Abstract
The aim of this study was to investigate the effect of ingesting sodium bicarbonate (SB) and sodium citrate (SC) on 400 m high-intensity swimming performance and blood responses. Six nationally ranked male swimmers (20.7 ± 2.1 yrs; 184 ± 6 cm; 79.9 ± 3.9 kg; 10.6 ± 1% body fat) participated in a double blinded, placebo controlled crossover trial. Ninety minutes after consuming SB (0.3 g·kg-1), SC (0.3 g·kg-1) or a placebo (PL) participants completed a single 400-m freestyle maximal test on three consecutive days. The order of the supplementation was randomized. Capillary blood samples were collected on 4 occasions: at rest (baseline), 60 min post-ingestion, immediately post-trial and 15 min post-trial. Blood pH, HCO3- concentration and base excess (BE) were determined. Blood pH, HCO3-, BE were significantly elevated from before loading to the pre-test (60 min post-ingestion) (p < 0.05) after SB ingestion, but not after SC ingestion (p > 0.05). Performance times were improved by 0.6% (p > 0.05) after supplementation of SB over PL in 5 out of 6 participants (responders). In contrast, ingestion of SC decreased performance by 0.2% (p > 0.05). No side effects were observed in either trial. Delayed blood response was observed after SC ingestion compared to SB and this provided no or modest ergogenic effect, respectively, for single bout high-intensity swimming exercise. Monitoring the magnitude of the time-to-peak level rise in alkalosis may be recommended in order to individualize the loading time accordingly before commencement of exercise.
Key words: dietary supplements, ergogenic aid, performance, nutrition
Introduction
Many athletes use dietary supplements in an effort to maximize performance. It is widely accepted that ingestion of dietary supplements that may nutritionally affect intracellular and extracellular buffering capacity are an evidence-based strategy for improving sports performance (Maughan, 2014).
Consumption of dietary sodium bicarbonate (SB) prior to exercise induces alkalosis by increasing blood bicarbonate pool and pH. This in turn enhances the buffering capacity of the extracellular space in the working muscle by influencing the efflux of the H+ into the extracellular space for disposal. During intensive submaximal exercise, one of the major cause of fatigue is believed to be metabolic acidosis caused by high rates of anaerobic glycolysis, which results in the accumulation of H+ ions in excess of intracellular buffering capacity (Plowman and Smith, 2013).
Ingestion of SB has previously been found to enhance performance in repeated bouts of exercise (Gao et al., 1988; Goods, 2014; Zajac et al., 2009) rather than in a single, short-term or high-intensity swimming exercise bout (Joyce et al., 2012).
The use of sodium citrate (SC) has been introduced as an alternative to SB due to the perception that it may elicit less gastrointestinal (GI) discomfort (Carr et al., 2011). The performance benefits of SC ingestion before high-intensity exercise still appear to be limited in the literature (Russell et al., 2014; Van Montfoort et al., 2004). In contrast, one study indicated that SC was not an effective ergogenic aid for high-intensity exercise (Someren et al., 1998).
Due to ergogenic properties, the use of SB is widely recommended for swimmers competing in high-intensity events lasting 1-7 min (Lindh et al., 2008). Ingestion of 0.3 g SB or SC/kg/body mass 60-90 min before exercise is commonly recommended (Siegler et al., 2016). This is believed to improve performance in high-intensity sprint-based events of short duration (1 min) by 1.7% (Carr et al., 2011).
However, supplementation of alkalizing agents such as SB or SC is challenged in recent studies, especially due to the inconsistency in performance effects. A dose response study of SB ingestion recently revealed large inter-individual variability in the magnitude of the increase in blood HCO3- concentrations over a 3-h period post-ingestion, which may partly explain negative consequences and non-ergogenic outcomes (Jones et al., 2016). Additionally, within-subject variability ergogenic effect of SB has been recently documented despite intra-individual blood responses to SB ingestion being consistent (Froio de Araujo Dias et al., 2015). Inter-individually different magnitude of changes in blood concentration for HCO3- and pH has been documented both in SB (Jones et al., 2016; Sparks et al., 2017; Stannard et al., 2016) and CS studies (Urwin et al., 2016). High intra-individual variability in the ergogenic response to SB appears to limit ergogenic benefits and performance increases therefore may not be induced during every exercise bout (McNaughton et al., 2016). Whether the accepted recommendation (loading time, dose) verified in SB trials, with expected physiological response, is applicable to SC ingestion, remains unclear. No clear recommendation for SC ingestion have been proposed yet.
Therefore, the aim of this study was to investigate the effect of ingesting sodium bicarbonate (SB) and sodium citrate (SC) on 400 m high-intensity swimming performance and blood responses using an accepted pre-loading protocol for SB.
Methods
Participants
Six elite level Czech male swimmers (20.7 ± 2.1 yr; 79.9 ± 3.9 kg; 50-60 km·week) volunteered to participate in this study (Table 1). All participants were nationally ranked with 200 and 400 m free-style personal best (long course) ~1:58 min and 4:14 min, respectively. After an explanation of all experimental procedures, each participant signed a written informed consent form to take part in this research. The Ethics Board of the Masaryk University approved the study.
Table 1.
Characteristics of participants
| Participants | Mean (SD) |
|---|---|
| Age | 20.7 ± 2.1 |
| Body mass (kg) | 79.9 ± 3.9 |
| Body height (cm) | 184 ± 6 |
| BMI (kg·m-2) | 23.6 ± 1.1 |
| Fat free mass (kg) | 71.4 ± 3.5 |
| Total body fat (%) | 10.6 ± 1 |
| VO2max (ml·min·kg-1) | 62.0 ± 4.5 |
| Steady-state haemoglobin level (g·l-1)* | 149.9 ± 19 |
*measured in a resting state during the trials
Procedures
A detailed experimental design is shown in Figure 1. A double blinded and placebo controlled design was used. Participants ingested identical gelatine capsules containing either SB (0.3 mg·kg-1 of body mass, BM), SC (0.3 mg·kg-1 of BM) or the placebo (PL). A starch, 0.18 mg·kg-1 of BM, was used for the PL. The capsules were professionally prepared by an experienced pharmacist to ensure that each participant was given the same amount. The amount of capsules distributed to participants varied (23-26) and were ingested within 5 min of distribution. Ingestion of the capsules was not associated with digestive problems. Following supplementation a single 400 m freestyle time-trial was completed. Participants completed three performance tests on three consecutive days following randomized supplementation order.
Figure 1.
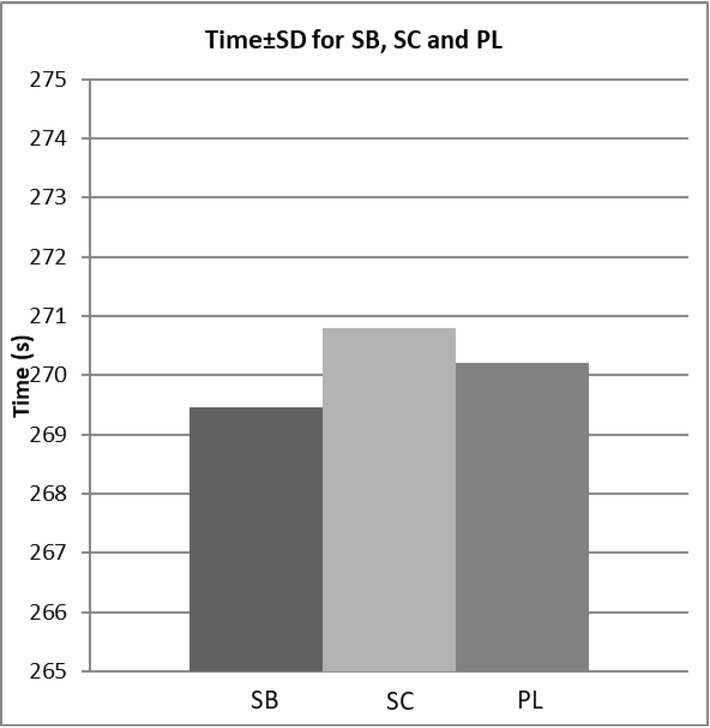
Change in performance time comparing between placebo (PL), sodium bicarbonate (SB) and sodium citrate (SC) supplementation trials.
Picture 1.
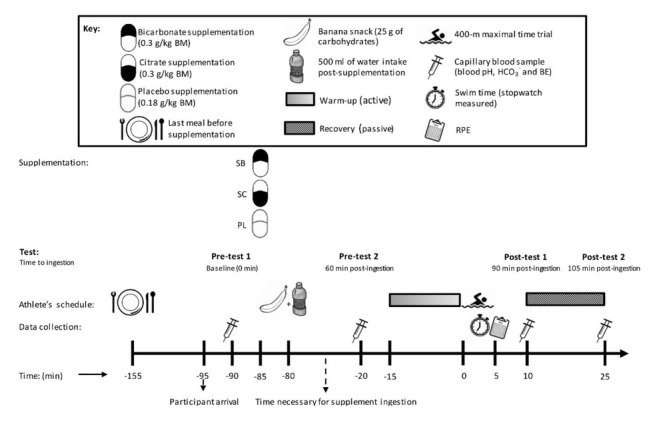
Experimental protocol
Test day
The swim tests took place in a 50-m pool between 14.00 -16.00 pm. All pre and post testing sessions (blood samples, data collection, GI discomfort questionnaire, supplement ingestion, resting between blood sampling) took place in a calm, thermoneutral warm-up room next to the pool.
After reporting to the warm-up room, resting blood samples were obtained (~2 h postprandially). After that, participants were administered SB, SC or the PL in random order. Participants were instructed to consume the capsules with a piece of banana and ad libitum water (minimum of 500 ml) to combat GI symptoms.
An acute-GI-discomfort questionnaire was administered to participants on the experimental day. The questionnaire consisted of a simple close-ended question: “Did you suffer from any of the following gastrointestinal distress (belching, heartburn, stomach ache, bloating, nausea, vomiting, diarrhoea)?” A Likert’s scale was used and included five responses on a linear scale (1 – strongly disagree, 2 – disagree, 3 – neither/nor agree, 4 – agree, 5 – strongly agree). Questionnaire was completed at 2 time points: 60 min post-ingestion (rest) and immediately after the trial. GI distress was identified when reporting options 4 and 5 on the Likert scale for each of the respondent.
Following supplement ingestion, participants rested under the control of researchers for 60 min and were allowed to ingest water ad libitum. This time period was controlled by a stopwatch so that exactly 60 min post-ingestion second blood samples were obtained.
After 15 min of the predefined warm up session (5 min out of water stretching followed by 800 m swim), an individual 400 m freestyle time-trial test was performed. Each participant was instructed to approach the race pace. Every swim was started from water and timed by an experienced coach using a stopwatch (Quartz, model 898). Afterwards, the participants immediately went back to the warm-up room for post-test blood sampling and acute GI discomfort questionnaires completion.
Blood sampling
Fingertip capillary blood samples were obtained on four occasions: at rest before participants ingested the tested supplement (baseline) and after 60 min of rest (post-ingestion), immediately after swimming (post-trial I) and 15 min after the cessation of the 400 m trial (post-trial II). Whole blood samples were collected by finger pricking using a sterile single use lancing device with 2.3 mm penetration depth. Amount of ~60 μl of blood was collected into plain heparinized capillary tubes, immediately injected into sensor cards and analysed for pH, HCO3- and base excess (BE). The device used for blood analysis was an electro-chemical apparatus Gastat Navi (Techno MedicaCo., Ltd.). Time needed for blood sample collection was ~2-3 min depending on each participants finger prick bleeding. Each blood analysis took 165 s before results were automatically printed. The blood collection was carried out by the same examiner in all trials.
Nutrition
Nutritional supplements containing creatine and beta-alanine were not allowed to be used for at least four weeks prior to or during the study. Caffeine intake was forbidden only during the testing period. A 4-day prospective food intake record was collected from participants at least one week before the start of the study. Participants were given an individualized nutritional plan that ensured a minimum of 7-8 g carbohydrates (CHO)·kg-1 of BM. All participants were instructed to eat according to the prescribed daily plan during all three consecutive testing days to ensure as similar nutritional condition as possible. This was analysed for adherence with non-significant difference between prescribed (~7.9 g CHO·kg-1 of BM) and adhered (~7.4 g CHO·kg-1 of BM) diet (Table 2). NutriPro software (Fitsport-komplex s.r.o., Czech Republic) was used to analyse energy intake and macronutrient distribution.
Table 2.
Nutrition prescription and control for adherence during testing days. Prescribed nutrition scheme/day in accordance with predicted energy expenditure. Adherence was monitored during 3 consecutive testing days. CHO, carbohydrates; PRO, protein. Values are Mean ± SD
| Prescribed | Adhered | |
|---|---|---|
| Energy (kcal·kg-1) | 54 | 52.8 ± 4.5 |
| CHO (g·kg-1) | 7.9 | 7.4 ± 1 |
| PRO (g·kg-1) | 2.5 | 2.6 ± 0.3 |
| FAT (g·kg-1) | 1.3 | 1.3 ± 0.1 |
Statistical analyses
All statistical analyses were conducted using Statistica (StatSoft CR s.r.o., Czech Republic) software and Microsoft Excel (Microsoft Inc., USA). Normality was assessed by the Shapiro-Wilk test. Paired t tests were used to compare blood measures (pH, HCO3- and BE) under experimental conditions at different sampling times. Performance differences between SC, SB and PL conditions were analysed by one-way ANOVA with LSD post hoc analysis. Cohen´s d was computed to determine effects size. Statistical significance was accepted at p ≤ 0.05 with data presented as mean ± standard deviation (SD). A coefficient of variance was used to assess inter-variable changes.
Results
There were no significant differences in the time of the 400-m test between SB, SC and PL conditions (p = 0.973, f = 0.02). However, supplementation with SB improved performance times over the placebo in 5 out of 6 participants (responders) by ~1% (time improvement of 0.11-1.84%, p = 0.79, f = 0.07). In contrast, the SC condition decreased performance over the PL in 5 out of 6 participants (time impairment of 0.26-1.01%, 0.72-2.71 s) (Figure 1).
Blood pH, HCO3-, BE were significantly elevated from before loading to pre-test (60 min post-ingestion) (p < 0.05) after SB ingestion, but not after SC ingestion (p > 0.05). The magnitude of the change in blood pH, HCO3-, BE concentration from post-trial I to post-trial II was significantly greater in all conditions (SB, SC, PL).
Post hoc analysis showed that baseline of all blood analyses was not significantly different between conditions. There were significant main effects for HCO3- concentration and BE values both in SB and SC condition over PL in all post-ingestion measurement points (p < 0.05).
There was a significant main effect in HCO3-concentrations, BE and pH in all time points for both SB and SC over PL (p < 0.05) except for non-significant difference in pH concentration 60 min post-ingestion between SC and PL. The only significant difference was found in BE response 60 min post-ingestion between the SC and SB trial (p < 0.05). Blood analyses response over sampling time points and experimental conditions are presented in Figure 2 (a, b, c).
Figure 2 a, b, c.
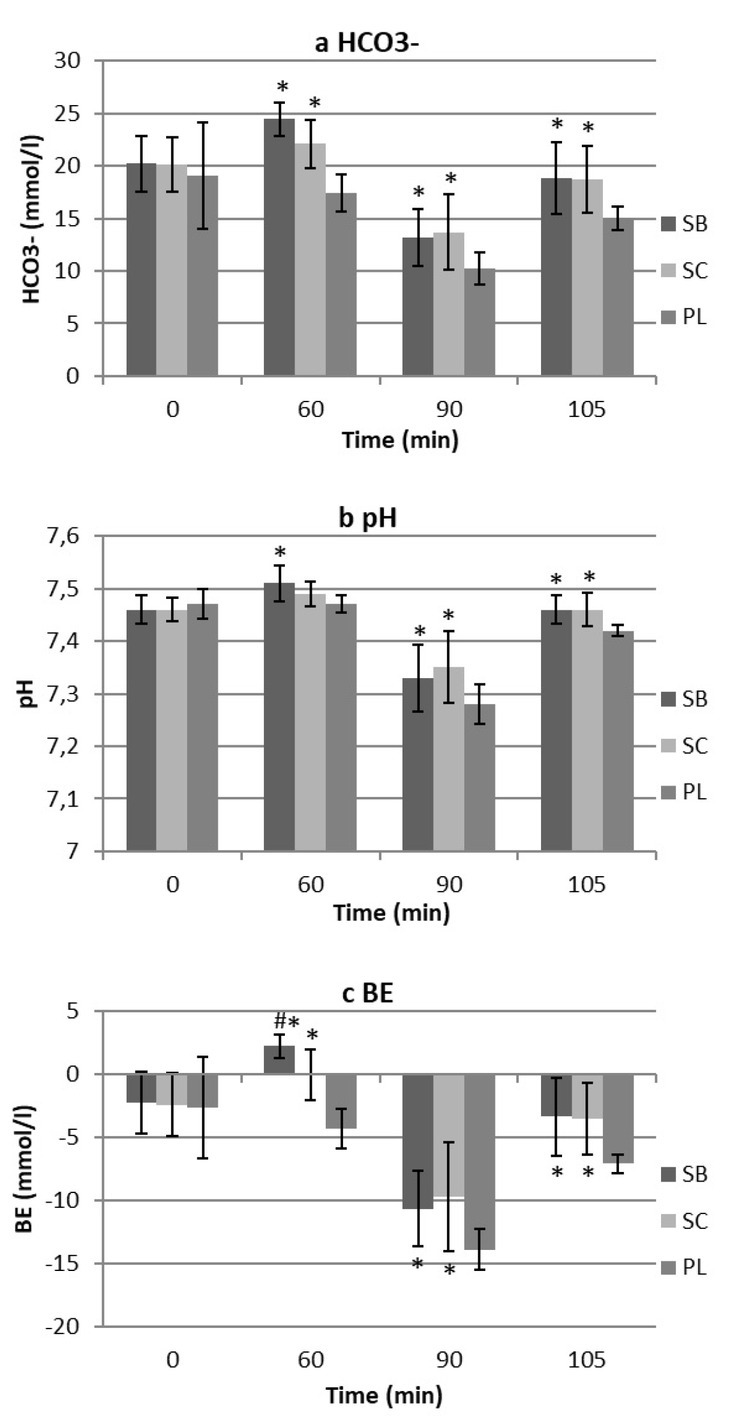
Blood HCO3-, pH and Base Excess (BE) responses for sodium bicarbonate (SB), sodium citrate (SC) and placebo (PL) in baseline (0 min), post-ingestion (60 min), post-trial I (90 min) and post-trial II (105 min). Significant effects (p < 0.05) between SB, SC vs. PL (*) and SC vs. SB (#) in pre-trial (0, 60 min) and post-trial (90, 105 min). Values are Mean ± SD.
Discussion
We showed that acute supplementation with 0.3 g·kg-1 of BM of sodium bicarbonate 90 min before the ~4.5 min maximum single swimming task, has only a moderate ergogenic effect. We found ~0.6% improvement in 400 m swimming performance in 5 out of 6 six elite similarly trained individuals (VO2max 62 ± 4.5 ml·min·kg-1). This translated to non-significant performance enhancement of 0.3-5.0 s (responders) (little effect, Cohen´s d = 0.28). Ergogenic properties of SB supplementation may differ from training status with moderate performance enhancement of 1.7% and 1.1% found in better trained individuals and a nonathletic population, respectively (Siegler et al., 2016). In contrast, we found that even under alkalotic conditions, ingestion of SC led to performance impairment in 5 participants by - 0.26-1.01% (Cohen´s d = 0.29). Both the SC and SB trials were effective in inducing alkalosis during baseline to the pre-test period, though in the SC trial this was not significant. Both SB and SC significantly attenuated the decline in blood pH compared with the placebo during the 15 min post-test period.
Our research indicates that despite the increase in blood concentrations of HCO3- and BE and attenuation of blood pH, the response seen after performance trials, an ergogenic aspect over PL of SB and SC supplementation was negated. The same trend was clear even when corrected for responders.
We think that our results may be viewed from several aspects that have been questioned in recent literature. Are the metabolic conditions within 400 m freestyle swimming profitably affected? Does the loading time before exercise have an ergogenic effect? Finally, to what extent is the performance affected by variability in blood response? This may be discussed when examining why an individual cannot always make full use of an enhanced buffering capacity and why this is not translated into better performance.
Although we focused on a single performance, the ingestion of alkalizing agents has previously been found to attenuate the performance decrease in a repeated high-intensity 100 m swimming exercise bout with no ergogenic effect seen in the first trial (Pruscino et al., 2008). Similarly Zajac et al. (2009) found that SB ingestion improved the total time of the 4 x 50 m repeated front crawl sprints by ~1.5 s, although only the first sprint was significantly improved. A relatively minor contribution of the non-oxidative glycolytic energy metabolism (~10%) to the total energy output during a 400 m swim has been found (Rodriguez and Mader, 2003). If correct, these metabolic conditions cannot be considered advantageous, when regarding SB supplementation as a crucial exogenous technique for promoting body’s buffering capacity.
Based on the scenario of a high aerobic proportion of energy supply (83.2-85.5%) for a 400 m swim and a minor proportion of energy provision derived from anaerobic lactate energy pathways (~10%) to the total energy output reported by Rodriguez and Mader (2003), we may speculate to what extent nutritionally induced alkalosis might affect 400 m performance. Even in a 182 m freestyle maximal test in a study of Capelli et al. (1998), a relatively high aerobic (61.5%) and low glycolytic metabolism (~25% anaerobic lactate) was shown. Therefore, high contribution of the oxidative sources not affecting the anaerobic site of the performance may partly account for the little effect we observed (Cohen´s d = 0.2) in those who responded to supplement by improving their time over the placebo. Moreover, our participants were rather sprinters (100 m freestyle personal best 52.6 ± 2.4 s) with a well-developed glycolytic metabolism. The modest mean performance time improvement of ~0.6% in our group of responders (n = 5) may be therefore explained by the fact that only 10% of the metabolism may benefit from the increased buffering capacity. This is in agreement with the results reported by Russell et al. (2014), who similarly identified a subcategory of swimmers, as responders, who improved their single 200 m swimming performance time by 1.03% after acute supplementation of SC. The present study appears to indicate that highly sprint-trained individuals are unable to take advantage of an increased extracellular buffering capacity.
According to Robergs et al. (2005), consumption of 0.3 g·kg-1 of BM of SB typically increases blood HCO3- concentration by 5-6 mmol/l from the baseline. Jones et al. (2016) in a dose response study found a highly variable increase of 6-12.3 mmol/l similar to our study (1.3 – 11 mmol, mean 4.25 mmol). The highest variability in blood HCO3- concentration increase was found between 20-75 min post ingestion (Jones et al., 2016). Generally, high inter-individual variability was found independent of the dose ingested with the coefficient of variation (CV) to be 29, 32 and 44% in 0.1, 0.2 and 0.3 g·kg-1 of BM of SB, respectively. This variability corresponds with the huge variation in the time window between ingestion and peak response of blood bicarbonate (75-180 min) for 0.3 mg/kg reported by the same authors.
In our study a 400 m time-trial started 85-90 min post-ingestion with the last time point (peak levels) of HCO3- measured 60 min post-ingestion with inter-individual CV of 39% suggesting our participants may not reach peak levels. In a study of Mero et al. (2013), a similar pattern in blood HCO3- concentration and pH changes was found. However, ingestion of SB even 60 min before onset of repeated 100 m performance improved performance.
Carr et al. (2011) suggested an elevation in a blood HCO3- concentration of 5-6 mmol·l-1 to be a “zone of potential ergogenic effect” and a “zone of almost certain ergogenic effect” was hypothesized to be an increase of > 6 mmol·l-1. In our group of performance responders, mean blood HCO3- elevations were 4.74 ± 3.28 mmol·l-1. Not surprisingly, the second lowest increase over the baseline levels (1.8 mmol·l-1) in our study was found in the participant who concurrently was the only non-responder. Therefore, low pre-exercise blood variables and variable response in our group can explain a lack of an ergogenic effect. Froio de Araujo Dias et al. (2015) found that blood response levels (absolute increases in blood pH, bicarbonate and base excess) prior to exercise were extremely intra-individually similar in repeated administration of SB. However, this did not necessarily translate into an improved exercise capacity. Probably loading time before exercise may therefore be a crucial individual factor for little improvements in performance after SB ingestion.
In contrast to Russell et al. (2014) and others, we found no improvement of performance after SC ingestion. No significant changes in resting pH, blood concentrations of HCO3- and BE were observed in the SC trial 60 min post-ingestion compared to the baseline levels. Additionally the mean blood increase of HCO3- was only 1.98 mmol which was a 13.5% lower increase compared to SB (p = 0.24). This is consistent with low blood kinetics of HCO3- after SC ingestion recently published by Urwin et al. (2016). As such, administration of SC ~90 min before the exercise is not as sufficient to induce improvements in buffering potential as we observed in the SB trial. Russell et al. (2014) administered 0.5 g·kg-1 of SC 120 min pre-trial and found a modest time improvement in 5 out of 10 participants. According to this, it may advisable to prolong the time between SC ingestion and the performance trial.
According to Siegler et al. (2016), it may be recommended to individually monitor the time-to-peak level rise in HCO3- at doses between 0.2 and 0.3 g·kg−1 and to adapt the supplement loading time accordingly before the commencement of exercise. A blood acid base response study of Gough et al. (2017) also supports an individualised NaHCO3 ingestion strategy as they found time-to-peak and absolute change in HCO3- to be a more reliable determinant than pH when inducing pre-exercise alkalosis.
Many studies fail to confirm the use of SB mainly due to the gastrointestinal discomfort observed in the majority of studies and nearly in all known supplementation protocols (Kahle et al., 2013). The inter-individual variability in the magnitude of change in Na concentration might explain why some individuals report distress, while others do not, even at the same SB dose. We did not measure Na concentrations, however, according to Carr et al. (2011), we strictly regulated nutrition on testing days in order to combat GI distress. A detailed nutritional plan was provided prior to the testing days and was controlled for adherence by examiners (participants prospectively recorded a dietary intake throughout the testing days). A prescribed nutrition intake was therefore precisely controlled and there were non-significant changes between prescribed and adhered nutrition (e.g. energy intake, CHO intake) during the testing days (Table 2) (Jeacocke and Burke, 2010). An acute GI-discomfort questionnaire revealed that only one participant recorded GI distress in a SB trial, with no side effects observed in SC trials. Therefore, we managed to eliminate side effect usually associated with SB or SC supplementation.
Conclusion
We showed that acute supplementation with sodium bicarbonate 90 min before a 400 m swimming task increased performance by ~0.6%. The ergogenic effect of sodium citrate ingestion was not confirmed. Despite clear changes in blood concentrations of pH, bicarbonate and BE observed after both the SB and SC trials, a significant ergogenic effect of supplementation prior to 400 m swimming is challenged. SC ingestion induces different blood response levels, which should be taken into account when applying guidelines for SB ingestion. Athletes are currently expected to individualize their ingestion timing to maximize peak pH or blood bicarbonate in order to maximize the performance effect. This may allow individuals to attain the ergogenic benefits of SB more consistently.
Acknowledgements
This article was written at the Masaryk University as part of the project: An effect of ergogenic aids on elite swimming performance 1085/2015 with the support of the Grant Agency of the Masaryk University. Preliminary results have been submitted in the 8th International Scientific Conference on Kinesiology Proceedings.
References
- Capelli C, Pendergast DR, Termin B. Energetics of swimming at maximal speeds in humans. Eur J Appl Physiol. 1998;78:385–393. doi: 10.1007/s004210050435. [DOI] [PubMed] [Google Scholar]
- Carr AJ, Hopkins WG, Gore CJ. Effects of Acute Alkalosis and Acidosis on Performance. Sports Med. 2011;41:801–814. doi: 10.2165/11591440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Carr AJ, Slater GJ, Gore CJ, Dawson B, Burke LM. Effect of Sodium Bicarbonate on [HCO3−], pH, and Gastrointestinal Symptoms. Int J Sport Nutr Exerc Metab. 2011;21:189–194. doi: 10.1123/ijsnem.21.3.189. [DOI] [PubMed] [Google Scholar]
- Froio de Araujo Dias G, da Eira Silva V, de Salles Painelli V, Sale C, Giannini Artioli G, Gualano B, Saunders B. (In)Consistencies in Responses to Sodium Bicarbonate Supplementation: A Randomised, Repeated Measures, Counterbalanced and Double-Blind Study. PloS One. 2015;10(11):e0143086. doi: 10.1371/journal.pone.0143086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JP, Costill DL, Horswill CA, Park SH. Sodium bicarbonate ingestion improves performance in interval swimming. Eur J Appl Physiol. 1988;58:171–174. doi: 10.1007/BF00636622. [DOI] [PubMed] [Google Scholar]
- Goods PSR. Sodium Bicarbonate and Repeated Swimming Sprints. J Aust Strength Cond. 2014;22:91–95. [Google Scholar]
- Gough LA, Deb SK, Sparks AS, McNaughton LR. The Reproducibility of Blood Acid Base Responses in Male Collegiate Athletes Following Individualised Doses of Sodium Bicarbonate: A Randomised Controlled Crossover Study. Sports Med. 2017:1–11. doi: 10.1007/s40279-017-0699-x. [DOI] [PubMed] [Google Scholar]
- Jeacocke NA, Burke LM. Methods to Standardize Dietary Intake before Performance Testing. Int J Sport Nutr Exerc Metab. 2010;20:87–103. doi: 10.1123/ijsnem.20.2.87. [DOI] [PubMed] [Google Scholar]
- Jones RL, Stellingwerff T, Artioli GG, Saunders B, Cooper S, Sale C. Dose-Response of Sodium Bicarbonate Ingestion Highlights Individuality in Time Course of Blood Analyte Responses. Int J Sport Nutr Exerc Metab. 2016;26:445–453. doi: 10.1123/ijsnem.2015-0286. [DOI] [PubMed] [Google Scholar]
- Joyce S, Minahan C, Anderson M, Osborne M. Acute and chronic loading of sodium bicarbonate in highly trained swimmers. Eur J Appl Physiol. 2012;112:461–469. doi: 10.1007/s00421-011-1995-z. [DOI] [PubMed] [Google Scholar]
- Kahle LE, Kelly PV, Eliot KA, Weiss EP. Acute sodium bicarbonate loading has negligible effects on resting and exercise blood pressure but causes gastrointestinal distress. Nutr Res. 2013;33:479–486. doi: 10.1016/j.nutres.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh AM, Peyrebrune MC, Ingham SA, Bailey DM, Folland JP. Sodium bicarbonate improves swimming performance. Int J Sports Med. 2008;29:519–523. doi: 10.1055/s-2007-989228. [DOI] [PubMed] [Google Scholar]
- Maughan RJ. The Encyclopaedia of Sports Medicine: An IOC Medical Commission Publication, Sports Nutrition. John Wiley & Sons; 2014. pp. 324–335. [Google Scholar]
- McNaughton LR, Gough L, Deb S, Bentley D, Sparks SA. Recent Developments in the Use of Sodium Bicarbonate as an Ergogenic Aid. Curr Sports Med Rep. 2016;15:233–244. doi: 10.1249/JSR.0000000000000283. [DOI] [PubMed] [Google Scholar]
- Mero AA, Hirvonen P, Saarela J, Hulmi JJ, Hoffman JR, Stout JR. Effect of sodium bicarbonate and beta-alanine supplementation on maximal sprint swimming. J Int Soc Sports Nutr. 2013;10:52. doi: 10.1186/1550-2783-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman SA, Smith DL. Exercise Physiology for Health Fitness and Performance. Lippincott Williams & Wilkins; 2013. pp. 55–59. [Google Scholar]
- Pruscino CL, Ross MLR, Gregory JR, Savage B, Flanagan TR. Effects of Sodium Bicarbonate, Caffeine, and Their Combination on Repeated 200-m Freestyle Performance. Int J Sport Nutr Exerc Metab. 2008;18:116–130. doi: 10.1123/ijsnem.18.2.116. [DOI] [PubMed] [Google Scholar]
- Robergs R, Hutchinson K, Hendee S, Madden S, Siegler J. Influence of Pre-Exercise Acidosis and Alkalosis on the Kinetics of Acid-Base Recovery Following Intense Exercise. Int J Sport Nutr Exerc Metab. 2005;15:59–74. doi: 10.1123/ijsnem.15.1.59. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, Mader A. Chatard JC. Biomechanics and medicine in swimming IX. Saint-Etienne: University of Saint-Etienne; 2003. “Energy metabolism during 400 and 100-m crawl swimming: computer simulation based on free swimming measurement”; pp. 373–378. [Google Scholar]
- Russell C, Papadopoulos E, Mezil Y, Wells GD, Plyley MJ, Greenway M, Klentrou P. Acute versus chronic supplementation of sodium citrate on 200 m performance in adolescent swimmers. J Int Soc Sports Nutr. 2014;11:26. doi: 10.1186/1550-2783-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler JC, Marshall PWM, Bishop D, Shaw G, Green S. Mechanistic Insights into the Efficacy of Sodium Bicarbonate Supplementation to Improve Athletic Performance. Sports Med – Open. 2016;2:41. doi: 10.1186/s40798-016-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someren K van, Fulcher K, McCarthy J, Moore J, Horgan G, Langford R.. An Investigation into the Effects of Sodium Citrate Ingestion on High-Intensity Exercise Performance. Int J Sport Nutr. 1998;8:356–363. doi: 10.1123/ijsn.8.4.356. [DOI] [PubMed] [Google Scholar]
- Sparks A, Williams E, Robinson A, Miller P, Bentley DJ, Bridge C, Mc Naughton LR. Sodium bicarbonate ingestion and individual variability in time-to-peak pH. Res Sports Med. 2017;25:58–66. doi: 10.1080/15438627.2016.1258645. [DOI] [PubMed] [Google Scholar]
- Stannard RL, Stellingwerff T, Artioli GG, Saunders B, Cooper S, Sale C. Dose-Response of Sodium Bicarbonate Ingestion Highlights Individuality in Time Course of Blood Analyte Responses. Int J Sport Nutr Exerc Metab. 2016;26:445–453. doi: 10.1123/ijsnem.2015-0286. [DOI] [PubMed] [Google Scholar]
- Urwin CS, Dwyer DB, Carr AJ. Induced Alkalosis and Gastrointestinal Symptoms After Sodium Citrate Ingestion: a Dose-Response Investigation. Int J Sport Nutr Exerc Metab. 2016;26:542–548. doi: 10.1123/ijsnem.2015-0336. [DOI] [PubMed] [Google Scholar]
- Van Montfoort MCE, Van Dieren L, Hopkins WG, Shearman JP. Effects of ingestion of bicarbonate, citrate, lactate, and chloride on sprint running. Med Sci Sports Exerc. 2004;36:1239–1243. doi: 10.1249/01.mss.0000132378.73975.25. [DOI] [PubMed] [Google Scholar]
- Zajac A, Cholewa J, Poprzecki S, Waskiewicz Z, Langfort J. Effects of sodium bicarbonate ingestion on swim performance in youth athletes. J Sports Sci Med. 2009;8:45–50. [PMC free article] [PubMed] [Google Scholar]


