Abstract
Background:
The second-generation ALK inhibitor alectinib recently demonstrated superior efficacy compared to the first-generation ALK inhibitor crizotinib in advanced anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC), establishing alectinib as the new standard first-line therapy. Brigatinib, another second-generation ALK inhibitor, has demonstrated substantial activity in patients with crizotinib-refractory ALK-positive NSCLC; however, its activity in the alectinib-refractory setting is unknown.
Methods:
A multicenter, retrospective study was performed at three institutions. Patients were eligible if they had advanced, alectinib-refractory ALK-positive NSCLC and were treated with brigatinib. Medical records were reviewed to determine clinical outcomes.
Results:
Twenty-two patients were eligible for this study. Confirmed objective responses to brigatinib were observed in 3 of 18 patients (17%) with measurable disease. Nine patients (50%) had stable disease on brigatinib. The median progression-free survival was 4.4 months [95% confidence interval (CI), 1.8–5.6 months] with a median duration of treatment of 5.7 months (95% CI, 1.8–6.2 months). Among nine patients in this study who underwent post-alectinib/pre-brigatinib biopsies, five had an ALK I1171X or V1180L resistance mutation; of these, one had a confirmed partial response and three had stable disease on brigatinib. One patient had an ALK G1202R mutation in a post-alectinib/pre-brigatinib biopsy, and had progressive disease as the best overall response to brigatinib.
Conclusions:
Brigatinib has limited clinical activity in alectinib-refractory ALK-positive NSCLC. Additional studies are needed to establish biomarkers of response to brigatinib and to identify effective therapeutic options for alectinib-resistant ALK-positive NSCLC patients.
Keywords: alectinib, brigatinib, ALK, NSCLC, resistance
INTRODUCTION
Anaplastic lymphoma kinase (ALK) gene rearrangements lead to expression of potent oncogenic fusion proteins and are found in approximately 5% of non-small cell lung cancer (NSCLC).1–4 Advanced NSCLC harboring an ALK rearrangement (ALK-positive NSCLC) can be effectively treated with small-molecule tyrosine kinase inhibitors (TKIs) that target ALK. Until recently, the standard first-line therapy for patients with advanced ALK-positive NSCLC was crizotinib, with an objective response rate (ORR) of 74% and a median progression-free survival (PFS) of 10.9 months.5 As responses to crizotinib are often short-lived due to acquired resistance, numerous next-generation ALK TKIs have been developed including second-generation TKIs such as ceritinib,6–8 alectinib,9–11 and brigatinib,12, 13 and the third-generation TKI lorlatinib.14 These next-generation ALK TKIs are more potent and central nervous system (CNS)-penetrant compared to crizotinib and retain variable activity against different crizotinib-resistant ALK mutations.4, 15
Brigatinib is a second-generation ALK inhibitor that was developed to overcome resistance to crizotinib. In preclinical studies, brigatinib potently inhibited the majority of crizotinib-resistant ALK mutations, including the most common gatekeeper mutation, L1196M.16 In a multicenter phase II study, brigatinib was highly active in patients with crizotinib-refractory ALK-positive NSCLC. Among 222 patients receiving one of two dosing regimens of brigatinib (90 mg once daily versus 180 mg once daily with a 7-day lead-in at 90 mg), the ORRs were 45% and 54%, with a median PFS of 9.2 month and 16.7 months, respectively.13, 17 These findings led to accelerated approval of brigatinib by the United States Food and Drug Administration for the treatment of crizotinib-refractory ALK-positive NSCLC.
Recent randomized trials have established a new role for second-generation TKIs, specifically alectinib and ceritinib, as first-line therapy for advanced ALK-positive NSCLC.18, 19 For example, the global randomized phase III ALEX trial demonstrated that alectinib was significantly superior to crizotinib in terms of efficacy and toxicity in untreated ALK-positive NSCLC.18 Alectinib has since received approval for the initial treatment of patients with ALK-positive NSCLC and has now been widely adopted as the standard of care in this setting. Nonetheless, for patients receiving alectinib either as first- or second-line therapy, resistance invariably develops, and the optimal treatment after alectinib has not been established. In particular, whether other second-generation TKIs may be effective after alectinib is unknown.
Preclinical studies suggest that brigatinib could represent a potentially effective treatment option for alectinib-refractory patients. Brigatinib has been shown to harbor significant activity against certain alectinib-resistant ALK mutations such as I1171N, I1171S, and V1180L.16 However, the degree of its preclinical and clinical activity against the highly recalcitrant solvent front mutation, ALK G1202R, has not been as well defined.15, 16 ALK G1202R is the most common resistance mutation after failure of alectinib.15 This mutation has also been detected in repeat tumor biopsies obtained from patients progressing on brigatinib,15 suggesting suboptimal inhibitory activity against G1202R. Thus, the activity of brigatinib in alectinib-resistant patients may be impacted by the presence of specific ALK resistance mutations.
Here, based on a multicenter, retrospective analysis of 22 ALK-positive patients treated with alectinib followed by brigatinib, we report the efficacy and safety of brigatinib in the setting of alectinib resistance.
MATERIALS AND METHODS
Study population
Patients were identified at three participating institutions: Massachusetts General Hospital (MGH; n = 11), Memorial Sloan Kettering Cancer Center (n = 6), and University of California-Irvine (n = 5). All patients had advanced NSCLC with an ALK rearrangement identified by local molecular profiling [e.g., fluorescent in situ hybridization, immunohistochemistry, DNA-based next-generation sequencing (NGS), or targeted RNA sequencing]. Patients had to have received alectinib (as any line of systemic therapy) with progression of disease prior to receiving brigatinib. Brigatinib was prescribed either commercially or on an expanded access protocol. This study was approved by the Institutional Review Board (IRB) at each participating institution.
Data collection
Medical records were retrospectively reviewed, and data were extracted on clinical, pathologic, and molecular features as well as treatment histories. Overall and intracranial responses to therapy were determined using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 based on investigator assessment. PFS was measured from the time of brigatinib or alectinib treatment initiation to clinical/radiographic progression or death. Patients without documented disease progression were censored on the date of last follow-up. Duration of treatment was measured from the time of brigatinib or alectinib initiation to the date that the drug was discontinued, or—if continuing on brigatinib at the time of data analysis—censored on the date of last follow-up. All data were updated as of April 15, 2018.
ALK mutation genotyping
A subset of the patients included in this study underwent repeat tumor or liquid biopsies at the time of progression on alectinib and prior to starting brigatinib, under IRB-approved protocols. Five patients underwent a tumor biopsy of the progressing lesion followed by targeted NGS using the commercially available FoundationOne platform (n = 2; Foundation Medicine, Inc.) or the MGH SNaPshot NGS platform (n = 3), as previously described.20 Four patients underwent a liquid biopsy using either the commercially available Guardant360 cell-free DNA (Guardant Health, Inc.) or FoundationACT platform (Foundation Medicine, Inc.). Additionally, five patients underwent a tumor (FoundationOne, n = 2), liquid (Guardant360, n = 1; FoundationACT, n = 1), or both tumor and liquid (MGH SNaPshot NGS and Guardant360, n = 1) biopsy after progression on brigatinib.
Statistical analysis
PFS and duration of treatment endpoints were estimated using the Kaplan-Meier method. 95% confidence intervals (CIs) were calculated using the log-log transformation. Data analysis was performed using SAS 9.4.
RESULTS
Patient characteristics
We identified 22 patients with advanced ALK-positive NSCLC who were treated with alectinib followed by brigatinib. The baseline clinicopathologic features of these patients are summarized in Table 1. The median age was 55 years (range, 22–76 years), and 59% were women. The majority of patients were never smokers (77%) with adenocarcinoma histology (86%). Most (19 of 22; 86%) patients received brigatinib as the immediate next line of therapy following alectinib. The median number of intervening lines of therapy between alectinib and brigatinib was 0 (range, 0–5). At the time of starting brigatinib, five patients (23%) had received one prior ALK TKI (alectinib); 13 (59%) had received two prior ALK TKIs (crizotinib and alectinib); and four (18%) had received three prior ALK TKIs (crizotinib, ceritinib, and alectinib) (Table 1).
Table 1.
Baseline clinical and pathologic features of patients enrolled in the study.
| Characteristic | All patients (N = 22) |
|---|---|
| Age at diagnosis, years | |
| Median | 55 |
| Range | 22–76 |
| Sex | |
| Male | 9 (41%) |
| Female | 13 (59%) |
| Race | |
| White | 18 (82%) |
| Asian | 3 (14%) |
| Unknown | 1 (5%) |
| Smoking history | |
| Never | 17 (77%) |
| Light (<10 pack-years) | 3 (14%) |
| Heavy (>10 pack-years) | 2 (9%) |
| Histology | |
| Adenocarcinoma | 19 (86%) |
| Other | 1 (5%) |
| Not specified | 2 (9%) |
| Stage at diagnosis# | |
| Stage I-III | 7 (32%) |
| Stage IV | 15 (68%) |
| Brain metastases at diagnosis | |
| Present | 8 (36%) |
| Absent | 12 (55%) |
| Not assessed^ | 2 (9%) |
| Brain metastases at the start of brigatinib therapy | |
| Present | 18 (82%) |
| Absent | 4 (18%) |
| Lines of systemic therapy before alectinib | |
| 0 | 3 (14%) |
| 1 | 12 (55%) |
| 2 | 6 (27%) |
| ≥3 | 1 (5%) |
| Intervening lines of therapy between alectinib and brigatinib | |
| 0 | 19 (86%) |
| 1 | 2 (9%) |
| 5 | 1 (6%) |
| Number of ALK TKIs prior to brigatinib | |
| 1 | 5 (23%) |
| 2 | 13 (59%) |
| 3 | 4 (18%) |
Staging based on the AJCC TNM 7th edition.
No baseline MRI brain or CT head with contrast obtained at the time of advanced disease diagnosis.
TKI, tyrosine kinase inhibitor.
Outcomes on alectinib
Three patients received alectinib as first-line therapy, whereas the remainder received alectinib as second-line therapy or beyond. All patients discontinued alectinib because of disease progression (intracranial, n = 7; extracranial, n = 9; both intra- and extracranial, n = 6). The median PFS on alectinib was 10.4 months (95% CI, 5.4–13.5 months), and median duration of alectinib treatment was 12.4 months (95% CI, 9.6–17.1 months).
Of note, two patients had undergone dose reduction(s) of alectinib. In one patient, the alectinib dose was decreased to 450 mg twice a day after four months of therapy due to cumulative fatigue, myalgias, and CPK elevation; this patient experienced disease progression six months later. The second patient received alectinib 300 mg twice a day because of marked fatigue, which developed after seven months on the standard dose. His intra- and extracranial disease progressed shortly thereafter. The alectinib dose was then gradually re-escalated to 600 mg twice a day without significant response, and alectinib was therefore discontinued.
Outcomes of brigatinib treatment post-alectinib
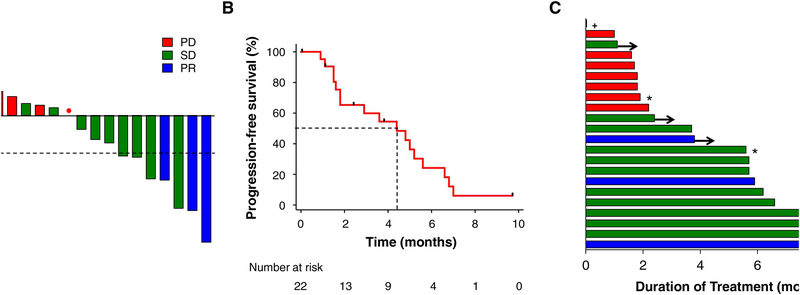
Eighteen of 22 patients had measurable disease at baseline and underwent at least one set of imaging for tumor response evaluation. A confirmed partial response (PR) per RECIST version 1.1 was observed in three patients (17%). Nine patients (50%) experienced stable disease (SD) on brigatinib (Fig. 1A), of which one was an unconfirmed PR. Three additional patients had non-measurable disease at baseline but were evaluable for tumor response. Of these, two (67%) had non-complete response/non-progressive disease and one (33%) had progressive disease (PD) as their best overall response. Additionally, among four of 22 patients who had measurable intracranial disease at baseline, one patient (25%) had an unconfirmed intracranial PR but experienced PD extracranially, leading to the termination of brigatinib treatment at 1.7 months. Three patients (75%) had PD as the best intracranial response.
Figure 1. Brigatinib activity in alectinib-refractory ALK-positive NSCLC.
(A) Best confirmed tumor responses of 18 ALK-positive patients who received brigatinib and had baseline measurable disease. All patients received and progressed on prior alectinib. The bars show best percent change in the target tumor burden from baseline. The dotted horizontal line shows the 30% threshold for partial response. The red dot indicates a patient who had best overall change from baseline of 0%, with new lesions. PD, progressive disease; SD, stable disease; PR, partial response. (B) Progression-free survival (PFS) on brigatinib for 22 patients. Vertical tick marks on the PFS curve indicate censoring of data. Dotted lines show the median PFS. (C) Swimmer plots demonstrating the duration of brigatinib treatment for each patient in the study cohort. Arrows indicate patients continuing on brigatinib at the time of data cutoff. * indicates patients with evaluable but non-measurable disease. + indicates a patient who required early permanent discontinuation of brigatinib due to pneumonitis and was therefore not evaluable for response to brigatinib.
The median PFS on brigatinib was 4.4 months (95% CI, 1.8–5.6 months), with five of 22 patients censored (Fig. 1B). Of the 17 patients who experienced disease progression on brigatinib, seven had CNS-only progression. The median duration of treatment was 5.7 months (95% CI, 1.8–6.2 months), with five patients continuing on brigatinib at the time of data cutoff (Fig. 1C).
All patients started brigatinib at the lead-in dose of 90 mg once daily. The median interval from the start of brigatinib 90 mg daily to the start of 180 mg daily dosing was 7 days (range, 6–47 days). One patient continued brigatinib 90 mg daily without dose escalation due to creatine phosphokinase (CPK) elevation [grade 3 per the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03] which had persisted from prior alectinib therapy. Another patient started brigatinib 90 mg daily and developed drug-related grade 3 pneumonitis within two days (Supplemental Figure), requiring permanent discontinuation of brigatinib. There was no escalation of brigatinib dosing beyond 180 mg daily.
The most common (>10%) treatment-related CTCAE grade 1/2 adverse events on brigatinib in this study included CPK elevation (27%), increase in alanine aminotransferase and/or aspartate aminotransferase (18%), diarrhea (14%), and fatigue (14%) (Table 2). One patient experienced CTCAE grade 3 pneumonitis as noted above, and one patient required two dose reductions for grade 3 mucositis. Two patients underwent a dose interruption without subsequent dose reductions. One patient experienced grade 2 pneumonitis after four days of brigatinib therapy at the 90 mg daily dosing (Supplemental Figure), requiring a drug hold for four days. She tolerated the re-challenge of brigatinib 90 mg daily, and was able to escalate to 180 mg daily six weeks later without recrudescence of pneumonitis. The second patient experienced grade 3 liver function test abnormalities attributed to brigatinib. She held brigatinib for three weeks with improvement in the liver function tests and subsequently resumed the drug with gradual re-escalation to the full dose (180 mg daily) without recurrence of this adverse event.
Table 2.
Treatment-related adverse events in all patients (N = 22).
| Grade 1–2* | Grade 3* | |
|---|---|---|
| CPK increased | 6 (27%) | 1 (5%) |
| AST/ALT increased | 4 (18%) | 1 (5%) |
| Diarrhea | 3 (14%) | 0 |
| Fatigue | 3 (14%) | 0 |
| Myalgia | 2 (9%) | 0 |
| Lipase increased | 2 (9%) | 0 |
| Amylase increased | 2 (9%) | 0 |
| Constipation | 1 (5%) | 0 |
| Pneumonitis | 1 (5%) | 1 (5%) |
| Rash | 1 (5%) | 0 |
| Nausea | 1 (5%) | 0 |
| Dyspnea | 1 (5%) | 0 |
| Cough | 1 (5%) | 0 |
| Fever | 1 (5%) | 0 |
| Mucositis | 0 | 1 (5%) |
Grading per the Common Terminology Criteria for Adverse Events version 4.0.
CPK, creatine phosphokinase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Molecular characteristics of post-alectinib/pre-brigatinib specimens
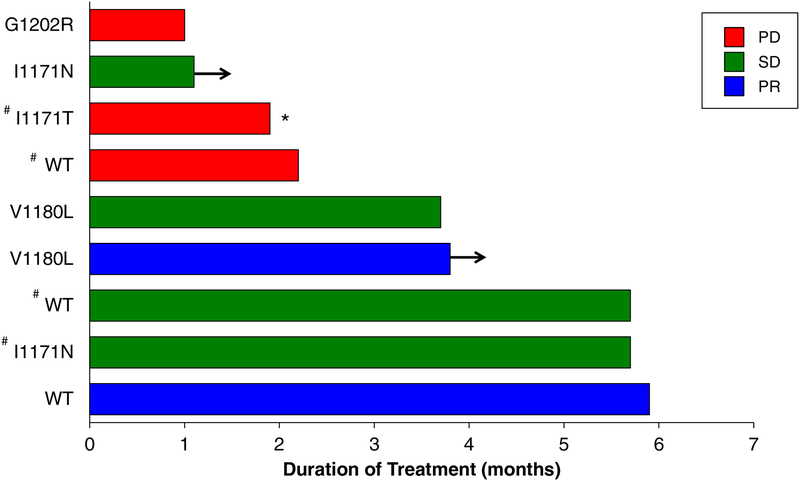
Nine patients underwent a repeat biopsy (tumor, n = 5; liquid, n = 4) at the time of progression on alectinib prior to switching to brigatinib. ALK resistance mutations were identified in six (67%) cases. The spectrum of resistance mutations included ALK I1171N (n = 2), V1180L (n = 2), I1171T (n = 1), and G1202R (n = 1). Data on the individual response and duration of treatment based on ALK resistance mutation for those patients who underwent a post-alectinib/prebrigatinib biopsy are shown in Fig. 2. Among five patients with an I1171N/T or V1180L mutation in the post-alectinib/pre-brigatinib biopsy, one achieved a confirmed PR (shown in Fig. 3A) and three had SD as the best overall response. The one patient with a known G1202R mutation had PD on the first tumor re-assessment (Fig. 3B). Among three patients who did not have an ALK resistance detected on biopsy (tumor, n = 1; liquid, n = 2), one patient each had a PR, SD, and PD.
Figure 2. Individual duration of brigatinib treatment in patients with post-alectinib/prebrigatinib biopsies.
Patients who achieved a confirmed partial response (PR) are represented in blue; stable disease (SD), in green; progressive disease (PD), in red. One patient (marked with *) had evaluable but non-measurable disease. WT indicates wild-type ALK (no ALK mutation identified in the resistant specimen). # indicates testing by liquid (rather than tumor) biopsy; all cases not marked with # underwent a tumor biopsy. Arrows indicate patients still receiving brigatinib at the time of data cutoff.
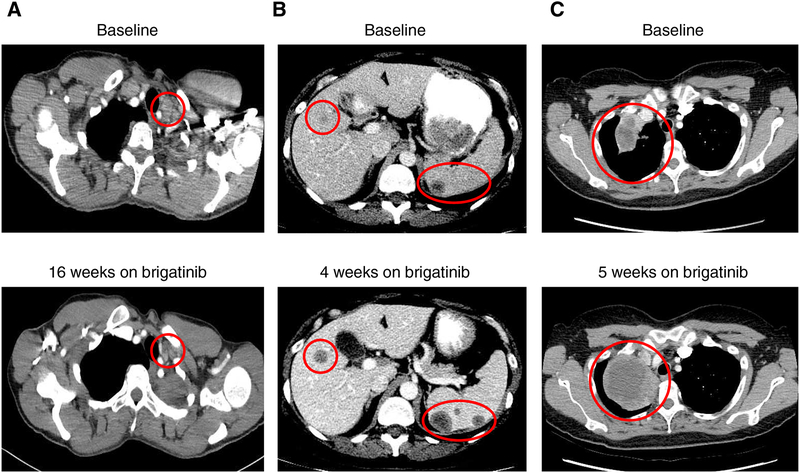
Figure 3. Examples of tumor responses to brigatinib in ALK-positive cases with ALK resistance mutations.
(A) Confirmed response of a supraclavicular lymph node to brigatinib in a patient with a V1180L resistance mutation detected in the post-alectinib/pre-brigatinib biopsy. This patient remained on treatment at the time of data cutoff. (B) Progressive disease with enlarging and new hepatic and splenic metastases after one month of treatment in a patient with ALK G1202R detected in the post-alectinib/pre-brigatinib biopsy. (C) Progressive disease with an enlarging right lung mass in a patient who did not undergo a post-alectinib/pre-brigatinib biopsy. This patient experienced disease relapse after approximately one month of therapy, and had a post-brigatinib liquid biopsy revealing a G1202R mutation (allele frequency of 3.2%).
Clinical outcomes after brigatinib
Five of the 17 patients in this cohort who experienced disease progression on brigatinib underwent a tumor or liquid biopsy at the time of progression; three of these patients also had a paired pre-brigatinib/post-alectinib biopsy (Supplemental Table). In one patient who had PD as the best overall response to brigatinib, the ALK G1202R mutation was detected on liquid biopsy at the time of progression (Fig. 3C). This patient had not undergone a pre-brigatinib biopsy; thus, we were unable to determine whether this mutation emerged in the setting of alectinib (i.e., prior to brigatinib exposure) or de novo during the course of brigatinib treatment. Another patient with an ALK V1180L mutation in the post-alectinib/pre-brigatinib tumor biopsy had SD on brigatinib, but experienced extracranial disease progression after 3.6 months of treatment. A liquid biopsy at the time of progression on brigatinib revealed an ALK G1202R resistance mutation [allele fraction (AF) of 7.9%] in addition to L1196M (AF 0.5%) and V1180L (AF 0.04%) mutations (Supplemental Table). Both of these patients with ALK G1202R in the post-brigatinib biopsy (in addition to the patient mentioned above who had PD on brigatinib with a pre-brigatinib ALK G1202R) were subsequently treated with lorlatinib and responded, confirming that their tumors did remain ALK dependent.
A third patient was found to have ALK D1203N, also a solvent front resistance mutation, after experiencing disease progression on brigatinib. This patient’s post-alectinib/pre-brigatinib liquid biopsy had revealed an ALK fusion without kinase domain mutations (Supplemental Table). In the remaining two patients, post-brigatinib biopsies revealed an ALK L1196M mutation (no prebrigatinib biopsy), and MET amplification (by NGS) along with ALK I1171N (which was present in the post-alectinib/pre-brigatinib biopsy), respectively.
DISCUSSION
In this multicenter retrospective analysis, we evaluated a cohort of patients with alectinib-refractory ALK-positive NSCLC treated with brigatinib. We found that brigatinib demonstrates limited clinical activity in this context, with an ORR of 17% and median PFS of 4.4 months. Brigatinib was generally well tolerated by patients with a safety profile largely consistent with what has been reported.12, 13
The standard treatment approach to advanced ALK-positive NSCLC continues to evolve. While crizotinib was established as the standard first-line therapy in 2014,5 more recent studies have evaluated (or are evaluating) the role of more potent and CNS-penetrant next-generation ALK TKIs such as ceritinib, alectinib, and brigatinib as first-line therapy.18, 19, 21 Most notably, in the global randomized phase III ALEX trial, alectinib was significantly superior to crizotinib in treatment-naïve ALK-positive NSCLC, demonstrating a 53% reduction in the risk of cancer progression or death (median PFS not reached for alectinib versus 11.1 months for crizotinib).18 These data have now effectively established alectinib as the standard initial treatment for patients diagnosed with advanced ALK-positive lung cancer.
As a result of this shift from first- to second-generation ALK TKIs as initial therapy, new questions have emerged. Perhaps most urgently as alectinib moves into the front-line setting, what are the most effective treatment options for patients who develop resistance to alectinib, and is there still a role for sequential ALK TKIs? Previous work has shown that brigatinib is highly effective in crizotinib-refractory ALK-positive NSCLC, with an ORR of 45–54% and median PFS of 9.2–16.7 months.12, 13, 17 To the best of our knowledge, however, no study has yet evaluated the clinical activity of brigatinib in the alectinib-refractory setting. Our analysis provides the first insight into this question, helping inform how to conceptualize the sequential treatment approach for ALK-positive patients whose disease progresses on alectinib as either the initial or later-line ALK TKI. Importantly, in this study, the efficacy of brigatinib in alectinib-refractory disease was substantially lower than what has been reported in the crizotinib-refractory context.12, 13 This finding may not be entirely surprising given the comparable ALK-inhibitory potencies and excellent CNS penetration of brigatinib and alectinib.15, 16 Nevertheless, the potential role for brigatinib in certain alectinib-refractory settings should not be underemphasized. Indeed, we observed confirmed responses in three (17%) of 18 patients with baseline measurable disease who previously progressed on alectinib, with stable disease in an additional nine (50%) patients. Moreover, six (27%) of 22 patients had a duration of brigatinib treatment lasting longer than 6 months, with one patient continuing on brigatinib at almost 10 months at the time of the manuscript submission.
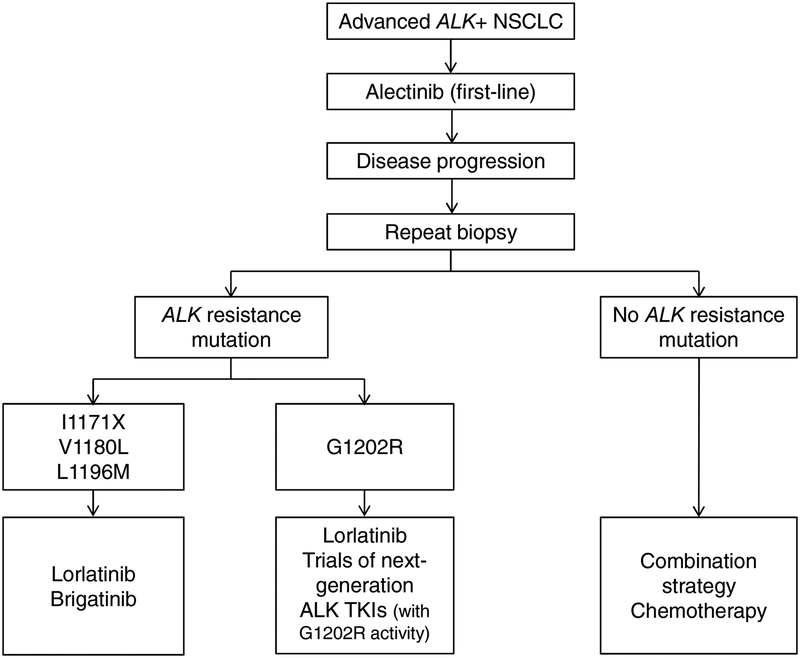
We speculate that the presence of specific ALK resistance mutations may influence responses of alectinib-refractory tumors to brigatinib. Prior studies have shown that while ALK resistance mutations are present in only ~20% of crizotinib-refractory tumors, they are significantly more common (in ~50–60%) following second-generation ALK TKIs.15 Of note, each second-generation ALK TKI gives rise to a distinct spectrum of resistance mutations. In the case of alectinib, the most common resistance mutations include G1202R (identified in ~30% of cases), I1171N and V1180L. In preclinical models, brigatinib retains activity against I1171X and V1180L, although it is less potent against G1202R.15, 16 In this study, of the seven of 22 evaluable patients who had progressive disease as the best overall response to brigatinib, one had a known G1202R mutation in the post-alectinib/pre-brigatinib biopsy, and another was found to have G1202R in a post-brigatinib biopsy (and did not have a pre-brigatinib biopsy). Of the 14 patients who had disease control on brigatinib, six underwent a post-alectinib/prebrigatinib biopsy, of which four had a known I1171N (n = 2) or V1180L (n = 2) mutation. Collectively, our findings begin to suggest that brigatinib may represent a viable therapeutic option in a small subset of alectinib-refractory patients with tumors harboring I1171X or V1180L, but may not be as effective otherwise, including in those with G1202R (Fig. 4). Given the small number of patients and the limited pre-brigatinib biopsy data in this cohort, larger studies are required to establish the activity of brigatinib in alectinib-refractory NSCLC based on ALK mutations.
Figure 4. Proposed sequential treatment approach to ALK-positive patients with acquired resistance to alectinib.
This schema is based on the available preclinical and clinical data. Once patients experience disease progression on first-line alectinib, repeat biopsies should be pursued, if feasible, in order to determine the ALK resistance mutation status. Cases with an ALK resistance mutation can be treated with sequential lorlatinib therapy. In a specific subset of cases with an I1171X, V1180L, or L1196M mutation, brigatinib may serve as an additional potential option; ceritinib could also be considered in this setting, although not preferred given its lower CNS activity. In the absence of an ALK resistance mutation, patients may be treated with chemotherapy or combination strategies.
The potential role of ALK resistance mutations as a molecular biomarker of response to ALK inhibitors is becoming increasingly appreciated, underscoring the importance of pursuing repeat biopsies in patients progressing on alectinib to inform the choice of next-line therapy. This was recently highlighted in the phase I trial of lorlatinib. Lorlatinib previously demonstrated potent preclinical activity against all single ALK resistance mutations including I1171X, V1180, and notably, G1202R.14, 15 This finding was recapitulated in the phase I study with tumor regression in all patients whose tumors harbored ALK resistance mutations including G1202R. In contrast, no tumor regression was observed in patients whose tumors lacked ALK resistance mutations and were presumably ALK-independent.22 In the subsequent phase II study, lorlatinib has demonstrated activity in patients who failed prior second-generation ALK TKIs including alectinib, with a confirmed ORR of 39%.23 Altogether, the growing body of data support a sequential TKI approach to ALK-positive NSCLC wherein the initial treatment with alectinib should be followed by a repeat biopsy at the time of disease relapse if feasible, with the selection of subsequent therapy tailored to the presence or absence of specific ALK resistance mutations (Fig. 4).
Our study has several notable limitations. First, this was a retrospective analysis with a relatively small number of patients and lacking a comparator cohort. While we performed a multicenter analysis in order to identify more patients eligible for the study, all participating centers were highly specialized academic institutions, incurring the possibility of a referral bias. The duration of follow-up was also limited. Another important limitation of this analysis is the small number of patients who had post-alectinib/pre-brigatinib biopsies, rendering it challenging to draw robust conclusions regarding the efficacy of brigatinib on the basis of ALK resistance mutations.
Finally, a majority of patients in this study received alectinib as a second- or greater-line therapy for ALK-positive NSCLC. We cannot exclude the possibility that the exposure to additional TKIs (e.g., crizotinib or ceritinib) prior to alectinib may have resulted in a lower efficacy of brigatinib than what may be observed in patients who receive alectinib as the first and only TKI before brigatinib. It is theoretically conceivable that sequential TKI treatment with multiple ALK inhibitors including alectinib may have resulted in more complex resistance mechanisms than would have emerged with alectinib only as the prior therapy. Ultimately, larger prospective studies in ALK-positive patients—ideally those treated with alectinib as the first and only prior ALK TKI—will be needed to confirm and extend our findings. A phase II study investigating the activity of brigatinib in patients whose disease has progressed on prior next-generation ALK TKIs is ongoing (ClinicalTrials.gov identifier NCT02706626).
In conclusion, this study provides the first insight into the clinical activity of brigatinib in alectinib-refractory, ALK-positive NSCLC. We found that the overall efficacy of brigatinib in this setting was limited. Responses were noted in 17% of patients, highlighting the potential utility of brigatinib in a small subset of alectinib-resistant patients. These findings help refine a tailored sequential treatment approach to ALK-positive NSCLC in patients who relapse on alectinib.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by a grant from the National Cancer Institute (R01CA164273, to A.T.S.), by Be a Piece of the Solution, and by the Targeting a Cure for Lung Cancer Research Fund at MGH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175–180. [DOI] [PubMed] [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–566. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov 2017;7:137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874–886. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661–668. [DOI] [PubMed] [Google Scholar]
- 11.Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinibpretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683–1696. [DOI] [PubMed] [Google Scholar]
- 13.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490–2498. [DOI] [PubMed] [Google Scholar]
- 14.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Anjum R, Squillace R, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 2016;22:5527–5538. [DOI] [PubMed] [Google Scholar]
- 17.Camidge DR, Tiseo M, Ahn M-J, et al. P3.02a-013 Brigatinib in Crizotinib-Refractory ALK+ NSCLC: Central Assessment and Updates from ALTA, a Pivotal Randomized Phase 2 Trial. Journal of Thoracic Oncology 2017;12:S1167–S1169. [Google Scholar]
- 18.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829–838. [DOI] [PubMed] [Google Scholar]
- 19.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med 2014;20:1479–1484. [DOI] [PubMed] [Google Scholar]
- 21.Popat S, Tiseo M, Gettinger S, et al. ALTA-1L (ALK in lung cancer trial of BrigAtinib in 1st Line): A randomized, phase 3 trial of brigatinib (BRG) versus crizotinib (CRZ) in tyrosine kinase inhibitor (TKI)–naive, advanced anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC). Annals of Oncology 2016;27:1289TiP–1289TiP. [Google Scholar]
- 22.Shaw AT, Ou S-HI, Felip E, et al. Efficacy and safety of lorlatinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) with one or more prior ALK tyrosine kinase inhibitor (TKI): A phase I/II study. Journal of Clinical Oncology 2017;35:9006–9006. [Google Scholar]
- 23.Solomon B, Shaw A, Ou S, et al. OA 05.06 Phase 2 Study of Lorlatinib in Patients with Advanced ALK+/ROS1+ Non-Small-Cell Lung Cancer. Journal of Thoracic Oncology 2017;12:S1756. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.