Abstract
Background:
Patients with schizophrenia were found to be less successful at emotion recognition tasks (ERTs) than healthy individuals. There is a debate surrounding whether this deficit is permanent or temporary. The current study aims to assess how emotion recognition skills are affected by treatment processes and during the course of the disease and also to determine the relation of this change with clinical assessment scales, other cognitive functions, and quantitative electroencephalography (QEEG).
Materials and Methods:
Twenty-four inpatients with treatment-resistant schizophrenia have been included in the study. Patients were assessed before beginning clozapine and 6 months later. During both assessments, clinical evaluation scales (Positive and Negative Syndrome Scale and Global Assessment of Functioning), Cambridge Neuropsychological Test Automated Battery (CANTAB) for schizophrenia which is used for assessment of cognitive functions were used. Electroencephalography (EEG) monitorings were performed only once before treatment. In this study, CANTAB ERT was used for emotion recognition.
Results:
There was no statistically significant change in the emotion recognition when the first and final ERTs were compared. There was a moderately positive relationship between emotional recognition and functioning (r = 0.65, P < 0.05). Cognitive functions such as visual memory, attention, flexible thinking, and planning were found to be in correlation with emotion recognition. Furthermore, slow waves such as delta and theta activities obtained from frontal, temporoparietal, and occipital regions were associated with emotion recognition.
Conclusion:
The current study supports that emotion recognition deficits are long-term stable features of schizophrenia, slow-wave electrical activity in the frontal, temporoparietal, and occipital areas in QEEG, and cognitive functions such as visual memory, attention, flexible thinking, and planning are found to be correlated with emotion recognition.
Keywords: Clinical psychopathology, facial emotion recognition, psychoneurobiology, quantitative electroencephalography, schizophrenia, social cognition
INTRODUCTION
Social cognition is defined as the ability to make sense of other people's behavior, to recognize and predict that they have a different mind than one's own, and to interact with complex social environments through understanding other people's beliefs and intentions.[1,2,3] The National Institute of Mental Health suggested that social cognition should be studied in five main areas: emotion perception, social perception, social knowledge, attributional bias, and theory of mind.[4] The inability to recognize emotional facial expressions is thought to be a key indicator of poor communication and deterioration in adaptive behaviors that cause impairment in social cognition.[5]
In patients with schizophrenia, the limitation of emotional expression was found to be an important feature of the disorder,[6] and such patients were found to be significantly less successful at emotion recognition tasks (ERTs) than healthy individuals.[7] The limitation is found to be present in every stage of schizophrenia, and emotion recognition deficits have been detected in risk groups and individuals at early stages[8] and in first-degree relatives.[9] Findings from the studies on the course of the facial emotion recognition deficits in patients with schizophrenia are inconsistent. There are also contradictory findings in the results of studies on how antipsychotics or other drugs affect on social task performance in schizophrenia. While some authors suggest that medications such as risperidone, quetiapine, modafinil, and intranasal oxytocin provide an improvement in social task performance;[10,11] some authors suggest that drugs such as clozapine and risperidone do not provide an improvement.[12]
The nature of facial processing inability is not clearly understood. Some authors hypothesize that facial processing inability is related to emotion-related content, some hypothesize that it is a disorder in processing social and biological stimuli, while other researchers hypothesize that it is related with a general deficit in visual attention and the processing of visual stimuli.[8,13] While there are studies supporting the description of a general defect, supporters of emotion-specific deficit approaches are gradually increasing.[7,14] Brain areas related to social cognition are as follows: the frontal lobe, temporal lobe, anterior cingulate cortex, fusiform gyrus, amygdala, and posterior association cortex as well as their inner relationships.[15,16,17,18] Although quantitative electroencephalography (QEEG) has poor spatial resolution, it is a functional brain imaging test with ideal temporal resolution of a millisecond.[19]
While, in schizophrenia, all cognitive functions explain social functioning with a 6% variance, social cognition explains social functioning with a 16% variance.[20] The purpose of this study is to assess how emotion recognition skills through facial expressions in patients with schizophrenia are affected by treatment processes and during the course of the disease, also to determine the relation of this change with clinical assessment scales, other cognitive functions, and QEEG. Before the study, we hypothesized that emotion recognition deficits would continue unchanged even if patients were in remission in schizophrenia, and emotion recognition ability would be associated with slow-wave activities in electroencephalography (EEG) and visual information processing, attention, and executive functions.
MATERIALS AND METHODS
This study is a part of a project examining the relation between electrophysiological variables and functioning, cognitive ability, and clinical presentation (treatment response/clinical severity) in schizophrenia patients. Twenty-four patients who have been hospitalized in the UHS Erenkoy Psychiatric and Neurological Diseases Research and Training Hospital between April 2015 and March 2016 were included in the study. These patients met both the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for schizophrenia and the Kane criteria for treatment-resistant schizophrenia, began clozapine treatment, and are right handed. The following patients were excluded: those younger than 18 and older than 60 years old, those determined to have sensory (hearing) problems that cause problems in understanding instructions, those with intellectual limitations or cognitive disabilities, those determined to have diseases related to general medical condition, those with a history of epilepsy or other neurologic disease, those with a history of alcohol/substance abuse in the recent year, and those who have been treated with electroconvulsive therapy in the last 6 months. Written informed consent was obtained from all patients participating in the study as well as from their relatives. Approvals from the Maltepe University Medical Faculty Clinical Trials Ethical Committee, the Turkish Ministry of Health, and the Turkish Medicines and Medical Devices Agency (due to the study being an observational drug study) were obtained.
Application
Patients were assessed before beginning clozapine and 6 months after clozapine treatment was initiated. During each assessments, the following assessment tools were employed: clinical evaluation scales (Positive and Negative Syndrome Scale [PANSS], Global Assessment of Functioning), and Cambridge Neuropsychological Test Automated Battery [CANTAB] for schizophrenia (used for assessment of cognitive functions including attention, visual memory, visual working memory, executive functions, and emotion recognition using facial expressions). However, electrophysiological evaluation was performed only once before the treatment. In this study, the CANTAB ERT was used for emotion recognition using facial expressions.
The patients started taking a potent dose of antipsychotic as of the 1st day of admission. Antipsychotic doses were optimized within 15–30 days and antipsychotic treatment continued for 6 months in the effective dose range.
Acquisition of electrophysiological records
Electrophysiological assessments of all participants were carried out early in the morning in a room isolated from light and electrical activity in the UHS Erenkoy Psychiatric and Neurological Diseases Research and Training Hospital male ward. EEG monitorings were carried out through a Neuron-Spectrum 5/P 19 channel digital EEG device (Neurosoft Inc., Ivanova, Russia). A room temperature of 23°C was maintained to avoid excessive perspiration. During the recordings, the participants were seated on a comfortable chair, and their vigilance level was kept as constant as possible by verbal commands. Nineteen channel computerized EEG recordings were acquired through Ag-AgCl plated disk electrodes placed on 19 scalp points according to international 10–20 nomenclature, acquiring maximal resting state of the patients. Active electrodes were placed on frontal (FP1, FP2, F3, F4, F7, F8, FZ), central (C3, C4, CZ), parietal (P3, P4, PZ), temporal (T3, T4, T5, T6), and occipital (O1, O2) areas. An average of A1 and A2 was employed as an acceptable reference electrode. Care was taken to ensure that all the electrode impedances were below 5 kΩ. The sampling rate was set to 512 Hz, with 0.05 Hz as the high-frequency filter and 70 Hz as the low-frequency filter. For the analyses of EEG signals, programs working through MATLAB named EEGLAB (version 13) and Darbeliai were used. The data acquired from EEG monitorization were transferred to EEGLAB by converting them into edf format. After re-filtering the data with 1 Hz as the low-frequency filter and 35 Hz as the high-frequency filter for determining and erasing epochs containing eye, muscle, and motion artifacts, a cleaning process was carried out using the ± 70-μV basic voltage method. Later, the records were re-scanned for artifacts, and subsequently, 60-s EEG traces have been obtained. Activities below 2 Hz were excluded to avoid misleading results due to eye movements. Then, fast Fourier transform process was applied using the named the Darbeliai computer program, and absolute frequency powers were obtained from each electrode. The following frequency bands of the mean absolute power spectra were considered: delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–16 Hz). For each global basal EEG activity (alpha, beta, theta, and delta), the average absolute power of the electrodes at the anatomically appropriate locations were calculated. The anatomical regions were examined by dividing the left frontal area (FP1, F3, F7), right frontal area (FP2, F4, F8), total frontal area (FP1, FP2, F3, F4, F7, F8, FZ), central area (C3, C4, CZ), left temporal area (T3, T5), right temporal area (T4, T6), parietal area (P3, P4, PZ), and occipital area (O1, O2).
Assessment of cognitive functions was carried out using the CANTAB. During all tests, participants were accompanied by one of the investigators (GS) and all tests were performed by the same person. Seven tests from the CANTAB schizophrenia battery were applied. The completion time of the tests was approximately 1.5 h. CANTAB Reaction Time was used for assessing attention, Paired Associate Learning for visual memory, Rapid Visual Information Processing for sustained attention, One Touch Stockings of Cambridge for planning and executive functions, Intra-Extra Dimensional Set Shift for flexible thinking and executive functions, and Spatial Working Memory for executive functions.
Social cognition was assessed by the ERT test. During the ERT test, participants were asked to identify the emotions contained in the facial expressions appearing on the screen. The test does not have an exercise phase. After the test began, participants were asked to look at the cross sign in the middle of the screen continuously so that it was easier to detect the facial expression that appeared on the screen for only 250 ms. After being visible for a short amount of time, the facial expression disappears and six boxes appear on the screen: anger, disgust, fear, surprise, happiness, and sadness. Participants are asked to touch The box that the best describes the emotion in the facial expression presented. The test consists of 2 blocks. Each block has a total of 90 facial expressions. While it is easier to understand the feelings of some of the expressions, it is harder to understand some others. As a result of the test, the response latencies of the patients, the correct response rates for both the total and for each emotion, and the frequency of emotions chosen by the patients can be evaluated.
Statistical analysis
For the statistical analysis of the findings obtained in the study, the Statistical Packages for the Social Sciences (SPSS) 16.0 software package for Windows (SPSS Inc., Chicago, IL, US) was used. Descriptive statistical methods (frequency, percentage, mean, and standard deviation) were used while evaluating the study data. The Wilcoxon test was used to compare quantitative data from repeated measures. The relationship between quantitative data was made by Pearson and Spearman correlations. The results were evaluated at a 95% confidence interval and a P < 0.05 significance level.
When the GPower 3.1.9.2 version is used (effect size: 0.8, significant tail: 2), the effect size of the research is calculated as 0.73.[21,22]
RESULTS
Sociodemographic and clinical data
The mean age of the 24 patients included in the study (19 males and 5 females) was 35.79 ± 8.93. The education level of the patients was 10.41 ± 3.48 years, the age of disease onset was 22.4 ± 5.54 years, the onset duration of the disease was 13.3 ± 7.5 years, and the number of hospitalizations was 4.2 ± 3.5. About 83% of the patients did not work regularly and 79.2% were single.
Nine patients received clozapine monotherapy, 4 received amisulpride, 2 received risperidone depot form, 2 received paliperidone palmitate, 2 received risperidone oral form, 4 received aripiprazole, 4 received haloperidol, 1 received olanzapine, 1 received quetiapine, and 1 received zuclopenthixol depot form in addition to clozapine.
Evaluation of emotion recognition tests
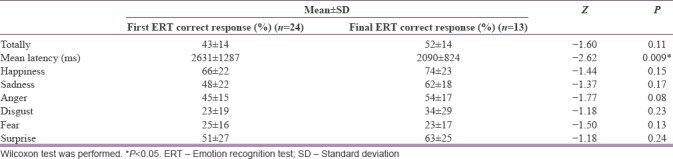
The evaluation of emotion recognition skills in the first and the final ERT tests are shown in Table 1.
Table 1.
Comparison of emotion recognition skills in the first and final emotion recognition tests
When the responses in the first emotional recognition test were examined, it was found that the average frequency of happiness responses was 42 ± 23, the average frequency of sadness responses was 25 ± 15, the average frequency of anger responses was 24 ± 20, the average frequency of disgust responses was 13 ± 17, the average frequency of fear responses was 19 ± 16, and the average frequency of surprise responses was 37 ± 21.
When the responses in the final emotional recognition test were examined, it is found that the average frequency of happiness responses was 42 ± 19, the average frequency of sadness responses was 35 ± 14, the average frequency of anger responses was 24 ± 9, the average frequency of disgust responses was 23 ± 36, the average frequency of fear responses was 13 ± 6, and the average frequency of surprise responses was 40 ± 14.
Changes in the emotion recognition test
There was no statistically significant change in the recognition of six basic emotions when the first and the final emotion recognition tests were compared. When the mean response time of the patients was compared, it was found that the response time decreased statistically in the final test (P = 0.009). When the frequency of patients respond to the emotional stimuli was examined, a statistically significant increase was found in the frequency of choosing sadness (P = 0.03). There was no statistically significant change in the frequency of choosing other emotions.
Evaluation of the relationship between emotion recognition ability and other clinical and electrophysiological data
When the relationship between scores on the first emotional recognition test and the age, education level, age at the time of disease onset, total disease duration, and total number of hospitalizations was examined, it was found that there was a statistically significant negative correlation between the rate of recognition of anger and the total number of hospitalizations (r = −0.41, P < 0.05). The correlation between the scores on the first emotional recognition test and clinical scales is shown in Table 2. The correlation between the scores on the first emotional recognition test and the scores obtained from the cognitive tests is shown in Table 3, and the correlation between the scores on the first emotional recognition test and the data obtained from the QEEG appears in Table 4.
Table 2.
Evaluation of correlation between scores on the first emotional recognition test and clinical scales
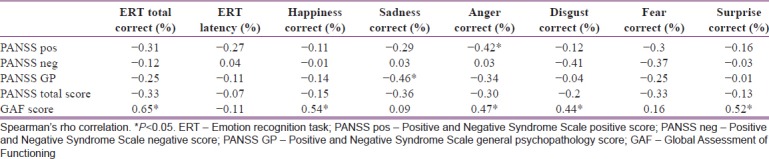
Table 3.
Evaluation of correlation between scores on the first emotional recognition test and other cognitive tests
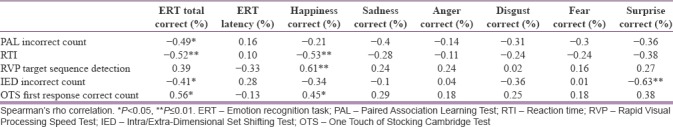
Table 4.
Evaluation of correlation between scores on the first emotional recognition test and quantitative electroencephalography
DISCUSSION
In the current study, both before and at the end of the treatment period, patients were found to have the most difficulty recognizing the negative emotions such as fear and disgust and had a tendency to respond to emotional stimuli with happiness, sadness, or surprise. In a study carried out among patients with frontal, parietal, and temporal lesions, while happiness recognition rates were found to be highest, fear and anger recognition rates were found to be lowest.[23] While some studies carried out among patients with schizophrenia have suggested that the emotion recognition deficits are emotion specific,[24,25] some researchers believe that the disorder includes all emotions.[26,27] It is noted that the recognition of negative emotion is more difficult than neutral or positive emotions and that negative emotions can be confused with other negatives, while this was not observed for positive emotions.[28] In our study, it was observed that schizophrenia patients tend to distort other expression categories, identifying them as happiness. This tendency reflects impairment in their empathy skills and impairment in their recognition of the distinction between the emotional states of others and their own.[29] Another reason is that the expression of happiness is more recognizable due to the employment of larger muscle groups and expressions; by its nature, such expression is more communicative than emotional.[30,31]
In the current study, there was no significant improvement in the emotion recognition skills of the patients at the end of the treatment period, but the response time was significantly shortened. However, there are studies reporting that in schizophrenia patients, impairment in emotional recognition skills is more evident in the acute phase and that impairment in the remission phase is not significant.[32] Similar to the current study, there are also researches that report that the degree of impairment remained the same during the remission period.[33] In a study conducted by Penn et al., it is shown that most schizophrenia patients spend less time than healthy control groups evaluating emotional expressions and that this is due to their limitations in visually scanning face expressions; as a result, schizophrenia patients misevaluate emotional expressions.[8] Findings from our study support the conclusion that emotional recognition difficulties in schizophrenia patients are not situational but are rather a permanent or long-term stable feature of schizophrenia.
Relationship between emotion recognition skills and other clinical data
When the correlation between the Positive and Negative Syndrome Scale (PANSS) and emotion recognition abilities is evaluated, a relation between the PANSS positive subscale and anger recognition and a relation between the PANSS general psychopathology subscale and sadness recognition were found, while the PANSS negative subscale was found not to be related with any emotion recognition. However, Kohler et al. also found that positive symptoms such as hallucinations, thought disorders, and negative symptoms such as alogia were associated with emotion recognition.[34]
When we investigated the relationship between emotion recognition and functionality in our study, it was found that there was a moderate positive correlation between emotion recognition and functionality. Emotions play a very important role in the successes and struggles of human life. The ability to perceive and identify emotions leads to increased success in job interviews and in social relationships such as interaction with family members and colleagues. In a review article by Couture et al.[35] that examined 22 studies related to social cognition and functional outcome, emotion perception, social perception, and theory of mind were found to be in correlation with social functioning, social behavior in society, social problem solving, and social competence. Emotion perception is related with social functioning, social skills, and occupational functioning. Social perception is related to social behavior in society and occupational functionality.[35] The concept of social functioning is of great importance for the life quality of patients with schizophrenia. For this reason, factors such as negative symptoms and cognitive functions are being studied. Deterioration in cognitive functions is predictive for a future change of 20%–60% in patients’ psychosocial functioning levels.[36] Researchers continue to study other co-factors for the remaining percentage. Hence, in recent years, social cognition has emerged as a possible factor. In a recent meta-analysis,[20] the effect of all types of cognitive functions in social functioning can be explained by a 6% variance, while the effect of social cognition domains is explained by a 16% variance. For this reason, in recent years, social cognition as a therapeutic goal came to be recognized as more important than other impairments in cognitive functions.
Other cognitive functions and quantitative electroencephalography correlates of emotion recognition
In our study, cognitive functions related to frontal and temporal functions such as visual memory, attention focus, flexible thinking, and planning are found to be in correlation with emotion recognition. During the face-processing, recognition of the identity of the person to whom the face belongs through its structural characteristics, as well as recognition of emotional facial expressions in the eyes, mouth and eyebrows occurs.[37] Thus, the face recognition process involves not only the face recognition function but also the recognition of the emotional expression. The quality of visual attention to specific areas of the face enhances emotion recognition performance.[38] Gallese et al. reported that problems related to working memory and attention in schizophrenia patients may have negative effects on emotion recognition.[13] Kohler et al. also reported that emotion discrimination has a significant and high correlation with cognitive functions such as attention, memory (verbal and spatial), flexible thinking, language, and abstract thinking.[34] Thus, the correlations identified support the idea that the impairment in frontotemporal functioning extends to the limbic region associated with emotional processing.[34]
When the relationship between QEEG data and emotion recognition is evaluated, emotion recognition was associated with slow waves such as delta and theta, rather than fast waves such as alpha and beta. Particularly, a significant correlation was found between delta and theta activities obtained from all cortical regions and emotion recognition. Delta-waves occur in Stages 3 and 4 of sleep. According to Knyazev, brain functional delta-oscillations are associated with autonomic functions, motivation processes associated with rewards, defensive mechanisms, higher emotional involvement, and cognitive processes related to attention and the detection of motivationally salient stimuli in the environment.[39] As for the theta-wave, it is thought to reflect the activity in the limbic system and hippocampus. It is reported to be related with anxiety, subconscious fear and night terrors. It is suggested that in normal functioning, the theta rhythm may mediate complex and adaptive behaviors such as short-term memory and learning.[40,41]
EEG studies have reported abnormal slowing in posterior, frontal-midline, and frontal-temporal regions in patients with schizophrenia.[39,40,41,42,43,44] Given increased slow-wave activity during the waking state, it has been suggested that part of the brain in schizophrenia might be in an inactive “sleep-like state” or that slow-wave activity in schizophrenia reflects subtle brain pathology.[45] In this context, increased slow-wave activity may reflect the neural network dysfunction that negatively affects social cognition, both by distorting cognition and directly impairing facial recognition.
Due to the small sample size of our study, studies in larger sample groups are needed. In addition, the QEEG data obtained in resting state are a limiting factor for our study. The electrophysiological evaluations performed during the facial ERT may provide more detailed data on the neural network and cortical structures related to emotion recognition. Moreover, our study is a 6-month prospective follow-up study; longer follow-up studies may provide more reliable information about the extent to which emotion recognition may be affected by treatment in the long term.
CONCLUSION
Our study supports the idea that emotion recognition deficits are long-term stable features of schizophrenia and thus that emotional recognition difficulties in schizophrenic patients are not situational but rather a permanent feature of the disease. The current study supports the view that antipsychotic drugs are inadequate to enhance the development of emotion recognition skills and social abilities in schizophrenia and that, therefore, different treatment approaches are needed. In addition, it was found that there is a strong correlation between emotion recognition and functionality and that cognitive functions related to frontal and temporal functioning such as visual memory, attention focusing, flexible thinking, and planning are correlated with emotion recognition. In accordance with these findings, electrical activity in the frontal, temporoparietal, and occipital areas in QEEG is found to be correlated with emotion recognition.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank MD Calli SY and MD Sever Fidan Y for their important contributions during data collection.
REFERENCES
- 1.Grady CL, Keightley ML. Studies of altered social cognition in neuropsychiatric disorders using functional neuroimaging. Can J Psychiatry. 2002;47:327–36. doi: 10.1177/070674370204700403. [DOI] [PubMed] [Google Scholar]
- 2.Sayın A, Candansayar S. Theory of mind in schizophrenia. New Symposium. 2008;46:74–80. [Google Scholar]
- 3.Yıldırım E, Alptekin O. A new featured dimension in schizophrenia: Social cognition. Düşünen Adam J Psychiatry Neurol Sci. 2012;25:368–75. [Google Scholar]
- 4.Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, et al. Social cognition in schizophrenia: An NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–20. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marwick K, Hall J. Social cognition in schizophrenia: A review of face processing. Br Med Bull. 2008;88:43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- 6.Ünal S. Personal experiences and expression of feelings in schizophrenia. Turk J Clin Psychiatry. 2000;3:131–6. [Google Scholar]
- 7.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: A meta-analytic review. Schizophr Bull. 2010;36:1009–19. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: An overview. Schizophr Bull. 2008;34:408–11. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eack SM, Mermon DE, Montrose DM, Miewald J, Gur RE, Gur RC, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr Bull. 2010;36:1081–8. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey PD, Patterson TL, Potter LS, Zhong K, Brecher M. Improvement in social competence with short-term atypical antipsychotic treatment: A randomized, double-blind comparison of quetiapine versus risperidone for social competence, social cognition, and neuropsychological functioning. Am J Psychiatry. 2006;163:1918–25. doi: 10.1176/ajp.2006.163.11.1918. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld AJ, Lieberman JA, Jarskog LF. Oxytocin, dopamine, and the amygdala: A neurofunctional model of social cognitive deficits in schizophrenia. Schizophr Bull. 2011;37:1077–87. doi: 10.1093/schbul/sbq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gultekin G, Yuksek E, Kalelioglu T, Bas A, Ocek Bas T, Duran A. Differential effects of clozapine and risperidone on facial emotion recognition ability in patients with treatment-resistant schizophrenia. Psychiatry Clin Psychopharmacol. 2017;27:19–23. [Google Scholar]
- 13.Gallese V. The roots of empathy: The shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003;36:171–80. doi: 10.1159/000072786. [DOI] [PubMed] [Google Scholar]
- 14.Tsoi DT, Lee KH, Khokhar WA, Mir NU, Swalli JS, Gee KA, et al. Is facial emotion recognition impairment in schizophrenia identical for different emotions? A signal detection analysis. Schizophr Res. 2008;99:263–9. doi: 10.1016/j.schres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL, et al. The functional organization of human extrastriate cortex: A PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336–53. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–40. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 17.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99:11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. Philos Trans R Soc Lond B Biol Sci. 1992;335:23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- 19.Coburn KL, Lauterbach EC, Boutros NN, Black KJ, Arciniegas DB, Coffey CE, et al. The value of quantitative electroencephalography in clinical psychiatry: A report by the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2006;18:460–500. doi: 10.1176/jnp.2006.18.4.460. [DOI] [PubMed] [Google Scholar]
- 20.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L, et al. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci Biobehav Rev. 2011;35:573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 22.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 23.Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001;48:235–53. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- 24.Dougherty FE, Bartlett ES, Izard CE. Responses of schizophrenics to expressions of the fundamental emotions. J Clin Psychol. 1974;30:243–6. doi: 10.1002/1097-4679(197407)30:3<243::aid-jclp2270300304>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Muzekari LH, Bates ME. Judgment of emotion among chronic schizophrenics. J Clin Psychol. 1977;33:662–6. doi: 10.1002/1097-4679(197707)33:3<662::aid-jclp2270330312>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 26.Novic J, Luchins DJ, Perline R. Facial affect recognition in schizophrenia. Is there a differential deficit? Br J Psychiatry. 1984;144:533–7. doi: 10.1192/bjp.144.5.533. [DOI] [PubMed] [Google Scholar]
- 27.Feinberg TE, Rifkin A, Schaffer C, Walker E. Facial discrimination and emotional recognition in schizophrenia and affective disorders. Arch Gen Psychiatry. 1986;43:276–9. doi: 10.1001/archpsyc.1986.01800030094010. [DOI] [PubMed] [Google Scholar]
- 28.Johnston PJ, Katsikitis M, Carr VJ. A generalised deficit can account for problems in facial emotion recognition in schizophrenia. Biol Psychol. 2001;58:203–27. doi: 10.1016/s0301-0511(01)00114-4. [DOI] [PubMed] [Google Scholar]
- 29.Schneider F, Gur RC, Gur RE, Shtasel DL. Emotional processing in schizophrenia: Neurobehavioral probes in relation to psychopathology. Schizophr Res. 1995;17:67–75. doi: 10.1016/0920-9964(95)00031-g. [DOI] [PubMed] [Google Scholar]
- 30.Borod JC. Interhemispheric and intrahemispheric control of emotion: A focus on unilateral brain damage. J Consult Clin Psychol. 1992;60:339–48. doi: 10.1037//0022-006x.60.3.339. [DOI] [PubMed] [Google Scholar]
- 31.Mandal MK, Bryden MP, Bulman-Fleming B. Similarities and variations in facial expressions of emotions: Cross-cultural evidence. Int J Psychol. 1996;31:41–58. [Google Scholar]
- 32.Weniger G, Lange C, Rüther E, Irle E. Differential impairments of facial affect recognition in schizophrenia subtypes and major depression. Psychiatry Res. 2004;128:135–46. doi: 10.1016/j.psychres.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Penn DL, Addington J, Pinkham A. Social cognitive impairments. In: Lieberman JA, Stroup TS, Perkins DS, editors. American Psychiatric Association Textbook of Schizophrenia. Arlington: American Psychiatric Publishing Press; 2006. pp. 261–74. [Google Scholar]
- 34.Kohler CG, Bilker W, Hagendoorn M, Gur RE, Gur RC. Emotion recognition deficit in schizophrenia: Association with symptomatology and cognition. Biol Psychiatry. 2000;48:127–36. doi: 10.1016/s0006-3223(00)00847-7. [DOI] [PubMed] [Google Scholar]
- 35.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophr Bull. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez N, Metzger A, Magné R, Bonnet-Brilhault F, Roux S, Barthelemy C, et al. Exploration of core features of a human face by healthy and autistic adults analyzed by visual scanning. Neuropsychologia. 2009;47:1004–12. doi: 10.1016/j.neuropsychologia.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW, et al. Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. J Autism Dev Disord. 2010;40:358–70. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- 39.Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36:677–95. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Buzsáki G. Theta rhythm of navigation: Link between path integration and Landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–40. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 41.Lisman JE, Idiart MA. Storage of 7+/- 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–5. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 42.Fernández A, Maestú F, Amo C, Gil P, Fehr T, Wienbruch C, et al. Focal temporoparietal slow activity in Alzheimer's disease revealed by magnetoencephalography. Biol Psychiatry. 2002;52:764–70. doi: 10.1016/s0006-3223(02)01366-5. [DOI] [PubMed] [Google Scholar]
- 43.Nakashima K, Sato H. The effects of various mental tasks on appearance of frontal midline theta activity in EEG. J Hum Ergol (Tokyo) 1992;21:201–6. [PubMed] [Google Scholar]
- 44.Chen YH, Stone-Howell B, Edgar JC, Huang M, Wootton C, Hunter MA, et al. Frontal slow-wave activity as a predictor of negative symptoms, cognition and functional capacity in schizophrenia. Br J Psychiatry. 2016;208:160–7. doi: 10.1192/bjp.bp.114.156075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: What causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22:537–44. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]



