth muscle actin and desmin were overexpressed in the cytoplasm of PSCs. An MTT assay revealed that PSCs promoted the viability of pancreatic cancer cells. However, the viability of pancreatic cancer cells promoted by PSCs was partially blocked by SB525334. Cellular invasion analysis demonstrated that PSCs promoted the invasion ability of pancreatic cancer cells. An apoptosis assay indicated that PSCs decreased the level of apoptosis induced by gemcitabine. In vivo experiments consisting of mice bearing MIA-PaCa-2 and PSCs demonstrated an increase in the rate of tumor growth compared with MIA-PaCA-2 alone, whereas SB525334 may delay the tumor progression induced by PSCs. The present findings indicated that PSCs promoted the viability and invasion of pancreatic cancer cells, and decreased the apoptosis of pancreatic cancer cells induced by gemcitabine.
Keywords: pancreatic cancer, pancreatic stellate cells, proliferation, invasion
Introduction
Pancreatic cancer is an aggressive and lethal disease with a poor prognosis, representing the fourth leading cause of cancer-associated mortality worldwide (1). The overall prognosis of pancreatic cancer has remained unchanged even though advances have been made in surgical techniques and adjuvant treatment regimens (2). The biological features of pancreatic cancer and the hypoxic environments surrounding cancer cells often lead to resistance to radiotherapy and chemotherapy (3). The tumor microenvironment has an active role in the progression of pancreatic cancer, and targeting the components of this microenvironment may denote a novel therapeutic strategy (4). Pancreatic cancer is characterized by an abundant desmoplastic/stromal reaction. It is reported that pancreatic stellate cells (PSCs) are responsible for the development of the desmoplastic reaction (5). As one of the most important types of stromal cell in the microenvironment of pancreatic cancer cells, PSCs have become notable (6,7).
Normally, PSCs are in their quiescent state, characterized by abundant vitamin A stored in lipid droplets in the cytoplasm (8). In response to pancreatic injury or pancreatic inflammation, PSCs are activated, transforming from quiescent phenotypes into myofibroblast-like cells that express the cytoskeletal protein α-smooth muscle actin (α-SMA), and synthesize excessive amounts of extracellular matrix (ECM) proteins leading to fibrosis (9,10). The interaction between pancreatic cancer cells and PSCs is the focus of an increasing number of studies. There is accumulating evidence that PSCs have an important role in the progression of pancreatic fibrosis in chronic pancreatitis, as well as in pancreatic cancer (11–13). PSCs interact with pancreatic cancer cells to create a fibrotic and hypoxic microenvironment, which promotes the initiation, development of and resistance to chemoradiation therapy. However, the specific role of PSCs in pancreatic cancer is currently unclear, and the association between PSCs and pancreatic cancer cells is under discussion. The present study was performed in order to explore the effect of PSCs on the viability of pancreatic cancer cells, and to elucidate the potential molecular mechanisms underlying the interaction between PSCs and pancreatic cancer cells.
Materials and methods
Cell culture
The PANC-1 and MIA-PaCa-2 human pancreatic cancer cell lines (American Type Culture Collection, Manassas, VA, USA) were grown at 37°C in Dulbeccos modified Eagles medium (DMEM) supplemented with high glucose, 1.5 g/l sodium bicarbonate, 100 U/ml penicillin, 100 mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 10% fetal calf serum (FCS; HyClone; GE Healthcare, Logan, UT, USA). AsPC-1 cells (American Type Culture Collection) were maintained in RPMI-1640 (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% FCS, penicillin (100 U/ml) and streptomycin (100 mg/ml). Cells were maintained in a 5% CO2 humidified incubator at 37°C.
Human PSCs were isolated as previously described (14). The protocols used in the present study were approved by the Ethics Committee of the Affiliated Hospital of Qingdao University, and conducted in full accordance with ethical principles. All patients gave informed consent to use excess pathological specimens for research and human pancreatic cancer tissues were collected with written informed consent from patients prior to participation in the study. The resected pancreatic tissues from patients who had undergone surgery for pancreatic cancer were digested with a mixture of collagenase P, pronase and DNase in Geys balanced salt solution (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). The suspension of cells was centrifuged in a 28.7% Nycodenz gradient at 1,400 × g for 23 min at room temperature. Stellate cells separated into a hazy band just above the interface of the Nycodenz solution and the aqueous buffer. This band was harvested, and the cells were washed and resuspended in Iscove's modified Dulbecco's medium (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS (Hyclone; GE Healthcare, Life Sciences, Logan, UT, USA), 4 mmol/l glutamine (Life Technologies; Thermo Fisher Scientific, Inc.) and antibiotics (Invitrogen; Thermo Fisher Scientific, Inc.). The cultured PSCs were used with their second-fourth passages. The expression of glial fibrillary acidic protein (GFAP) and α-SMA was examined by immunofluorescent staining. Briefly, PSCs were incubated without serum for 24 h at 37°C and fixed in 4% paraformaldehyde for 30 min at room temperature. Following blocking with 1% normal bovine serum albumin (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 1 h at room temperature, cells were incubated with mouse anti α-SMA antibody (Sigma-Aldrich, Merck KGaA; catalog number: A5228) or anti-GFAP antibody (Abcam; catalog number: ab7260) at 1:400 dilution overnight at 4°C. Following washing with PBS, PSCs cells were incubated with anti-rabbit Alexa 488Yconjugated IgG at 1:100 dilution (Abcam; catalog number: ab150077) and Alexa 555Y labeled anti-mouse IgG antibody (Abcam; catalog number: AB_2563179) at 1:100 dilution for 1 h at 4°C and washed again with PBS, and then samples were analyzed for fluorescence under a confocal laser scanning microscope (Nikon A1/C1, Tokyo, Japan). For the negative control, the primary antibody was replaced with 2% bovine serum albumin or polyclonal rabbit IgG at 1:200 dilution (Abcam; catalog number: ab6721).
Co-culture of pancreatic cancer cells and PSCs
Pancreatic cancer cells were seeded at a density of 2×105 cells/well into 6-well culture plates (BD Biosciences, Franklin Lakes, NJ, USA). Human PSCs were seeded at 4×105 cells/culture insert into culture inserts with 1.0 µm pores (BD Biosciences). The subsequent day, the culture inserts seeded with PSCs were placed into the 6-well plates containing pancreatic cancer cells, and incubation was continued for ≤3 days at 37°C.
Cellular viability assay
PANC-1, MIA-PaCa-2 and AsPC-1 pancreatic cancer cell lines in the exponential phase of growth were seeded into a 96-well tissue culture plate with (1×104)/well and incubated for 24 h at 37°C. The cells were co-cultured with medium alone (control group) or PSCs or SB525334, an inhibitor for TGF-beta1 receptor, (Abmole Bioscience Inc., Huston, TX, USA; Catalog number: M2108) for 12, 24 and 48 h for evaluation. Cell viability was determined using an MTT assay, according to the manufacturer's protocol (kit CGD1, Sigma-Aldrich,). Briefly, cells were grown in media supplements with 10% FBS, harvested using trypsin (Thermo Fisher Scientific, Inc.; Catalog number: 25200056) and counted using Trypan blue and a hemocytometer. Cells were then serially diluted in a clear cell culture plate and incubated for 3 h with MTT reagent at 37°C. Following this incubation, cells were treated with MTT solvent for 15 min at room temperature and measured with spectrophotometer (SKU: S500, Parco Scientific Company, USA). Absorbance was measured at OD=590 nm.
Cellular invasion analysis
A cellular migration assay was performed using modified Boyden Chambers consisting of Transwell pre-coated Matrigel membrane filter inserts with 8 µm pores in 24-well tissue culture plates (BD Biosciences). Briefly, PSCs were seeded at 5×104 cells/well into the plates. Media supplemented with 10% FCS in the lower chamber served as the chemoattractant. The following day, pancreatic cancer cells were seeded at 2×105 cells/insert into the culture inserts (8 µm pores) and placed on the 24-well plate containing human PSCs or SB525334. After a 24-h incubation at 37°C, the stained cells were counted by inverted microscopy. Briefly, cells were removed from the upper surface of the membranes with a cotton swab, and cells that migrated to the lower surface were stained with 0.2% (w/v) crystal violet in 2% ethanol for 15 min at room temperature and were washed with water. Dried membranes were cut out and mounted on glass slides in immersion oil. At least 10 random high-power fields from each of triplicate membranes were counted for each experimental condition at ×200 magnification. Each experiment was performed in triplicate.
Apoptosis assay
The apoptosis of MIA-PaCa-2 and AsPC-1 pancreatic cancer cells, with or without human PSCs, was induced by gemcitabine and detected by flow cytometry using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China) according to the manufacturer's protocol. Briefly, the cells were harvested and stained with propidium iodide (PI) and Annexin V-FITC. The apoptosis rate was assayed using a FACSCalibur flow cytometer (BD Biosciences and Beckman-Coulter, Inc., San Jose, CA, USA) at a 488-nm wavelength.
In vivo experiments
BALB/c female nude mice at the age of 6–8 weeks (weighing, 18–22 g) were used. BALB/c mice were purchased from the Center of Experimental Animals, Affiliated Hospital of Medical College, Qingdao University, and maintained in a pathogen-free facility at room temperature. Mice had free access to food and water and were kept in a 12 h light/dark cycle. Female BALB/c nude mice were divided into the following groups: MIA-PaCa-2 alone as the control group; co-transplantation of MIA-PaCa-2 and PSCs group; co-transplantation of MIA-PaCa-2, PSCs and SB525334 group. MIA-PaCa-2 (1×105) and PSCs (5×104) or SB525334 were suspended in 100 µl PBS, and then subcutaneously co-transplanted with PSCs (5×104) into the right flank of the mice. In total, 10 mice were used in each group, and the number of mice used in the experiment is 30. Tumor sizes and body weight were measured every week by external caliper, and the average tumor volumes were calculated as (length × weight2). A total of 2.5 weeks subsequent to implantation, mice were humanely euthanized with 3% pentobarbital sodium (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and tumors were harvested. All protocols were approved by the Animal Ethics Committee of the Affiliated Hospital of Medical College, Qingdao University.
Statistical analysis
Results are presented as the mean ± standard deviation of triplicate cultures, and statistical differences were assessed using the Student's t-test or two-way analysis of variance. P<0.05 was considered to indicate a statistically significant difference. Data were analyzed using SPSS version 16.0 (SPSS, Chicago, IL, USA).
Results
Isolation and identification of PSCs
PSCs from human pancreatic cancer tissues were isolated by the aforementioned enzymatic digestion-gradient centrifugation method. Attached cells exhibited an activated phenotype, characterized by a myofibroblast-like appearance and a shortening of double time. Under cytoimmunochemistry examination, the PSC markers α-SMA and GFAP were overexpressed in the cytoplasm of the PSCs.
In vitro proliferation assay
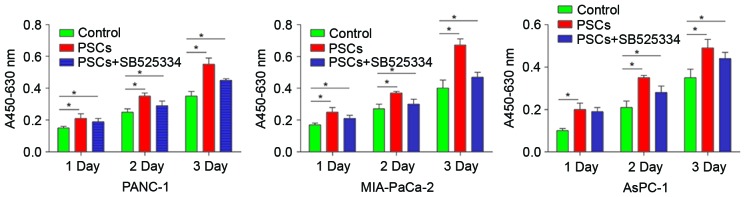
The AsPC-1, PANC-1 and MIA-PaCa-2 (1×105) pancreatic cancer cell lines and PSCs (5×104) were seeded into the upper and lower chambers, respectively. The cells were then co-cultured for 72 h. Subsequently, an MTT assay was performed to determine the number of pancreatic cancer cells in the upper chamber, and the results were expressed as the absorbance at 490 nm in a spectrophotometer. The MTT assay revealed that, subsequent to a 72-h co-culture with PSCs, the viability of cancer cells significantly increased, compared with those in the control group without co-culture (P<0.05). The effect of PSCs on the viability of AsPC-1 was significantly higher compared with the control (140.13±12.50% vs. 104.21±8.9%; P=0.014). However, the proliferation of pancreatic cancer cells promoted by PSCs was partially blocked by the transforming growth factor-β (TGF-β) inhibitor, SB525334 (125.71±11.30% vs. 140.13±12.50%; P=0.036; Fig. 1). The viability of PANC-1 in control, PSCs and SB525334 group was 65.06±10.9, 137.13±11.30 and 99.78±13.11% respectively (P=0.017). The viability of MIA-PaCa-2 in control, PSCs and SB525334 group was 87.26±11.1, 165.73±15.35 and 95.18±11.09% (P=0.008), demonstrating that PANC-1 and MIA-PaCa-2 also exhibited similar results. These findings demonstrated that PSCs promoted the viability of pancreatic cancer cells, and the effects may be partially inhibited by SB525334.
Figure 1.
PSCs promote the viability of AsPC-1, PANC-1 and MIA-PaCa-2 cells, and these effects may be partially inhibited by SB525334. The viability of cancer cells significantly increased, compared with those in the control group without co-culture (P<0.05). The effect of PSCs on the viability of AsPC-1 was significantly higher compared with the control (140.13±12.50%; P=0.014). However, the proliferation of pancreatic cancer cells promoted by PSCs was partially blocked by the transforming growth factor-β (TGF-β) inhibitor, SB525334. *P<0.05. PSCs, pancreatic stellate cells.
Cells migration analysis
A Matrigel invasion assay was performed to evaluate the effect of PSCs on the invasion ability of pancreatic cancer cells. With the presence of PSCs in the lower chambers, the invasion capacity of AsPC-1 cells was prone to increase as compared with the control (437.50±21.61%; P<0.001). A significant increase in the migration of MIA-PaCa-2 cells was also induced in the presence of PSCs, as compared with the control (353.85±19.16%; P=0.013; Fig. 2).
Figure 2.
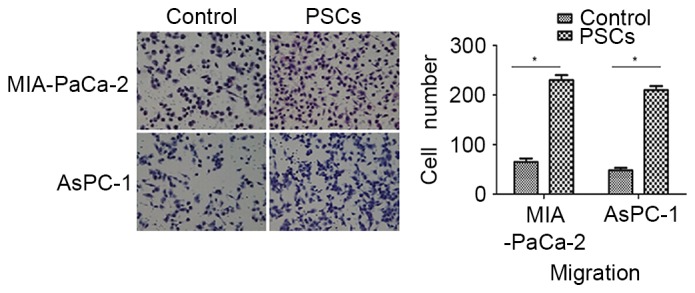
PSCs increase the invasive ability of AsPC-1 and MIA-PaCa-2 cells. With the presence of PSCs in the lower chambers, the invasion capacity of AsPC-1 cells was prone to increase as compared with the control (437.50±21.61%; P<0.001). A significant increase in the migration of MIA-PaCa-2 cells was also induced in the presence of PSCs, as compared with the control (353.85±19.16%; P=0.013). *P<0.05. The number of invasive cells was calculated by counting the cells from 10 randomly selected fields of view at ×200 magnification. PSCs, pancreatic stellate cells.
Apoptosis assay
The percentage of apoptotic cells in the two groups was increased with the extension of the intervention time, and the percentage of apoptotic cells was significantly different (P<0.05). The percentage of apoptotic cells for AsPC-1 in the PSCs group was decreased compared with that in control group at 48 h subsequent to intervention (32.7±6.1% vs. 55.3±9.1%; P=0.027; Fig. 3). The percentage of apoptotic cells for MIA-PaCa-2 cells in PSCs group and in control group was 39.6±7.3% vs. 68.3±8.7% (P=0.013), a similar result being observed for MIA-PaCa-2 cells.
Figure 3.
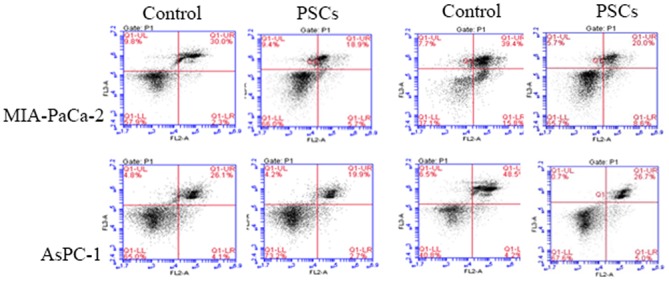
The percentage of apoptotic cells in the pancreatic cancer cell population induced by gemcitabine was decreased by PSCs. The percentage of apoptotic cells for AsPC-1 in the PSCs group was decreased compared with that in control group at 48 h subsequent to intervention (32.7±6.1% vs. 55.3±9.1%; P=0.027). PSCs, pancreatic stellate cells.
In vivo experiments
To evaluate the effects of PSCs on in vivo tumor growth, MIA-PaCa-2 cells and PSCs or SB525334 were co-transplanted into nude mice. The results demonstrated that PSCs significantly enhanced the growth of MIA-PaCa-2 cells compared with the control group (P=0.003). Mice bearing MIA-PaCa-2 cells and PSCs exhibited an increase in the rate of tumor growth compared with MIA-PaCA-2 cells alone, whereas SB525334 delayed the tumor progression induced by PSCs. The tumor volumes in the control group and co-transplantion group differed significantly at 3, 4 and 5 weeks subsequent to implantation (the P-values were 0.02, 0.013 and 0.007 respectively). In total, 5 weeks subsequent to implantation, the mean tumor volume changed to 1,374.6±195.8 mm3 from 109.0±14.7 mm3 in the control group, compared with in the mice with co-transplanted MIA-PaCa-2 cells and PSCs (2,548.6±142.9 mm3) or with co-transplanted MIA-PaCa-2 cells and SB525334 (2,015.7±202.3 mm3; Fig. 4). Body weight was not significantly different among the three groups.
Figure 4.
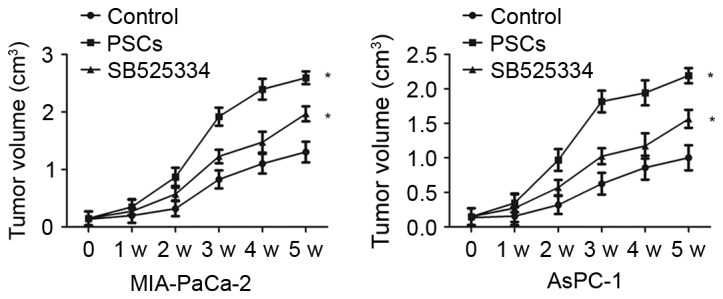
PSCs significantly enhances the growth of MIA-PaCa-2 and AsPC-1 cells, as compared with the control group in vivo. The mean tumor volume changed to 1,374.6±195.8 mm3 from 109.0±14.7 mm3 in the control group, compared with in the mice with co-transplanted MIA-PaCa-2 cells and PSCs (2,548.6±142.9 mm3) or with co-transplanted MIA-PaCa-2 cells and SB525334 (2,015.7±202.3 mm3 after 5 weeks implantation. *P<0.05. PSCs, pancreatic stellate cells.
Discussion
The incidence of pancreatic cancer is gradually increasing and the mortality rate has not reduced significantly (1). The pathogenesis of pancreatic cancer has primarily focused on the molecular biology of the tumor cells (15). Previously, it has been reported that the initiation, growth and progression of cancer is associated with intricate crosstalk between the tumor and stroma (16). The interaction between pancreatic cancer cells and the surrounding microenvironment, which consists of ECM proteins, growth factors and constituent non-tumor cells, remains largely ignored (17). In 2004, PSCs were revealed to produce the ECM proteins and were the principal source of collagen in the fibrotic matrix, which comprised the pancreatic tumor stroma (18). Additionally, the presence of activated PSCs in surrounding human pancreatic intraepithelial neoplastic lesions (Pan-INs) demonstrated that they may function during the early stages of cancer development (10). Therefore, the interaction between pancreatic cancer cells and PSCs is receiving increasing attention.
The present study isolated, identified and cultured human PSCs. The role of PSCs in stimulating pancreatic cancer cell migration, invasion and resistance to chemotherapy was determined. The findings of the present study may be summarized as follows: Activated PSCs are present in pancreatic cancer tissues; PSCs promote the viability of pancreatic cancer cells; the viability induced by PSCs may be blocked by SB525334, an inhibitor of TGF-β; PSCs increase the invasive ability of pancreatic cancer cells; PSCs decrease the degree of apoptosis induced by gemcitabine, indicating that PSCs have an important role in the resistance of pancreatic cancer cells to gemcitabine; co-injection of pancreatic cancer cells with PSCs in an orthotopic model resulted in increased tumor volume.
TGF-β1 is a pleiotropic cytokine that controls cellular proliferation, differentiation, adhesion, migration, apoptosis and the epithelial-mesenchymal transition (19). TGF-β1 also has a vital role in the interaction between cellular and non-cellular components of the tumor microenvironment (20). It has previously been demonstrated that TGF-β1 promotes the activation and proliferation of PSCs, as well as the epithelial-mesenchymal transition of pancreatic cancer cells (21,22). SB525334 is a selective inhibitor of TGF-β1 (23). In the present study, SB525334 partially blocked the increased proliferation and the invasive ability of pancreatic cancer cells induced by PSCs. Additionally, SB525334 reduced the resistance of pancreatic cancer cells to gemcitabine caused by PSCs.
In the present study, the association between pancreatic cancer cells and PSCs has been determined. However, the mechanism by which PSCs regulate the proliferation and apoptosis of pancreatic cancer cells remains unclear. In particular, signaling pathways involved in genetic alterations during the process of PSC activation must be characterized. The origin of activated PSCs arising from the existing pool of quiescent PSCs or from mesenchymal stem cells remains controversial (24,25). An improved understanding of the pathophysiology of pancreatic cancer is possible following the resolution of current challenges in the field. PSCs are a potential novel therapeutic target, which may improve the outcome of pancreatic cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81802888; and 81572314) and the Key Research and Development Project of Shandong Province (grant no. 2018GSF118088).
Availability of data and materials
The datasets used and analyzed for the present study are available from the corresponding author upon reasonable request.
Authors' contributions
SL, YL and YZ designed the experiments, contributed to the analysis and interpretation of data, and wrote the initial draft of the manuscript. SC and BS performed the experiments. DC and DW revised the paper, assisted with the experiments and supervised the experimental work. All authors approved the final manuscript.
Ethics approval and consent to participate
Tissue samples were collected once ethical approval was obtained from the Ethics Committee of the Affiliated Hospital of Qingdao University. In all cases written informed consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Dai MH, Liu SL, Chen NG, Zhang TP, You L, Q Zhang F, Chou TC, Szalay AA, Fong Y, Zhao YP. Oncolytic vaccinia virus in combination with radiation shows synergistic antitumor efficacy in pancreatic cancer. Cancer Lett. 2014;344:282–290. doi: 10.1016/j.canlet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Ma Q, Xu Q, Duan W, Lei J, Wu E. Targeting the cancer-stroma interaction: A potential approach for pancreatic cancer treatment. Curr Pharm Des. 2012;18:2404–2415. doi: 10.2174/13816128112092404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vonlaufen A, Phillips PA, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV. Pancreatic stellate cells and pancreatic cancer cells: An unholy alliance. Cancer Res. 2008;68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 6.McCarroll JA, Naim S, Sharbeen G, Russia N, Lee J, Kavallaris M, Goldstein D, Phillips PA. Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front Physiol. 2104;5:141. doi: 10.3389/fphys.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hata T, Kawamoto K, Eguchi H, Kamada Y, Takamatsu S, Maekawa T, Nagaoka S, Yamada D, Iwagami Y, Asaoka T, et al. Fatty acid-mediated stromal reprogramming of pancreatic stellate cells induces inflammation and fibrosis that fuels pancreatic cancer. Pancreas. 2017;46:1259–1266. doi: 10.1097/MPA.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 8.Pang TCY, Wilson JS, Apte MV. Pancreatic stellate cells: What's new? Curr Opin Gastroenterol. 2017;33:366–373. doi: 10.1097/MOG.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 9.Apte MV, Wilson JS. Dangerous liaisons: Pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012;27(Suppl 2):S69–S74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 10.Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 12.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J Clin Invest. 2007;117:150–159. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masamune A, Watanabe T, Kikuta K, Shimosegawa T. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7:S48–S54. doi: 10.1016/j.cgh.2009.07.038. (11 Suppl) [DOI] [PubMed] [Google Scholar]
- 14.Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Hamada S, Shimosegawa T. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2009;58:550–559. doi: 10.1136/gut.2008.154401. [DOI] [PubMed] [Google Scholar]
- 15.Ohnishi H, Miyata T, Yasuda H, Satoh Y, Hanatsuka K, Kita H, Ohashi A, Tamada K, Makita N, Iiri T, et al. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J Biol Chem. 2004;279:8873–8878. doi: 10.1074/jbc.M309698200. [DOI] [PubMed] [Google Scholar]
- 16.Mazzocca A, Fransvea E, Dituri F, Lupo L, Antonaci S, Giannelli G. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor beta blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology. 2010;51:523–534. doi: 10.1002/hep.23285. [DOI] [PubMed] [Google Scholar]
- 17.Dunér S, Lopatko Lindman J, Ansari D, Gundewar C, Andersson R. Pancreatic cancer: The role of pancreatic stellate cells in tumor progression. Pancreatology. 2010;10:673–681. doi: 10.1159/000320711. [DOI] [PubMed] [Google Scholar]
- 18.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Gupta DK, Singh N, Sahu DK. TGF-β mediated crosstalk between malignant hepatocyte and tumor microenvironment in hepatocellular carcinoma. Cancer Growth Metastasis. 2014;7:1–8. doi: 10.4137/CGM.S14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu FL, Mo EP, Yang L, Du J, Wang HS, Zhang H, Kurihara H, Xu J, Cai SH. Autophagy is involved in TGF-β1-induced protective mechanisms and formation of cancer-associated fibroblasts phenotype in tumor microenvironment. Oncotarget. 2016;7:4122–4141. doi: 10.18632/oncotarget.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Cao Y, Yang W, Duan C, Aronson JF, Rastellini C, Chao C, Hellmich MR, Ko TC. BMP2 inhibits TGF-β-induced pancreatic stellate cell activation and extracellular matrix formation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G804–G813. doi: 10.1152/ajpgi.00306.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S, Satoh K, Egawa S, Unno M, Shimosegawa T. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 23.Guan S, Xu W, Han F, Gu W, Song L, Ye W, Liu Q, Guo X. Ginsenoside Rg1 attenuates cigarette smoke-induced pulmonary epithelial-mesenchymal transition via inhibition of the TGF-β1/Smad pathway. Biomed Res Int. 2017;2017:7171404. doi: 10.1155/2017/6510198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian L, Lu Z, Miao Y. Primary cultures for pancreatic stellate cells (PSCs) Methods Mol Biol. 2019;1882:149–155. doi: 10.1007/978-1-4939-8879-2_13. [DOI] [PubMed] [Google Scholar]
- 25.Kawakubo K, Ohnishi S, Fujita H, Kuwatani M, Onishi R, Masamune A, Takeda H, Sakamoto N. Effect of fetal membrane-derived mesenchymal stem cell transplantation in rats with acute and chronic pancreatitis. Pancreas. 2016;45:707–713. doi: 10.1097/MPA.0000000000000541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed for the present study are available from the corresponding author upon reasonable request.