Abstract
Accelerated ubiquitination and subsequent endoplasmic reticulum (ER)-associated degradation (ERAD) constitute one of several mechanisms for feedback control of HMG CoA reductase, the rate-limiting enzyme in synthesis of cholesterol and nonsterol isoprenoids. This ERAD is initiated by the accumulation of certain sterols in ER membranes, which trigger binding of reductase to ER membrane proteins called Insigs. Insig-associated ubiquitin ligases facilitate ubiquitination of reductase, marking the enzyme for extraction across the ER membrane through a reaction that is augmented by nonsterol isoprenoids. Once extracted, ubiquitinated reductase becomes dislocated into the cytosol for degradation by 26S proteasomes. In this review, we will highlight several advances in the understanding of reductase ERAD, which includes the discovery for a role of the vitamin K2 synthetic enzyme UBIAD1 in the reaction and demonstration that sterol-accelerated ERAD significantly contributes to feedback regulation of reductase and cholesterol metabolism in livers of whole animals.
Introduction
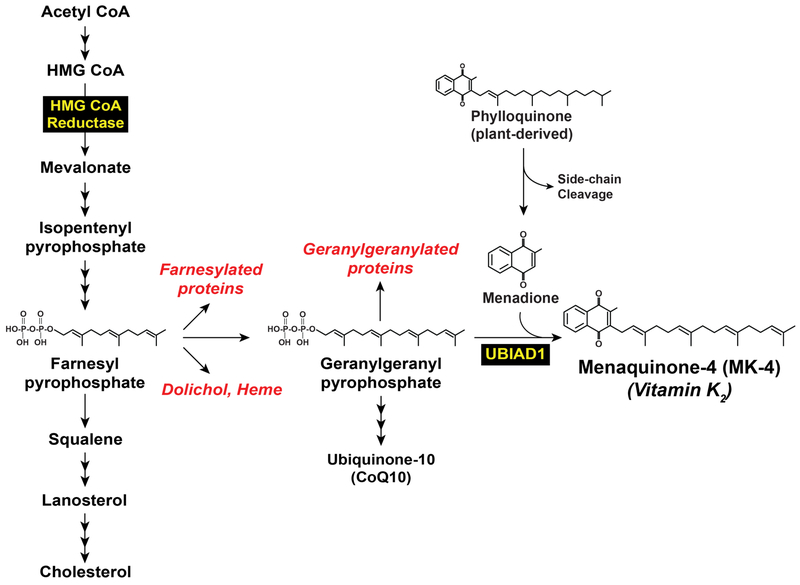
The endoplasmic reticulum (ER)-localized enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase catalyzes synthesis of mevalonate, a rate-limiting reaction in the branched pathway that produces sterols such as cholesterol and nonsterol isoprenoids including farnesyl pyrophosphate (Fpp), geranylgeranyl pyrophosphate (GGpp), dolichol, heme, ubiquinone, and the vitamin K2 subtype menaquinone-4 (MK-4) (Figure 1) (1,2). Cholesterol, the bulk end-product of the mevalonate pathway, plays a well-known role in the maintenance of membrane integrity. In addition, cholesterol is a precursor in synthesis of steroid hormones as well as vitamin D and is an important component of lipoproteins that ferry the sterol and other lipids throughout the body. Nonsterol end-products of mevalonate metabolism play crucial roles in a variety of cellular processes. These range from electron transport (heme and ubiquinone), asparagine-linked glycoprotein synthesis (dolichol), and coagulation (vitamin K2) to signal transduction, cell growth, and migration (Fpp and GGpp) (2). A constant supply of nonsterol isoprenoids is required for optimal cell function; however, cells must avoid the overaccumulation of cholesterol, which can form solid crystals that are highly toxic. This toxicity extends to the whole animal; excess levels of circulating cholesterol initiates atherosclerosis, a major risk factor for coronary artery disease (3). The delicate balance between synthesis of sterol and nonsterol isoprenoids is achieved through a multivalent feedback regulatory system that exerts stringent control on levels and activity of reductase (4).
Figure 1.
Biosynthesis of cholesterol and nonsterol isoprenoids in mammalian cells.
Results of studies employing compactin, a competitive inhibitor of reductase isolated from fungi by Endo and co-workers (5,6), first revealed the multivalent feedback regulation of reductase. When cells were cultured in medium supplemented with fetal calf serum (FCS), reductase levels were suppressed and cholesterol was synthesized at low rates owing to receptor-mediated uptake of low density lipoproteins (LDLs) (7). Cholesterol liberated from internalized LDL suppressed reductase through the multivalent feedback regulatory system. Depriving cells of cholesterol by incubating them in the absence of lipoproteins and the presence compactin disrupted the reductase regulatory system, eliciting a marked increase (up to 200-fold) in the amount of reductase protein (8). The addition to cells of LDL or oxysterols, which bypass the LDL-receptor for cellular uptake, partially suppressed reductase. However, complete reversal of the compactin-induced increase in reductase required treatment of cells with small amounts of mevalonate together in addition to exogenous sterols. Together, these findings provided a basis for the concept that sterol and nonsterol isoprenoids mediate multiple feedback mechanisms that govern the levels of reductase.
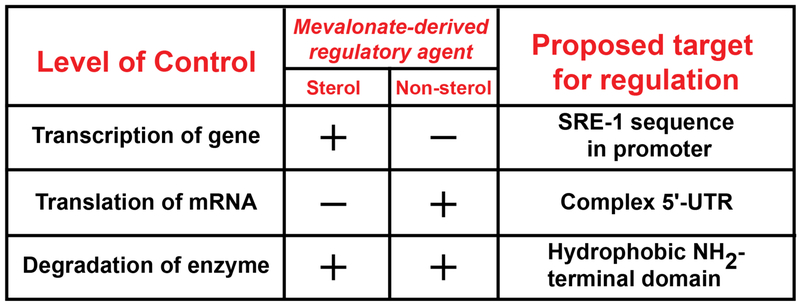
Sterol and nonsterol isoprenoids differentially inhibit reductase through multiple mechanisms including: 1) inhibiting transcription of the reductase gene; 2) blocking translation of the reductase mRNA; and 3) accelerating degradation of the reductase protein (Figure 2) (1). Sterols mediate transcriptional regulation of reductase by blocking proteolytic activation of sterol regulatory element-binding proteins (SREBPs). This family of membrane-bound transcription factors enhance transcription of genes encoding reductase and other cholesterol biosynthetic enzymes as well as the LDL-receptor (9).Translational regulation is mediated by an unknown nonsterol isoprenoid through a reaction that may be mediated by the complex 5’-untranslated region of the reductase mRNA (1). Sterol and nonsterol isoprenoids combine to accelerate ER-associated degradation (ERAD) of reductase through a mechanism mediated by the ubiquitin/proteasome system (10–12). This ERAD reduces the half-life of reductase protein from 12 hr in compactin-treated cells to less than 1 hr in sterol-replete cells.
Figure 2.
Mevalonate-mediated multivalent feedback regulation of mammalian HMG CoA reductase. Sterol and nonsterol isoprenoids differentially inhibit HMG CoA reductase through three mechanisms – transcription, translation, and protein degradation. Transcriptional regulation is mediated by the sterol-regulated transcription factors called SREBPs that bind to SRE-1 sequences in the promoter of the HMG CoA reductase gene. Translational regulation is mediated by an unidentified nonsterol isoprenoid through an unknown mechanism that may involve the complex 5-untranslated region (UTR) in the reductase mRNA. Sterol and nonsterol isoprenoids combine to accelerate degradation of reductase protein. This figure was adapted from Goldstein and Brown, 1980 (1).
Insigs, major players in transcriptional and post-transcriptional mechanisms for feedback regulation of HMG CoA reductase
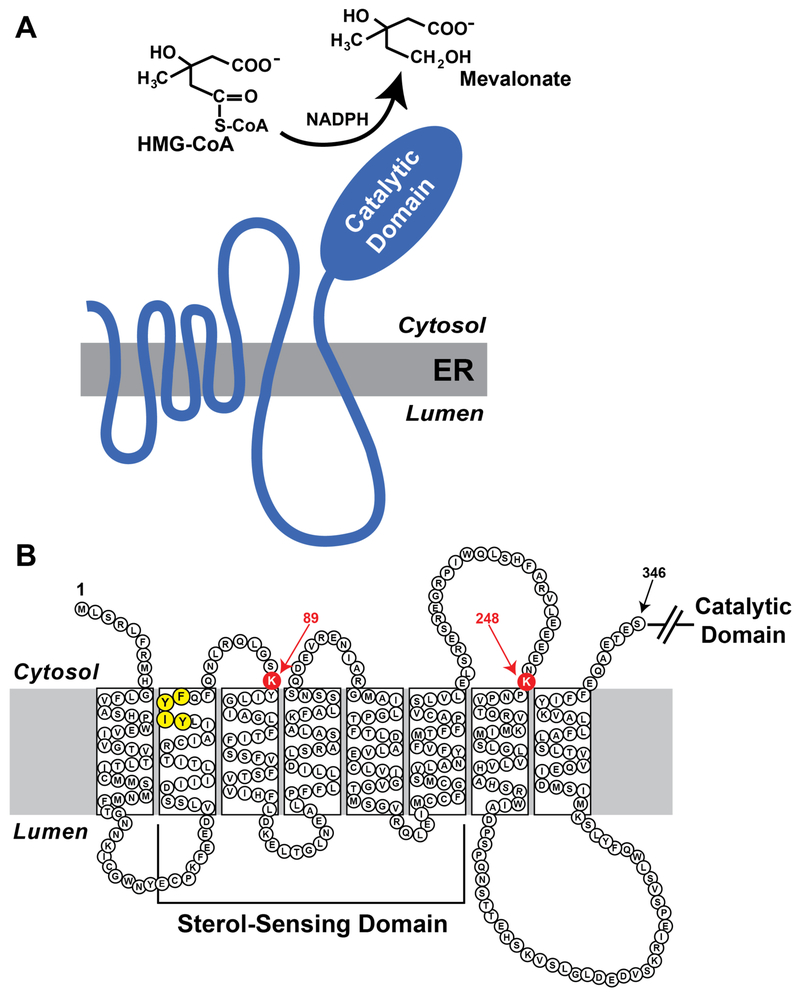
Mammalian HMG CoA reductase is comprised of 887 or 888 amino acids; the protein can be roughly divided into two distinct domains (Figure 3A). The N-terminal domain of reductase anchors the protein to ER membranes and consists of eight transmembrane domains separated by short hydrophilic loops (13). The hydrophilic C-terminal domain of reductase projects into the cytosol where it exerts all of the catalytic activity of the enzyme (14). Two early observations indicate that the membrane domain plays a key role in ERAD of reductase. First, expression of the truncated, soluble C-terminal domain of reductase rescues the cholesterol auxotrophy of reductase-deficient Chinese hamster ovary (CHO) cells (15) However, this protein was found to be very stable and sterols failed to accelerate its degradation. The second observation was provided by studies of a fusion protein between the membrane domain of reductase and soluble β-galactosidase (16). Degradation of the reductase-β-galactosidase fusion protein was accelerated by sterols in a manner similar to that of wild type, full-length reductase. Considered together, these key findings suggest a scenario in which the membrane domain of reductase directly or indirectly senses membrane-embedded sterols, triggering reactions that cause the entire enzyme to become susceptible to proteolytic degradation. This degradation can be blocked by inhibitors of the 26S proteasome, which leads to the accumulation of ubiquitinated forms of reductase (11).
Figure 3.
Domain structure of mammalian HMG CoA reductase. (A) As discussed in the text, HMG CoA reductase consists of two domains: an N-terminal domain with eight transmembrane helices that plays an essential role in sterol-accelerated ERAD and a C-terminal domain that exerts enzymatic activity. (B) Amino acid sequence and topology of HMG CoA reductase. Lysine residues that are required for Insig-mediated, sterol-induced ubiquitination are highlighted in red and denoted by arrows. The YIYF sequence required for sterol-regulated binding to Insigs is highlighted in yellow.
Insight into the mechanism for sterol-accelerated ERAD of reductase emerged from the transcriptional axis of the multivalent reductase regulatory system. Proteolytic activation of SREBPs requires Scap, which contains an N-terminal membrane domain with eight membrane-spanning helices followed by a cytosolic C-terminal domain that mediates association with SREBPs (17,18). In cholesterol-deprived cells, Scap escorts SREBPs from the ER to the Golgi where two proteases (designated site-1 and site-2 protease) release transcriptionally active fragments of SREBPs into the cytosol (19). These fragments migrate into the nucleus and stimulate transcription of target genes, resulting in the enhanced synthesis and uptake of cholesterol. When cholesterol accumulates to greater than 5 mol% of ER lipids, it binds to Scap, stimulating the protein to bind to ER membrane proteins called Insig-1 and Insig-2 (20,21). Insig binding blocks incorporation of Scap and its bound SREBP into COPII-coated vesicles that mediate transport of ER-derived proteins to the Golgi. Without transport to the Golgi, SREBPs are no longer activated, expression of SREBP target genes declines, and the rate of cholesterol synthesis/uptake falls. Scap-Insig binding is mediated by a segment of the membrane domain of Scap that comprises transmembrane helices 2–6. A similar region is found in several other polytopic membrane proteins (including Niemann Pick C1 protein, Patched, Dispatched, and reductase) that have been postulated to interact with sterols. The region has become known as the sterol-sensing domain (22) Mutations within the sterol sensing domain of Scap abrogates its binding to Insigs and renders the mutant Scap refractory to sterol-mediated retention in the ER, leading to constitutive activation of SREBPs (20).
The presence of a sterol-sensing domain in reductase led to an appraisal for a role for Insigs in the enzyme’s sterol-accelerated ERAD (12). Three observations that indicate that at least one Insig protein is required for reductase ERAD. First, sterols failed to accelerate ERAD of reductase when the protein was overexpressed in Chinese hamster ovary (CHO) cells by transfection. Co-expression of Insig-1 or Insig-2 restored sterol-accelerated ERAD, indicating saturation of endogenous Insigs by overexpressed reductase. Second, RNA interference (RNAi)-mediated knockdown of Insig-1 and Insig-2 abolished sterol-accelerated reductase ERAD (23). Finally, mutant CHO cells deficient in both Insig-1 and Insig-2 are refractory to sterol-regulated ERAD of reductase as well as sterol-mediated inhibition of SREBP processing (24). Sterol-accelerated ERAD coincided with sterol-induced binding of the reductase membrane domain to Insigs. This binding was abolished by mutation of a tetrapeptide sequence, YIYF, in the sterol-sensing domain of reductase; the mutant reductase was also refractory to sterol-accelerated ERAD. The YIYF sequence localizes to the second transmembrane domain of reductase (Figure 3B) and is also present in the sterol-sensing domain of Scap, where it mediates sterol-induced formation of the Scap-Insig complex. Moreover, overexpression of the sterol-sensing domain of Scap sequesters Insigs and blocks sterol-accelerated ERAD of reductase (12). Thus, Scap and reductase appear to bind the same site on Insigs and the two proteins may compete for limiting amounts of Insigs when intracellular levels of sterols rise.
Insig-mediated ubiquitination and geranylgeranyl-enhanced membrane extraction of HMG CoA reductase
A role for the ubiquitin/proteasome system in sterol-accelerated ERAD of reductase was first provided by the observation that proteasome inhibition blocked the reaction (10), leading to the accumulation of ubiquitinated forms of the enzyme on ER membranes (11). It was subsequently demonstrated that sterol-induced ubiquitination of reductase required Insigs and the reaction was found to be obligatory for ERAD (23). For example, RNAi-mediated knockdown or genetic deficiency of Insigs abrogated sterol-induced ubiquitination as well as degradation of reductase. In addition, mutation of the YIYF sequence not only abolishes Insig binding, but also prevents sterol-induced ubiquitination and ERAD of reductase. The membrane domain of reductase, which is both necessary and sufficient for sterol-accelerated ERAD, contains a pair of cytosolically exposed lysine residues at positions 89 and 248 (Figure 3B). Substitution of arginine residues for lysines 89 and 248 (K89R/K248R) in the membrane domain of reductase does not block its sterol-induced binding to Insigs, but rather blocks ubiquitination and subsequent degradation of the enzyme. These observations implicate lysines 89 and 248 as sterol-regulated, Insig-dependent sites of ubiquitination in reductase. Notably, the K89R and K248R mutations prevent reductase ubiquitination and degradation in the context of the full-length protein, which is consistent with the premise that the catalytic domain is dispensable for sterol-accelerated ERAD (15).
In vitro assays reconstituting sterol-induced ubiquitination of reductase indicated that the reaction was mediated by a membrane bound E3 ubiquitin ligase (25). Indeed, subsequent studies showed that at least two membrane-bound E3 ubiquitin ligases (designated gp78 and Trc8) associate with Insig-1 and Insig-2 (26,27). Co-immunoprecipitation experiments reveal that in the presence of sterols, Insigs mediate binding between reductase and gp78 or Trc8. RNAi-mediated knockdown of either gp78 or Trc8 blocked ubiquitination of reductase and inhibited sterol-accelerated ERAD by 50–60%. The combined knockdown of both gp78 and Trc8 produced a more complete inhibition of ERAD (>90%). Interestingly, binding of gp78 to Insig-1 stimulates its ubiquitination and degradation in sterol-deprived cells (28). Insig-1 degradation frees Scap and its bound SREBP to transport to the Golgi for proteolysis of SREBP and activation of target genes, which also includes the Insig-1 gene. When cholesterol levels are restored, Scap binds to Insig-1 and displaces gp78. As a result, Insig-1 becomes stabilized, binds to Scap-SREBP, and retains the complex in the ER. Collectively, these reactions are termed “convergent feedback inhibition” (29). Although Insig-1 mRNA is produced at high levels in the absence of cholesterol, Insig-1 protein is ubiquitinated and rapidly degraded. Insig-1 protein is only produced when sufficient levels of cholesterol are synthesized trigger binding to Scap. Thus, inhibiting of Golgi transport and SREBP processing requires the convergence of newly synthesized Insig-1 and newly acquired cholesterol.
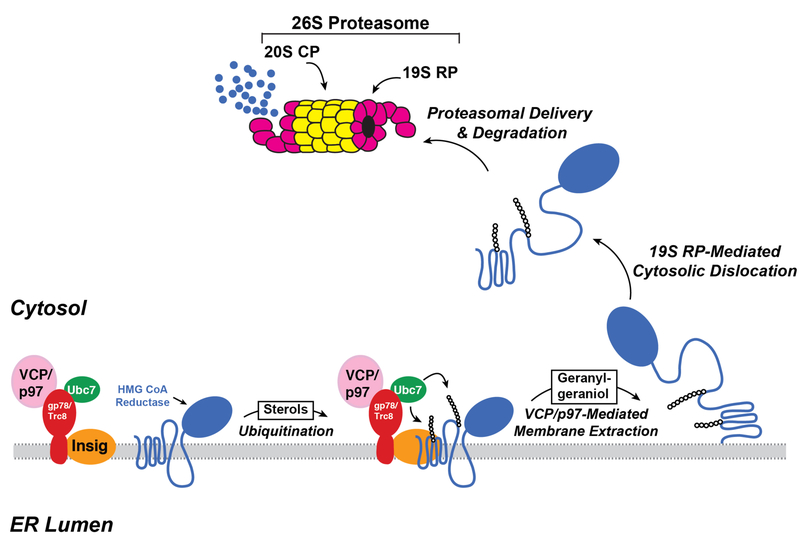
Two sequential post-ubiquitination steps have been identified in the reductase ERAD pathway (30). The first step is mediated by valosin-containing protein (VCP)/p97, a member of the ATPases associated with diverse cellular activities (AAA)-ATPase superfamily. VCP/p97 associates with two substrate recruitment factors, Npl4 and Ufd1, which bind polyubiquitin chains. Current evidence indicates that energy derived from VCP/p97-mediated hydrolysis of ATP drives extraction of ubiquitinated reductase across the ER membrane (31). In the second post-ubiquitination step, extracted reductase then becomes dislodged from the membrane into the cytosol by the 19S regulatory subunit of the proteasome, which also contains AAA-ATPase activity (30). Once dislocated into the cytosol, reductase is delivered into the proteolytic core of the 20S proteasome for degradation. The addition to cells of the 20-carbon nonsterol isoprenoid geranylgeraniol (GGOH), the alcohol derivative of GGpp, is required for maximal ERAD of reductase (23). We postulate that GGOH becomes converted to GGpp, which augments reductase ERAD by enhancing membrane extraction (32). The events that lead to sterol accelerated ERAD of reductase are depicted in Figure 4.
Figure 4.
Insig-mediated, sterol-accelerated ERAD of HMG CoA reductase.
Identification of UbiA prenyltransferase domain-containing protein-1 (UBIAD1) as the target of geranylgeraniol in HMG CoA reductase ERAD
UbiA prenyltransferase domain-containing protein-1 (UBIAD1) belongs to the UbiA superfamily of integral membrane prenyltransferases that catalyze transfer of isoprenyl groups to aromatic acceptors, producing molecules such as ubiquinones, hemes, chlorophylls, vitamin E, and vitamin K (33,34). In mammalian cells, UBIAD1 catalyzes the transfer of GGpp to menadione derived from plant-derived phylloquinone, producing the vitamin K2 subtype menaquinone-4 (MK-4) (Figure 1) (35). In 2007, two groups discovered that mutations in UBIAD1 are associated with Schynder corneal dystrophy (SCD), a rare autosomal dominant eye disease in humans characterized by progressive opacification of the cornea owing to the abnormal accumulation of cholesterol (36,37). In subsequent years, a total of 25 mutations have been identified in ~50 SCD families that alter 21 amino acid residues in UBIAD1 (38–40); several of these residues lie within or near the UBIAD1 active site (41,42). A possible link between UBIAD1 and cholesterol metabolism was provided by co-immunoprecipitation experiments that revealed an association of UBIAD1 with reductase (38). However, the relevance of this association to the regulation of cholesterol homeostasis and pathogenesis of SCD was unclear.
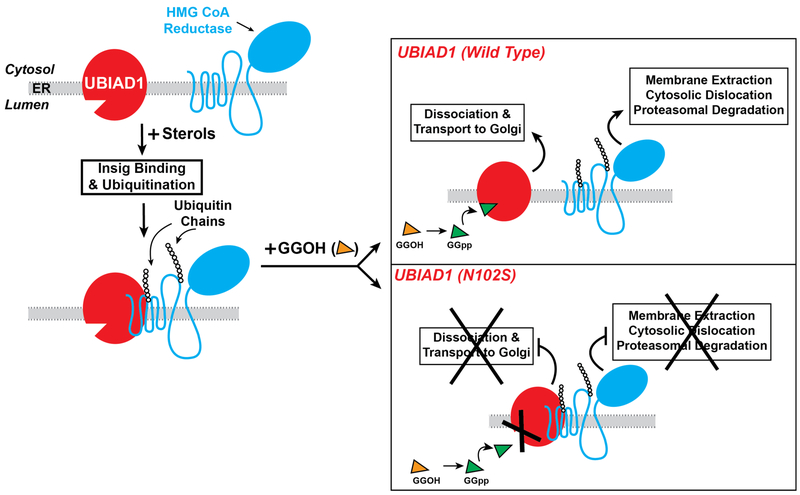
Recent studies discovered that sterols stimulate binding of UBIAD1 to a subset of reductase molecules (43). This binding was inhibited by GGpp, but not by Fpp, a 15-carbon nonsterol isoprenoid that does not augment reductase ERAD. CRISPR/Cas9-mediated knockout of UBIAD1 relieved the requirement of GGpp in the ERAD of reductase, indicating the prenyltransferase is an inhibitor of the reaction. Mutation of asparagine-102 to serine (N102S) is the most common UBIAD1 variant observed in SCD patients. UBIAD1 (N102S) resists GGpp-induced release from reductase and inhibits its ERAD in a dominant-negative fashion (43). It was subsequently determined that the remaining 20 SCD-associated mutants of UBIAD1 similarly block reductase ERAD (44).
In the course of investigation, it was discovered GGpp also stimulates translocation of UBIAD1 from the ER to the Golgi (43,44). Despite its steady-state localization to the Golgi of GGpp-replete cells, UBIAD1 continuously cycles between the Golgi and ER. Upon sensing depletion of GGpp in ER membranes, UBIAD1 becomes trapped in the organelle and inhibits ERAD of reductase to stimulate mevalonate synthesis for replenishment of GGpp. In contrast to its wild type counterpart, UBIAD1 (N102S) is refractory to GGpp-induced transport to the Golgi and remains sequestered in the ER where the mutant prenyltransferase blocks reductase ERAD. Structural analyses of archaeal UbiA prenyltransferases reveal that an asparagine residue corresponding to N102 in human UBIAD1 coordinates a Mg2+ ion that mediates association with the phosphate group of the isoprenyl substrate (41,42). Thus, it is reasonable speculate that defective Golgi transport of UBIAD1 (N102S) results from its reduced affinity for GGpp (Figure 5). Remarkably, the remaining SCD-associated UBIAD1 variants are similarly defective in Golgi transport and are sequestered in membranes of the ER (44). Whether this sequestration results from reduced affinity of these variants for GGpp remains to be determined. Together, these findings indicate that UBIAD1 mediates a novel sensing mechanism that monitors levels of GGpp embedded in membranes of the ER and modulates transport of the prenyltransferase between the ER and Golgi. This sensing mechanism appears to become disrupted in SCD and SCD-associated UBIAD1 remains sequestered in the ER where it binds to and inhibits ERAD of reductase. This Inhibition likely contributes to overaccumulation of cholesterol that characterizes SCD.
Figure 5.
Role of UBIAD1 in sterol-accelerated ERAD of HMG CoA reductase. The intracellular accumulation of sterols causes reductase to bind to Insigs, resulting in its ubiquitination by Insig-associated ubiquitin ligases and association with UBIAD1. Exogenously added GGOH becomes phosphorylated to produce GGpp, which enhances reductase ERAD by binding to UBIAD1 and stimulating release from reductase-Insig. This release allows for transport of UBIAD1 from ER to Golgi and membrane extraction, cytosolic dislocation, and proteasomal degradation of reductase. SCD-associated variants of UBIAD1 resist GGpp-induced release from reductase and remain in the ER to block reductase ERAD.
Physiologic significance of sterol-accelerated ERAD of HMG CoA reductase and implications for clinical medicine
Administration of the statin mevanolin to cholesterol-fed mice produced a 3- to 8-fold increase in the level of reductase protein in liver membranes (45). The elevated levels of reductase were rapidly suppressed by the subcutaneous injection of small amounts of mevalonate, suggesting full suppression of hepatic reductase required both sterol and nonsterol isoprenoids. These key early studies established that a multivalent feedback regulatory system similar to that described in cultured cells operates in the liver to control reductase so as to coordinate synthesis of sterol and nonsterol isoprenoids (4). The transcriptional component of the multivalent reductase regulatory system is mediated by SREBPs and has been subjected to intense scrutiny in livers of transgenic and knockout mice (9). In contrast, little is known about post-transcriptional mechanisms for feedback control of reductase in the liver and their contribution to the regulation of the enzyme and cholesterol metabolism. This is due in part to the inability to directly measure post-transcriptional parameters in vivo. For example, studies in the 1970s used indirect techniques such as measurement of enzymatic activity following cycloheximide treatment to assess the stability of reductase (46,47). Studies conducted when antibodies against reductase were available showed that the enzyme accumulated more than 20-fold in livers of mice with genetic deficiencies in both Insigs compared to their wild type counterparts (48). Considering that Insigs mediate both sterol-mediated inhibition of SREBP-2 activation and sterol-accelerated degradation of reductase, the accumulation resulted from the combination in defects in transcriptional and post-transcriptional regulation of the enzyme. However, the degree to which transcription and degradation contributed to regulation of reductase in the livers of Insig-deficient mice remained obscure.
The recent characterization of two lines of genetically-manipulated mice revealed that sterol-accelerated ERAD plays a significant role in feedback regulation of reductase and cholesterol metabolism in the liver (49). Tg-HMGCR (TM1–8) mice express in the liver, the membrane domain of reductase (a region both necessary and sufficient for sterol-accelerated ERAD (12)) under transcriptional control of a sterol-independent promoter. Subjecting Tg-HMGCR (TM1–8) mice to cholesterol feeding led to the disappearance of HMGCR (TM1–8) protein from hepatic membranes. Conversely, HMGCR (TM1–8) protein accumulated when diets of the transgenic mice were supplemented with the reductase inhibitor lovastatin. The mRNA encoding the HMGCR (TM1–8) transgene remained constant, regardless of feeding regimen, indicating that changes in the HMGCR (TM1–8) protein resulted from sterol-mediated modulation of its ERAD.
The analysis of Tg-HMGCR (TM1–8) mice described above was complemented by studies of knock-in mice (designated HmgcrKi/Ki) expressing the ubiquitination-resistant K89R/K248R form of endogenous reductase (Figure 3). The reductase failed to become ubiquitinated and accumulated in the liver and other tissues of HmgcrKi/Ki mice. This accumulation occurred despite reduced levels of reductase mRNA, which was attributable to reduced activation of SREBP-2 owing to accumulation of cholesterol. When normalized to the amount of hepatic reductase mRNA, it was estimated that reductase protein accumulated almost 14-fold in HmgcrKi/Ki mice compared to wild type controls. A similar disproportionate accumulation of reductase protein relative to its mRNA occurs in livers of mice deficient for gp78 (50). However, levels of Insig-2, and to a lesser extent, Insig-1, accumulates in gp78-deficient livers, whereas Insig protein levels remain unchanged in HmgcrKi/Ki livers. These results indicate that changes in cholesterol metabolism observed in HmgcrKi/Ki mice are solely attributable to inhibition of reductase ERAD.
The availability of HmgcrKi/Ki mice allowed the determination of the extent to which sterol-accelerated ERAD contributes to feedback regulation of reductase. The relative amount (normalized to reductase mRNA) of hepatic reductase protein was refractory to accelerated ERAD when knock-in mice were subjected to a diet supplemented with high levels (2%) of cholesterol. However, the absolute amount of reductase protein in knock-in mouse livers was markedly reduced by cholesterol feeding, which correlated with reduced amounts of reductase mRNA. Thus, it was concluded that 2% cholesterol feeding reduces levels of reductase primarily through inhibition of SREBP-2 activation. Cholesterol-depletion studies showed that lovastatin-induced accumulation of reductase was significantly blunted in HmgcrKi/Ki mice compared to wild type animals. Taken together, the characterization of Tg-HMGCR (TM1–8) and HmgcrKi/Ki mice demonstrate that sterol-induced ubiquitination and degradation play a direct and significant role in the feedback regulation of reductase and cholesterol homeostasis in vivo.
The significance of the multivalent reductase regulatory system is evidenced by the efficacy of statins in lowering plasma levels of LDL-cholesterol and reducing the incidence of atherosclerosis and associated coronary artery disease (3). However, statins trigger a markedly increase in the amount of hepatic reductase protein because they inhibit production of sterol and nonsterol isoprenoids that mediate the enzyme’s feedback regulation (1,45,51). This increase in reductase allows for continued synthesis of cholesterol and thereby blunts the cholesterol-lowering effects of statins (52–54). The finding that the statin-mediated increase in reductase is blunted (5-fold) in HmgcrKi/Ki vs. wild type mice indicates inhibition of ERAD significantly contributes to the increase. Thus, Tg-HMGCR (TM1–8) and HmgcrKi/Ki mice may prove useful in the development of new agents that accelerate reductase ERAD and improve the effectiveness of statins.
The discovery of a role for UBIAD1 in the ERAD of reductase was a major breakthrough in the understanding of the reaction. Current evidence suggest UBIAD1 is a novel sensor of membrane-embedded GGpp that modulates reductase ERAD to coordinate synthesis of sterol and nonsterol isoprenoids (44). This sensing mechanism becomes disrupted in SCD, leading to the inhibition of reductase ERAD and overaccumulation of cholesterol. Considering that studies supporting these conclusions were conducted exclusively in cultured cells, it is imperative that a role for UBIAD1 in reductase ERAD is established in vivo. This may set the stage for development of strategies that prevent or retard the corneal accumulation of cholesterol associated with SCD. In addition, these studies may reveal that statin-mediated accumulation of hepatic reductase results from GGpp depletion, which traps UBIAD1 in the ER where it inhibits reductase ERAD. Thus, preventing UBIAD1-mediated inhibition of reductase ERAD may emerge as a novel strategy to prevent statin-induced accumulation of reductase.
Acknowledgments
Work in the DeBose-Boyd laboratory is supported by National Institutes of Health grants HL020948 and GM112409 to R.D.B.
References
- 1.Goldstein JL, and Brown MS (1990) Regulation of the mevalonate pathway. Nature 343, 425–430 [DOI] [PubMed] [Google Scholar]
- 2.Edwards PA, and Ericsson J (1999) Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway 63. Annu.Rev.Biochem 68, 157–185 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JL, and Brown MS (2015) A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MS, and Goldstein JL (1980) Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res 21, 505–517 [PubMed] [Google Scholar]
- 5.Endo A, Kuroda M, and Tsujita Y (1976) ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J.Antibiot.(Tokyo) 29, 1346–1348 [DOI] [PubMed] [Google Scholar]
- 6.Endo A, Kuroda M, and Tanzawa K (1976) Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity 9. FEBS Lett. 72, 323–326 [DOI] [PubMed] [Google Scholar]
- 7.Brown MS, and Goldstein JL (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 [DOI] [PubMed] [Google Scholar]
- 8.Brown MS, Faust JR, and Goldstein JL (1978) Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J. Biol. Chem 253, 1121–1128 [PubMed] [Google Scholar]
- 9.Horton JD, Goldstein JL, and Brown MS (2002) SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J.Clin.Invest 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue S, Bar-Nun S, Roitelman J, and Simoni RD (1991) Inhibition of degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in vivo by cysteine protease inhibitors. J. Biol. Chem 266, 13311–13317 [PubMed] [Google Scholar]
- 11.Ravid T, Doolman R, Avner R, Harats D, and Roitelman J (2000) The ubiquitin-proteasome pathway mediates the regulated degradation of mammalian 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Biol. Chem 275, 35840–35847 [DOI] [PubMed] [Google Scholar]
- 12.Sever N, Yang T, Brown MS, Goldstein JL, and DeBose-Boyd RA (2003) Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol.Cell 11, 25–33 [DOI] [PubMed] [Google Scholar]
- 13.Roitelman J, Olender EH, Bar-Nun S, Dunn WA Jr., and Simoni RD (1992) Immunological evidence for eight spans in the membrane domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase: implications for enzyme degradation in the endoplasmic reticulum. J Cell Biol 117, 959–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liscum L, Finer-Moore J, Stroud RM, Luskey KL, Brown MS, and Goldstein JL (1985) Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J. Biol. Chem 260, 522–530 [PubMed] [Google Scholar]
- 15.Gil G, Faust JR, Chin DJ, Goldstein JL, and Brown MS (1985) Membrane-bound domain of HMG CoA reductase is required for sterol-enhanced degradation of the enzyme. Cell 41, 249–258 [DOI] [PubMed] [Google Scholar]
- 16.Skalnik DG, Narita H, Kent C, and Simoni RD (1988) The membrane domain of 3-hydroxy-3-methylglutaryl-coenzyme A reductase confers endoplasmic reticulum localization and sterol-regulated degradation onto beta-galactosidase. J. Biol. Chem 263, 6836–6841 [PubMed] [Google Scholar]
- 17.Nohturfft A, Brown MS, and Goldstein JL (1998) Topology of SREBP cleavage-activating protein, a polytopic membrane protein with a sterol-sensing domain. J. Biol. Chem 273, 17243–17250 [DOI] [PubMed] [Google Scholar]
- 18.Hua X, Nohturfft A, Goldstein JL, and Brown MS (1996) Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell 87, 415–426 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein JL, DeBose-Boyd RA, and Brown MS (2006) Protein sensors for membrane sterols. Cell 124, 35–46 [DOI] [PubMed] [Google Scholar]
- 20.Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, and Brown MS (2002) Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 110, 489–500 [DOI] [PubMed] [Google Scholar]
- 21.Radhakrishnan A, Goldstein JL, McDonald JG, and Brown MS (2008) Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab 8, 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwabara PE, and Labouesse M (2002) The sterol-sensing domain: multiple families, a unique role? Trends Genet. 18, 193–201 [DOI] [PubMed] [Google Scholar]
- 23.Sever N, Song BL, Yabe D, Goldstein JL, Brown MS, and DeBose-Boyd RA (2003) Insig-dependent ubiquitination and degradation of mammalian 3-hydroxy-3-methylglutaryl-CoA reductase stimulated by sterols and geranylgeraniol. J. Biol. Chem 278, 52479–52490 [DOI] [PubMed] [Google Scholar]
- 24.Sever N, Lee PC, Song BL, Rawson RB, and Debose-Boyd RA (2004) Isolation of mutant cells lacking Insig-1 through selection with SR-12813, an agent that stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem 279, 43136–43147 [DOI] [PubMed] [Google Scholar]
- 25.Song BL, and DeBose-Boyd RA (2004) Ubiquitination of 3-hydroxy-3-methylglutaryl-CoA reductase in permeabilized cells mediated by cytosolic E1 and a putative membrane-bound ubiquitin ligase. J. Biol. Chem 279, 28798–28806 [DOI] [PubMed] [Google Scholar]
- 26.Song BL, Sever N, and DeBose-Boyd RA (2005) Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol. Cell 19, 829–840 [DOI] [PubMed] [Google Scholar]
- 27.Jo Y, Lee PC, Sguigna PV, and DeBose-Boyd RA (2011) Sterol-induced degradation of HMG CoA reductase depends on interplay of two Insigs and two ubiquitin ligases, gp78 and Trc8. Proc. Natl. Acad. Sci. U.S.A 108, 20503–20508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JN, Song B, DeBose-Boyd RA, and Ye J (2006) Sterol-regulated degradation of Insig-1 mediated by the membrane-bound ubiquitin ligase gp78. J. Biol. Chem 281, 39308–39315 [DOI] [PubMed] [Google Scholar]
- 29.Gong Y, Lee JN, Lee PC, Goldstein JL, Brown MS, and Ye J (2006) Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab 3, 15–24 [DOI] [PubMed] [Google Scholar]
- 30.Morris LL, Hartman IZ, Jun DJ, Seemann J, and DeBose-Boyd RA (2014) Sequential Actions of the AAA-ATPase Valosin-containing Protein (VCP)/p97 and the Proteasome 19 S Regulatory Particle in Sterol-accelerated, Endoplasmic Reticulum (ER)-associated Degradation of 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase. J. Biol. Chem 289, 19053–19066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vij N (2008) AAA ATPase p97/VCP: cellular functions, disease and therapeutic potential. J.Cell Mol.Med 12, 2511–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elsabrouty R, Jo Y, Dinh TT, and DeBose-Boyd RA (2013) Sterol-induced dislocation of 3-hydroxy-3-methylglutaryl coenzyme A reductase from membranes of permeabilized cells. Mol. Biol. Cell 24, 3300–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonitz T, Alva V, Saleh O, Lupas AN, and Heide L (2011) Evolutionary relationships of microbial aromatic prenyltransferases. PLoS One 6, e27336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W (2016) Bringing Bioactive Compounds into Membranes: The UbiA Superfamily of Intramembrane Aromatic Prenyltransferases. Trends Biochem Sci 41, 356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, and Okano T (2010) Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 468, 117–121 [DOI] [PubMed] [Google Scholar]
- 36.Orr A, Dube MP, Marcadier J, Jiang H, Federico A, George S, Seamone C, Andrews D, Dubord P, Holland S, Provost S, Mongrain V, Evans S, Higgins B, Bowman S, Guernsey D, and Samuels M (2007) Mutations in the UBIAD1 gene, encoding a potential prenyltransferase, are causal for Schnyder crystalline corneal dystrophy. PLoS One 2, e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss JS, Kruth HS, Kuivaniemi H, Tromp G, White PS, Winters RS, Lisch W, Henn W, Denninger E, Krause M, Wasson P, Ebenezer N, Mahurkar S, and Nickerson ML (2007) Mutations in the UBIAD1 gene on chromosome short arm 1, region 36, cause Schnyder crystalline corneal dystrophy. Invest Ophthalmol Vis Sci 48, 5007–5012 [DOI] [PubMed] [Google Scholar]
- 38.Nickerson ML, Bosley AD, Weiss JS, Kostiha BN, Hirota Y, Brandt W, Esposito D, Kinoshita S, Wessjohann L, Morham SG, Andresson T, Kruth HS, Okano T, and Dean M (2013) The UBIAD1 prenyltransferase links menaquinone-4 [corrected] synthesis to cholesterol metabolic enzymes. Hum Mutat 34, 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowinska AK, Wylegala E, Teper S, Lyssek-Boron A, Aragona P, Roszkowska AM, Micali A, Pisani A, and Puzzolo D (2014) Phenotype-genotype correlation in patients with Schnyder corneal dystrophy. Cornea 33, 497–503 [DOI] [PubMed] [Google Scholar]
- 40.Lin BR, Frausto RF, Vo RC, Chiu SY, Chen JL, and Aldave AJ (2016) Identification of the First De Novo UBIAD1 Gene Mutation Associated with Schnyder Corneal Dystrophy. J Ophthalmol 2016, 1968493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang H, Levin EJ, Liu S, Bai Y, Lockless SW, and Zhou M (2014) Structure of a Membrane-Embedded Prenyltransferase Homologous to UBIAD1. PLoS biology 12, e1001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng W, and Li W (2014) Structural insights into ubiquinone biosynthesis in membranes. Science 343, 878–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumacher MM, Elsabrouty R, Seemann J, Jo Y, and DeBose-Boyd RA (2015) The prenyltransferase UBIAD1 is the target of geranylgeraniol in degradation of HMG CoA reductase. eLife 4, e05560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schumacher MM, Jun DJ, Jo Y, Seemann J, and DeBose-Boyd RA (2016) Geranylgeranyl-regulated transport of the prenyltransferase UBIAD1 between membranes of the ER and Golgi. J. Lipid Res. 57, 1286–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kita T, Brown MS, and Goldstein JL (1980) Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in livers of mice treated with mevinolin, a competitive inhibitor of the reductase. J.Clin.Invest 66, 1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards PA, and Gould RG (1972) Turnover rate of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase as determined by use of cycloheximide 245. J Biol.Chem 247, 1520–1524 [PubMed] [Google Scholar]
- 47.Edwards PA, Muroya H, and Gould RG (1972) In vivo demonstration of the circadian thythm of cholesterol biosynthesis in the liver and intestine of the rat 244. J. Lipid Res 13, 396–401 [PubMed] [Google Scholar]
- 48.Engelking LJ, Liang G, Hammer RE, Takaishi K, Kuriyama H, Evers BM, Li WP, Horton JD, Goldstein JL, and Brown MS (2005) Schoenheimer effect explained - feedback regulation of cholesterol synthesis in mice mediated by Insig proteins. J Clin.Invest 115, 2489–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang S, Hartman IZ, Calhoun LN, Garland K, Young GA, Mitsche MA, McDonald J, Xu F, Engelking L, and DeBose-Boyd RA (2016) Contribution of Accelerated Degradation to Feedback Regulation of 3-Hydroxy-3-methylglutaryl Coenzyme A Reductase and Cholesterol Metabolism in the Liver. J. Biol. Chem 291, 13479–13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu TF, Tang JJ, Li PS, Shen Y, Li JG, Miao HH, Li BL, and Song BL (2012) Ablation of gp78 in liver improves hyperlipidemia and insulin resistance by inhibiting SREBP to decrease lipid biosynthesis. Cell Metab 16, 213–225 [DOI] [PubMed] [Google Scholar]
- 51.Reihner E, Rudling M, Stahlberg D, Berglund L, Ewerth S, Bjorkhem I, Einarsson K, and Angelin B (1990) Influence of pravastatin, a specific inhibitor of HMG-CoA reductase, on hepatic metabolism of cholesterol. N.Engl.J.Med 323, 224–228 [DOI] [PubMed] [Google Scholar]
- 52.Schonewille M, de Boer JF, Mele L, Wolters H, Bloks VW, Wolters JC, Kuivenhoven JA, Tietge UJ, Brufau G, and Groen AK (2016) Statins increase hepatic cholesterol synthesis and stimulate fecal cholesterol elimination in mice. J Lipid Res 57, 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldberg IJ, Holleran S, Ramakrishnan R, Adams M, Palmer RH, Dell RB, and Goodman DS (1990) Lack of effect of lovastatin therapy on the parameters of whole-body cholesterol metabolism. J Clin Invest 86, 801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grundy SM, and Bilheimer DW (1984) Inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase by mevinolin in familial hypercholesterolemia heterozygotes: effects on cholesterol balance. Proc. Natl. Acad. Sci. U.S.A 81, 2538–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]