Abstract
Amphetamines (AMPHs) are potent psychostimulants that are widely used and abused, with profound medical and societal impact. Their actions at dopaminergic neurons are thought to mediate their therapeutic efficacy as well as their liability for abuse and dependence. AMPHs target the dopamine transporter (DAT), the plasmalemmal membrane protein that mediates the inactivation of released dopamine (DA) through its reuptake. AMPHs act as substrates for DAT and are known to cause mobilization of dopamine (DA) to the cell exterior via DAT-mediated reverse transport (efflux). It has become increasingly evident that the mechanisms that regulate AMPH-induced DA efflux are distinct from those that regulate DA uptake. Central to these mechanisms is the phosphorylation of the DAT amino (N)-terminus, which has been repeatedly demonstrated to facilitate DAT-mediated DA efflux, without impacting other aspects of DAT physiology. This review aims to summarize the current status of knowledge regarding DAT N-terminal phosphorylation and its regulation by protein modulators and the membrane microenvironment. A better understanding of these mechanisms may lead to the identification of novel therapeutic approaches that interfere selectively with the pharmacological effects of AMPHs without altering the physiological function of DAT.
1. INTRODUCTION
Prescribed and illicit amphetamines (AMPHs) are widely misused and abused with up to 56 million users worldwide (Vearrier, Greenberg, Miller, Okaneku, & Haggerty, 2012), often leading to aggression and psychosis (Dawe, Davis, Lapworth, & McKetin, 2009), cardiovascular damage, malnutrition, and a host of other medical and psychosocial complications (Heal, Smith, Gosden, & Nutt, 2013). AMPH and its derivatives are also in wide clinical use for the treatment of attention-deficit disorders (Sembower, Ertischek, Buchholtz, Dasgupta, & Schnoll, 2013; Sweeney, Sembower, Ertischek, Shiffman, & Schnoll, 2013). The action of these drugs at dopaminergic neurons is thought to be responsible, in major part, for their therapeutic efficacy as well as their liability for abuse and dependence (Sembower et al., 2013; Sulzer, Sonders, Poulsen, & Galli, 2005; Sweeney et al., 2013). AMPHs target the dopamine transporter (DAT), the plasmalemmal membrane protein that mediates the inactivation of released dopamine (DA) through its reuptake. AMPHs act as substrates for DAT; upon binding to the transporter they are transported into the neuron in a Na+-dependent manner, eliciting an inward current (Sonders, Zhu, Zahniser, Kavanaugh, & Amara, 1997; Sulzer et al., 2005; Zaczek, Culp, & De Souza, 1991). Once inside neurons, they are transported by the vesicular monoamine transporter (VMAT) into vesicles, causing the release of vesicular DA into the cytoplasm and its mobilization to the cell exterior via DAT through reverse transport (efflux) (Eshleman, Henningsen, Neve, & Janowsky, 1994; Freyberg et al., 2016; Sulzer, 2011; Sulzer et al., 1995). This nonexocytic efflux of DA through DAT results in a dramatic increase of synaptic DA and is believed to play a major role in the psychostimulatory and rewarding properties of AMPHs (Eshleman et al., 1994; Sulzer, 2011; Sulzer et al., 1995).
DAT belongs to the solute carrier 6 (SLC6) family of Na+/Cl−-dependent neurotransmitter transporters, which also includes the transporters for serotonin (SERT), norepinephrine, GABA, and glycine. DAT comprises 12 transmembrane segments, with cytoplasmic N-terminal and C-terminal domains oriented intracellularly (Amara & Kuhar, 1993; Chen & Reith, 2004; Penmatsa, Wang, & Gouaux, 2013; Torres, Carneiro, et al., 2003). \It primarily functions to regulate the strength and duration of the dopaminergic response by mediating the reuptake of DA from the synaptic cleft, thereby terminating neurotransmission. Accordingly, it plays a critical role in modulating the physiological functions of DA, which include motor control and arousal, as well as higher cognitive functions such as motivation and reward-seeking behavior (Iversen & Iversen, 2007).
Substrate translocation through DAT is driven by the electrochemical gradient generated by the plasma membrane Na+/K+ ATPase. It involves the sequential binding of Na+, Cl−, and DA, with the movement of Na+ down its concentration gradient providing the energy necessary to transport DA against its concentration gradient (Krueger, 1990; Torres, Gainetdinov, & Caron, 2003). DAT is thought to function via an alternating access mechanism, whereby binding of substrate and ions triggers a regulated transition of the transporter from an “outward-facing” conformation, in which the substrate binding site is exposed to the extracellular space, to an “inwardfacing” conformation, in which the substrate binding site is exposed to the intracellular milieu ( Jardetzky, 1966; Loland, Norgaard-Nielsen, & Gether, 2003). It was thus proposed that AMPH-induced DA efflux through DAT occurs via a facilitated exchange mechanism, whereby the transport of AMPH into the cell via DAT increases the number of transporters in the inward-facing conformation, leading to the transport of DA out of the cell by exchange (Fischer & Cho, 1979). However, later studies challenged this model and suggested that AMPH-induced efflux is at least partially uncoupled from uptake function (Pifl & Singer, 1999; Scholze et al., 2002). In fact, it has been proposed that the releasing properties of AMPH may be related to its ability to elicit DAT-mediated inward currents. Consistent with such a model, studies have shown that DAT-mediated DA efflux is voltage sensitive, is influenced by intracellular ionic concentrations, and might involve a channel-like mode of the transporter (Kahlig et al., 2005; Khoshbouei, Wang, Lechleiter, Javitch, & Galli, 2003; Pifl & Singer, 1999; Sitte et al., 1998). AMPH treatment increases intracellular Na+ and Ca2+ (Gnegy et al., 2004; Kantor et al., 2001) which in turn stimulate DA efflux in a concentration-dependent manner. Furthermore, studies over the last two decades have identified various mutations and modulators that differentially affect AMPH-induced DA efflux and DA uptake. Central to these mechanisms is the phosphorylation of the DAT N-terminus, which has been repeatedly demonstrated to promote DAT-mediated DA efflux, without affecting the uptake capacity of DAT, its trafficking, or oligomerization. This review aims to summarize the current status of knowledge regarding DAT N-terminal phosphorylation and its regulation by interacting partners and the membrane environment. A better understanding of these mechanisms may lead to the identification of novel therapeutic approaches that interfere selectively with the pharmacological effects of AMPHs without altering the physiological function of DAT.
2. A ROLE FOR DAT N-TERMINAL PHOSPHORYLATION IN DAT-MEDIATED DA EFFLUX
Analysis of the primary amino acid sequence of DAT (Kilty, Lorang, & Amara, 1991) reveals several putative phosphorylation sites for various kinases in the intracellular domains, and multiple kinases have been shown to regulate DAT function (Foster, Cervinski, Gorentla, & Vaughan, 2006; Foster & Vaughan, 2016; Ramamoorthy, Shippenberg, & Jayanthi, 2011). Early studies demonstrating the ability of DAT to undergo phosphorylation were performed using 32P metabolic labeling in heterologous expression systems (Granas, Ferrer, Loland, Javitch, & Gether, 2003; Huff, Vaughan, Kuhar, & Uhl, 1997) as well as in rat and mouse striatal tissue (Foster, Pananusorn, & Vaughan, 2002; Vaughan, Huff, Uhl, & Kuhar, 1997). More recently, our group developed phosphospecific anti-DAT antibodies to probe the phosphorylation of individual serine resides in DAT in response to various stimuli (Karam, Sen, & Javitch, 2017). These studies showed that DAT phosphorylation is rapidly elevated by activation of protein kinase C (PKC) with phorbol 12-myristate, 13-acetate (PMA) (Huff et al., 1997; Karam et al., 2017; Vaughan et al., 1997), diacylglycerol analogs (Vaughan et al., 1997), or Gq activation by G protein-coupled receptor agonists (Granas et al., 2003). DAT phosphorylation was also shown to be strongly enhanced by treating cells with protein phosphatase inhibitors such as okadaic acid (OA), indicating that the transporter is subject to ongoing kinase activity even in the absence of exogenous kinase activators (Foster, Pananusorn, Cervinski, Holden, & Vaughan, 2003; Foster et al., 2002; Huff et al., 1997; Karam et al., 2017; Vaughan et al., 1997). Critically, treatment with psychostimulants such as AMPH and methamphetamine (METH) stimulates an increase in DAT phosphorylation in both heterologously expressing cells and in rat brain tissue (Cervinski, Foster, & Vaughan, 2005; Karam et al., 2017). In contrast, the DAT blocker cocaine does not stimulate DAT phosphorylation, but it does prevent the phosphorylation increase induced by METH (Cervinski et al., 2005).
Enzymatic digestion of 32PO4-labeled DAT followed by epitope-specific immunoprecipitation showed that most basal, PMA-, and OA-stimulated phosphorylation of DAT occurs at the distal N-terminus in rat striatal tissue (Foster et al., 2002). Consistent with these data, deletion of the first 22 N-terminal residues of DAT (Δ1–22-hDAT) was shown to dramatically decrease incorporation of 32P in response to PKC activation in cultured cells, suggesting that transporter phosphorylation is almost entirely due to phosphorylation of the distal N-terminus, which includes five serine residues at positions 2, 4, 7, 12, and 13 (Granas et al., 2003). Surprisingly, however, truncation of the distal N-terminus (Δ1–22) does not affect surface localization or PKC-mediated internalization of DAT, as shown by surface biotinylation studies (Granas et al., 2003; Khoshbouei et al., 2004), nor does it impair DA reuptake or inhibitor binding in [3H]tyramine and [3H]dopamine uptake assays (Khoshbouei et al., 2004). The ability of the transporter to interact with itself and form oligomers in the plasma membrane is also unaffected, as shown by cysteine cross-linking analyses (Hastrup, Karlin, & Javitch, 2001; Hastrup, Sen, & Javitch, 2003). In marked contrast, deletion of the first 22 amino acids or simultaneous mutation of the five N-terminal serines to alanine (hDAT-StoA) dramatically inhibits AMPH-induced DA efflux in heterologous cells, as measured by superfusion of a population of cells or by amperometry combined with the patch-clamp technique in the whole-cell configuration. Mutation of these five serines instead to aspartate (hDAT-StoD), which mimics a constitutive phosphorylation state, preserves efflux (Khoshbouei et al., 2003).
Consistent with these findings, studies of a Drosophila melanogaster behavioral model have shown that DAT phosphorylation is also necessary for the stimulatory effects of AMPH in vivo (Pizzo et al., 2013). AMPH stimulates an increase in crawling velocity in Drosophila larvae in a DAT-dependent manner; null mutation in the Drosophila DAT (dDAT) gene inhibits this behavioral response. Expression of human DAT in a dDAT null mutant rescues the locomotor response to AMPH, demonstrating the ability of human DAT to substitute functionally for dDAT in the context of this behavioral model (Pizzo et al., 2013). Remarkably, expression of hDAT-StoA fails to rescue the AMPH-induced behavioral response, whereas expression of hDAT-StoD results in robust hyperlocomotion in response to AMPH (Pizzo et al., 2013), consistent with the finding in vitro that phosphorylation is required for AMPH-induced DA efflux (Khoshbouei et al., 2004). In contrast, expression of hDAT-StoA rescues the locomotor response to the psychostimulant methylphenidate (Ritalin®), a DA uptake blocker that does not induce efflux (Pizzo et al., 2013), consistent with the findings that inhibition of DAT phosphorylation does not impair DA reuptake function in vitro. Taken together, these data strongly support an essential and selective role for DAT phosphorylation in AMPH-induced DA efflux and associated behavior.
3. KINASES, PHOSPHATASES, AND SITES OF PHOSPHORYLATION IMPLICATED IN DAT-MEDIATED DA EFFLUX
3.1. Ca2+/Calmodulin-Dependent Protein Kinase Alpha
One critical aspect of AMPH-induced DA efflux through DAT is its dependence on the intracellular Ca2+ stores. AMPH treatment increases intracellular Ca2+, which stimulates DA efflux in a concentration-dependent manner (Gnegy et al., 2004; Kantor et al., 2001). Treatment with a Ca2+ chelator inhibits AMPH-induced voltage-sensitive currents through DAT, as well as AMPH-induced DA efflux in heterologous cells, and superfusion of rat striatal slices with the Ca2+ chelator BAPTA-AM also suppresses AMPH-induced efflux of endogenous DA, without affecting basal DA levels. Interestingly, although it is generally accepted that extracellular Ca2+ is not required for reverse transport, it was recently shown that AMPH-induced DAT-mediated currents can robustly activate the voltage-gated L-type Ca2+ channels Cav1.3 and Cav1.2 (Cameron, Solis, Ruchala, De Felice, & Eltit, 2015), suggesting that the increase in intracellular Ca2+ may be partially mediated by these channels. Consistent with such a model, the increase of intracellular Ca2+ in response to low concentrations (but not high concentrations) of AMPH can be inhibited by removal of Ca2+ from the extracellular milieu (Gnegy et al., 2004). Taken together, these data establish a fundamental role for Ca2+ in AMPH-induced signaling mechanisms, although the precise mechanisms by which AMPH treatment leads to an increase in its intracellular levels will require further investigation.
One cellular target for Ca2+ that is highly expressed in neurons is Ca2+/calmodulin-dependent protein kinase alpha (CaMKIIα), which was identified in a yeast two-hybrid screen as a direct interacting partner of DAT via its carboxy (C)-terminus. This interaction was further confirmed by GST-pulldown in vitro and by coimmunoprecipitation in heterologous cells (Fog et al., 2006) and in mouse striatum (Steinkellner et al., 2012). Furthermore, DAT was shown to colocalize with CaMKIIα in midbrain dopaminergic neurons in culture (Fog et al., 2006). Activation of CaMKIIα stimulates DA efflux in heterologous cells and in dopaminergic neurons in culture, most likely through direct interaction with and phosphorylation of DAT, as addition of a synthetic DAT C-terminal peptide that can compete with binding for CaMKIIα inhibits efflux (Fog et al., 2006). In contrast, pharmacological inhibition of CaMKIIα was shown to blunt AMPH-induced DAT phosphorylation and DA efflux in heterologous cells, as well as dopaminergic neurons in culture, striatal slices, and in the striatum of living mice (Fog et al., 2006). Similarly, DAT-mediated efflux is significantly reduced in CaMKIIα knockout (KO) mice, mice that express a permanently self-inhibited CaMKIIα mutant, as well as in a mouse model of Angelman syndrome known to exhibit reduced CaMKIIα activity (Steinkellner et al., 2014, 2012).
Importantly, this observed role for CaMKIIα in AMPH-induced DA efflux also manifests at the level of whole-animal behavior, in several experimental models. CaMKIIα KO mice exhibit a significantly blunted locomotor response to AMPH, as well as a markedly reduced behavioral sensitization to the psychostimulant, suggesting a role for CaMKIIα in both acute and chronic responses to AMPH (Steinkellner et al., 2015, 2014, 2012). Furthermore, expression of a CaMKIIα inhibitory peptide (CaMKIINtide) (Chang, Mukherji, & Soderling, 1998) selectively in DA neurons of Drosophila larvae prevents AMPH-induced hyperlocomotion (Pizzo et al., 2014), as does inhibiting DAT phosphorylation by expressing hDAT-StoA (Pizzo et al., 2013), demonstrating that the role of CaMKIIα in AMPH-stimulated behaviors is mediated in DA neurons. Critically, pseudophosphorylation of the hDAT N-terminus by mutation of the five serines to aspartates (hDAT-StoD) leads to robust hyperlocomotion when coexpressed with CaMKNtide, bypassing the need for CaMKIIα activity in AMPH-induced behavior (Pizzo et al., 2014), thereby demonstrating that the role of CaMKIIα in AMPH-induced efflux and behavior is directly related to its role in DAT N-terminal phosphorylation.
Delineating the role of CaMKIIα in DA efflux is especially critical to better understand a possible regulation of efflux by endogenous factors independently of AMPH. Of note, stimulation of the DA D2 receptor (D2R) has been shown to result in the elevation of intracellular Ca2+ concentration and activation of CaMKIIα, most likely through activation of phospholipase C β (PLCβ) and inositol triphosphate (IP3) production (Morris & Scarlata, 1997; Nishi, Snyder, & Greengard, 1997; Takeuchi, Fukunaga, & Miyamoto, 2002), suggesting that D2 autoreceptors may modulate DA efflux. Consistent with such a model, it was recently shown that treatment with D2R antagonists inhibits the AMPH-independent, anomalous DA efflux caused by the A559V mutation in hDAT (Bowton et al., 2010), which is associated with autism disorder and attention-deficit hyperactivity disorder (Bowton et al., 2014; Mazei-Robison, Couch, Shelton, Stein, & Blakely, 2005). Cells expressing hDAT-A559V also showed an increase in CaMKIIα activity compared to cells expressing wild-type hDAT, and the increase was reduced by treatment with the D2R antagonists (Bowton et al., 2010). Given that the anomalous DA efflux was found to be dependent on phosphorylation of N-terminal serines, these findings point to a role for D2R in regulating DA efflux in a CaMKIIα-dependent manner, although further investigation is needed to determine whether this effect is directly mediated through DAT phosphorylation. It must also be remembered that unlike heterologous cells loaded with DA that can efflux directly from the cytoplasm through DAT, in neurons DA is highly concentrated in synaptic vesicles and must be released from vesicles into the cytoplasm before it can efflux through DAT.
3.2. Protein Kinase C
PKC activators, such as PMA, stimulate N-terminal phosphorylation of DAT in cells in culture (Granas et al., 2003; Huff et al., 1997; Karam et al., 2017) and in rat striatum (Cowell, Kantor, Hewlett, Frey, & Gnegy, 2000; Foster et al., 2002; Granas et al., 2003), and treatment with PKC inhibitors attenuates OA-induced (Foster et al., 2002; Gorentla, Moritz, Foster, & Vaughan, 2009; Huff et al., 1997; Karam et al., 2017; Vaughan et al., 1997) and AMPH-induced DAT phosphorylation (Cervinski et al., 2005; Karam et al., 2017), suggesting a role for PKC in both basal and stimulant-induced DAT phosphorylation. Treatment with a PKC activator was also shown to elicit release of 3[H]dopamine from striatal synaptosomes that is cocaine-sensitive and independent of extracellular Ca2+ (Davis & Patrick, 1990). Furthermore, AMPH increases striatal particulate PKC activity (Giambalvo, 1992) in a manner dependent on intracellular Ca2+ (Giambalvo, 2003), implicating PKC as another target of Ca2+ signaling in response to AMPH. Furthermore, treatment with PKC inhibitors or down-regulation of PKC attenuates AMPH-induced DAT-mediated DA efflux in cells and in rat striatal tissue (Cowell et al., 2000; Kantor & Gnegy, 1998; Kantor et al., 2001; Zestos, Mikelman, Kennedy, & Gnegy, 2016), as well as AMPH-induced hyperlocomotion (Browman et al., 1998; Carpenter et al., 2017; Zestos et al., 2016) and AMPH-self administration (Carpenter et al., 2017). Investigations using isoform-specific inhibitors have implicated PKCβ as the mediator of DAT-mediated AMPH-induced DA efflux and psychomotor behavior ( Johnson, Guptaroy, Lund, Shamban, & Gnegy, 2005; Zestos et al., 2016). Consistent with these observations, PKCβ was also shown to associate in complex with DAT in rat striatal synaptosomal preparations and to colocalize with the transporter in mesencephalic neurons ( Johnson et al., 2005; O’Malley, Park, Isom, & Gnegy, 2010).
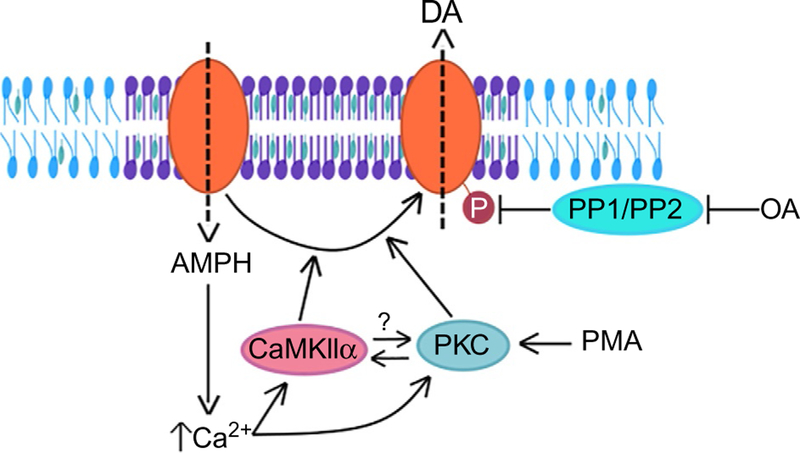
In summary, there is compelling evidence for a role for both PKC and CaMKIIα in regulating AMPH-induced DA efflux. However, whether these roles are mediated through direct phosphorylation of DAT in vivo remains to be determined. The role of PKC is complicated by its regulation of DAT trafficking (Cervinski et al., 2005; Chen et al., 2009; Foster et al., 2006; Gabriel et al., 2013; Granas et al., 2003), which is in part mediated through DAT interactions with the lipid raft protein Flotillin-1 (Flot1, see below) (Cremona et al., 2011) and the membrane-associated GTPase Rin, which interacts with DAT in membrane microdomains (Navaroli et al., 2011). The two kinases may act in parallel, although the behavioral data from Drosophila showing complete inhibition of AMPH-induced hyperlocomotion by CaMKIINtide suggest a more complex interaction (Pizzo et al., 2014). A PKC-mediated mechanism should not be blocked unless CaMKIIα functions downstream of the actions of PKC. Thus, the precise role of PKC activation in DAT phosphorylation and its functional interaction with CaMKIIα will require further study (Fig. 1).
Fig. 1.
Regulation of DAT phosphorylation. AMPH acts as a substrate for DAT (orange oval) and is transported into the neuron (dashed arrow pointing intracellularly), which leads to an increase in intracellular Ca2+ levels, an increase in CaMKIIα and PKC activity, and an increase in the levels of DAT phosphorylation, all of which have been demonstrated to facilitate AMPH-induced DAT-mediated DA efflux (dashed arrow pointing extracellularly). Activation of PKC by the phorbol ester PMA also increases DAT phosphorylation, as does inhibition of protein phosphatases by okadaic acid (OA). The mechanistic relationship between CaMKII and PKC, with respect to DAT phosphorylation, remains unclear (as indicated by the question mark). Localization of DAT to cholesterol-rich membrane microdomains (purple section of plasma membrane) facilitates its phosphorylation in response to AMPH, and thereby the consequent DA efflux and associated behaviors.
Other kinases, including protein kinase A (PKA), mitogen-activated protein kinase (MAPK) kinases (MEK1/2), and phosphatidylinositol 3-kinase (PI3K), have been shown to regulate the phosphorylation of the DAT N-terminus in vitro and/or in vivo (Batchelor & Schenk, 1998; Gorentla et al., 2009; Lin et al., 2003; Moritz et al., 2013; Page et al., 2004; Ramamoorthy et al., 2011; Vuorenpaa, Ammendrup-Johnsen, Jorgensen, & Gether, 2016). However, there is little evidence thus far to suggest that they play a selective role in AMPH-induced efflux in vivo, but rather have been shown to modulate DA uptake activity and/or DAT surface expression and trafficking.
3.3. Phosphatases
As noted earlier, treatment with protein phosphatase inhibitors results in an increase in DAT phosphorylation in the absence of exogenous kinase activation, suggesting that dephosphorylation is another regulatory mechanism of DAT function (Fig. 1). DAT phosphorylation is significantly increased following treatment with OA and other inhibitors of protein phosphatase 1 (PP1) and protein phosphatase 2 (PP2) (Huff et al., 1997; Karam et al., 2017; Vaughan et al., 1997). OA dose–response and peptide inhibitor experiments further suggest a primary role for PP1 in mediating 32P labeling (Foster et al., 2003; Gorentla et al., 2009; Vaughan et al., 1997). On the other hand, biochemical analyses have also identified the catalytic subunit of PP2A in complex with DAT (Bauman et al., 2000), suggesting that the transporter may be regulated by multiple phosphatases.
3.4. Serine Residues
As previously noted, the majority of basal, PMA-induced, and AMPH-induced DAT phosphorylation occurs at a cluster of five serine residues in the proximal N-terminus (S2, S4, S7, S12, and S13). Efforts to identify the specific residues that are phosphorylated have relied on metabolic phosphorylation of DAT with 32P, peptide mapping using specific proteases, and, more recently, immunolabeling of DAT with phosphospecific antibodies. Deletion of the first 22 amino acids of DAT or the simultaneous mutation of the five N-terminal serine residues to alanine eliminates the incorporation of 32P in response to PKC activation (Granas et al., 2003). Mutation of serines at positions 7, 12, and 13 was found to reduce phosphorylation of DAT by PKC, MEK1/2, and PI3K (Lin et al., 2003). In another study, a recombinant rat DAT N-terminal peptide was shown to be phosphorylated in vitro by PKA at S7, CaMKIIα at S13, and at multiple sites (S4, S7, and S13) by PKC (Moritz et al., 2013), while mass spectrometry pointed to S7 as a site for OA-induced phosphorylation of heterologously expressed hDAT (Moritz et al., 2013). Studies with two distinct phosphospecific antibodies that recognize DAT when phosphorylated at either S7 or S12 show that treatment of cells with either AMPH or OA can promote DAT phosphorylation at both these residues, in a PKC- and CaMKIIα-dependent manner (Karam et al., 2017). Consistent with these data, functional analyses of the efflux-deficient hDAT-StoA mutant show that single mutation to aspartate of the alanines at positions 7 and 12, but not at positions 2, 4, and 13, restores a substantial fraction of AMPH-induced DA efflux observed with aspartates at all five positions (hDAT-StoD) (~ 30% and 45%, respectively) (Fog et al., 2006; Khoshbouei et al., 2004).
4. REGULATION OF DAT-MEDIATED DA EFFLUX BY THE MEMBRANE MICROENVIRONMENT
Evidence has emerged for a critical role of the localization of DAT in the plasma membrane and the surrounding microenvironment in regulating DAT function, and specifically DAT phosphorylation and DAT-mediated DA efflux. Treatment with disruptors of lipid rafts such as nystatin, flipin, or methyl-β-cyclodextrin (MbC) leads to loss of DAT from cholesterol-rich regions in the plasma membrane (Cremona et al., 2011; Foster, Adkins, Lever, & Vaughan, 2008). Treatment with nystatin inhibits AMPH-induced DA efflux, without impairing DAT-mediated DA uptake in heterologous cells (Cremona et al., 2011). In contrast, treatment with MbC has been shown to inhibit transport by DAT and SERT (Adkins et al., 2007; Foster et al., 2008; Magnani, Tate, Wynne, Williams, & Haase, 2004), but this may be reconciled by the different mechanisms by which these drugs disrupt lipid rafts. MbC acts by extracting cholesterol from cell membranes (Ilangumaran & Hoessli, 1998), whereas nystatin and filipin directly insert into the membrane, bind to free cholesterol, and physically disrupt rafts, while maintaining tightly bound cholesterol within the membrane (Coutinho & Prieto, 1995). Given the findings with nystatin (Cremona et al., 2011) and those with filipin (Foster et al., 2008), the effect of MbC likely relates to the removal of cholesterol tightly bound to DAT (Penmatsa et al., 2013), as this could directly impact DAT’s ability to undergo the conformational changes associated with transport (Hong & Amara, 2010; Jones, Zhen, & Reith, 2012; North & Fleischer, 1983; Scanlon, Williams, & Schloss, 2001), in addition to the localization of DAT to rafts.
4.1. Flotillin-1
Consistent with a role for raft localization in the regulation of DAT function, we recently showed that the lipid raft-associated protein Flot1 is required to maintain the localization of DAT in cholesterol-rich regions in the plasma membrane and plays a role in regulating PKC-mediated DAT endocytosis, as well as AMPH-induced DA efflux (Cremona et al., 2011). Like nystatin treatment, knockdown of Flot1 also leads to loss of DAT from rafts. This is accompanied by a decrease in PKC-triggered internalization of DAT, although basal levels of DAT at the plasma membrane are unaffected, suggesting that Flot1 is not required for trafficking of DAT to the plasma membrane. Consistent with this, depletion of Flot1 has no effect on the uptake capacity of DAT. In contrast, depletion of Flot1 dramatically inhibits AMPH-induced DA efflux in primary cultures of DA neurons (Cremona et al., 2011). Furthermore, genetic ablation of Flot1, or knock-down of its expression selectively in DA neurons of Drosophila larvae, inhibits hyperlocomotion induced by AMPH but not that induced by methylphenidate (Pizzo et al., 2013). Therefore, like DAT phosphorylation, the localization of DAT to lipid rafts is required for its efflux capacity, but not for its ability to transport DA.
The role of raft localization in DA efflux is likely independent of its role in DAT endocytosis, given that inhibition of internalization would leave more DAT at the surface available to efflux DA, resulting in increased AMPH-induced amperometric currents in DA neuronal cultures, as well as enhanced AMPH-induced behavior in flies, whereas we see inhibition of both these measures. Since lipid rafts can scaffold interactions underlying key signaling events that are mediated by kinases, including CaMKIIα (Jayanthi, Samuvel, & Ramamoorthy, 2004; Suzuki, Du, Tian, Zhang, & Endo, 2008; Tsui, Inagaki, & Schulman, 2005; Weerth, Holtzclaw, & Russell, 2007) and PKC (Evans, Murray, Leslie, & Falke, 2006; Niggli, Meszaros, Oppliger, & Tornay, 2004), it is tempting to speculate that the distribution of DAT within the plasma membrane may regulate its phosphorylation and thereby AMPH-induced DA efflux and behavior. Consistent with such a model, basal and PMA-induced DAT phosphorylation levels are higher in cholesterol-rich fractions of the membrane (Foster et al., 2008), and treatment with nystatin inhibits PMA-induced phosphorylation of DAT at S7 (Karam et al., 2017). Critically, expression of hDAT-StoD in fly DA neurons bypasses the need for Flot1 in AMPH-induced hyperlocomotion (Pizzo et al., 2013), just as it bypasses the requirement for CaMKIIα (Pizzo et al., 2014). This observation that pseudophosphorylation of DAT is able to rescue the AMPH response that is lost despite the mislocalization of DAT away from lipid rafts supports the hypothesis that the role of Flot1 in efflux is to localize DAT to lipid rafts so it can be phosphorylated. These data provide a direct link between the localization of DAT to lipid rafts, its N-terminal phosphorylation, and the consequent behavioral response to AMPH. Furthermore, DAT phosphorylation has been shown to influence palmitoylation of DAT on Cys580, with increased phosphorylation leading to reduced palmitoylation, and vice versa (Moritz et al., 2015; Rastedt, Vaughan, & Foster, 2017). Lipid modification of proteins is thought to drive raft localization (Levental, Lingwood, Grzybek, Coskun, & Simons, 2010; Resh, 2006), suggesting a complex interplay between posttranslational modifications at the termini of DAT, although the mechanisms underlying the concerted regulation of these events remain unclear.
4.2. Phosphatidylinositol 4,5-Bisphosphate
A potential mechanism connecting membrane microdomain localization to DA efflux may relate to DAT N-terminal interactions with charged lipids. Molecular dynamics simulations have revealed the DAT N-terminus to be a highly dynamic domain that engages with lipid membranes through electrostatic interactions between charged residues in the N-terminus and the lipids phosphatidylinositol 4,5-bisphosphate (PIP2) (Khelashvili et al., 2015). PIP2 lipids are strongly anionic and represent a small fraction of the phospholipid composition of the inner leaflets of the plasma membrane (Balla, 2013; McLaughlin, Wang, Gambhir, & Murray, 2002). Yet, they regulate many cellular processes including transmembrane ion fluxes (Gamper & Shapiro, 2007; Hilgemann & Ball, 1996), by affecting the function and organization of various membrane proteins (Balla, 2013; McLaughlin et al., 2002; Suh & Hille, 2008). Ion fluxes have also been observed in neurotransmitter: sodium symporters and are required for AMPH-induced substrate efflux (Khoshbouei et al., 2003; Pifl & Singer, 1999; Sitte et al., 1998).
Association of PIP2 lipids with DAT was established by immunoprecipitation from heterologous cells expressing hDAT and from mouse striatal tissues, as well as by pulldown with a GST-fused hDAT N-terminal construct comprising the first 64 amino acids (Hamilton et al., 2014). Functional analyses further showed that PIP2 depletion or sequestration inhibits AMPH-induced DA efflux without affecting DAT-mediated DA reuptake (Hamilton et al., 2014), similar to its effects on AMPH-induced efflux through SERT (Buchmayer et al., 2013). Consistent with these findings, charge-neutralizing substitutions of lysines at positions 3 and 5 in the hDAT N-terminus to alanine or asparagine (K3A/K5A or K3N/K5N), which significantly inhibit its binding to PIP2 lipids, also blunt AMPH-dependent DA efflux without affecting DA reuptake in heterologous cells. Similarly, expression of hDAT-K3A/K5A in Drosophila dopaminergic neurons does not affect DA-dependent basal locomotor activity in adult flies, but rather diminishes AMPH-induced hyperlocomotion (Hamilton et al., 2014). Taken together, these studies support a role for direct electrostatic interactions between specific regions of the DAT N-terminus and PIP2 lipids in AMPH-induced DA efflux. It is possible that these interactions stabilize the N-terminus in a position that facilitates its phosphorylation. Alternatively, they might regulate dynamic intraprotein interactions between the N-terminus and other parts of hDAT to promote a conformation that is favorable for efflux. Curiously, molecular dynamics simulations of the hDAT N-terminus revealed that its phosphorylation disrupts its electrostatic interactions with the negatively charged membrane (Khelashvili et al., 2015), as does, deletion of the first 22 amino acids or the K3A/K5A mutation (Khelashvili et al., 2015). That such distinct manipulations with opposing effects on efflux (Fog et al., 2006; Hamilton et al., 2014) all decrease interactions with the membrane suggest that release of the N-terminus is not sufficient for efflux, and it is more likely that phosphorylation facilitates its interaction either with another part of hDAT, or possibly with another protein, to promote an efflux-competent conformation. Yet another possibility is that phosphorylation of the N-terminus acts as a “latch” that allows for communication between transporters to induce efflux, as proposed in the oligomer-based countertransport model (Seidel et al., 2005; Sitte & Freissmuth, 2010). Oligomerization of DAT has been recognized as an important prerequisite for exit from the endoplasmic reticulum, and DAT has been shown to exist as an oligomer in the plasma membrane (Farhan, Freissmuth, & Sitte, 2006; Hastrup et al., 2001; Kilic & Rudnick, 2000). Furthermore, studies have suggested that oligomerization might play a role in efflux, through a mechanism in which AMPH is taken up by one oligomeric moiety and DA is effluxed via another moiety within the same oligomer (Seidel et al., 2005; Sitte & Freissmuth, 2010). Based on this model, phosphorylation could act as the mechanism by which the two individual subunits communicate, although this remains speculative and has not been tested experimentally.
4.3. Syntaxin
The neuronal soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) protein syntaxin 1A (STX1A) plays a central role in vesicular fusion (Han, Pluhackova, & Bockmann, 2017) and is also known to regulate the function of various neurotransmitter transporters, including SERT and NET (Dipace, Sung, Binda, Blakely, & Galli, 2007; Quick, 2002a, 2002b, 2003, 2006). STX1A was identified in a yeast two-hybrid screen as an interacting partner of DAT (Lee, Kim, Kim, & Lee, 2004) and was later shown to coimmunoprecipitate with DAT from heterologous cells and mouse striatal synaptosomes via direct association with the DAT N-terminal tail (Binda et al., 2008; Carvelli, Blakely, & DeFelice, 2008; Cervinski, Foster, & Vaughan, 2010; Lee et al., 2004). This interaction is potentiated by AMPH treatment, both in cultured cells and in mouse striatal synaptosomes, in a manner dependent on CaMKIIα activity, pointing to a possible link between the DAT/STX1 interaction and AMPH-induced DAT phosphorylation. Consistent with this, treatment of rat striatal tissue with the STX1 protease Botulinum Neurotoxin C (BoNT/C) blunts DAT phosphorylation (Cervinski et al., 2010). Furthermore, perfusion of exogenous STX1A into heterologous cells, as well as DA neurons in culture, enhances AMPHinduced DA efflux, and this response is also dependent on CaMKIIα activity. These findings suggest that AMPH activation of CaMKIIα supports DAT/STX1A association, resulting in a mode of DAT that is more efflux competent.
A recent report described the identification and characterization of two independent autism-associated single nucleotide variations in the genes coding for DAT and STX1 (Cartier et al., 2015). Mechanistic examination showed that the functional effects of the two mutations converged to inhibit AMPH-induced DA efflux in heterologous cells, as well as AMPH-mediated hyperlocomotion in Drosophila, possibly by inhibiting the DAT/STX1 interaction. Interestingly, the STX1 variant exhibits decreased casein kinase (CK2)-mediated phosphorylation at S14 (Cartier et al., 2015), a modification known to regulate the interaction of STX1 with other proteins (Foletti, Lin, Finley, & Scheller, 2000; Hirling & Scheller, 1996; Khelashvili, Galli, & Weinstein, 2012). Indeed, mutation to alanine of this residue significantly decreases DAT/STX1 interaction, as does inhibition of CK2 activity. Consistent with this, CK2 inhibition also blunts AMPH-induced efflux without affecting DA reuptake in mouse striatal slices, and expression of a dominant-negative CK2 in DA neurons of flies also inhibits AMPH-induced hyperlocomotion (Cartier et al., 2015). Despite these advances, it remains unclear whether STX1 enhances efflux in vivo. The role of STX1 is further complicated by its effect on DAT surface levels; BoNT/C-mediated inhibition of STX1 leads to decreased levels of DAT at the plasma membrane, and thus lower levels of DAT-mediated uptake (Cervinski et al., 2010). Still, this and other recent discoveries of specific mutations in hDAT that affect DA efflux in particular and are linked to various neuropsychiatric disorders underscore the significance of the mechanisms that regulate efflux, and their potential relevance to disease beyond the response to psychostimulants (Hamilton et al., 2013; Hansen et al., 2014; Mazei-Robison et al., 2008; Mergy et al., 2014; Ng et al., 2014).
5. ACUTE ACTION OF AMPH AT THE VMAT
While DAT clearly mediates efflux of cytoplasmic DA across the plasma membrane, how AMPHs release DA from vesicles into the cytoplasm remains controversial. DA is synthesized in the cytosol and concentrated in synaptic vesicles by VMAT, which functions by exporting two H+ from the vesicle lumen for each cationic monoamine substrate it imports (Knoth, Zallakian, & Njus, 1981). This substrate antiport mechanism is driven by a vesicular pH gradient generated by the vacuolar H+-ATPase (Edwards, 2007), which also promotes the retention of dopamine in vesicles through its protonation (Maron, Stern, Kanner, & Schuldiner, 1983).
Given VMAT’s central role in maintaining DA stores, it follows that its function is essential for the actions of all DA-dependent psychostimulants. However, mechanisms by which AMPH acts directly at VMAT to redistribute DA from vesicles into the cytoplasm are not well defined. Indeed, studies in rodents have shown that the pharmacological inhibition of VMAT prior to treatment with AMPHs blunts the behavioral responses to these drugs (Harrod, Dwoskin, Crooks, Klebaur, & Bardo, 2001; Meyer et al., 2011; Miller et al., 2001; Neugebauer et al., 2007; Wilmouth, Zheng, Crooks, Dwoskin, & Bardo, 2013). These studies, however, were hindered by the fact that early VMAT inhibitors, primarily tetrabenazine, also target DAT (Meyer et al., 2011). This was addressed with the recent development of a novel, more selective VMAT inhibitor, (+)-CYY477, which, when administered ≤5 min preceding psychostimulant treatments, completely blocked locomotor and self-administration behaviors stimulated by AMPHs, without affecting those induced by cocaine (Freyberg et al., 2016). These data suggested that, under conditions where DA stores are still available to elicit a behavioral response to a DAT uptake blocker, VMAT function is required for the acute actions of AMPHs to release DA from intraluminal stores prior to its DAT-mediated efflux into the synaptic space.
In an effort to delineate the molecular mechanisms underlying the actions of AMPH at VMAT leading up to the release of DA from the vesicles, we monitored the dynamics of vesicular cargo with a fluorescent false neurotransmitter, as well as real-time changes in synaptic vesicle pH with a genetically encoded pH biosensor in an ex vivo Drosophila brain preparation, and showed that AMPH treatment diminishes the vesicular pH gradient and redistributes vesicular contents, in a manner dependent on both DAT and VMAT (Freyberg et al., 2016). Weak bases such as chloroquine can deacidify vesicles by diffusion of the neutral species across the vesicle membrane followed by protonation and trapping within the vesicle lumen (Maron et al., 1983; Sulzer et al., 1995), and it has been proposed that AMPH also acts through a weak base mechanism (Fleckenstein, Volz, Riddle, Gibb, & Hanson, 2007; Sulzer et al., 1995). However, genetic and pharmacological analyses using this optical model provided strong evidence that VMAT-mediated substrate-coupled H+ antiport itself provides the critical in vivo mechanism for AMPH-induced vesicular deacidification. Notably, the DAT and VMAT substrate 1-methyl-4-phenylpyridinium (MPP+), which has a fixed positive charge on its quaternary amine (Darchen, Scherman, Desnos, & Henry, 1988; Javitch, D’Amato, Strittmatter, & Snyder, 1985), also leads to vesicle deacidification, even though it is not a weak base. Moreover, chloroquine’s actions are not blocked by inhibition of VMAT, whereas vesicle deacidification in response to physiological concentrations of the DAT and VMAT substrates AMPH, DA, and MPP+ are blocked by selective VMAT inhibitors, consistent with transport by VMAT being the source of proton depletion and not a weak base effect (Freyberg et al., 2016).
Despite these advances, it remains unclear how AMPH transport into vesicles, and their concomitant deacidification, leads to redistribution of DA to the cytoplasm. One hypothesis is that in the presence of a diminished proton gradient, DA simply diffuses out of vesicles as a neutral species and also is less efficiently taken up without the driving force for VMAT transport. However, it has been shown that protonophores dramatically enhance the efflux of MPP+ from synaptic vesicles (Moriyama, Amakatsu, & Futai, 1993), which suggests that a mechanism other than diffusion may be at play. Computational analyses show a striking similarity between the structure-based properties of the N-terminus of the human VMAT2 and that of hDAT (Khelashvili & Weinstein, 2015), suggesting a potential role of N-terminal phosphorylation of VMAT in modulating DA efflux from synaptic vesicles. Consistent with such a model, it was recently shown that phosphorylation of serines in the N-terminus of VMAT is essential for AMPH-induced DA efflux (Torres & Ruoho, 2014), suggesting the exciting possibility that VMAT might actively mediate the exit of monoamines from vesicles via reverse transport in a phosphorylation-dependent manner, as does DAT at the plasma membrane. However, this hypothesis has not been explored mechanistically nor validated in an in vivo setting.
6. CONCLUSION
The findings reviewed here point to a complex regulatory network that modulates dopamine transporter phosphorylation in a manner that influences the transporter’s ability to efflux DA (Table 1). Why multiple transporters in the SLC6 family, including DAT and SERT (Sucic et al., 2010), and possibly VMAT in the SLC18 family, are regulated by N-terminal phosphorylation remains a mystery. Clearly this mode of regulation did not evolve in anticipation of psychostimulants such as AMPH that can coopt the pathway. Whether reverse transport is an alternative means of neurotransmitter release in the absence of psychostimulants but with activation of particular kinases is an intriguing question. One other speculative possibility is that this pathway serves as an “overflow valve” in pathological conditions when cytoplasmic transmitter might be high, particularly in the case of cytoplasmic DA, which can be quite toxic through its reactive species (Kita, Miyazaki, Asanuma, Takeshima, & Wagner, 2009; Segura-Aguilar et al., 2014). While these potential mechanisms are currently purely speculative, further study should reveal clues as to the function of this fascinating means of modulation.
Table 1.
Modulators of DAT Phosphorylation and AMPH-Induced DAT-Mediated DA Efflux
| Modulator | Direct Interaction | Functional Impact |
|---|---|---|
| CaMKIIα | With DAT C-terminus based on yeast two– hybrid interaction | Phosphorylates DAT N-terminus in vitro Supports DA efflux in vitro, ex vivo, and in vivo Functions in DA neurons of flies to facilitate AMPH-induced behavior; this can be bypassed by pseudophosphorylation of the DAT N-terminus Supports AMPH-induced behaviors in rodents |
| PKC | No evidence | Phosphorylates DAT N-terminus in vitro Supports DA efflux in vitro and ex vivo Supports AMPH-induced behavior in rodents |
| PP1 | No evidence | Mediates a major fraction of DAT dephosphorylation |
| PP2 | In complex with DAT, as observed by coimmunoprecipitation | Extent of direct role is unclear |
| Flotillin-1 | In complex with DAT, as observed by coimmunoprecipitation | Necessary for localization of DAT to lipid rafts, which in turn facilitates DAT phosphorylation Supports DA efflux in vitro and ex vivo Functions in DA neurons in flies to support AMPH-induced hyperlocomotion; this can be bypassed by pseudophosphorylation of the DAT N-terminus |
| PIP2 | Electrostatic interaction via DAT N-terminus (K3/K5), as observed by coimmunoprecipitation and GST-pulldown | Interaction with DAT supports DA efflux in vitro and AMPH-induced hyperlocomotion in flies |
| Syntaxin | With the DAT N-terminus as observed by yeast two-hybrid assay and by coimmunoprecipitation from synaptosomes Interaction is potentiated by AMPH treatment in a CaMKII– dependent manner Interaction is enhanced by CK2-mediated phosphorylation |
Interaction with DAT supports efflux in vitro and ex vivo Supports DAT phosphorylation in rat striatal tissue |
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants R01 DA041510 (J.A.J., C.S.K.) and K05 DA022413 (J.A.J.).
Dedication. Training with Solomon Snyder was extremely inspiring but also profoundly humbling. Sol’s interests and knowledge were protean, and his influence has been and continues to be wide ranging and profound, touching on a vast range of scientific topics and discoveries. Sol introduced me to the world of transporters as a graduate student in his lab nearly 40 years ago, and I have studied them ever since. In contrast, after a number of key discoveries, Sol moved on. Nonetheless, a Pubmed search of Sol Snyder and amphetamine leads to 36 hits, whereas a similar search for me, despite my focus in the area reveals just 31! Humbling indeed how great an influence Sol could have on an area in such a short period of time. I just reread Sol’s 1972 paper in the Archives of General Psychiatry in which he inferred, based on the lack of substantial stereospecificity, that amphetamine induced psychosis through its effects on dopamine and not norepinephrine (Snyder, 1972). This in part led to his discovery of the dopamine receptor as the target for antipsychotic actions (Creese, Burt, & Snyder, 1976), something the field studies to this day. Truly humbling but always inspiring. Sol’s influence on all of those he trained is eternal and without measure, and Caline, a scientific granddaughter he has not yet met, and I dedicate this review to him and congratulate him on his profound achievements, both as a scientist, a mentor, a friend, and a true mensch.
ABBREVIATIONS
- AMPH
amphetamine
- CaMKIIα
Ca2+/calmodulin-dependent protein kinase alpha
- CaMKIINtide
CaMKIIα inhibitor peptide
- Cav
voltage-gated Ca2+ channel
- CK2
casein kinase
- D2R
dopamine D2 receptor
- DA
dopamine
- DAT
dopamine transporter
- Flot1
Flotillin-1
- MbC
methyl-β-cyclodextrin
- N-terminus
amino terminus
- OA
okadaic acid
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKC
protein kinase C
- PMA
phorbol 12-myristate, 13-acetate
- PP
protein phosphatase
- SERT
serotonin transporter
- SLC
solute carrier
- STX1
syntaxin
- VMAT
vesicular monoamine transporter
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, et al. (2007). Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry, 46(37), 10484–10497. [DOI] [PubMed] [Google Scholar]
- Amara SG, & Kuhar MJ (1993). Neurotransmitter transporters: Recent progress. Annual Review of Neuroscience, 16, 73–93. 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Balla T (2013). Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiological Reviews, 93(3), 1019–1137. 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor M, & Schenk JO (1998). Protein kinase A activity may kinetically upregulate the striatal transporter for dopamine. The Journal of Neuroscience, 18(24), 10304–10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, & Blakely RD (2000). Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. The Journal of Neuroscience, 20(20), 7571–7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, et al. (2008). Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Molecular Pharmacology, 74(4), 1101–1108. 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N, et al. (2010). Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. The Journal of Neuroscience, 30(17), 6048–6057. 10.1523/JNEUROSCI.5094-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Reddy IA, Campbell NG, Hamilton PJ, Henry LK, et al. (2014). SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Translational Psychiatry 4, e464 10.1038/tp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman KE, Kantor L, Richardson S, Badiani A, Robinson TE, & Gnegy ME (1998). Injection of the protein kinase C inhibitor Ro31–8220 into the nucleus accumbens attenuates the acute response to amphetamine: Tissue and behavioral studies. Brain Research, 814(1–2), 112–119. [DOI] [PubMed] [Google Scholar]
- Buchmayer F, Schicker K, Steinkellner T, Geier P, Stubiger G, Hamilton PJ, et al. (2013). Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. Proceedings of the National Academy of Sciences of the United States of America, 110(28), 11642–11647. 10.1073/pnas.1220552110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Solis E Jr., Ruchala I, De Felice LJ, & Eltit JM (2015). Amphetamine activates calcium channels through dopamine transporter-mediated depolarization. Cell Calcium, 58(5), 457–466. 10.1016/j.ceca.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C, Zestos AG, Altshuler R, Sorenson RJ, Guptaroy B, Showalter HD, et al. (2017). Direct and systemic administration of a CNS-permeant tamoxifen analog reduces amphetamine-induced dopamine release and reinforcing effects. Neuropsychopharmacology, 42, 1940–1949. 10.1038/npp.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier E, Hamilton PJ, Belovich AN, Shekar A, Campbell NG, Saunders C, et al. (2015). Rare autism-associated variants implicate syntaxin 1 (STX1 R26Q) phosphorylation and the dopamine transporter (hDAT R51W) in dopamine neurotransmission and behaviors. eBioMedicine, 2(2), 135–146. 10.1016/j.ebiom.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Blakely RD, & DeFelice LJ (2008). Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proceedings of the National Academy of Sciences of the United States of America, 105(37), 14192–14197. 10.1073/pnas.0802214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, & Vaughan RA (2005). Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. The Journal of Biological Chemistry, 280(49), 40442–40449. 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, & Vaughan RA (2010). Syntaxin 1A regulates dopamine transporter activity, phosphorylation and surface expression. Neuroscience, 170(2), 408–416. 10.1016/j.neuroscience.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, & Soderling TR (1998). Characterization of a calmodulin kinase II inhibitor protein in brain. Proceedings of the National Academy of Sciences of the United States of America, 95(18), 10890–10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau RWT, Leitges M, et al. (2009). Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. The Journal of Pharmacology and Experimental Therapeutics, 328(3), 912–920. 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, & Reith ME (2004). Interaction between dopamine and its transporter: Role of intracellular sodium ions and membrane potential. Journal of Neurochemistry, 89(3), 750–765. 10.1111/j.1471-4159.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- Coutinho A, & Prieto M (1995). Self-association of the polyene antibiotic nystatin in dipalmitoylphosphatidylcholine vesicles: A time-resolved fluorescence study. Biophysical Journal, 69(6), 2541–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, & Gnegy ME (2000). Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. European Journal of Pharmacology, 389(1), 59–65. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, & Snyder SH (1976). Dopamine receptors and average clinical doses. Science, 194(4264), 546 10.1126/science.194.4264.546. [DOI] [PubMed] [Google Scholar]
- Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, et al. (2011). Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nature Neuroscience, 14(4), 469–477. 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darchen F, Scherman D, Desnos C, & Henry JP (1988). Characteristics of the transport of the quaternary ammonium 1-methyl-4-phenylpyridinium by chromaffin granules. Biochemical Pharmacology, 37(22), 4381–4387. [DOI] [PubMed] [Google Scholar]
- Davis ME, & Patrick RL (1990). Diacylglycerol-induced stimulation of neurotransmitter release from rat brain striatal synaptosomes. Journal of Neurochemistry, 54(2), 662–668. [DOI] [PubMed] [Google Scholar]
- Dawe S, Davis P, Lapworth K, & McKetin R (2009). Mechanisms underlying aggressive and hostile behavior in amphetamine users. Current Opinion in Psychiatry, 22(3), 269–273. 10.1097/YCO.0b013e32832a1dd4. [DOI] [PubMed] [Google Scholar]
- Dipace C, Sung U, Binda F, Blakely RD, & Galli A (2007). Amphetamine induces a calcium/calmodulin-dependent protein kinase II-dependent reduction in norepinephrine transporter surface expression linked to changes in syntaxin 1A/transporter complexes. Molecular Pharmacology, 71(1), 230–239. 10.1124/mol.106.026690. [DOI] [PubMed] [Google Scholar]
- Edwards RH (2007). The neurotransmitter cycle and quantal size. Neuron, 55(6), 835–858. 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Henningsen RA, Neve KA, & Janowsky A (1994). Release of dopamine via the human transporter. Molecular Pharmacology, 45(2), 312–316. [PubMed] [Google Scholar]
- Evans JH, Murray D, Leslie CC, & Falke JJ (2006). Specific translocation of protein kinase C alpha to the plasma membrane requires both Ca2 + and PIP2 recognition by its C2 domain. Molecular Biology of the Cell, 17(1), 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan H, Freissmuth M, & Sitte HH (2006). Oligomerization of neurotransmitter transporters: A ticket from the endoplasmic reticulum to the plasma membrane. Handbook of Experimental Pharmacology, 175, 233–249. [DOI] [PubMed] [Google Scholar]
- Fischer JF, & Cho AK (1979). Chemical release of dopamine from striatal homogenates: Evidence for an exchange diffusion model. The Journal of Pharmacology and Experimental Therapeutics, 208(2), 203–209. [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, & Hanson GR (2007). New insights into the mechanism of action of amphetamines. Annual Review of Pharmacology and Toxicology, 47, 681–698. 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, et al. (2006). Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron, 51(4), 417–429. 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Foletti DL, Lin R, Finley MA, & Scheller RH (2000). Phosphorylated syntaxin 1 is localized to discrete domains along a subset of axons. The Journal of Neuroscience, 20(12), 4535–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, & Vaughan RA (2008). Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. Journal of Neurochemistry, 105(5), 1683–1699. 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Cervinski MA, Gorentla BK, & Vaughan RA (2006). Regulation of the dopamine transporter by phosphorylation. Handbook of Experimental Pharmacology, 175, 197–214. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Cervinski MA, Holden HE, & Vaughan RA (2003). Dopamine transporters are dephosphorylated in striatal homogenates and in vitro by protein phosphatase 1. Brain Research. Molecular Brain Research, 110(1), 100–108. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, & Vaughan RA (2002). Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. The Journal of Biological Chemistry, 277(28), 25178–25186. 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Foster JD, & Vaughan RA (2016). Phosphorylation mechanisms in dopamine transporter regulation. Journal of Chemical Neuroanatomy, 83–84, 10–18. 10.1016/j.jchemneu.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J, et al. (2016). Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nature Communications, 7, 10652 10.1038/ncomms10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel LR, Wu S, Kearney P, Bellve KD, Standley C, Fogarty KE, et al. (2013). Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: Differential dependence on dynamin and the actin cytoskeleton. The Journal of Neuroscience, 33(45), 17836–17846. 10.1523/JNEUROSCI.3284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, & Shapiro MS (2007). Regulation of ion transport proteins by membrane phosphoinositides. Nature Reviews. Neuroscience, 8(12), 921–934. 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT (1992). Protein kinase C and dopamine transport—1. Effects of amphetamine in vivo. Neuropharmacology, 31(12), 1201–1210. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT (2003). Differential effects of amphetamine transport vs. dopamine reverse transport on particulate PKC activity in striatal synaptoneurosomes. Synapse, 49(2), 125–133. 10.1002/syn.10223. [DOI] [PubMed] [Google Scholar]
- Gnegy ME, Khoshbouei H, Berg KA, Javitch JA, Clarke WP, Zhang M, et al. (2004). Intracellular Ca2 + regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Molecular Pharmacology, 66(1), 137–143. 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- Gorentla BK, Moritz AE, Foster JD, & Vaughan RA (2009). Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry, 48(5), 1067–1076. 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granas C, Ferrer J, Loland CJ, Javitch JA, & Gether U (2003). N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. The Journal of Biological Chemistry, 278(7), 4990–5000. 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- Hamilton PJ, Belovich AN, Khelashvili G, Saunders C, Erreger K, Javitch JA, et al. (2014). PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nature Chemical Biology, 10(7), 582–589. 10.1038/nchembio.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, et al. (2013). De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Molecular Psychiatry, 18(12), 1315–1323. 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Pluhackova K, & Bockmann RA (2017). The multifaceted role of SNARE proteins in membrane fusion. Frontiers in Physiology, 8, 5 10.3389/fphys.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen FH, Skjorringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, et al. (2014). Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. The Journal of Clinical Investigation, 124(7), 3107–3120. 10.1172/JCI73778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, & Bardo MT (2001). Lobeline attenuates d-methamphetamine self-administration in rats. The Journal of Pharmacology and Experimental Therapeutics, 298(1), 172–179. [PubMed] [Google Scholar]
- Hastrup H, Karlin A, & Javitch JA (2001). Symmetrical dimer of the human dopa mine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proceedings of the National Academy of Sciences of the United States of America, 98(18), 10055–10060. 10.1073/pnas.181344298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastrup H, Sen N, & Javitch JA (2003). The human dopamine transporter forms a tetramer in the plasma membrane: Cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. The Journal of Biological Chemistry, 278(46), 45045–45048. 10.1074/jbc.C300349200. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Gosden J, & Nutt DJ (2013). Amphetamine, past and present—A pharmacological and clinical perspective. Journal of Psychopharmacology, 27(6), 479–496. 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, & Ball R (1996). Regulation of cardiac Na+, Ca2 + exchange and KATP potassium channels by PIP2. Science, 273(5277), 956–959. [DOI] [PubMed] [Google Scholar]
- Hirling H, & Scheller RH (1996). Phosphorylation of synaptic vesicle proteins: Modulation of the alpha SNAP interaction with the core complex. Proceedings of the National Academy of Sciences of the United States of America, 93(21), 11945–11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong WC, & Amara SG (2010). Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. The Journal of Biological Chemistry, 285(42), 32616–32626. 10.1074/jbc.M110.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff RA, Vaughan RA, Kuhar MJ, & Uhl GR (1997). Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. Journal of Neurochemistry, 68(1), 225–232. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, & Hoessli DC (1998). Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. The Biochemical Journal, 335(Pt. 2), 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, & Iversen LL (2007). Dopamine: 50 Years in perspective. Trends in Neurosciences, 30(5), 188–193. 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Jardetzky O (1966). Simple allosteric model for membrane pumps. Nature, 211(5052), 969–970. [DOI] [PubMed] [Google Scholar]
- Javitch JA, D’Amato RJ, Strittmatter SM, & Snyder SH (1985). Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proceedings of the National Academy of Sciences of the United States of America, 82(7), 2173–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, & Ramamoorthy S (2004). Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. The Journal of Biological Chemistry, 279(18), 19315–19326. 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, & Gnegy ME (2005). Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. The Journal of Biological Chemistry, 280(12), 10914–10919. 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Jones KT, Zhen J, & Reith ME (2012). Importance of cholesterol in dopamine transporter function. Journal of Neurochemistry, 123(5), 700–715. 10.1111/jnc.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, et al. (2005). Amphetamine induces dopamine efflux through a dopamine transporter channel. Proceedings of the National Academy of Sciences of the United States of America, 102(9), 3495–3500. 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor L, & Gnegy ME (1998). Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. The Journal of Pharmacology and Experimental Therapeutics, 284(2), 592–598. [PubMed] [Google Scholar]
- Kantor L, Hewlett GH, Park YH, Richardson-Burns SM, Mellon MJ, & Gnegy ME (2001). Protein kinase C and intracellular calcium are required for amphetamine-mediated dopamine release via the norepinephrine transporter in undifferentiated PC12 cells. The Journal of Pharmacology and Experimental Therapeutics, 297(3), 1016–1024. [PubMed] [Google Scholar]
- Karam CS, Sen N, & Javitch JA (2017). Phospho-specific antibodies targeting the amino terminus of the human dopamine transporter. Journal of Chemical Neuroanatomy, 83–84, 91–98. 10.1016/j.jchemneu.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelashvili G, Doktorova M, Sahai MA, Johner N, Shi L, & Weinstein H (2015). Computational modeling of the N-terminus of the human dopamine transporter and its interaction with PIP2-containing membranes. Proteins, 83(5), 952–969. 10.1002/prot.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelashvili G, Galli A, & Weinstein H (2012). Phosphatidylinositol 4,5-biphosphate (PIP(2)) lipids regulate the phosphorylation of syntaxin N-terminus by modulating both its position and local structure. Biochemistry, 51(39), 7685–7698. 10.1021/bi300833z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelashvili G, & Weinstein H (2015). Functional mechanisms of neurotransmitter transporters regulated by lipid-protein interactions of their terminal loops. Biochimica et Biophysica Acta, 1848(9), 1765–1774. 10.1016/j.bbamem.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. (2004). N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biology, 2(3), E78 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, & Galli A (2003). Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. The Journal of Biological Chemistry, 278(14), 12070–12077. 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- Kilic F, & Rudnick G (2000). Oligomerization of serotonin transporter and its functional consequences. Proceedings of the National Academy of Sciences of the United States of America, 97(7), 3106–3111. 10.1073/pnas.060408997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilty JE, Lorang D, & Amara SG (1991). Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science, 254(5031), 578–579. [DOI] [PubMed] [Google Scholar]
- Kita T, Miyazaki I, Asanuma M, Takeshima M, & Wagner GC (2009). Dopamine-induced behavioral changes and oxidative stress in methamphetamine-induced neurotoxicity. International Review of Neurobiology, 88, 43–64. 10.1016/S0074-7742(09)88003-3. [DOI] [PubMed] [Google Scholar]
- Knoth J, Zallakian M, & Njus D (1981). Stoichiometry of H+-linked dopamine transport in chromaffin granule ghosts. Biochemistry, 20(23), 6625–6629. [DOI] [PubMed] [Google Scholar]
- Krueger BK (1990). Kinetics and block of dopamine uptake in synaptosomes from rat caudate nucleus. Journal of Neurochemistry, 55(1), 260–267. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim MY, Kim DH, & Lee YS (2004). Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochemical Research, 29(7), 1405–1409. [DOI] [PubMed] [Google Scholar]
- Levental I, Lingwood D, Grzybek M, Coskun U, & Simons K (2010). Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proceedings of the National Academy of Sciences of the United States of America, 107(51), 22050–22054. 10.1073/pnas.1016184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Zhang PW, Zhu X, Melgari JM, Huff R, Spieldoch RL, et al. (2003). Phosphatidylinositol 3-kinase, protein kinase C, and MEK1/2 kinase regulation of dopamine transporters (DAT) require N-terminal DAT phosphoacceptor sites. The Journal of Biological Chemistry, 278(22), 20162–20170. 10.1074/jbc.M209584200. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norgaard-Nielsen K, & Gether U (2003). Probing dopamine transporter structure and function by Zn2+-site engineering. European Journal of Pharmacology, 479(1–3), 187–197. [DOI] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, & Haase J (2004). Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. The Journal of Biological Chemistry, 279(37), 38770–38778. 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- Maron R, Stern Y, Kanner BI, & Schuldiner S (1983). Functional asymmetry of the amine transporter from chromaffin granules. The Journal of Biological Chemistry, 258(19), 11476–11481. [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, et al. (2008). Anomalous dopamine release associated with a human dopamine transporter coding variant. The Journal of Neuroscience, 28(28), 7040–7046. 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Couch RS, Shelton RC, Stein MA, & Blakely RD (2005). Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology, 49(6), 724–736. 10.1016/j.neuropharm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, & Murray D (2002). PIP(2) and proteins: Interactions, organization, and information flow. Annual Review of Biophysics and Biomolecular Structure, 31, 151–175. 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- Mergy MA, Gowrishankar R, Gresch PJ, Gantz SC, Williams J, Davis GL, et al. (2014). The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proceedings of the National Academy of Sciences of the United States of America, 111(44), E4779–4788. 10.1073/pnas.1417294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AC, Horton DB, Neugebauer NM, Wooters TE, Nickell JR, Dwoskin LP, et al. (2011). Tetrabenazine inhibition of monoamine uptake and methamphetamine behavioral effects: Locomotor activity, drug discrimination and self-administration. Neuropharmacology, 61(4), 849–856. 10.1016/j.neuropharm.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, et al. (2001). Lobeline inhibits the neurochemical and behavioral effects of amphetamine. The Journal of Pharmacology and Experimental Therapeutics, 296(3), 1023–1034. [PubMed] [Google Scholar]
- Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, et al. (2013). Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. The Journal of Biological Chemistry, 288(1), 20–32. 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AE, Rastedt DE, Stanislowski DJ, Shetty M, Smith MA, Vaughan RA, et al. (2015). Reciprocal phosphorylation and palmitoylation control dopamine transporter kinetics. The Journal of Biological Chemistry, 290(48), 29095–29105. 10.1074/jbc.M115.667055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Amakatsu K, & Futai M (1993). Uptake of the neurotoxin, 4-methylphenylpyridinium, into chromaffin granules and synaptic vesicles: A proton gradient drives its uptake through monoamine transporter. Archives of Biochemistry and Biophysics, 305(2), 271–277. 10.1006/abbi.1993.1422. [DOI] [PubMed] [Google Scholar]
- Morris AJ, & Scarlata S (1997). Regulation of effectors by G-protein alpha- and beta gamma-subunits. Recent insights from studies of the phospholipase c-beta isoenzymes. Biochemical Pharmacology, 54(4), 429–435. [DOI] [PubMed] [Google Scholar]
- Navaroli DM, Stevens ZH, Uzelac Z, Gabriel L, King MJ, Lifshitz LM, et al. (2011). The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking. The Journal of Neuroscience, 31(39), 13758–13770. 10.1523/JNEUROSCI.2649-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, & Bardo MT (2007). Lobelane decreases methamphetamine self-administration in rats. European Journal of Pharmacology, 571(1), 33–38. 10.1016/j.ejphar.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Zhen J, Meyer E, Erreger K, Li Y, Kakar N, et al. (2014). Dopamine transporter deficiency syndrome: Phenotypic spectrum from infancy to adulthood. Brain, 137(Pt. 4), 1107–1119. 10.1093/brain/awu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli V, Meszaros AV, Oppliger C, & Tornay S (2004). Impact of cholesterol depletion on shape changes, actin reorganization, and signal transduction in neutrophil-like HL-60 cells. Experimental Cell Research, 296(2), 358–368. [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, & Greengard P (1997). Bidirectional regulation of DARPP-32 phosphorylation by dopamine. The Journal of Neuroscience, 17(21), 8147–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North P, & Fleischer S (1983). Alteration of synaptic membrane cholesterol/phospholipid ratio using a lipid transfer protein. Effect on gamma-aminobutyric acid uptake. The Journal of Biological Chemistry, 258(2), 1242–1253. [PubMed] [Google Scholar]
- O’Malley HA, Park Y, Isom LL, & Gnegy ME (2010). PKCbeta co-localizes with the dopamine transporter in mesencephalic neurons. Neuroscience Letters, 480(1), 40–43. 10.1016/j.neulet.2010.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page G, Barc-Pain S, Pontcharraud R, Cante A, Piriou A, & Barrier L (2004). The up-regulation of the striatal dopamine transporter’s activity by cAMP is PKA-, CaMK II-and phosphatase-dependent. Neurochemistry International, 45(5), 627–632. 10.1016/j.neuint.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Penmatsa A, Wang KH, & Gouaux E (2013). X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature, 503(7474), 85–90. 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C, & Singer EA (1999). Ion dependence of carrier-mediated release in dopamine or norepinephrine transporter-transfected cells questions the hypothesis of facilitated exchange diffusion. Molecular Pharmacology, 56(5), 1047–1054. [DOI] [PubMed] [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Ma CL, McCabe BD, & Javitch JA (2014). Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Molecular Psychiatry, 19(3), 279–281. 10.1038/mp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, et al. (2013). The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Molecular Psychiatry, 18(7), 824–833. 10.1038/mp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW (2002a). Role of syntaxin 1A on serotonin transporter expression in developing thalamocortical neurons. International Journal of Developmental Neuroscience, 20(3–5), 219–224. [DOI] [PubMed] [Google Scholar]
- Quick MW (2002b). Substrates regulate gamma-aminobutyric acid transporters in a syntaxin 1A-dependent manner. Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5686–5691. 10.1073/pnas.082712899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW (2003). Regulating the conducting states of a mammalian serotonin transporter. Neuron, 40(3), 537–549. [DOI] [PubMed] [Google Scholar]
- Quick MW (2006). The role of SNARE proteins in trafficking and function of neurotransmitter transporters. Handbook of Experimental Pharmacology, 175, 181–196. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, & Jayanthi LD (2011). Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacology & Therapeutics, 129(2), 220–238. 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastedt DE, Vaughan RA, & Foster JD (2017). Palmitoylation mechanisms in dopamine transporter regulation. Journal of Chemical Neuroanatomy, 83–84, 3–9. 10.1016/j.jchemneu.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD (2006). Palmitoylation of ligands, receptors, and intracellular signaling molecules. Science’s STKE, 2006(359), re14 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- Scanlon SM, Williams DC, & Schloss P (2001). Membrane cholesterol modulates serotonin transporter activity. Biochemistry, 40(35), 10507–10513. [DOI] [PubMed] [Google Scholar]
- Scholze P, Norregaard L, Singer EA, Freissmuth M, Gether U, & Sitte HH (2002). The role of zinc ions in reverse transport mediated by monoamine transporters. The Journal of Biological Chemistry, 277(24), 21505–21513. 10.1074/jbc.M112265200. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Paris I, Munoz P, Ferrari E, Zecca L, & Zucca FA (2014). Protective and toxic roles of dopamine in Parkinson’s disease. Journal of Neurochemistry, 129(6), 898–915. 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]
- Seidel S, Singer EA, Just H, Farhan H, Scholze P, Kudlacek O, et al. (2005). Amphetamines take two to tango: An oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Molecular Pharmacology, 67(1), 140–151. 10.1124/mol.67.1. [DOI] [PubMed] [Google Scholar]
- Sembower MA, Ertischek MD, Buchholtz C, Dasgupta N, & Schnoll SH (2013). Surveillance of diversion and nonmedical use of extended-release prescription amphetamine and oral methylphenidate in the United States. Journal of Addictive Diseases, 32(1), 26–38. 10.1080/10550887.2012.759880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, & Freissmuth M (2010). The reverse operation of Na(+)/cl(−)-coupled neurotransmitter transporters—Why amphetamines take two to tango. Journal of Neurochemistry, 112(2), 340–355. 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, & Pifl C (1998). Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. Journal of Neurochemistry, 71(3), 1289–1297. [DOI] [PubMed] [Google Scholar]
- Snyder SH (1972). Catecholamines in the brain as mediators of amphetamine psychosis. Archives of General Psychiatry, 27(2), 169–179. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, & Amara SG (1997). Multiple ionic conductances of the human dopamine transporter: The actions of dopamine and psychostimulants. The Journal of Neuroscience, 17(3), 960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner T, Montgomery TR, Hofmaier T, Kudlacek O, Yang JW, Rickhag M, et al. (2015). Amphetamine action at the cocaine- and antidepressant-sensitive serotonin transporter is modulated by alphaCaMKII. The Journal of Neuroscience, 35(21), 8258–8271. 10.1523/JNEUROSCI.4034-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner T, Mus L, Eisenrauch B, Constantinescu A, Leo D, Konrad L, et al. (2014). In vivo amphetamine action is contingent on alphaCaMKII. Neuropsychopharmacology, 39(11), 2681–2693. 10.1038/npp.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner T, Yang JW, Montgomery TR, Chen WQ, Winkler MT, Sucic S, et al. (2012). Ca(2 +)/calmodulin-dependent protein kinase IIalpha (alphaCaMKII) controls the activity of the dopamine transporter: Implications for Angelman syndrome. The Journal of Biological Chemistry, 287(35), 29627–29635. 10.1074/jbc.M112.367219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucic S, Dallinger S, Zdrazil B, Weissensteiner R, Jorgensen TN, Holy M, et al. (2010). The N terminus of monoamine transporters is a lever required for the action of amphetamines. The Journal of Biological Chemistry, 285(14), 10924–10938. 10.1074/jbc.M109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, & Hille B (2008). PIP2 is a necessary cofactor for ion channel function: How and why? Annual Review of Biophysics, 37, 175–195. 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D (2011). How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron, 69(4), 628–649. 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, & Ewing A (1995). Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. The Journal of Neuroscience, 15(5 Pt. 2), 4102–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, & Galli A (2005). Mechanisms of neurotransmitter release by amphetamines: A review. Progress in Neurobiology, 75(6), 406–433. 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Du F, Tian QB, Zhang J, & Endo S (2008). Ca2 +/calmodulin-dependent protein kinase IIalpha clusters are associated with stable lipid rafts and their formation traps PSD-95. Journal of Neurochemistry, 104(3), 596–610. 10.1111/j.1471-4159.2007.05035.x. [DOI] [PubMed] [Google Scholar]
- Sweeney CT, Sembower MA, Ertischek MD, Shiffman S, & Schnoll SH (2013). Nonmedical use of prescription ADHD stimulants and preexisting patterns of drug abuse. Journal of Addictive Diseases, 32(1), 1–10. 10.1080/10550887.2012.759858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Fukunaga K, & Miyamoto E (2002). Activation of nuclear Ca(2 +)/calmodulin-dependent protein kinase II and brain-derived neurotrophic factor gene expression by stimulation of dopamine D2 receptor in transfected NG108–15 cells. Journal of Neurochemistry, 82(2), 316–328. [DOI] [PubMed] [Google Scholar]
- Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, et al. (2003). Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. The Journal of Biological Chemistry, 278(4), 2731–2739. 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, & Caron MG (2003). Plasma membrane monoamine transporters: Structure, regulation and function. Nature Reviews. Neuroscience, 4(1), 13–25. 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Torres B, & Ruoho AE (2014). N-terminus regulation of VMAT2 mediates methamphetamine-stimulated efflux. Neuroscience, 259, 194–202. 10.1016/j.neuroscience.2013.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui J, Inagaki M, & Schulman H (2005). Calcium/calmodulin-dependent protein kinase II (CaMKII) localization acts in concert with substrate targeting to create spatial restriction for phosphorylation. The Journal of Biological Chemistry, 280(10), 9210–9216. 10.1074/jbc.M407653200. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, & Kuhar MJ (1997). Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. The Journal of Biological Chemistry, 272(24), 15541–15546. [DOI] [PubMed] [Google Scholar]
- Vearrier D, Greenberg MI, Miller SN, Okaneku JT, & Haggerty DA (2012). Methamphetamine: History, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Disease-a-Month, 58(2), 38–89. 10.1016/j.disamonth.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Vuorenpaa A, Ammendrup-Johnsen I, Jorgensen TN, & Gether U (2016). A kinome wide screen identifies novel kinases involved in regulation of monoamine transporter function. Neurochemistry International, 98, 103–114. 10.1016/j.neuint.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Weerth SH, Holtzclaw LA, & Russell JT (2007). Signaling proteins in raft-like microdomains are essential for Ca2 + wave propagation in glial cells. Cell Calcium, 41(2), 155–167. 10.1016/j.ceca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Zheng G, Crooks PA, Dwoskin LP, & Bardo MT (2013). Oral administration of GZ-793A, a VMAT2 inhibitor, decreases methamphetamine selfadministration in rats. Pharmacology, Biochemistry, and Behavior, 112, 29–33. 10.1016/j.pbb.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaczek R, Culp S, & De Souza EB (1991). Interactions of [3H]amphetamine with rat brain synaptosomes. II. Active transport. The Journal of Pharmacology and Experimental Therapeutics, 257(2), 830–835. [PubMed] [Google Scholar]
- Zestos AG, Mikelman SR, Kennedy RT, & Gnegy ME (2016). PKCbeta inhibitors attenuate amphetamine-stimulated dopamine efflux. ACS Chemical Neuroscience, 7(6), 757–766. 10.1021/acschemneuro.6b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]