Abstract
Multidrug resistance (MDR) is a serious problem that hampers the success of cancer pharmacotherapy. A common mechanism is the overexpression of ATP-binding cassette (ABC) efflux transporters in cancer cells such as P-glycoprotein (P-gp/ABCBl), multidrug resistance-associated protein 1 (MRP1/ABCC1) and breast cancer resistance protein (BCRP/ABCG2) that limit the exposure to anticancer drugs. One way to overcome MDR is to develop ABC efflux transporter inhibitors to sensitize cancer cells to chemotherapeutic drugs. The complete clinical trials thus far have showen that those tested chemosensitizers only add limited or no benefits to cancer patients. Some MDR modulators are merely toxic, and others induce unwanted drug-drug interactions. Actually, many ABC transporters are also expressed abundantly in the gastrointestinal tract, liver, kidney, brain and other normal tissues, and they largely determine drug absorption, distribution and excretion, and affect the overall pharmacokinetic properties of drugs in humans. In addition, ABC transporters such as P-gp, MRP1 and BCRP co-expressed in tumors show a broad and overlapped specificity for substrates and MDR modulators. Thus reliable preclinical assays and models are required for the assessment of transporter-mediated flux and potential effects on pharmacokinetics in drug development. In this review, we provide an overview of the role of ABC efflux transporters in MDR and pharmacokinetics. Preclinical assays for the assessment of drug transport and development of MDR modulators are also discussed.
Keywords: Cancer therapy, multidrug resistance, ABC transporter, Pharmacokinetics, Drug development
1. INTRODUCTION
Resistance to structurally and functionally related or unrelated medications is a major impediment to successful cancer pharmacotherapy [1–6], Intrinsic or primary multidiug resistance (MDR) occurs among a small population of tumor cells such as “cancer stem cells” or malignant tumor cells that are “inherently” resistant to anticancer drugs from the very beginning of drug treatment. In contrast, acquired or secondary MDR happens in survived tumor cells that develop drug resistance capacity during pharmacotherapy. A number of mechanisms have been identified for MDR, which could be drag-dependent, target-dependent and drag/target-independent [7]. Drag-dependent MDR is caused by the alternation of cellular drag disposition, particularly the overexpression of efflux drag transporters and drag-metabolizing enzymes in cancer cells. Target-dependent MDR is attributable to the desensitization of drug targeting, which includes mutation, translocation, deletion and amplification of the target. Drag- or target-independent MDR is due to the escape from drag targeting through genetic or epigenetic alternation of cell signaling pathways. It is noteworthy that these mechanisms are recognized for the resistance to both cytotoxic agents and targeted anticancer drags, and multiple mechanisms may be involved in drag resistant tumor cells. In addition, drug resistance is shown for other types of therapeutic agents such as antibiotics and antimalarials [8–11]. In this review, we focus on the role of ATP-binding cassette (ABC) transporters in MDR of cancer cells and the development of ABC efflux transporter inhibitors as chemosensitizing agents towards improved chemotherapy, as well as the importance of ABC transporters in pharmacokinetics and preclinical assays for the evaluation of drag transport in drug development.
2. CANCER PHARMACOTHERAPY AND MULTIDRUG RESISTANCE
Cancer disease is a leading cause of death worldwide. The number of cancer deaths is estimated to increase from 12 million new cases and 7 million deaths in 2008 to 26 million new cases and 11.4 million deaths in 2030, respectively [12]. The high incidence and mortality are likely due to the complex interactions of non-modifiable (e.g., genetic susceptibility and aging) and modifiable risk factors (e.g., tobacco, infectious agents, diet, and physical activity) [13].
2.1. Cancer Pharmacotherapy
Standard managements of cancer diseases include surgery, radiotherapy and pharmacotherapy, which may be used alone or in combination, depending upon cancer type, disease status, pathological and molecular characteristics as well as patient’s condition. Surgery represents the first line of therapy and benign tumors may be curable by surgery. Radiation therapy is commonly applied to kill or control malignant cells and it may be curative in some cancers localized to specific areas of human body. Pharmacotherapy is the use of a variety of anticancer drags including chemotherapeutics, hormonal and immunotherapeutic agents (Table 1) to manage cancer cell proliferation or apoptosis. Among them, the small-molecule chemotherapeutic agents exhibit a broad spectrum of cytotoxic activities and represent the most commonly used drags for cancer therapy. Based on their mechanistic actions, small-molecule anticancer drags may be divided into three major types, cytotoxic drags (antimetabolites, genotoxic drags and mitotic spindle inhibitors), protein kinase inhibitors and hormonal agents (Table 2) [5, 14, 15].
Table 1.
Major classes of anticancer drugs.
| Pharmacotherapy | Agents |
|---|---|
| Chemotherapy | Antimetabolites |
| Genotoxic drugs | |
| Mitosis inhibitors | |
| Endocrine therapy | Steroids |
| Inhibitors of hormone synthesis | |
| Hormone receptor antagonists | |
| Immunotherapy | Antibodies |
| Vaccines |
Table 2.
Common small-molecule cytotoxic drugs, protein kinase inhibitors and hormonal agents used for cancer treatment.
| Group | Sub-group | Example drugs |
|---|---|---|
|
Anti-metabolites (inhibit the syntheses of building blocks required for DNA replication) |
Pyrimidine antagonists | 5-Fluorouracil |
| Gemcitabine | ||
| Arabinosylcytosine | ||
| Purine antagonists | 6-Mercaptopurine | |
| 6-Thioguanine | ||
| Fludarabine | ||
| Folate antagonists | Acyclovir | |
| Methotrexate | ||
| Trimethoprim | ||
| Pyrimethamine | ||
| Hydroxyurea | ||
|
Genotoxic agents (directly or indirectly damage DNA in the nucleus of the cell) |
Alkylating agents | Busulfan |
| CisplatinCyclophosphamide | ||
| Mechlorethamine | ||
| Ifosfamide | ||
| Chlorambucil | ||
| Intercalating agents | Actinomycin D Doxorubicin | |
| Daunorubicin | ||
| Epirubicin | ||
| Topoisomerase inhibitors | Irinotecan | |
| Topotecan | ||
| Etoposide | ||
| Teniposide | ||
| Mitoxantrone | ||
|
Mitotic spindle inhibitors (inhibit mitosis bv disruDtins microtubules) |
Vinca alkaloids | Vinblastine |
| Vincristine | ||
| Taxanes | Paclitaxell | |
| Docetaxel | ||
| Others | Colchicine | |
|
Protein kinase inhibitors (block the actions of protein kinase enzymes or receptors) |
Bcr-Abl inhibitors | Imatinib |
| Nilotinib | ||
| EGFR inhibitors | Gefitinib | |
| Afatinib | ||
| Erlotinib | ||
| Lanatinib | ||
| Others | Dasatinib | |
| Flavopiridol | ||
| Sorafenib | ||
| Axitinib | ||
|
Hormones (modulate endocrine system) |
SERMs | Tamoxifen |
| Raloxifene | ||
| Aromatase inhibitors | Anastrozole Letrozole | |
| Letrozole | ||
| Aminoglutethimide | ||
| Exemestone | ||
| Androgen antagonists | Flutamide | |
| Bicalutamide | ||
| Cyproterone | ||
| Niltamide | ||
| GnRH analogs | Leunrolide | |
| Goserelin | ||
| Others | Abiraterone | |
| Dutasteride | ||
| Finasteride | ||
| Megestrol |
Antimetabolites are structurally similar to the metabolites, e.g., heterocyclic bases and nucleosides needed for the biosyntheses of DNA and RNA, so that they prevent the growth and division of tumor cells. Antimetabolite drags include folate, purine and pyrimidine antagonists (Table 2). Pyrimidine antagonists such as 5-fluorouracil, gemcitabine and arabinosylcytosine block the production of pyrimidine nucleotide or cause a premature termination when the drugs themselves are incorporated into DNA [5, 16]. This process stops DNA replication and arrests cell growth. Gemcitabine also irreversibly inhibits the enzyme ribonucleotide reductase (RNR) required for DNA replication and repair, and induces apoptosis [17], It is widely used in the treatment of various carcinomas including pancreatic, bladder, breast and non-small cell lung cancers. Likewise, purine antagonists such as 6-mercaptopurine, 6-thioguanine, fludarabine and acyclovir inhibit the syntheses of adenine and guanine necessary for DNA replication [18]. Folate antagonists include methotrexate, trimethoprim and pyrimethamine, which block the use of folic acid. In particular, these antifolates inhibit the enzyme dihydrofolate reductase (DHFR) that is required for methylation and formation of purine and pyrimidine for DNA/RNA/protein production [5, 19]. Note that methotrexate is a competitive inhibitor of DHFR, and overexpression of DHFR may explain the resistance to methotrexate.
Genotoxic agents bind to DNA directly or damage DNA through interfering critical enzymes in control of the changes of DNA structure [5]. Genotoxic drugs include alkylating agents (e.g., cisplatin and cyclophosphamide), intercalating agents (e.g., doxorubicin and daunorubicin) and topoisomerase inhibitors (e.g., irinotecan and topotecan) (Table 2). Alkylating agents add an alkyl group to the DNA and prevent cells from proper DNA replication and transcription. Intercalating agents bind to the DNA directly and inhibit DNA replication in cancer cells [5, 20], The inhibition of topoisomerase I or II, enzymes in control of the DNA structure, prevents the general functions such as transcription, replication and repair of DNA [21]. Topoisomerase I inhibitors include topotecan, irinotecan and rubitecan, and topoisomerase II inhibitors consist of etoposide and teniposide. Interestingly, the anthracenedione anticancer drug mitoxantrone not only acts as a topoisomerase II inhibitor but also participates in intercalation.
Mitotic spindle inhibitors are mainly natural compounds such as vinca alkaloids (e.g., vinblastine and vincristine) and taxanes (e.g., paclitaxel and docetaxel) [22] (Table 2). Mitosis is the final process in cell cycle when the chromosomes in the nucleus are separated into two identical sets for the formation of two nuclei/cells. Microtubules, assembled by tubulin components, play a central role in the separation of chromosomes to the opposite ends of a mitosing cell during the anaphase. Mitotic inhibitors block the polymerization of tubulin monomers, and thus prevent cancerous cells from division and tumorigenesis [5, 22].
Hormonal agents are a big group of anticancer drugs in treating hormone-dependent cancers (e.g., breast and prostate cancer) [15, 23–25], which interfere with the endocrine systems. Hormonal anticancer drugs include selective estrogen receptor modulators (SERMs; e.g., tamoxifen and raloxifene), aromatase CYP19A1 inhibitors (e.g., anastrozole, letrozole, aminoglutethimide and exemestone), androgen antagonists (e.g., flutamide, bicalutamide, cyproterone and niltamide) and gonadotropin-releasing hormone (GnRH) analogues (e.g., leuprolide and goserelin) (Table 2). Aromatase inhibitors block the action of aromatase in converting testosterone to estrogen, and they are often used for treating estrogen-sensitive breast cancers in postmenopausal women. SERMs act as antagonists of estrogen receptor in breast tissues; thus SERMs are widely used for the treatment of estrogen receptor positive breast cancers in both pre- and post-menopausal women. Androgen antagonists such as flutamide and bicalutamide inhibit the androgen receptors and suppress testosterone promoted cell proliferation. Therefore antiandrogen drugs are primarily employed to treat prostate cancers. The GnRH analogs act as pituitary GnRH antagonists and reduce the production of sex hormones (both testosterone and estrogen). GnRH analog therapy is frequently used in the treatment of prostate cancer. In addition, inhibitors of CYP17A1 (e.g., abiraterone) or 5α-reductase (dutasteride and finasteride) involved in steroid metabolism have been approved by the US Food and Drug Administration (FDA) for the treatment of prostate cancer or prostatic hyperplasia, and many others are under development.
Protein kinase inhibitors (Table 2) are a newer group of anticancer drugs that selectively block the actions of protein kinases critical for the metabolism, proliferation, migration, invasion and apoptosis of cancer cells. The approval of imatinib by FDA in 2001 as the first Bcr-Abl tyrosine-kinase inhibitor for the treatment of chronic myelogenous leukemia (CML) represents a successful and revolutionized “targeted” or “rational” therapy for cancer disease [26–28]. Besides Bcr-Abl, many protein kinases such the epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF) and mitogen activated protein kinase (MAPK) involved in different or the same cell signaling pathways are commonly overexpressed or activated in cancer cells, and are proven to promote tumorigenesis. Targeting these protein kinase oncogenes with antibodies or small molecules, either by inhibiting the ATP binding sites or disrupting the kinase-substrate interactions through other means, is an effective way to manage tumor growth. Indeed, many kinase inhibitors such as nilotinib (for the treatment of CML), gefitinib (for treatment of lung and colorectal cancer), erlotinib (for the treatment of non-small cell lung cancer) and dasatinib (for the treatment of CML) (Table 2) have been developed and approved for cancer treatment in the past decade [28–30]. In addition, there are lots of other selective protein kinase inhibitors currently under clinical tests of their potential applications to cancer treatments.
2.2. Multidrug Resistance and the Common Echanisms
The ability of cancer cells that are inheritably resistant to or develop a resistance afterwards to the same drug or different drugs causes a persistent problem in cancer pharmacotherapy [31]. Since the development of the remarkable protein kinase inhibitor imatinib for the treatment of CML, MDR was once regarded as a vanishing problem specifically associated with cytotoxic drugs, and it would not be a problem for “targeted” therapeutics [32]. Soon it was found that some CML patients, especially the most advanced-phase patients, showed relapse on imatinib therapy despite many CML patients demonstrated durable responses [33–36]. So were other protein kinase inhibitors such as gefitinib and erlotinib [37–39], although some drugs (e.g., gefitinib, erlotinib and nilotinib) inhibit the function of ABC efflux transporters [40–44], Therefore, understanding how MDR occurs is critical for the prediction of MDR and development of possible strategies to overcome MDR towards improved pharmacotherapy.
MDR can arise as a result of the changes of cellular or non-cellular processes [45]. Non-cellular drug resistance is caused by a higher vascular permeability and the absence of functional lymphatic system that leads to a reduced nutrient, oxygen and drug access to tumor cells [46, 47]. For instance, the generation of lactic acid by hypoxic tumor cells provides acidic environments and MDRs [48], A cellular drug resistance may be classified as classical and non-classical MDR phenotypes [2]. Classical MDR, known as transport-based MDR, includes a decreased uptake of water-soluble drugs (e.g., folate inhibitors and nucleotide analogues) and increased energy-dependent efflux of hydrophobic drugs [4, 5]. Non-classical MDR is accompanied by the alteration of specific enzyme systems (e.g., glutathione S-transferases and/or topoisomerases) and imbalance of proteins, which control drug metabolism, apoptosis and membrane permeability [2,49–51].
Alternations of drug processing proteins and changes of drug targeted proteins or cell signaling pathways involve multiple molecular mechanisms such as gene mutation, translocation, deletion and amplification as well as epigenetic regulation [32, 52]. The mutational status of p53 was revealed to be closely related to the response of ovarian cancer to platinum-based therapy [53] and the response of ovarian cancer to platinum-based therapy [53] and the response of breast cancer to anthracycline treatment [54]. The point mutations found in the Bcr-Abl kinase domain might limit its ability to form an inactive conformation required for the inhibition by imatinib, and confer the resistance to imatinib [33, 55], Gene amplification is another common mechanism of MDR, which is exemplified by the amplification of DHFR gene in methotrexate resistance cells [56]. The aberrant epigenetic regulation such as methylation of CpG island, histone modification and noncoding microRNAs alters a large number of genes and impacts on the chemosensitivities of cancer cells [57–59].
The most commonly encountered MDRs are associated with the alternations of ABC efflux transporters that actively pump various anticancer drugs out of tumor cells [60, 61]. Overexpression of ABC drug transporters is remarkably related to gene amplification [62], transcriptional and epigenetic changes [59, 63]. In addition, the discovery of cancer stem cells in solid tumors and the finding on ABC transporter overexpression in cancer stem cells are also revolutionizing the study on drug resistance [64].
3. ABC EFFLUX TRANSPORTERS UNDERLYING MULTIDRUG RESISTANCE
The human ABC transporters consists of 48 members that are classified into seven subfamilies designated ABCA to ABCG according to the similarity of their amino acid sequences [61]. Most human ABC transporters are located on the brush border membrane of enterocytes, biliary canalicular membrane of hepatocytes, luminal membrane in the proximal tubules of kidney and the epithelium cells at blood-brain barrier. Catalyzing the ATP-dependent transport of structurally diverse compounds across cellular membranes, ABC transporters are critical elements for the cells in protection against xenobiotics [65]. Some ABC transporters are also responsible for the homeostasis of endogenous agents, and people carrying defected ABC genes may be more susceptible to specific diseases such as the Tangier’s disease, Stargardt’s disease and adrenoleukodystrophy [66–68].
3.1. General Properties of ABC Transporters
A typical ABC transporter is composed of two distinct domains, transmembrane domain (TMD) and nucleotide (ATP) binding domain (NBD). The hydrophilic NBD is located within cytoplasm for ATP binding and hydrolysis to harness energy for the transport of substrates across membrane [69]. The NBD is highly conservative, consisting of the Walker A (GXXGXGKS/T where X represents any amino acid) and Walker B (ΦΦΦΦD where Φ is hydrophobic) motifs that are separated by the ABC signature motif (LSGGQ). The serine residue in ABC signature sequence is critical for the interactions between Walker A and ABC signature motifs to form the so-called ATP sandwich and to warrant the consequent ATP hydrolysis. The TMD spans the membrane and forms channels. The hydrophobic TMDs are structurally diverse, which recognize and translocate a broad variety of substrates upon conformational changes. Therefore, the TMDs determine the characteristics of transported substrates. In addition, most ABC efflux transporters (e.g., P-glycoprotein or P-gp/MDRl/ABCBl) consist of two N-terminal TMDs and two C-terminal NBDs (TMD1-NBD1-TMD2-NBD2), and each TMD generally contains six transmembrane segments (α-helices). By contrast, breast cancer resistance protein (BCRP/ABCG2) is a half-transporter that only has one TMD at the C-terminal end and one NBD at the N-terminal end (NBD-TMD). Nevertheless, ABCG2 forms a homodimer through the disulfide bonds towards the extrusion of its substrates [70].
ABC efflux transporter-mediated drug translocation may be exemplified by simple kinetic mechanisms. Generally, substrate binding initiates the transport cycle and ATP binding induces NBD dimerization and configuration of the ATP sandwich. Although changes of transporter structures at different stages are not elucidated exclusively, substrates seem to be bound at the high-affinity site within the TMDs. The conformational changes by binding and hydrolysis of ATP or movement of proton via the electrochemical gradient converts the high-affinity site to low-affinity site in the membrane and the alternative side of the membrane is released [71, 72]. These conformational changes can be transmitted between domains of ABC transporters. Substrates cross the bilayer within the core of the transporter, largely shielded from the surrounding lipid phase. ABC transporters extract their substrates from the inner leaflet of the bilayer to phospholipid flippases and eventually pump them out of the cells [73], Hydrolysis of the second ATP molecule and release of Pj separate the NBDs and restore the stable conformational state for the binding of another substrate.
3.2. Multidrug Resistance ABC Efflux Transporters
The MDR phenotype is often linked to the overexpression of ABC efflux transporters such as P-gp, multidrug resistance-associated proteins (MRPs/ABCCs) and BCRP. P-gp is the first ABC efflux transporter found to be responsible for the sensitivity of cells to chemotherapeutic agents [74, 75]. The second member of ABC efflux transporter revealed to confer MDR is MRP1, which was over-expressed in cancer cells whose P-gp levels were not increased [76, 77], The third ABC efflux transporter critical for MDR is BCRP [78–80], which is a half-transporter exhibiting a very broad specificity for substrates like P-gp and MRP1 (Table 3). While there are many other ABC transporters important in MDR, we will limit our discussion to more extensively-studied P-gp, MRP1 and BCRP that also share the specificity for substrates.
Table 3.
Common drugs transported by P-gp, MRP1 and BCRP.
| P-gp (ABCB1) | Anticancer drues: doxorubicin, daunorubicin. epirubicin. colchicine, antinomvcin D. etoposide. teniposide. methotrexate, mitocvcin C, paclitaxel, mitoxantrone, docetaxel, vinblastine, vincristine |
| Antihvnertensives: losartan. celiorolol. reseroine. talinolol. Nicardipine | |
| Antiarrhvthimics: dieoxin. propafenone, auinidine. verapamil, amiodarone | |
| Antibiotics: ervthromvcin. rifampin, levofloxacin. clarithromycin, tetracycline | |
| Antivirals: amorenavir. indinavir, nelfinair. ritonavir, saauinavir | |
| Antidepressants: amitrintvline. fluoxetine, paroxetine, sertraline | |
| Immunosuppressants: cyclosporine A. sirolimus. tacrolimus, valspodar | |
| Opioids: methadone, morphine | |
| Lipid lowerine drues: atorvastatin. lovastatin | |
| Glucocorticoids: aldosterone, cortisol, dexamethasone. Methvlprednisolone | |
| Antihistamines: fexofenadine, terfenadine | |
| Others: oroeesterone. itraconazole, phenobarbital. phenvtoin. cimetidine | |
| MRP1 (ABCC1) |
Anticancer drues: doxorubicin, daunorubicin. colchicine, toootecan. irinotecan. SN-38. methotrexate, etoposide. teniposide. vincristine. vinblastine, imatinib, gefitinib |
| Antivirals: indinavir, ritonavir, saauinavir | |
| Antibiotics: ciprofloxacin, difloxacin. ereoafloxacin. pirarbicin | |
| BCRP (ABCG2) | Anticancer drues: doxorubicin, daunorubicin. epirubucin. methotrexate, toootecan. irinotecan, SN-38. etoposide. teniposide. imatinib, gefitinib |
| Antibiotics: ciprofloxacin, norfloxacin, ofloxacin | |
| Lipid lowerine drues: cerivastatin. pravastatin, rosuvastatin | |
| Antivirals: lamivudine. Zidovudine | |
| Antihvoertensives: reseroine | |
| Others: azidothvmidine. lamivudine |
P-gp, a 170 kDa glycoprotein, is abundantly expressed on the intestinal mucosal membrane, the luminal blood-brain barrier and the apical membranes of hepatocytes and kidney proximal tubule epithelia [81]. P-gp substrates include amphipathic compounds (unmodified drugs and drug conjugates), lipid soluble compounds (molecular weights in the range of 300 to 1000) and compounds with aromatic rings and a positive charge at physiological pH [82], P-gp transports a broad range of therapeutic drugs (Table 3) including anticancer drugs (e.g., vinca alkaloids, anthracyclines, epipodophyllotoxin and taxanes), HIV-protease inhibitors, analgesics, antihistamines, immunosuppressive agents, cardiac glycosides, calcium-channel blockers, calmodulin inhibitors, antiemetics, antihelmintics, antibiotics and steroids [83–85]. In addition, P-gp transports many endogenous compounds such as steroid hormones, lipids, peptides and small cytokines [86–88].
MRP1, a 190 kDa polypeptide, is composed of three TMDs, two NBDs and one N-terminal intracellular linker region (L0) (arranged as TMD0-L0-TMD1-NBD1-TMD2-NBD2). The characteristic TMD0 appeared to be critical for subcellular distribution of MRP1, despite it was not required for its basolateral trafficking [89]. Like P-gp, MRP I can confer the resistance to many chemotherapeutic agents such as the folate antagonist methotrexate, and MRP1 substrates comprise a variety of hydrophobic compounds, organic anion conjugates and anionic nonconjugated agents (Table 3). One difference between MRP1 and P-gp specificity for substrates is that taxanes are poor substances for MRP1 [90]. In addition, MRP1 transports many endogenous compounds including free glutathione, glutathione-conjugated leukotrienes, glucuronate and sulfate conjugates, as well as heavy metal oxyanions such as arsenite and trivalent antimonite [91, 92], which are different from those transported by P-gp.
BCRP is a 72 kDa half-ABC transporter, consisting of 655 amino acids and two TMDs. The human BCRP/ABCG2 gene is to 532 bp) and 15 introns and spanning over 66 kb [93, 94], BCRP is mainly expressed in the gastrointestinal tract, liver, kidney, brain, endothelium, mammary tissue, testis and placenta. Overexpression of BCRP in cancer cells can confer MDR besides that BCRP affects dmg absorption, distribution and excretion [81], BCRP actively extrudes a board range of endogenous and exogenous substrates (Table 3) across biological membranes, which include sulfate conjugates, taxanes, carcinogens, glutamated folates and porphyrins [84, 85].
Clinical investigations have shown a potential relationship between these ABC efflux transporters and tumor drug responses or patient’s survival, although some might not be conclusive. A meta-analysis of thirty-one clinical studies indicated that P-gp was expressed in 41% of breast tumors, and patients with P-gp-expressing tumors were three times more likely to fail to respond to chemotherapy [95], The association of P-gp expression with a poor survival rate was more striking when only considering patients whose tumor P-gp expression was measured after chemotherapy. Indeed, P-gp expression was rapidly activated in human tumors in vivo following a transient exposure to doxorubicin [96], supporting the role for P-gp induction in the acquired MDR during cancer chemotherapy.
Schaich et al [97] examined the utility of P-gp and MRP1 expression as prognostic biomarker in 331 adult acute myeloid leukaemia (AML) patients. While P-gp was an independent prognostic factor for the outcome of induction therapy and overall survival, MRP1 was turned out to be an independent predictor for disease-free survival. The latter is different from the observation that MRP1 was not a significant factor in MDR in 352 newly diagnosed AML patients [98]. In another study, MRP1 expression was revealed to be detectable in all of the 209 primary neuroblastoma analyzed, and high levels of MRP 1 were highly predictive of both event-free survival and overall survival of the patients [99]. In contrast, P-gp was of no prognostic significance for neuroblastoma
BCRP expression was observed in all types of tumors, and it was more frequent in endothelial cells of the adenocarcinomas of digestive tract, endometrium and lung [100]. Despite the mRNA and protein levels and function of BCRP were not correlated well, an exploratory study indicated that BCRP protein might be predictive of shorter disease-free survival for adult acute lymphoblastic leukaemia (ALL) patients [101], In another study, BCRP appeared to be a predictor of survival in patients with advanced non-small cell lung cancer (NSCLC) [102]. Further, BCRP was found to be over-expressed in 24 of a total of 73 consecutive AML patients and it was significantly co-expressed with P-gp [103]. Interestingly, P-gp expression predicts the achievement of complete remission, and BCRP-positive cases showed an increased risk of relapse and a shorter disease-free survival. The latter finding suggests that BCRP may be regarded as a prognostic factor in AML patients with normal karyotype.
4. ABC TRANSPORTERS AS TARGETS TO COMBAT MULTIDRUG RESISTANCE
One way to overcome ABC transporter-controlled MDR is to use ABC transporter inhibitors to sensitize tumor cells to chemotherapeutic agents. The rationale for combined use of ABC transporter inhibitors with anticancer drugs towards an improved drug response is clear, and large efforts have been made to develop chemosensitizers (Table 4). While the clinical trials of combined therapies showed some encouraging outcomes, no effective MDR-reversing agent has been developed and approved to date for an appreciable sensitization of malignant tumorsto chemotherapeutic drugs without toxic effects.
Table 4.
ABC transporter inhibitors as chemosensitizers.
| MDR1 | Amlodipine, cyclosporine, quinidine, quinine, verapamil, nifedipine, dexniguldipine, PSC-833, VX-710, GF120918, LY475776, LY335979, XR-9576, V-104, R101933, disulfiram, pluronic L61 |
| MRP1 | Cyclosporine, quinidine, quinine, verapamil, VX-710, LY475776, V-104, disulfiram, MK571, tricyclic isoxazoles |
| BCRP | Cyclosporine, VX-710, GF120918, XR-9576, ftimi-tremorgin C |
Combined use of the first-generation MDR inhibitors (e.g., verapamil, quinine and cyclosporine A) with anticancer drugs (e.g., mitoxantrone, daunorubicin and etoposide) produced toxic side effects and showed only limited or no benefits [4, 104, 105], Actually, the first-generation inhibitors are pharmaceutical agents themselves and they are not specifically developed for the modulation of ABC transporters. Many of the first-generation inhibitors (e.g., verapamil and cyclosporine A) are also substrates for ABC transporters (e.g., P-gp) and thus the use of high doses of chemosensitizers to inhibit the activity of ABC transporter inevitably leads to unwanted toxicities.
The second-generation MDR inhibitors were designed to reduce possible primary toxicities. While R-verapamil and PSC-833 (Valspodar) inhibit the function of P-gp, the MDR modulators exhibit no or minimal activity in blocking calcium channel and suppressing immune system, respectively. Combined use of some MDR inhibitors (e.g., PSC-833) with anticancer drugs seemed to provide some advantage for some AML patients [106]. However, co-administration of the MDR modulators induces pharmacokinetic interactions. In particular, concurrent MDR inhibitors elevated the systemic exposure to anticancer drugs by altering the absorption, distribution, metabolism and excretion (ADME) of anticancer drugs [107] and thus led to an increased toxicity in patients [108–110]. On the other hand, VX-710 did not alter the pharmacokinetics of doxorubicin [111] and it did increase the chemosensitivity of MDR cells overexpressing ABC transporters [112], However, coadministration of VX-710 did not significantly increase the benefits of anticancer drugs, which may be due to the existence of other mechanisms of MDR besides the overexpression of ABC transporters [111, 113]
The third-generation MDR inhibitors such as laniquidar (R101933), ONT-093 (OC14–093), zosuqiodar (LY335979), elacridar (GF120918) and tariquidar (XR9576) have a high affinity to ABC transporters and a low pharmacokinetic interaction due to a limited CYP3A inhibition [114, 115]. Although many in vitro studies have demonstrated an enhancement of chemosensitivity by the third-generation MDR inhibitors, clinical trials revealed that the outcomes of chemotherapeutic agents were not improved by coadministration of the third-generation MDR modulators. For example, the addition of zosuqiodar did not increase the overall survival rate of older patients with newly diagnosed AML [116], and concurrent tariquidar did not appear to prolong the overall survival of patients with metastatic cancers [117], albeit a potent inhibition of P-gp did not produce any toxicity.
There are many possible reasons for the failure of clinical trials of three generations of ABC transporter inhibitors to overcome MDR, besides the inherited side effects (first-generation), unexpected drug-drug interactions (second-generation) and presence of other mechanisms of MDR in tumors. Given the fact that the tumors are highly heterogeneous, a linear distribution of drugs from blood to tumor cells might be absent. Without measuring the actual concentrations of chemosensitizers and anticancer drugs within tumor cells in a whole body system, one is merely unaware of the level of MDR modulators to which tumors are exposed, or the extent of inhibition of MDR transporters within the tumor cells. Indeed, a novel approach that employs biodegradable polymersome to carry both anticancer drug and MDR inhibitor is proven to be an effective and practical means to combat MDR [118]. In addition, the ABC transporters P-gp, MRPs and BCRP are often coexpressed in tumors and they also have an overlapped specificity for a variety of substrates (Table 3). Selective inhibition of one or two ABC efflux transporters could be compensated by the remaining transporters. Nevertheless, the notion that targeting ABC efflux transporters may overcome MDR is still strong, and new chemosensitizers as well as novel approaches such as targeted downregulation of MDR genes using small molecule dmgs or RNA interference [4, 119] are in development.
5. ABC EFFLUX TRANSPORTERS IN PHARMACOKINETICS
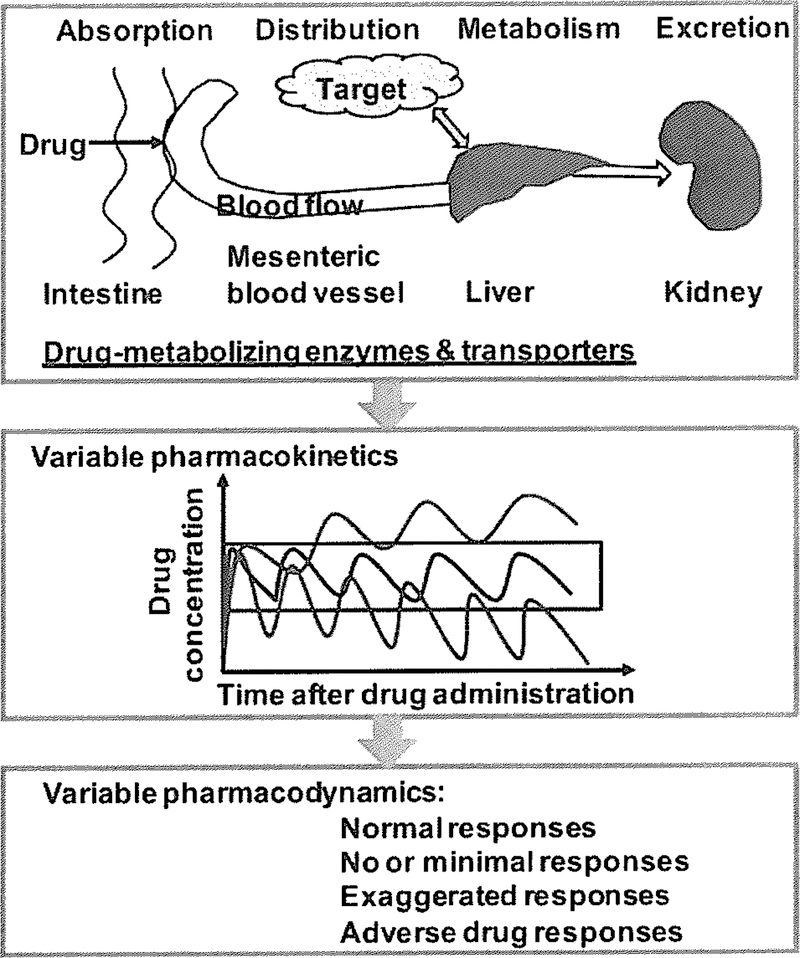
ADME processes may limit or enhance the extent of dmgs to the target tumor cells. ADME is mechanistically mediated by drug-metabolizing enzymes and transporters expressed in different tissues including small intestine, liver, target site (e.g., tumor) and kidney. In particular, xenobiotic-metabolizing enzymes such as cytochrome P450 (CYP or P450) isoforms play a critical role in metabolic elimination of dmgs, and transporters such as ABC and solute carrier (SLC) transporters have high impact on dmg absorption, distribution and excretion [81, 120–122], The interplay of enzymes and transporters and the interactions between drags and enzymes/transporters ultimately determine the pharmacokinetics of dmgs and consequently, affect the pharmacodynamics (Fig. 1).
Fig. (1).
Considerable variations in pharmacokinetics and pharmacodynamics may occur when a drug is processed in different ways in humans, which are determined by the functions and extents of xenobiotic-metabolizing enzymes and transporters expressed in various tissues.
The role of P-gp in pharmacokinetics is one of the most documented. Digoxin and talinolol are two dmgs that are often used for the assessment of P-gp-mediated changes. A comprehensive analysis of 123 clinical studies [123] revealed that the degree of changes in digoxin pharmacokinetics, as manifested by AUC or Cmax ratio, was relatively small (< 3-fold increase) when a P-gp inhibitor was co-administered. Indeed, the most strikingly change in digoxin pharmacokinetics (3.05-fold increase of AUC ratio) was caused by valspodar [107], a second-generation MDR modulator. Furthermore, induction of P-gp (and P450 enzymes) through transcriptional or other mechanisms is expected to decrease the systemic dmg exposure. The co-administration of rifampicin resulted in a 30–54% decrease in AUC of digoxin [124] and a 21–35% decrease in AUC of talinolol [125], which was associated with a 3.5- to 4.2-fold increase in intestinal P-gp protein expression. It is also noteworthy that a small change in dmg concentration or exposure may cause significant alternation of dmg response, when the dmg has a narrow therapeutic window. As an example, acute rejection in organ transplant patients was reported, due to the interaction of St. John’s Wort and cyclosporin that might involve the induction of both P-gp transporter and P450 enzymes [126].
Similar to the effects of ABC transporters over-expressed in tumor cells on intracellular dmg accumulation, ABC transporters expressed on the luminal side of the endothelial cells to form blood-brain barrier may influence the blood-brain dmg distribution and consequently dmg concentration within the brain. It was shown that the AUC of 11C-verapamil between human brain and blood compartment was increased around 90% after intravenous administration of P-gp inhibitor cyclosporine [127]. In contrast, the use of Mdrla/lb gene knockout mouse models revealed a strikingly higher extent of contribution of P-gp to blood-brain dmg distribution in vivo [128]. The difference between clinical and animal data may be attributed not only to the distinct physiology of animal models but also to the technique in measuring dmg concentrations. Nevertheless, it is certainly clear that ABC transporters play an important role in dmg distribution.
There are also increased clinical studies on the role of BCRP in pharmacokinetics. Co-administration of elacridar (GF120918), a potent inhibitor of BCRP and P-gp, significantly elevated the systemic exposure to orally-administered topotecan, as indicated by the increase of oral bioavailability from 40.0% to 97.1% [129]. Further investigations on BCRP pharmacogenetics also support the importance of BCRP in pharmacokinetics. For instance, the BCRP 421C>A polymorphism appeared to influence the pharmacokinetics of rosuvastatin (−45% lower AUC among 42ICC homozygotes) in healthy Chinese males, whereas SLCOIBI and CYP2C9 genetic polymorphism showed no impact [130]. Another study among 660 healthy Finnish volunteers showed consistent findings on the impact of 421C>A polymorphism on pharmacokinetics of BCRP substrates, atorvastatin and rosuvastatin [131]. The systemic exposure to atorvastatin was about 70% higher in subjects carrying the C.421AA allele, and the systemic exposure to rosuvastatin in subjects carrying the C.421AA allele was over 100% higher. Different pharmacokinetics may be translated into a significant change in dmg response. Indeed, the 421C>A polymorphism in ABCG2 gene was significantly associated with diarrhea in non-small-cell lung cancer patients treated with oral gefitinib [132], and irinotecan-induced severe myelosuppression [133].
6. PRECLINICAL EVALUATION OF ABC TRANSPORTER FUNCTION
A number of assays and models are available for the assessment of functions of ABC transporters, prediction of cellular and tissue drug absorption or disposition, and development of MDR modulators in the preclinical setting. Based on the model systems utilized in the assays, they are classified into in vitro (e.g., membrane-based ATPase activity assay and vesicular uptake assay, and cell-based monolayer drug efflux assay and uptake assay), ex vivo (e.g., everted gut sacs), in situ (e.g., in situ organ perfusion), and in vivo assays (e.g., genetically modified animal models) [3, 122, 134].
6.1. Membrane Vesicular Uptake Assay
This assay employs inside-out membrane vesicles to directly assess the uptake of drugs mediated by membrane transporters. The membrane vesicles may be prepared from a variety of tissues [135–138], cells [139–141] and transfected cells [142–145]. Since ATP-dependent ABC proteins do not traverse the lipid membrane, in-side-out membrane vesicles bind to ATP and pump substrates into the vesicles. Therefore, this assay directly determines transporter function and provides good estimation of kinetic parameters such as the Km for substrates and IC50 or Ki for inhibitors.
Membrane vesicles require ATP-binding and -hydrolysis factors including ATP, phosphocreatine, magnesium and creatine phosphokinase to complete uptake process. It often involves the utilization of radioactive [138, 140, 141, 145] or fluorescent compounds [146, 147]. Membrane vesicular transport study is able to distinguish the inhibitors from substrates and it can be developed as high-throughput assay. It has been used to define the localization, function and regulation of ABC transporters, as well as interspecies difference [148, 149], pharmacogenetics [150, 151] and specificity of substrates or inhibitors for a given transporters [152]. One drawback of this assay is that membrane vesicle preparation is time-consuming and requires some skill. In addition, a non-specific binding or passive diffusion can lead to false negative data, highlighting the importance of critical controls for membrane vesicular uptake study.
6.2. ATPase Activity Assay
ABC transporter-mediated substrate translocation against a concentration gradient requires the energy produced by ATP hydrolysis. Based on the transport ATPase cycle [153], the membrane ATPase activity assays have been developed to indirectly evaluate the function of ABC transporters in cellular drug disposition [143, 154–157]. Colorimetric measuring the amount of inorganic phosphate released by ATP hydrolysis, which is proportional to the rate of ATP hydrolysis, allows investigators to assess the interactions of substrates/inhibitors with ABC transporters. Similar to other AT-Pases [158–160], ABC transporters can be trapped by vanadate or beryllium fluoride. Therefore, a test in the presence and absence of vanadate or beryllium fluoride would help define the transporter selective activity.
One major drawback of ATPase activity study is that utilization of this assay is unable to distinguish between inhibitor and substrate of ABC transporter. The reason is that any compounds that can bind to transporters will stimulate the ATPase cycle. There are also some compounds that actually affect ATPase activity, whereas they do not have any direct interactions with ABC transporters; thus this indirect ATPase assay would give false positive results. Moreover, crude membrane and even purified transporter preparations are known to have a high baseline vanadate-sensitive ATPase activity. This basal ATPase activity is likely caused by endogenous lipids or other substrates, and sometimes it may not be further stimulated by substrates of ABC transporters.
6.3. Trans-cellular Drug Transport Assay
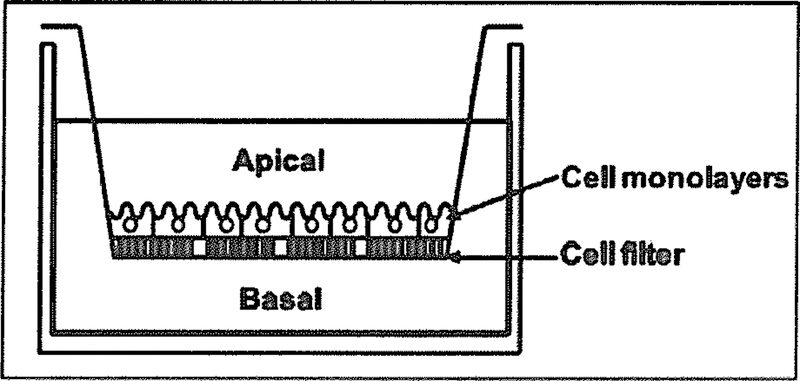
This is a direct assay of the translocation of a test compound across polarized epithelial or endothelial cell monolayer grown on permeable supports (Fig. 2) to mimic the biological barrier in vivo. It is regarded as a standard assay to assess drug transport and permeability. Cell monolayers are expected to form tight junctions to prevent passive paracellular transport. The test compound may be added to the apical (upper chamber) or basolateral (lower chamber), and drug concentrations in the opposite side are monitored over time, the apparent permeability coefficient (Papp in cm/sec) is calculated according to the equation Papp (cm/min) = dQ/(dt × A × C0), where dQ/dt is the flux rate (pmol/mon, slope of the cumulative amount (pmol) of transported drug (Q) in the receiver compartment versus time (min) curve), A (cm2) is the surface area of the filter, and C0 is the initial drug concentration (pmol/mL or µM) applied to the donor chamber. The efflux ratio of basal-to-apical (B-to-A) to apical-to-basal (A-to-B) is further calculated from the formula Papp, B-to-A/Papp for various studies such as the determination of the direction of drug transport, role of specific transporter, substrate specificity and inhibition potency, the screening of transporter modulators and the prediction of drug permeability in vivo. For instance, a high efflux ratio (e.g., > 2) will support the potential role of an efflux transporter in transporting the test drug across cell monolayers.
Fig. (2).
Cell monolayer grown on permeable support for drug transport assay. Apical-to-basal or basal-to-apical drug translocation can be evaluated separately.
The polarized, wild-type and engineered Caco-2 and MDCK cells are commonly used for cell monolayer drug efflux studies [138, 161–164], Primary cultured cells from brain endothelial cells have also been applied to monolayer efflux assay [165]. Given the complexity of intestinal drug absorption such as the interplay between metabolism and efflux, a possible involvement of carrier-mediated transport and a low or an absent expression of drug-metabolizing enzymes in stable cell lines, a high efflux ratio obtained from monolayer efflux study does not always mean a poor oral absorption for the test drug. Since cell culture is required, this assay is time consuming and labor intensive when compared to membrane-based ATPase activity and vesicular transport assays [134].
6.4. Cell-based Drug Uptake Assay/Intracellular Drug Accumulation
This uptake assay measures the amount of drugs accumulated within the cells following the exposure to a test compound for a certain period. Research often takes advantage of the fluorescence or radioactivity of ABC transporter substrates (or their metabolites) for the evaluation of intracellular drug accumulation by using flow cytometry or radioactivity counter. Calcein-AM is widely used in the assays for assessing the interactions of drugs and ABC transporters. Calcein-AM can easily get into the cells through passive diffusion and it is cleaved irreversibly to a hydrophilic, non-permeable and fluorescent calcein free acid by endogenous esterases. Because calcein-AM is a good substrate for ABC efflux transporters (e.g., P-gp and MRP1), intracellular accumulation of the fluorescent calcein is inversely related to ABC efflux transporter-mediated cellular drug disposition; thus calcein AM uptake assays are good for high-throughput screening of ABC efflux transporter inhibitors [157, 166], By contrast, some drugs are fluorescent compounds themselves (e.g., doxorubicin for P-gp and MRP1, and mitoxantrone for BCRP), and drug accumulation can be measured directly for assessing the function or regulation of corresponding transporters [167–169]. While this cell-based uptake assay is easy to use, studies are generally limited to the use of fluorescent or radioactive substrates, screening of inhibitors or evaluation of transporter function/regulation.
6.5. Perfusion of Isolated Tissue
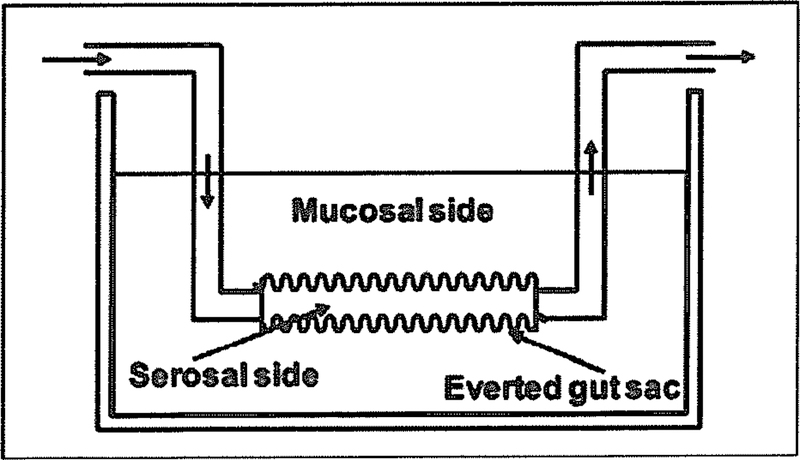
The perfusion of an isolated organ such as intestine, liver and kidney has been employed for the assessment of drug uptake or disposition in a specific organ [170–173], Prediction of oral drug absorption commonly involves the use of isolated human and animal intestinal segments, which are either mounted in a diffusion apparatus (e.g., Ussing chamber) or everted to produce the everted gut sacs (Fig. 3) [174, 175]. In these studies, a test compound is administered to a donor compartment (serosal or mucosal) and drug concentrations in the receiver compartment (mucosal or serosal) are determined over time. As an example, the study with intestinal perfusion isolated from wild-type versus Bcrpl/Abcg2 knockout mice revealed the important role for Bcrpl in the extrusion of glucuronide and sulfate conjugates formed in the enterocytes to intestinal lumen [176]. Compared to the studies with in vitro and whole animal models, use of excised tissues for drug transport studies, in the presence and absence of selective inhibitor, may provide a clear understanding of transporter function in the given organ. Nevertheless, a possible involvement of drug metabolism or other compounding factors can complicate the prediction of intestinal drug absorption.
Fig. (3).
Everted intestinal sac for the assessment of directional drug transport (e.g., serosal-to-mucosal).
6.6. In situ Organ Perfusion
In situ organ perfusion with live animals allows a more accurate determination of the transporter functions in intestinal absorption, biliary elimination, renal excretion and brain penetration under a physiologically relevant environment. These studies include the perfusion of intestine [177, 178], liver [179–181], kidney [173, 182] and brain [183–186]. For example, in situ brain perfusion was employed to investigate the role of P-gp at blood-brain barrier in protection against xenobiotic drugs [187, 188], as well as the impact of P-gp modulators [189, 190]. This assay was also used, along with the Mdrla knockout mouse models, for the evaluation of P-gp-mediated drug transport across blood-brain barrier [191]. As another example, in situ intestinal perfusion studies in wild-type versus Mdrla/Ib knockout mice nicely demonstrated the impact of intestinal P-gp on the absorption of a number of P-gp substrates [178], These in situ organ perfusion techniques are powerful tools to define drug transport and role of ABC transporters in an intact organ system. However, in situ organ perfusion requires special surgical skills and sophisticated equipment. In addition, the anesthetic agents used in these studies may affect the results [192].
6.7. Genetically Modified Animal Models
ABC transporter genetically modified mouse models are unique addition to wild-type iaboratorial animals for evaluation of the role of a specific ABC transporter in pharmacokinetics in a whole body system [193–195], Mice deficient in Mdrla [196] or both Mdrla and Mdrlb [197] were developed, and comparative studies in wild-type versus knockout mice demonstrated the importance of P-gp in brain uptake, pharmacokinetics and toxicity of a number of P-gp substrates such as ivermectin, vinblastine, dexamethasone, digoxin, cyclosporine A, ondansetron, loperamide and rhodamine [196–199], Meanwhile, two Mrpl knockout mouse lines were generated through targeted disruption of the exons encoding the first NBD of mouse Mrpl gene [200] and part of the second putative NBD [201], Many studies were conducted with Mrpl knockout mouse models, demonstrating the role of MRP1 in limiting tissue distribution of MRP1 substrates [202–204], In addition, two Bcrpl knockout mouse models were created to investigate the in vivo function of BCRP/ABCG2 [205, 206]. An early study using Bcrpl knockout mouse models revealed a critical role for Bcrpl in protection of diet-dependent photoxocity [205]. Since then, many studies with the Bcrpl knockout mice have been conducted to demonstrate the effects of Bcrpl expression on absorption, distribution, and elimination of dietary carcinogens (e.g., PhIP), medications (e.g., nitrofurantoin) and other agents [207–215], These genetically modified mice are useful animal models to evaluate the importance of ABC transporters at the systemic level. Nevertheless, an altered expression of other ADME genes [196, 216] reminds that one need be cautious when translating data from transgenic mice into humans [193,217].
7. CONCLUSION
Overexpression of ABC efflux transporters such as P-gp, MRP1 and BCRP in tumor cells is recognized as an important mechanism for MDR that hampers the success of cancer pharmacotherapy, besides genetic mutations of target proteins and changes of cell signaling pathways and epigenetic regulations. Many ABC efflux transporters are also expressed ubiquitously in normal human tissues, and their interactions with drug-metabolizing enzymes and other transporters in the gut, liver and kidney may largely affect the overall pharmacokinetic properties of drugs. Meanwhile, P-gp, MRP1 and BCRP have an overlapped specificity for many anticancer drugs. These factors may complicate the development and use of ABC transporter inhibitors as MDR-reversing agents. Indeed, many clinically-tested chemosensitizers have been proved to be unbeneficial for cancer patients due to inherited toxicities or pharmacokinetic interactions. Nevertheless, the enthusiasm is still high for targeting ABC efflux transporters to overcome MDR, which includes the development of new chemosensitizers and novel approaches. In addition, the lessons learned from MDR modulators are helpful for the development of novel anticancer drugs that would ideally bypass transporter-mediated efflux. Therefore, understanding the impact of transporters on drug flux is critical for drug development. Presently there are a number of preclinical assays and models available for the evaluation of transporter-controlled flux, prediction of pharmacokinetic outcomes and screening of MDR modulators. Understanding the limitations as well as the benefits of individual preclinical assays and models is necessary for proper data analysis and interpretation in drug development.
ACKNOWLEDGEMENTS
A-M. Yu wants to thank the support (award number R01DA021172) from the National Institute On Drug Abuse, National Institutes of Health (NIH).
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- [1].Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature 2007; 446: 749–57. [DOI] [PubMed] [Google Scholar]
- [2].Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 2000; 1 1: 265–83. [DOI] [PubMed] [Google Scholar]
- [3].Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. ABC drug transporters as molecular targets for the prevention of multidrug resistance and drug-drug interactions. Current drug delivery 2007; 4: 324–33. [DOI] [PubMed] [Google Scholar]
- [4].Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006;5:219–34. [DOI] [PubMed] [Google Scholar]
- [5].Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract 2005; 14 Suppl 1: 35–48. [DOI] [PubMed] [Google Scholar]
- [6].Tamaki A, Ierano C, Szakacs G, Robey RW, Bates SE. The controversial role of ABC transporters in clinical oncology. Essays in biochemistry 2011; 50: 209–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rochat B importance of influx and efflux systems and xenobiotic metabolizing enzymes in intratumoral disposition of anticancer agents. Curr Cancer Drug Targets 2009; 9: 652–74. [DOI] [PubMed] [Google Scholar]
- [8].Tegos GP, Haynes M, Strouse JJ, et al. Microbial efflux pump inhibition: tactics and strategies. Current pharmaceutical design 2011; 17: 1291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sa JM, Chong JL, Wellems TE. Malaria drug resistance: new observations and developments. Essays in biochemistry 2011; 51: 137–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Petersen I, Eastman R, Lanzer M. Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS letters 2011; 585: 1551–62. [DOI] [PubMed] [Google Scholar]
- [11].Nikaido H Multidrug resistance in bacteria. Annual review of biochemistry 2009; 78: 119–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].O’Callaghan T Introduction: The prevention agenda. Nature 2011; 471: S2–4. [DOI] [PubMed] [Google Scholar]
- [13].Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24: 2137–50. [DOI] [PubMed] [Google Scholar]
- [14].Zamble DB, Lippard SJ. Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem Sci 1995; 20: 435–9. [DOI] [PubMed] [Google Scholar]
- [15].Espinosa E, Zamora P, Feliu J, Gonzalez Baron M. Classification of anticancer drugs--a new system based on therapeutic targets. Cancer treatment reviews 2003; 29: 515–23. [DOI] [PubMed] [Google Scholar]
- [16].Grem JL. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Investigational new drugs 2000; 18:299–313. [DOI] [PubMed] [Google Scholar]
- [17].Cerqueira NM, Fernandes PA, Ramos MJ. Understanding ribonucleotide reductase inactivation by gemcitabine. Chemistry 2007; 13: 8507–15. [DOI] [PubMed] [Google Scholar]
- [18].Allegra CJ, Grem JL, Yeh GC, Chabner BA. Antimetabolites. Cancer chemotherapy and biological response modifiers 1988; 10: 1–22. [PubMed] [Google Scholar]
- [19].Walling J From methotrexate to pemetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates. Investigational new drugs 2006; 24: 37–77. [DOI] [PubMed] [Google Scholar]
- [20].Snyder RD, Amone MR. Putative identification of functional interactions between DNA intercalating agents and topoisomerase II using the V79 in vitro micronucleus assay. Mutation research 2002;503:21–35. [DOI] [PubMed] [Google Scholar]
- [21].Sinha BK. Topoisomerase inhibitors. A review of their therapeutic potential in cancer. Drugs 1995; 49: 11–9. [DOI] [PubMed] [Google Scholar]
- [22].Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature reviews. Cancer 2004; 4: 253–65. [DOI] [PubMed] [Google Scholar]
- [23].Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nature reviews. Urology 2011; 8: 29–41. [DOI] [PubMed] [Google Scholar]
- [24].Dunn BK, Ford LG. Hormonal interventions to prevent hormonal cancers: breast and prostate cancers. European J cancer prevention : the official JEur Cancer Prevention Organisation 2007; 16: 232–42. [DOI] [PubMed] [Google Scholar]
- [25].Lumachi F, Luisetto G, Basso SM, Basso U, Brunello A, Camozzi V. Endocrine therapy of breast cancer. Current medicinal chemistry 2011; 18: 513–22. [DOI] [PubMed] [Google Scholar]
- [26].Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature medicine 1996; 2: 561–6. [DOI] [PubMed] [Google Scholar]
- [27].Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 2005; 105:2640–53. [DOI] [PubMed] [Google Scholar]
- [28].Noble ME, Endicott JA, Johnson LN. Protein kinase inhibitors: insights into drug design from structure. Science 2004; 303: 1800–5. [DOI] [PubMed] [Google Scholar]
- [29].Chahrour O, Cairns D, Omran Z. Small molecule kinase inhibitors as anti-cancer therapeutics. Mini Rev Med Chem 2012. [DOI] [PubMed]
- [30].Dancey J, Sausville EA. Issues and progress with protein kinase inhibitors for cancer treatment. Nature reviews. Drug Discov 2003; 2:296–313. [DOI] [PubMed] [Google Scholar]
- [31].Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002; 2: 48–58. [DOI] [PubMed] [Google Scholar]
- [32].Fojo T Multiple paths to a drug resistance phenotype: mutations, translocations, deletions and amplification of coding genes or promoter regions, epigenetic changes and microRNAs. Drug Resist Updat 2007; 10: 59–67. [DOI] [PubMed] [Google Scholar]
- [33].Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293: 876–80. [DOI] [PubMed] [Google Scholar]
- [34].Druker BJ, Sawyers CL, Kantaijian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. New Eng J Med 2001; 344: 1038–42. [DOI] [PubMed] [Google Scholar]
- [35].Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 2002; 99: 1928–37. [DOI] [PubMed] [Google Scholar]
- [36].O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. New Eng J Med 2003; 348: 994–1004. [DOI] [PubMed] [Google Scholar]
- [37].Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. New Eng J Med 2005; 352: 786–92. [DOI] [PubMed] [Google Scholar]
- [38].Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine 2005; 2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316: 1039–43. [DOI] [PubMed] [Google Scholar]
- [40].Kitazaki T, Oka M, Nakamura Y, et al. Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells. Lung Cancer 2005; 49:337–43. [DOI] [PubMed] [Google Scholar]
- [41].Morita H, Koyama K, Sugimoto Y, Kobayashi J. Antimitotic activity and reversal of breast cancer resistance protein-mediated drug resistance by stilbenoids from Bletilla striata. Bioorganic Med Chem letters 2005; 15: 1051–4. [DOI] [PubMed] [Google Scholar]
- [42].Shi Z, Peng XX, Kim IW, et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res 2007; 67: 1 1012–20. [DOI] [PubMed] [Google Scholar]
- [43].Tiwari AK, Sodani K, Wang SR, et al. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCBl/Pgp and ABCG2/BCRP/MXR transporters. Biochemical Pharmacol 2009; 78: 153–61. [DOI] [PubMed] [Google Scholar]
- [44].K S, K TA, S S, A P, J XZ, J CJ, L SY, T TT, Chen ZS. GW583340 and GW2974, human EGFR and HER-2 inhibitors, reverse ABCG2-and ABCB1-mediated drug resistance Biochem Pharmacol 2012: (in press). [DOI] [PMC free article] [PubMed]
- [45].Fan D, Poste G, Seid C, et al. Reversal of multidrug resistance in murine fibrosarcoma cells by thioxanthene flupentixol. Invest New Drugs 1994; 12: 185–95. [DOI] [PubMed] [Google Scholar]
- [46].Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res 1987; 47: 3039–51. [PubMed] [Google Scholar]
- [47].Jain RK. Determinants of tumor blood flow: a review. Cancer Res 1988;48:2641–58. [PubMed] [Google Scholar]
- [48].Demant EJ, Sehested M, Jensen PB. A model for computer simulation of P-glycoprotein and transmembrane delta pH-mediated anthracycline transport in multidrug-resistant tumor cells. Biochim Biophys Acta 1990; 1055: 117–25. [DOI] [PubMed] [Google Scholar]
- [49].Kuzmich S, Tew KD. Detoxification mechanisms and tumor cell resistance to anticancer drugs. Med Res Rev 1991; 11: 185–217. [PubMed] [Google Scholar]
- [50].Onishi Y, Azuma Y, Sato Y, Mizuno Y, Tadakuma T, Kizaki H. Topoisomerase inhibitors induce apoptosis in thymocytes. Biochim Biophys Acta 1993; 1175: 147–54. [DOI] [PubMed] [Google Scholar]
- [51].Tew KD, Bomber AM, Hoffman SJ. Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res 1988; 48: 3622–5. [PubMed] [Google Scholar]
- [52].Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer 2002; 2: 188–200. [DOI] [PubMed] [Google Scholar]
- [53].Richardson A, Kaye SB. Drug resistance in ovarian cancer: the emerging importance of gene transcription and spatio-temporal regulation of resistance. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy 2005; 8:311–21. [DOI] [PubMed] [Google Scholar]
- [54].Anelli A, Brentani RR, Gadelha AP, Amorim De Albuquerque A, Soares F. Correlation of p53 status with outcome of neoadjuvant chemotherapy using paclitaxel and doxorubicin in stage 1I1B breast cancer. Annals of oncology : official J the European Society for Medical Oncology / ESMO 2003; 14: 428–32. [DOI] [PubMed] [Google Scholar]
- [55].Burgess MR, Skaggs BJ, Shah NP, Lee FY, Sawyers CL. Comparative analysis of two clinically active BCR-ABL kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc Natl Acad Sci USA 2005; 102: 3395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Biedler JL, Spengler BA. A novel chromosome abnormality in human neuroblastoma and antifolate-resistant Chinese hamster cell lives in culture. J Natl Cancer Inst 1976; 57: 683–95. [DOI] [PubMed] [Google Scholar]
- [57].Glasspool RM, Teodoridis JM, Brown R. Epigenetics as a mechanism driving polygenic clinical drug resistance. Br J Cancer 2006; 94: 1087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gregory RI, Shiekhattar R. MicroRNA biogenesis and cancer. Cancer research 2005; 65: 3509–12. [DOI] [PubMed] [Google Scholar]
- [59].Meng F, Henson R, Lang M, et al. Involvement of human micro RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 2006; 130: 2113–29. [DOI] [PubMed] [Google Scholar]
- [60].van Veen HW, Konings WN. The ABC family of multidrug transporters in microorganisms. Biochim Biophys Acta 1998; 1365: 31–6. [DOI] [PubMed] [Google Scholar]
- [61].Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annual review of biochemistry 2002; 71: 537–92. [DOI] [PubMed] [Google Scholar]
- [62].Roninson IB, Abelson HT, Housman DE, Howell N, Varshavsky A. Amplification of specific DNA sequences correlates with multidrug resistance in Chinese hamster cells. Nature 1984; 309: 626–8. [DOI] [PubMed] [Google Scholar]
- [63].Soengas MS, Capodieci P, Polsky D, et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 2001; 409:207–11. [DOI] [PubMed] [Google Scholar]
- [64].Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nature reviews. Cancer 2005; 5:275–84. [DOI] [PubMed] [Google Scholar]
- [65].Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 2003; 55: 3–29. [DOI] [PubMed] [Google Scholar]
- [66].Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science 2001; 291: 1304–51. [DOI] [PubMed] [Google Scholar]
- [67].Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature 2001; 409: 860–921. [DOI] [PubMed] [Google Scholar]
- [68].Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 2001; 11: 1 156–66. [DOI] [PubMed] [Google Scholar]
- [69].Rosenberg MF, Kamis AB, Callaghan R, Higgins CF, Ford RC. Three-dimensional structures of the mammalian multidrug resistance P-glycoprotein demonstrate major conformational changes in the transmembrane domains upon nucleotide binding. J Biol Chem 2003; 278: 8294–9. [DOI] [PubMed] [Google Scholar]
- [70).Kage K, Tsukahara S, Sugiyama T, et al. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. International J cancer. J lnt Cancer 2002; 97: 626–30. [DOI] [PubMed] [Google Scholar]
- [71].Loo TW, Clarke OM. The packing of the transmembrane segments of human multidrug resistance P-glycoprotein is revealed by disulfide cross-linking analysis. J Biological Chem 2000; 275: 5253–6. [DOI] [PubMed] [Google Scholar]
- [72].Pinkett HW, Lee AT, Lum P, Locher KP, Rees DC. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science 2007; 315: 373–7. [DOI] [PubMed] [Google Scholar]
- [73].Bolhuis H, van Veen HW, Molenaar D, Poolman B, Driessen AJ, Konings WN. Multidrug resistance in Lactococcus lactis: evidence for ATP-dependent drug extrusion from the inner leaflet of the cytoplasmic membrane. EMBO J 1996; 15: 4239–45. [PMC free article] [PubMed] [Google Scholar]
- [74).Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et biophysica acta 1976; 455: 152–62. [DOI] [PubMed] [Google Scholar]
- [75].Chen CJ, Chin JE, Ueda K, et al. Internal duplication and homology with bacterial transport proteins in the mdrl (P-glycoprotein) gene from multidrug-resistant human cells. Cell 1986; 47: 381–9. [DOI] [PubMed] [Google Scholar]
- [76].McGrath T, Center MS. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res 1988; 48: 3959–63. [PubMed] [Google Scholar]
- [77].Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human Jung cancer cell line. Science 1992; 258: 1650–4. [DOI] [PubMed] [Google Scholar]
- [78).Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 1998; 95: 15665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 1998; 58: 5337–9. [PubMed] [Google Scholar]
- [80].Miyake K, Mickley L, Litman T, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res 1999; 59: 8–13. [PubMed] [Google Scholar]
- [81].Giacomini KM, Huang SM, Tweedie DJ, et al. Membrane transporters in drug development. Nat Rev Drug Discov 2010; 9: 215–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Aller SG, Yu J, Ward A, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009; 323: 1718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 1999; 39: 361–98. [DOI] [PubMed] [Google Scholar]
- [84].Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 2008; 9: I 05–27. [DOI] [PubMed] [Google Scholar]
- [85].Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCBI (MDRI ), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol 2006; 25: 231–59. [DOI] [PubMed] [Google Scholar]
- [86].Wolf DC, Horwitz SB. P-glycoprotein transports corticosterone and is photoaffinity-labeled by the steroid. Int J Cancer 1992; 52: 141–6. [DOI] [PubMed] [Google Scholar]
- [87].Liu Y, Huang L, Hoffman T, Gosland M, Vore M. MDR I substrates/modulators protect against beta-estradiol-l 7beta-D-glucuronide cholestasis in rat liver. Cancer Res 1996; 56: 4992–7. [PubMed] [Google Scholar]
- [88].King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nature Neurosci 2001; 4: 268–74. [DOI] [PubMed] [Google Scholar]
- [89].Westlake CJ, Cole SP, Deeley RG. Role of the NH2-terminal membrane spanning domain of multidrug resistance protein l/ABCCI in protein processing and trafficking. Mol Biol cell 2005; 16: 2483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mechanisms Ozben T. and strategies to overcome multiple drug resistance in cancer. FEBS letters 2006; 580: 2903–9. [DOI] [PubMed] [Google Scholar]
- [91).Cole SP, Sparks KE, Fraser K, et al. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res 1994; 54: 5902–10. [PubMed] [Google Scholar]
- [92].Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein l (MRPl/ABCC l ). Pflugers Arch 2007; 453: 621–41. [DOI] [PubMed] [Google Scholar]
- [93].Staud F, Pavek P. Breast cancer resistance protein (BCRP/ABCG2). Int J Biochem Cell Biol 2005; 37: 720–5. [DOI] [PubMed] [Google Scholar]
- [94].Ozvegy C, Litman T, Szakacs G, et al. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun 2001; 285: 1 11–7. [DOI] [PubMed] [Google Scholar]
- [95].Track BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR J /gp 170 expression and its possible functional significance. J the National Cancer Institute 1997; 89: 917–31. [DOI] [PubMed] [Google Scholar]
- [96].Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW. Rapid activation of MDR I gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clinical cancer research : an official J Am Assoc Cancer Res 1999; 5: 3352–6. [PubMed] [Google Scholar]
- [97].Schaich M, Soucek S, Thiede C, Ehninger G, Illmer T. MDR I and MRPl gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. Br J Haematol 2005; 128: 324–32. [DOI] [PubMed] [Google Scholar]
- [98].Leith CP, Kopecky KJ, Chen JM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR l /P-glycoprotein, MRPl, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood 1999; 94: 1086–99. [PubMed] [Google Scholar]
- [99].Haber M, Smith J, Bordow SB, et al. Association of high-level MRP l expression with poor clinical outcome in a large prospective study of primary neuroblastoma. J Clin Oncol : official J Am Soc Clin Oncol 2006; 24: 1546–53. [DOI] [PubMed] [Google Scholar]
- [100].Diestra JE, Scheffer GL, Catala l, et al. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol 2002; 198: 213–9. [DOI] [PubMed] [Google Scholar]
- [101].Suvannasankha A, Minderman H, O’Loughlin KL, et al. Breast cancer resistance protein (BCRP/MXR/ABCG2) in adult acute lymphoblastic leukaemia: frequent expression and possible correlation with shorter disease-free survival. Br J Haematol 2004; 127: 392–8. [DOI] [PubMed] [Google Scholar]
- [102].Yoh K, Ishii G, Yokose T, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clinical cancer research : an officia!J Am Association for Cancer Res 2004; 10: 1691–7. [DOI] [PubMed] [Google Scholar]
- [103].Damiani D, Tiribelli M, Calistri E, et al. The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica 2006; 91: 825–8. [PubMed] [Google Scholar]
- [104].Daenen S, van der Holt B, Verhoef GE, et al. Addition of cyclosporin A to the combination of mitoxantrone and etoposide to overcome resistance to chemotherapy in refractory or relapsing acute myeloid leukaemia: a randomised phase 11 trial from HOVON, the Dutch-Belgian Haemato-Oncology Working Group for adults. Leuk Res 2004; 28: 1057–67. [DOI] [PubMed] [Google Scholar]
- [105].List AF, Kopecky KJ, Willman CL, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood 2001; 98: 3212–20. [DOI] [PubMed] [Google Scholar]
- [106].Kolitz JE, George SL, Dodge RK, et al. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol 2004; 22: 4290–301. [DOI] [PubMed] [Google Scholar]
- [107].Kovarik JM, Rigaudy L, Guerret M, Gerbeau C, Rost KL. Longitudinal assessment of a P-glycoprotein-mediated drug interaction of valspodar on digoxin. Clin Pharmacol Therapeut 1999; 66: 391–400. [DOI] [PubMed] [Google Scholar]
- [108].Baer MR, George SL, Dodge RK, et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B Study 9720. Blood 2002; l 00: 1224–32. [PubMed] [Google Scholar]
- [109].Lhomme C, Joly F, Walker JL, et al. Phase Ill study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage Ill epithelial ovarian cancer or primary peritoneal cancer. J Clin Oneal : official J Am Soc Clin Oneal 2008; 26: 2674–82. [DOI] [PubMed] [Google Scholar]
- [110].Kolitz JE, George SL, Marcucci G, et al. P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients younger than age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood 2010; 1 16: 1413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bramwell VH, Morris D, Ernst DS, et al. Safety and efficacy of the multidrug-resistance inhibitor biricodar (VX-710) with concurrent doxorubicin in patients with anthracycline-resistant advanced soft tissue sarcoma. Clin Cancer Res : an official J Am Assoc Cancer Res 2002; 8: 383–93. [PubMed] [Google Scholar]
- [112].Minderman H, O’Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res 2004; 10: 1826–34. [DOI] [PubMed] [Google Scholar]
- [113].Rago RP, Einstein A Jr., Lush R, et al. Safety and efficacy of the MDR inhibitor Ince] (biricodar, VX-710) in combination with mitoxantrone and prednisone in hormone-refractory prostate cancer. Cancer Chemotherap Pharmacol 2003; 51: 297–305. [DOI] [PubMed] [Google Scholar]
- [114].Guns ES, Denyssevych T, Dixon R, Bally MB, Mayer L. Drug interaction studies between paclitaxel (Taxol) and OC 144–093--a new modulator of MOR in cancer chemotherapy. Eur J Drug Metab Pharmacokinet 2002; 27: 1 19–26. [DOI] [PubMed] [Google Scholar]
- [115].Stewart A, Steiner J, Mellows G, Laguda B, Norris D, Bevan P. Phase I trial of XR9576 in healthy volunteers demonstrates modulation of P-glycoprotein in CD56+ lymphocytes after oral and intravenous administration. Clin Cancer Res 2000; 6: 4186–91. [PubMed] [Google Scholar]
- [116].Cripe LD, Uno H, Paietta EM, et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 2010; 1 16: 4077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kelly RJ, Draper D, Chen CC, et al. A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer. Clinical cancer research : an official J Am Assoc Cancer Res 2011; 17: 569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Pang Z, Feng L, Hua R, et al. Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Molecular pharmaceutics 2010; 7: 1995–2005. [DOI] [PubMed] [Google Scholar]
- [119].] Yu AM. Small interfering RNA in drug metabolism and transport. Current drug metabolism 2007; 8: 700–8. [DOI] [PubMed] [Google Scholar]
- [120].Yu AM. Role of microRNAs in the regulation of drug metabolism and disposition. Expert opinion on drug metabolism & toxicology 2009; 5: 1513–28. [DOI] [PubMed] [Google Scholar]
- [121].Yu AM, Pan YZ. Noncoding microRNAs: Small RNAs play a big role in regulation of ADME? Acta Pharmaceutica Sinica B 2012; 2: 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lu C, Liao M, Cohen L, Xia CQ. Emerging in vitro tools to evaluate cytochrome P450 and transporter-mediated drug-drug interactions. Current drug discovery technologies 2010; 7: 199–222. [DOI] [PubMed] [Google Scholar]
- [123].Fenner KS, Troutman MD, Kempshall S, et al. Drug-drug interactions mediated through P-glycoprotein: clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug. Clinical pharmacology and therapeutics 2009; 85: 173–81. [DOI] [PubMed] [Google Scholar]
- [124].Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest 1999; 104: 147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Westphal K, Weinbrenner A, Zschiesche M, et al. Induction of P-glycoprotein by rifampin increases intestinal secretion of talinolol in human beings: a new type of drug/drug interaction. Clinical pharmacology and therapeutics 2000; 68: 345–55. [DOI] [PubMed] [Google Scholar]
- [126].Ruschitzka F, Meier PJ, Turina M, Luscher TF, Noll G. Acute heart transplant rejection due to Saint John’s wort. Lancet 2000; 355: 548–9. [DOI] [PubMed] [Google Scholar]
- [127].Sasongko L, Link JM, Muzi M, et al. Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography. Clin Pharmacol Therapeut 2005; 77: 503–14. [DOI] [PubMed] [Google Scholar]
- [128].Schinkel AH, Mol CA, Wagenaar E, van Deemter L, Smit JJ, Borst P. Multidrug resistance and the role of P-glycoprotein knockout mice. Eur J Cancer 1995; 31 A: 1295–8. [DOI] [PubMed] [Google Scholar]
- [129].Kruijtzer CM, Beijnen JH, Rosing H, et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GFl 20918. J Clin Oncol : official J Am Soc Clin Oncol 2002; 20: 2943–50. [DOI] [PubMed] [Google Scholar]
- [130].Zhang W, Yu BN, He YJ, et al. Role of BCRP 42I C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clinica chimica acta; lnt J Clin Chem 2006; 373: 99–103. [DOI] [PubMed] [Google Scholar]
- [131].Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Therapeut 2009; 86: 197–203. [DOI] [PubMed] [Google Scholar]
- [132].Cusatis G, Gregorc V, Li J, et al. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst 2006; 98: 1739–42. [DOI] [PubMed] [Google Scholar]
- [133].Cha PC, Mushiroda T, Zembutsu H, et al. Single nucleotide polymorphism in ABCG2 is associated with irinotecan-induced severe myelosuppression. J Human Genet 2009; 54: 572–80. [DOI] [PubMed] [Google Scholar]
- [134].Xia CQ, Milton MN, Gan LS. Evaluation of drug-transporter interactions using in vitro and in viva models. Curr Drug Metab 2007; 8: 341–63. [DOI] [PubMed] [Google Scholar]
- [135].Kamimoto Y, Gatmaitan Z, Hsu J, Arias IM. The function of Gp 170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biological Chem 1989; 264: 11693–8. [PubMed] [Google Scholar]
- [136].Sinicrope FA, Dudeja PK, Bissonnette BM, Safa AR, Brasitus TA. Modulation of P-glycoprotein-mediated drug transport by alterations in lipid fluidity of rat liver canalicular membrane vesicles. J Biological Chem 1992; 267: 24995–5002. [PubMed] [Google Scholar]
- [137].Hsing S, Gatmaitan Z, Arias IM. The function of Gp! 70, the multidrug-resistance gene product, in the brush border of rat intestinal mucosa. Gastroenterology 1992; 102: 879–85. [DOI] [PubMed] [Google Scholar]
- [138].Makhey VD, Guo A, Norris DA, Hu P, Yan J, Sinko PJ. Characterization of the regional intestinal kinetics of drug efflux in rat and human intestine and in Caco-2 cells. Pharm Res 1998; 15: 1160–7. [DOI] [PubMed] [Google Scholar]
- [139].Lew VL, Hockaday A, Freeman CJ, Bookchin RM. Mechanism of spontaneous inside-out vesiculation of red cell membranes. J Cell Biol 1988; 106: 1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Horio M, Gottesman MM, Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci USA 1988; 85: 3580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Karlsson JE, Heddle C, Rozkov A, et al. High-activity p-glycoprotein, multidrug resistance protein 2, and breast cancer resistance protein membrane vesicles prepared from transiently transfected human embryonic kidney 293-epstein-barr virus nuclear antigen cells. Drug metabolism and disposition: the Biological Fate Chemicals 2010; 38: 705–14. [DOI] [PubMed] [Google Scholar]
- [142].Reid G, Wielinga P, Zelcer N, et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol 2003; 63: 1094–103. [DOI] [PubMed] [Google Scholar]
- [143].Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem 1992; 267: 4854–8. [PubMed] [Google Scholar]
- [144].Wu CP, Woodcock H, Hladky SB, Barrand MA. cGMP (guanosine 3’,5’-cyclic monophosphate) transport across human erythrocyte membranes. Biochemical Pharmacol 2005; 69: 1257–62. [DOI] [PubMed] [Google Scholar]
- [145].Miliotis T, Ali L, Palm JE, et al. Development of a highly sensitive method using liquid chromatography-multiple reaction monitoring to quantify membrane P-glycoprotein in biological matrices and relationship to transport function. Drug metabolism and disposition: the Biological Fate Chemicals 2011; 39: 2440–9. [DOI] [PubMed] [Google Scholar]
- [146).Shapiro AB, Fox K, Lam P, Ling V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur J Biochem / FEBS 1999; 259: 841–50. [DOI] [PubMed] [Google Scholar]
- [147].Shapiro AB, Corder AB, Ling V. P-glycoprotein-mediated Hoechst 33342 transport out of the lipid bilayer. Eur J Biochem / FEBS 1997; 250: 115–21. [DOI] [PubMed] [Google Scholar]
- [148].N inomiya M, Ito K, Horie T. Functional analysis of dog multidrug resistance-associated protein 2 (Mrp2) in comparison with rat Mrp2. Drug metabolism and disposition: Biological Fate Chemicals 2005; 33: 225–32. [DOI] [PubMed] [Google Scholar]
- [149].lshizuka H, Konno K, Shiina T, et al. Species differences in the transport activity for organic anions across the bile canalicular membrane. J Pharmacol Exp Therapeut 1999; 290: 1324–30. [PubMed] [Google Scholar]
- [150].Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol 2005; 68: 800–7. [DOI] [PubMed] [Google Scholar]
- [151].Center MS, Zhu Q, Sun H. Mutagenesis of the putative nucleotide-binding domains of the multidrug resistance associated protein (MRP). Analysis of the effect of these mutations on MRP mediated drug resistance and transport. Cytotechnology 1998; 27: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res 2003; 63: 5538–43. [PubMed] [Google Scholar]
- [153].Rosenberg MF, Velarde G, Ford RC, et al. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J 2001; 20: 5615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Mao Q, Leslie EM, Deeley RG, Cole SP. ATPase activity of purified and reconstituted multidrug resistance protein MRPl from drug-selected H69AR cells. Biochimica et Biophysica acta 1999; 1461: 69–82. [DOI] [PubMed] [Google Scholar]
- [155].Glavinas H, Kis E, Pal A, et al. ABCG2 (breast cancer resistance protein/mitoxantrone resistance-associated protein) ATPase assay: a useful tool to detect drug-transporter interactions. Drug metabolism and disposition: Biological Fate Chemicals 2007; 35: 1533–42. [DOI] [PubMed] [Google Scholar]
- [156].Hassan HE, Myers AL, Lee IJ, Chen H, Coop A, Eddington ND. Regulation of gene expression in brain tissues of rats repeatedly treated by the highly abused opioid agonist, oxycodone: microarray profiling and gene mapping analysis. Drug metabolism and disposition: Biological Fate Chemicals 2010; 38: 157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Abraham I, Jain S, Wu CP, et al. Marine sponge-derived sipholane triterpenoids reverse P-glycoprotein (ABCB I )-mediated multidrug resistance in cancer cells. Biochem Pharmacol 2010; 80: 1497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].al-Shawi MK, Senior AE. Characterization of the adenosine triphosphatase activity of Chinese hamster P-glycoprotein. J Biological Chem 1993; 268: 4197–206. [PubMed] [Google Scholar]
- [159].Hamada H, Tsuruo T. Purification of the I 70-to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170-to 180-kilodalton membrane glycoprotein is an ATPase. J Biological Chem 1988; 263: 1454–8. [PubMed] [Google Scholar]
- [160].Urbatsch IL, Sankaran B, Weber J, Senior AE. P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J Biological Chem 1995; 270: 19383–90. [DOI] [PubMed] [Google Scholar]
- [161].Taipalensuu J, Tornblom H, Lindberg G, et al. Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Therapeut 2001; 299: 164–70. [PubMed] [Google Scholar]
- [162].Xia CQ, Liu N, Yang D, Miwa G, Gan LS. Expression, localization, and functional characteristics of breast cancer resistance protein in Caco-2 cells. Drug metabolism and disposition: Biological Fate Chemicals 2005; 33: 637–43. [DOI] [PubMed] [Google Scholar]
- [163].Calcagno AM, Ludwig JA, Fostel JM, Gottesman MM, Ambudkar SY. Comparison of drug transporter levels in normal colon, colon cancer, and Caco-2 cells: impact on drug disposition and discovery. Mol Pharm 2006; 3: 87–93. [DOI] [PubMed] [Google Scholar]
- [164].Xie J, Lei C, Hu Y, Gay GK, Bin Jamali NH, Wang CH. Nanoparticulate formulations for paclitaxel delivery across MOCK cell monolayer. Curr Pharm Des 2010; 16: 2331–40. [DOI] [PubMed] [Google Scholar]
- [165].Gaillard PJ, van der Sandt IC, Voorwinden LH, et al. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood-brain barrier. Pharm Res 2000; 17: 1198–205. [DOI] [PubMed] [Google Scholar]
- [166].Wang EJ, Casciano CN, Clement RP, Johnson WW. In vitro flow cytometry method to quantitatively assess inhibitors of P-glycoprotein. Drug metabolism and disposition: Biological Fate Chemicals 2000; 28: 522–8. [PubMed] [Google Scholar]
- [167].Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328, −5 I 9c and −520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol 2011; 81: 783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol 2009; 75: 1374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Shukla S, Schwartz C, Kapoor K, Kouanda A, Ambudkar SV. Use of baculovirus BacMam vectors for expression of ABC drug transporters in mammalian cells. Drug metabolism and disposition: Biological Fate Chemicals 2012; 40: 304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Artursson P, Ungell AL, Lofroth JE. Selective paracellular permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments. Phann Res 1993; 10: 1 123–9. [DOI] [PubMed] [Google Scholar]
- [171].Wei Y, Neves LA, Franklin T, Klyuchnikova N, Placzek B, Hughes HM, Curtis CG. Vascular perfused segments of human intestine as a tool for drug absorption. Drug metabolism and disposition: Biological Fate Chemicals 2009; 37: 731–6. [DOI] [PubMed] [Google Scholar]
- [172].Booth CL, Brouwer KR, Brouwer KL. Effect of multidrug resistance modulators on the hepatobiliary disposition of doxorubicin in the isolated perfused rat liver. Cancer Res 1998; 58: 3641–8. [PubMed] [Google Scholar]
- [173].Hori R, Okamura N, Aiba T, Tanigawara Y. Role of P-glycoprotein in renal tubular secretion of digoxin in the isolated perfused rat kidney. J Pharmacol Exp Therapeut 1993; 266: 1620–5. [PubMed] [Google Scholar]
- [174].Emi Y, Tsunashima D, Ogawara K, Higaki K, Kimura T. Role of P-glycoprotein as a secretory mechanism in quinidine absorption from rat small intestine. J Phann Sci 1998; 87: 295–9. [DOI] [PubMed] [Google Scholar]
- [175].Su SF, Huang JD. Inhibition of the intestinal digoxin absorption and exsorption by quinidine. Drug metabolism and disposition: Biological Fate Chemicals 1996; 24: 142–7. [PubMed] [Google Scholar]
- [176].Adachi Y, Suzuki H, Schinkel AH, Sugiyama Y. Role of breast cancer resistance protein (Bcrpl/Abcg2) in the extrusion of glucuronide and sulfate conjugates from enterocytes to intestinal lumen. Mo! Pharmacol 2005; 67: 923–8. [DOI] [PubMed] [Google Scholar]
- [177].Saitoh H, Hatakeyama M, Eguchi O, Oda M, Takada M. Involvement of intestinal P-glycoprotein in the restricted absorption of methylprednisolone from rat small intestine. J Pharm Sci 1998; 87: 73–5. [DOI] [PubMed] [Google Scholar]
- [178].Adachi Y, Suzuki H, Sugiyama Y. Quantitative evaluation of the function of small intestinal P-glycoprotein: comparative studies between in situ and in vitro. Phann Res 2003; 20: 1163–9. [DOI] [PubMed] [Google Scholar]
- [179].Watanabe T, Miyauchi S, Sawada Y, et al. Kinetic analysis of hepatobiliary transport of vincristine in perfused rat liver. Possible roles of P-glycoprotein in biliary excretion of vincristine. J Hepatol 1992; 16: 77–88. [DOI] [PubMed] [Google Scholar]
- [180].Liu L, Pang KS. The roles of transporters and enzymes in hepatic drug processing. Drug Metab Dispos 2005; 33: 1–9. [DOI] [PubMed] [Google Scholar]
- [181].Lau YY, Okochi H, Huang Y, Benet LZ. Multiple transporters affect the disposition of atorvastatin and its two active hydroxy metabolites: application of in vitro and ex situ systems. J Pharmacol Exp Therapeut 2006; 316: 762–71. [DOI] [PubMed] [Google Scholar]
- [182].Okamura N, Hirai M, Tanigawara Y, Tanaka K, Yasuhara M, Ueda K, Komano T, Hori R. Digoxin-cyclosporin A interaction: modulation of the multidrug transporter P-glycoprotein in the kidney. J Pharmacol Exp Therapeut 1993; 266: 1614–9. [PubMed] [Google Scholar]
- [183].Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. A J Physiol 1984; 247: H484–93. [DOI] [PubMed] [Google Scholar]
- [184].Smith QR, Takasato Y. Kinetics of amino acid transport at the blood-brain barrier studied using an in situ brain perfusion technique. Ann New York Acad Sci 1986; 481: 186–20I. [DOI] [PubMed] [Google Scholar]
- [185].Cistemino S, Rousselle C, Dagenais C, Scherrmann JM. Screening of multidrug-resistance sensitive drugs by in situ brain perfusion in P-glycoprotein-deficient mice. Phann Res 2001; 18: 183–90. [DOI] [PubMed] [Google Scholar]
- [186].Zhao R, Pollack GM. Regional differences in capillary density, perfusion rate, and P-glycoprotein activity: a quantitative analysis of regional drug exposure in the brain. Biochemical Pharmacol 2009; 78: 1052–9. [DOI] [PubMed] [Google Scholar]
- [187].Drion N, Lemaire M, Lefauconnier JM, Scherrmann JM. Role of P-glycoprotein in the blood-brain transport of colchicine and vinblastine. J Neurochem 1996; 67: 1688–93. [DOI] [PubMed] [Google Scholar]
- [188].Drion N, Risede P, Cholet N, Chanez C, Scherrmann JM. Role of P-170 glycoprotein in colchicine brain uptake. J Neurosci Res 1997; 49: 80–8. [PubMed] [Google Scholar]
- [189].Chikhale EG, Burton PS, Borchardt RT. The effect of verapamil on the transport of peptides across the blood-brain barrier in rats: kinetic evidence for an apically polarized efflux mechanism. The J Pharmacol Exp Therapeut 1995; 273: 298–303. [PubMed] [Google Scholar]
- [190].Lemaire M, Bruelisauer A, Guntz P, Sato H. Dose-dependent brain penetration of SDZ PSC 833, a novel multidrug resistance-reversing cyclosporin, in rats. Cancer Chemotherap Pharmacol 1996; 38: 481–6. [DOI] [PubMed] [Google Scholar]
- [191].Dagenais C, Rousselle C, Pollack GM, Scherrmann JM. Development of an in situ mouse brain perfusion model and its application to mdrla P-glycoprotein-deficient mice. J cerebral blood flow and metabolism : official J Int Soc Cerebral Blood Flow Metabol 2000; 20: 381–6. [DOI] [PubMed] [Google Scholar]
- [192].Regev R, Assaraf YG, Eytan GD. Membrane fluidization by ether, other anesthetics, and certain agents abolishes P-glycoprotein ATPase activity and modulates efflux from multidrug-resistant cells. Eur J Biochem / FEBS 1999; 259: 18–24. [DOI] [PubMed] [Google Scholar]
- [193].Jiang XL, Gonzalez FJ, Yu AM. Drug-metabolizing enzyme, transporter, and nuclear receptor genetically modified mouse models. Drug Metabol Rev 2011; 43: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Schellens JH, Malingre MM, Kruijtzer CM, et al. Modulation of oral bioavailability of anticancer drugs: from mouse to man. Eur J Phann Sci : official J Eur Fed Phann Sci 2000; 12: 103–10. [DOI] [PubMed] [Google Scholar]
- [195].van Waterschoot RA, Schinkel AH. A critical analysis of the interplay between cytochrome P450 3A and P-glycoprotein: recent insights from knockout and transgenic mice. Pharmacol Rev 2011; 63: 390–410. [DOI] [PubMed] [Google Scholar]
- [196].Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdrla P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 1994; 77: 491–502. [DOI] [PubMed] [Google Scholar]
- [197].Schinkel AH, Mayer U, Wagenaar E, et al. Normal viability and altered pharmacokinetics in mice lacking mdrl-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci USA 1997; 94: 4028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin investigation 1996; 97: 251 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdrl a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin investigation 1995; 96: 1698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Wijnholds J, Evers R, van Leusden MR, et al. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nature Med 1997; 3: 1275–9. [DOI] [PubMed] [Google Scholar]
- [201].Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res 1997; 57: 5238–42. [PubMed] [Google Scholar]
- [202].Wijnholds J, deLange EC, Scheffer GL, et al. Multidrug resistance protein I protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J Clin Investigation 2000; 105: 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Sugiyama D, Kusuhara H, Lee YJ, Sugiyama Y. Involvement of multidrug resistance associated protein I (Mrp I ) in the efflux transport of I 7beta estradiol-D-1 7beta-glucuronide (E2 l 7betaG) across the blood-brain barrier. Pharrn Res 2003; 20: 1394–400. [DOI] [PubMed] [Google Scholar]
- [204].Wang F, Zhou F, Kruh GD, Gallo JM. Influence of blood-brain barrier efflux pumps on the distribution of vincristine in brain and brain tumors. Neuro-Oncol 2010; 12: 1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Jonker JW, Buitelaar M, Wagenaar E, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA 2002; 99: 15649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp I gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci USA 2002; 99: 12339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].van Herwaarden AE, Jonker JW, Wagenaar E, et al. The breast cancer resistance protein (Bcrp I IAbcg2) restricts exposure to the dietary carcinogen 2-amino-l-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res 2003; 63: 6447–52. [PubMed] [Google Scholar]
- [208].Breedveld P, Pluim D, Cipriani G, et al. The effect of Bcrpl (Abcg2) on the in viva pharrnacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 2005; 65: 2577–82. [DOI] [PubMed] [Google Scholar]
- [209].Jonker JW, Merino G, Musters S, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nature Med 2005; 11: 127–9. [DOI] [PubMed] [Google Scholar]
- [210].Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol 2005; 67: 1 758–64. [DOI] [PubMed] [Google Scholar]
- [211].Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug metabolism and disposition: Biological Fate Chemicals 2006; 34: 690–5. [DOI] [PubMed] [Google Scholar]
- [212].Salphati L, Lee LB, Pang J, Plise EG, Zhang X. Role of P-glycoprotein and breast cancer resistance protein-I in the brain penetration and brain pharmacodynamic activity of the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug metabolism and disposition: Biological Fate Chemicals 2010; 38: 1422–6. [DOI] [PubMed] [Google Scholar]
- [213].Polli JW, Olson KL, Chism JP, et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{ 3-chloro-4-[(3-fluorobenzyl)oxy ]phenyl}−6-[5-( {[2-(methylsulfonyl)ethyl]amino }methyl)-2-furyl]-4-quinazolinamine; GW57201 6). Drug metabolism and disposition: Biological Fate Chemicals 2009; 37: 439–42. [DOI] [PubMed] [Google Scholar]
- [214].Zhou L, Naraharisetti SB, Wang H, Unadkat JO, Hebert MF, Mao P. The breast cancer resistance protein (Bcrpl /Abcg2) limits feta! distribution of glyburide in the pregnant mouse: an Obstetric-Feta! Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol 2008; 73: 949–59. [DOI] [PubMed] [Google Scholar]
- [215].Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Influence of breast cancer resistance protein (Abcg2) and p-glycoprotein (Abcbla) on the transport of imatinib mesylate (Gleevec) across the mouse blood-brain barrier. J Neurochem 2007; 102: 1749–57. [DOI] [PubMed] [Google Scholar]
- [216].Schuetz EG, Umbenhauer DR, Yasuda K, et al. Altered expression of hepatic cytochromes P-450 in mice deficient in one or more mdrl genes. Mol Pharmacol 2000; 57: 188–97. [PubMed] [Google Scholar]
- [217].Shen HW, Jiang XL, Gonzalez FJ, Yu AM. Humanized transgenic mouse models for drug metabolism and pharmacokinetic research. Curr Drug Metabol 2011; 12: 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]