Abstract
Background and Aims:
Bupivacaine is the most frequently used local anaesthetic for spinal anaesthesia, however, use of levobupivacaine in clinical practice has advanced recently. This study aimed to compare the anaesthetic potency and haemodynamic effects of intrathecal bupivacaine with buprenorphine versus levobupivacaine with buprenorphine in infraumbilical surgeries.
Methods:
This prospective randomised double blind study was conducted in seventy patients aged 18–65 years, American Society of Anesthesiologists grade I-II, scheduled for lower abdominal and lower limb surgery under spinal anaesthesia. The patients either received 0.5% isobaric racemic bupivacaine 3 ml with 2 μg/kg of buprenorphine (Group B) or 0.5% isobaric levobupivacaine 3 ml with 2 μg/kg of buprenorphine (Group L). The time for onset of sensory block between the two groups was the priomary end-point. Other measurements included haemodynamic variables, sensory and motor blockade characteristics, postoperative analgesia, and complications in the first 24 h.
Results:
There was no significant difference in the onset of sensory block between the two groups. Sensory and motor blockade characteristics were similar between the two groups. However, there was significant fall in the heart rate at 5 min in Group B compared to Group L. There was statistically significant fall in systolic blood pressure in group B compared to Group L from 5 min up to 60 min and fall in diastolic blood pressure from 10 min to 45 min.
Conclusion:
Our study showed that onset of sensory block is similar between isobaric levobupivacaine with buprenorphine 37 38 and isobaric bupivacaine with buprenorphine.
Key words: Buprenorphine, intrathecal, levobupivacaine
INTRODUCTION
Spinal anaesthesia with bupivacaine is the most common technique used in patients undergoing lower limb and lower abdominal surgeries.[1] However, bradycardia and systemic hypotension are the most common side-effects seen with this technique. Marked hypotension may be harmful, particularly in elderly patients with limited cardiac reserve.[2] Levobupivacaine, an amide local anaesthetic agent, is the isolated S-enantiomer of racaemic bupivacaine. It is the most recent long acting local anaesthetic agent to have been introduced for clinical use.[3] Reports of toxicity with levobupivacaine are scarce, and occasional toxic symptoms are usually reversible with minimal treatment without any fatal outcome. However, levobupivacaine has not entirely replaced bupivacaine in clinical practice.[4] In comparative trials, although its clinical effects were not significantly different from those of bupivacaine, there was some variability in efficacy findings in different clinical populations.[5] The clinical studies available on intrathecal anaesthesia with levobupivacaine suggest that it achieves satisfactory surgical anaesthesia but with an unpredictable spread of sensory blockade.[4] To improve the block characteristics of intrathecally administered local anaesthetics, addition of adjuvant is widely in practice. Neuraxial opioids are widely used for providing intraoperative and postoperative analgesia without prolonging motor and sympathetic block.[6,7] However, literature on the anaesthetic potency of intrathecally administered levobupivacaine with buprenorphine is less, which prompted us to conduct this study. Therefore, we designed a double blinded clinical trial to compare the anaesthetic efficacy and haemodynamic effects of levobupivacaine (15 mg) and isobaric bupivacaine (15 mg) with buprenorphine (2 μg/kg) for spinal anaesthesia.
METHODS
Seventy patients aged 18-65 years with American Society of Anesthesiologists (ASA) class I and II were enrolled in the study after ethical committee approval and written informed consent. Patients posted for elective lower abdominal and lower limb surgeries under spinal anaesthesia formed the study population. Seventy patients were selected from the study population using computer generated random table (https://www.randomizer.org/). Patients were excluded if they were ASA class III and IV, known hypersensitivity to amide local anaesthetics, general contraindications against spinal anaesthesia, and morbidly obese (150% the ideal weight or >130 kg). The patients were randomly allocated into one of the two study groups using sealed envelope technique. Group B received 3 ml of preservative free bupivacaine 0.5% isobaric (Anawin™, bupivacaine hydrochloride, Neon laboratories, India) with buprenorphine 2 μg/kg, and group L received 3 ml levobupivacaine 0.5% isobaric (Levoanawin™, levobupivacaine hydrochloride, Neon laboratories, India) with buprenorphine 2 μg/kg for spinal anaesthesia. Buprenorphine (Buprigesic™, buprenorphine hydrochloride, Neon laboratories, India) was loaded undiluted from the ampoule of buprenorphine containing 300 μg/ml.
A day before surgery detailed preanaesthetic check-up was done. General physical examination along with proper systemic examination, assessment of airway, and local examination of lumbar spine was done. Relevant investigations were reviewed. Numerical Rating Scale (NRS) was explained to the patients to determine the level of analgesia in the postoperative period. It was carried out on a straightwith a 0–10 cm line (no pain at all - maximum pain imaginable). Patients were asked to restrict solids and fluids by mouth 8 h before surgery. Oral premedication with 0.5 mg of alprazolam was given on previous night of the surgery.
On the day of surgery, patients were shifted to the operation theater, and multipara monitor was attached. Baseline respiratory rate, heart rate (HR), non-invasive systolic and diastolic blood pressure (SBP and DBP), peripheral oxygen saturation (SpO2), and electrocardiography (ECG) were recorded, and continuous monitoring was started. Intravenous (IV) line was secured with 18-gauge Intracath™ and patients were preloaded with 10 ml/kg body weight of Ringer lactate solution over 15–20 min. Under strict aseptic conditions and with the patients in a sitting position, through a 25-gauge Quincke needle in the midline at L3-4 intervertebral space study drug was administered intrathecally. Study drug was prepared in similar syringes keeping the drug volume constant by an anaesthesiologist, who then handed over the syringe to another anaesthesiologist who was unaware of the contents of the syringe and performed the spinal block and also monitored all the patient variables. Immediately after administration, the patients were turned into supine position.
Sensory block was assessed by loss of sensation to pinprick in the midline with an 18 G blunt needle from below upwards. It was performed every 2 min for first 10 min and then at an interval of 5 min until no change in level occurred. Onset of sensory block (when patient does not feel pinprick at T10 level), highest level of sensory block achieved, time to maximum sensory block, and total duration of sensory block (regression to T10 dermatome) was noted.
Motor blockade was assessed according to a modified Bromage scale[8] (0 = no paralysis, able to flex hips/knees/ankles; 1 = able to move knees, unable to raise extended legs; 2 = able to flex ankles, unable to flex knees; and 3 = unable to move any part of the lower limb). These tests were performed every 2 min for up to 10 min after spinal anaesthesia. Maximum motor block achieved, time to maximum motor block, and total duration of motor block (from the time of intrathecal administration of the drug to motor recovery to Bromage 0) was noted. The surgical procedure was started 10 min after initiation of spinal anaesthesia. If the level of analgesia was inadequate, the regimen was switched to general anaesthesia. The haemodynamic variables and SpO2 were recorded before spinal anaesthesia and thereafter every 5 min until the end of the procedure. A decrease >25% from baseline, or to <60 mm Hg, in mean arterial pressure, was defined as hypotension and treated with mephentermine bolus 6 mg; a HR <50 bpm was defined as bradycardia and treated with 0.6 mg of atropine; and a decrease in SpO2 to <93% was defined as hypoxia and treated with supplemental oxygen using a face mask.
In postoperative unit, patients were monitored for haemodynamic parameters every 30 min until the sensory and motor variables were back to normal. The patients were asked to assess their level of pain according to the VAS every 15 min for 120 min, then half hourly for 180 min, hourly for 12 h, and thereafter every 3 h till 24 h of surgery in both groups. Rescue analgesia in the form of injection tramadol hydrochloride (2 mg/kg) IV was supplemented on complaining of pain (NRS >3) in both groups. Total duration of analgesia was considered from the time of subarachnoid administration of the drug to the time at which patient demanded first dose of rescue analgesia. Patients were monitored for any side effects or complications such as hypotension, bradycardia, nausea, vomiting, sedation, urinary retention, pruritus, headache, backache, and neurological changes for 24 h.
The primary outcome of the study was to compare the two groups in terms of time for onset of sensory blockade. The secondary outcome was to assess motor blockade, peak sensory/motor level, time to reach peak sensory/motor block, and degree of motor block of the two groups. Intraoperative haemodynamic effects and duration of analgesia were also assessed between the groups.
The sample size has been estimated based on the study by Glaser et al.[9] considering the difference between two means, substituting the values in standard deviation (SD) in the first group - 6, SD in the second group - 8, precision 10%, desired confidence level 95%, the sample size was calculated to be 30 in each group to provide power of 80%. To compensate for any losses, 35 patients/group were assigned as shown in the consort diagram [Figure 1].
Figure 1.
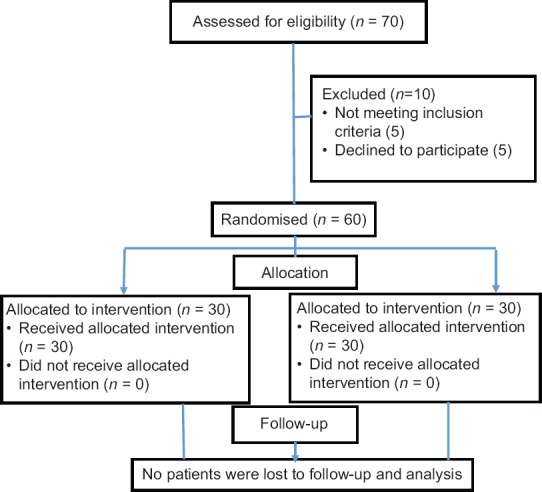
Consort diagram
Statistical Package for Social Sciences 20 software was used for statistical calculation. The statistical evaluation was performed using paired and unpaired t test and analysis of variance. Data are presented as mean ± standard deviation, and P < 0.05 was considered significant. The categorical data were analyzed using the Chi-square test.
RESULTS
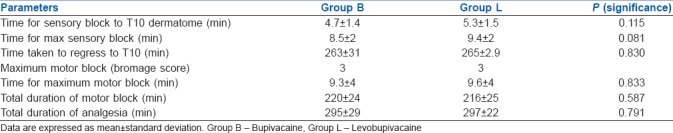
In the present study, both groups were comparable with respect to demographic characteristics as shown in Table 1. There were no significant differences between the two groups with respect to type of surgery, baseline haemodynamic parameters, or American Society of Anesthesiologists classification. After administering the study drug intrathecally, the mean time taken for onset of sensory block to T10 dermatome was 4.7 min and 5.3 min in Group B and Group L, respectively. The time taken to achieve maximum sensory block was comparable between the two groups. Twenty percent of patients in Group B reached T6 sensory level, whereas 43% of patients in Group L achieved T6 sensory level. Median maximum motor block achieved in both the groups was Bromage 3. Time for maximum motor block and total duration of motor block was also comparable between the two groups. There was no statistically significant difference in the total duration of analgesia between the two groups. Motor and sensory block parameters are shown in Table 2.
Table 1.
Comparison of demographic characteristics of patients among groups
Table 2.
Sensory and motor block characteristics in both the groups
However, there was statistically significant difference in the haemodynamic effects between the two groups. Graph 1 shows the comparison of HR between the two groups. There was statistically significant decrease in the HR in Group B compared to Group L at 5 min (P = 0.041). Graph 2 depicts the comparison between the two groups, both the SBP and DBP from baseline up to 300 min after spinal anaesthesia. As seen in the graph, there is statistically significant decrease in SBP in Group B when compared with Group L from 5 min up to 60 min (P < 0.001). The decrease in DBP in Group B compared to Group L was also statistically significant from 10 min (P = 0.001) up to 45 min (P < 0.001).
Graph 1.
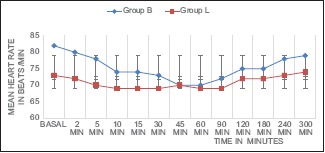
Comparison of mean heart rate before and up to 300 min after spinal anaesthesia. Bupivacaine (Group B) and Levobupivacaine (Group L). Data expressed as mean ± S.D
Graph 2.
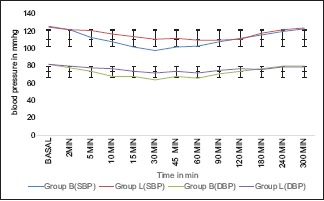
Comparison of systolic and diastolic blood pressure before and up to 300 min after spinal anaesthesia. Systolic blood pressure (SBP) and diastolic blood pressure (DBP); bupivacaine (Group B); levobupivacaine (Group L). Data expressed as mean ± S.D
Anaesthesia was adequate in all patients, and none of the patients needed general anaesthesia or airway management. Three patients in Group B had bradycardia at 5 min after spinal anaesthesia requiring treatment with injection atropine. Two patients in Group B had hypotension 5 min after spinal anaesthesia requiring treatment with injection mephentermine 6 mg and IV fluids. The patients were monitored for side effects and complications for 24 h. None of the patients had nausea, vomiting, sedation, headache, or backache in the postoperative period.
DISCUSSION
Levobupivacaine is a relatively new long acting local anaesthetic, with a pharmacological activity very similar to that of racaemic bupivacaine but less cardiotoxic and neurotoxic than the racaemic bupivacaine.[4,10,11] The quest for safer anaesthesia procedure with reduction of local anaesthetic dose by addition of adjuvants seems to be never ending. A large array of opioids ranging from morphine, fentanyl, and sufentanil to hydromorphone, buprenorphine, and tramadol has been used with varying success.[12] Buprenorphine is an opioid of the phenanthrene morphine class with extremely high binding affinity at the μ and kappa receptors.[7] This high-affinity of buprenorphine for opioid receptors produces a longer duration of action.[13] It is a centrally acting partial opioid agonist with both spinal and supraspinal component of analgesia. It is compatible with cerebrospinal fluid and produces no adverse reactions when administered intrathecally. It is highly lipid soluble and diffuses quickly into neural tissue, decreasing the extent of rostral spread leading to minor risk of respiratory depression in the postoperative period.[14,15,16] It has been used intrathecally in a dose of 75–150 μg with reasonable efficacy.[12] In the present study, we administered 2 μg/kg of buprenorphine through intrathecal route. There is paucity of literature evaluating the effect of buprenorphine on intrathecal levobupivacaine. The results of the current study established that the addition of buprenorphine to intrathecal levobupivacaine produced similar onset of sensory block compared to racemic bupivacaine with buprenorphine but with better preserved haemodynamics in the former group.
Behr et al. added buprenorphine (0.15 mg) to levobupivacaine for brachial plexus block. There were significant (P < 0.05) differences in the onset and the duration of the sensory block and in the duration of postoperative analgesia. Epineural buprenorphine prolonged postoperative analgesia more effectively than intramuscular buprenorphine, which suggests that buprenorphine acts at a peripheral nervous system site of action.[17]
Attri et al. compared levobupivacaine (Group L) and levobupivacaine with fentanyl (Group LF) in infraumbilical surgeries under spinal anaesthesia. Onset of sensory block was rapid in Group LF (4.8 ± 1.5 min) compared to Group L (7.6 ± 1.5 min).[6] Similarly in our study, addition of buprenorphine offset the slower onset of levobupivacaine. Singh et al. studied intrathecal buprenorphine versus fentanyl as adjuvants to 0.75% ropivacaine in lower limb surgeries and concluded that buprenorphine is better as compared to fentanyl in prolonging the duration of sensory block and achieving a better outcome in terms of pain relief.[18] Thus, buprenorphine was the prefered adjuvant in our study.
Monica et al. conducted a study between isobaric levobupivacaine and isobaric bupivacaine for spinal anaesthesia. They concluded that sensory and motor blockade onset was faster in the bupivacaine group, and greater sensory blockade with a longer postoperative painless period was achieved.[3] To offset this, slower sensory and motor onset of levobupivacaine with buprenorphine was added in our study. Thus, levobupivacaine with buprenorphine produced anaesthetic and analgesic effects similar to bupivacaine but with a better preserved haemodynamic profile in the former group.
However, Sathitkarnmanee et al. and Glaser et al. found no clinical differences in spinal blockade characteristics between isobaric levobupivacaine and isobaric bupivacaine.[9,11]
Sahin et al. conducted a study comparing the spinal characteristics of bupivacaine and levobupivacaine but found no significant differences between the two groups in haemodynamic characteristics unlike our study. In addition, the recovery time of sensory and motor blockade was shorter in levobupivacaine group (175, 216 min), and maximum level of sensory block was also higher with levobupivacaine unlike our study.[5]
Fattorini et al. studied clinical and anaesthetic features of intrathecal levobupivacaine and racaemic bupivacaine in patients undergoing major orthopedic surgical procedures. They concluded that, notwithstanding the complete absence of any significant haemodynamic complications in the patients of levobupivacaine group, further and larger studies are needed to assess if levobupivacaine is preferable to bupivacaine for minimizing the possible cardiovascular impact of spinal anaesthesia. Our study reinforces the results of Fattorini et al. as HR and blood pressure remained more stable in the levobupivacaine group when compared to bupivacaine.[19]
According to our study, Herrara et al. showed that incidence of hypotension was statistically significantly higher with bupivacaine (38.3%) compared to levobupivacaine (13.3%). There was a decrease in SBP and DBP at 30 min intraoperatively.[20]
Considering the limitations of our study, we have not included elderly age groups who are more prone for haemodynamic instability, and also we have not considered pregnant patients as well as ASA III and ASA IV patients in our study. There is no control group in our study.
CONCLUSION
Addition of buprenorphine to intrathecal levobupivacaine produced similar onset of sensory block compared to racemic bupivacaine with buprenorphine but with better preserved haemodynamics in the former group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bidikar M, Mudakanagoudar MS, Santhosh MC. Comparison of intrathecallevobupivacaine and levobupivacaine plus fentanyl for cesarean section. Anesth Essays Res. 2017;11:495–8. doi: 10.4103/aer.AER_16_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erdil F, Bulut S, Demirbilek S, Gedik E, Gulhas N, Ersoy MO, et al. The effects of intrathecallevobupivacaine and bupivacaine in the elderly. Anaesthesia. 2009;64:942–6. doi: 10.1111/j.1365-2044.2009.05995.x. [DOI] [PubMed] [Google Scholar]
- 3.del-Rio-Vellosillo M, Garcia-Medina JJ, Abengochea-Cotaina A, Pinazo-Duran MD, Barbera-Alacreu M. Spinal anesthesia for knee arthroscopy using isobaric bupivacaine and levobupivacaine: Anesthetic and neuroophthalmological assessment. Bio Med ResInt. 2014;2014:349034. doi: 10.1155/2014/349034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burlacu CL, Buggy DJ. Update on local anesthetics: Focus on levobupivacaine. Ther Clin Risk Manag. 2008;4:381–92. doi: 10.2147/tcrm.s1433. doi: 10.1155/2014/349034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin AS, Turker G, Bekar A, Bilgin H, Korfali G. A comparison of spinal anesthesia characteristics following intrathecal bupivacaine or levobupivacaine in lumbar disc surgery. Eur Spine J. 2014;23:695–700. doi: 10.1007/s00586-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attri JP, Kaur G, Kaur S, Kaur R, Mohan B, Kashyap K, et al. Comparison of levobupivacaine and levobupivacaine with fentanyl in infraumbilical surgeries under spinal anaesthesia. Anesth Essays Res. 2015;9:178–84. doi: 10.4103/0259-1162.152148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ipe S, Korula S, Varma S, George GM, Abraham SP, Koshy LR, et al. A comparative study of intrathecal and epidural buprenorphine using combined spinal-epidural technique for caesarean section. Indian J Anaesth. 2010;54:205–9. doi: 10.4103/0019-5049.65359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A, T Baig. Incidence of lower limb motor weakness in patients receiving postoperative epidural analgesia and factors associated with it: An Observational Study. Saudi J Anaesth. 2016;10:149–53. doi: 10.4103/1658-354X.168806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glaser C, Marhofer P, Zimpfer G, Heinz MT, Sitzwohl C, Kapral S, et al. Levobupivacaine versus racemic bupivacaine for spinal anesthesia. Anesth Analg. 2002;94:194–8. doi: 10.1097/00000539-200201000-00037. [DOI] [PubMed] [Google Scholar]
- 10.Bajwa SJS, Kaur J. Clinical profile of levobupivacaine in regional anesthesia: A systematic review. J Anaesthesiol Clin Pharmacol. 2013;29:530–9. doi: 10.4103/0970-9185.119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathitkarnmanee T, Thongrong C, Tribuddharat S, Bn MT, Bn KP, Bn RK. A comparison of spinal isobaric levobupivacaine and racemic bupivacaine for lower abdominal and lower extremity surgery. J Med Assoc Thai. 2011;94:716–20. [PubMed] [Google Scholar]
- 12.Swain A, Nag DS, Sahu S, Samaddar DP. Adjuvants to local anesthetics: Current understanding and future trends. World JClin Cases. 2017;5:307–23. doi: 10.12998/wjcc.v5.i8.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marappa P, Chikkapillappa MA, Chennappa NM, Pujari VS. A Comparative study of analgesic efficacy of intrathecal buprenorphine with ultrasound-guided transversusabdominis plane block for postcesarean delivery analgesia. Anesth, Essays Res. 2017;11:376–9. doi: 10.4103/0259-1162.206279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixit S. Post-operative analgesia after caesarean section: An experience with intrathecal buprenorphine. Indian J Anaesth. 2007;51:515–8. [Google Scholar]
- 15.Rabiee SM, Alijanpour E, Jabbari A, Rostami S. Benefits of using intrathecal buprenorphine. Caspian J Intern Med. 2014;5:143–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Shaikh SI, Kiran M. Intrathecal buprenorphine for post-operative analgesia: A prospective randomised double blind study. J Anaesthesiol Clin Pharmacol. 2010;26:35–8. [Google Scholar]
- 17.Behr A, Freo U, Ori C, Westermann B, Alemanno F. Buprenorphine added to levobupivacaine enhances postoperative analgesia of middle interscalene brachial plexus block. J Anesth. 2012;26:746–51. doi: 10.1007/s00540-012-1416-4. [DOI] [PubMed] [Google Scholar]
- 18.Singh AP, Kaur R, Gupta R, Kumari A. Intrathecal buprenorphine versus fentanyl as adjuvant to 0.75% ropivacaine in lower limb surgeries. J Anaesthesiol Clin Pharmacol. 2016;32:229–33. doi: 10.4103/0970-9185.182107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fattorini F, Ricci Z, Rocco A, Romano R, Pascarella MA, Pinto G, et al. Levobupivacaine versus racemic bupivacaine for spinal anaesthesia in orthopaedic major surgery. Minerva Anestesiol. 2006;72:637–44. [PubMed] [Google Scholar]
- 20.Herrera R, Andrés JD, Estañ L, Olivas FJM, Martínez-Mir I, Steinfeldt T, et al. Haemodynamic impact of isobaric levobupivacaine versus hyperbaric bupivacaine for subarachnoid anesthesia in patients aged 65 and older undergoing hip surgery. BMC Anesthesiology. 2014;14:97. doi: 10.1186/1471-2253-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]


