Abstract
Objective:
Benefit finding (BF) has exhibited a salutary effect on psychological adjustment to cancer. However, few studies have examined its relationship with physiology or have examined BF in men with cancer. This study investigated whether BF is associated with hypothalamic-pituitary- adrenal axis activity (ie, diurnal salivary cortisol) in men treated for prostate cancer. Positive affect (PA) is proposed as a potential pathway linking BF to diurnal salivary cortisol.
Methods:
A sample of 66 men treated for localized prostate cancer within the prior 2 years completed questionnaires and collected salivary cortisol 3 times per day over 3 consecutive days. Hierarchical linear modeling was used for estimating the effects of BF and PA on cortisol responses as measured by diurnal slope and area under the curve (AUCg). Confidence intervals for indirect effects were estimated using the Monte Carlo method for mediation testing.
Results:
BF was significantly associated with diurnal cortisol slope, controlling for body mass index and age (B = −.12, P = .03), such that greater BF was associated with steeper cortisol slope. Analyses revealed that PA mediated the effect of BF on cortisol slope (Monte Carlo estimation 95% CI = −0.087, −0.001); negative affect did not mediate this relationship. BF was not significantly associated with AUCg.
Conclusions:
Deriving more benefit from one’s experience with prostate cancer is associated with a healthier diurnal cortisol rhythm. Through its potential to enhance PA, the relationship of BF and physiological processes underscores the health relevant value of BF in prostate cancer survivors.
Keywords: benefit finding, diurnal cortisol, HPA axis, oncology, positive affect, prostate cancer
1. INTRODUCTION
Cancer diagnosis and treatment can cause emotional distress and even lead one to confront mortality and the inevitability of death.1 Moreover, prostate cancer is not a single acute event, but rather is an unfolding process across the disease trajectory. After treatment, survivors typically face a host of difficult circumstances including physical side effects (eg, sexual dysfunction, incontinence, sleep disruption), social role changes, and existential concerns that can persist indefinitely.1 Behavioral and emotional responses to such situations may be inextricably linked to physical health. In tandem with the difficulties of cancer, many survivors also report benefit from their experiences (eg, enhanced self-perceptions, clarification of personal goals, improved family and social relations, and changed life priorities). In fact, there is growing recognition that stressful experiences associated with cancer might catalyze positive changes, such as benefit finding (BF),2 meaning-focused coping,3 or posttraumatic growth.4 Although different terms are used across the research literature to describe such change, we use benefit finding to indicate perceptions of positive life changes following cancer.
BF exerts a positive impact on psychological adjustment to cancer including better psychological well-being5,6 and improved subjective physical health.7,8 Finding benefit has potential to bolster positive intrapersonal and interpersonal coping resources, such as positive appraisals, emotion regulation skills, likelihood of goal attainment, and stronger interpersonal relationships.3,5,9,10 Such resources likely modulate physiological stress responses, including hypothalamic-pituitary-adrenal (HPA) axis regulation.10,11
Research on BF and HPA activity is limited, but there is some evidence to indicate that BF is related to better cortisol functioning in non-cancer populations. For instance, BF is related to decreased 24-hour urinary-free cortisol output in HIV-positive persons12 and steeper diurnal cortisol slope in maternal caregivers.13 The pattern of cortisol secretion across the day can provide insight into HPA axis dysregulation. In particular, flatter slopes (or low diurnal variation) have been associated with various disorders and poorer health.14 In a study examining cortisol reactivity to a laboratory induced stressor, healthy women who reported higher BF from a past stressful event exhibited faster cortisol resolution from consecutive laboratory stress tasks.11 BF has also been examined in the context of cancer, though primarily breast cancer. Studies in women with breast cancer have shown that greater BF is correlated with lower serum cortisol,15 steeper diurnal cortisol slope,16 and increased lymphocyte proliferation.17 In fact, observations from cancer patients have exclusively been from women with breast cancer. Further, only one of these16 examined diurnal cortisol patterns.
The psychological pathway by which BF affects physical health remains largely theoretical, although empirical evidence is building. Bower10 proposed that BF exerts its influence on physical health either directly, or indirectly, through positive affect (PA). Similarly, Folkman’s model of meaning-focused coping3 proposed that BF represents 1 type of meaning-based coping that contributes to the generation of PA. Consistent with these theoretical perspectives, perceiving benefits from cancer increases overall PA.6–8 Moreover, PA exerts beneficial effects on neuroendocrine functioning18 suggesting a plausible pathway by which BF relates to HPA activity.
In addition to increasing PA, there’s potential that BF reduces negative affect (NA); however, meta-analytic reviews evidence that BF is less consistently associated with NA and is found to have lower effect sizes than PA.7,8 Given that PA and NA represent 2 distinct (though related) dimensions of affect each with adaptive significance in dealing with cancer stress19 and that NA has been demonstrated to dysregulate cortisol among cancer patients,20,21 we tested NA as an alternative pathway through which BF may be related to diurnal cortisol.
The goal of this study is to examine potential biological benefits of BF, with particular focus on cortisol slope and daily cortisol output, in prostate cancer survivors. We speculated that survivors who reported higher levels of BF would show a steeper decline in diurnal cortisol across the day and lower daily output, than those reporting lower levels of BF (Hypothesis 1). Second, we tested a model that specifies PA as a mediator of BF’s relationship with cortisol (Hypothesis 2). An alternative pathway, NA, was also tested as a possible mediator of BF’s relationship with cortisol.
2. METHODS
2.1. Participants and procedures
Men who completed radical prostatectomy or radiation therapy for localized prostate cancer within the prior 2 years were recruited to take part in a study on “health-related quality of life after prostate cancer.” Men were excluded for the presence of medical comorbidities (eg, active infection, autoimmune disorder) or medications/substances (eg, steroids, cigarette use, excessive alcohol) that could confound cortisol assessment. Sixty-six English-speaking men were recruited over 24 months via physician (n = 4), community outreach (n = 12), advertisement (n = 3), and an institutional tumor registry database (n = 47).
After providing written informed consent, participants completed questionnaires and were trained on saliva collection procedures in person. Height and weight were measured onsite. Participants provided saliva samples 3 times per day over 3 consecutive days. Men received $50 compensation for their participation in the larger study. All procedures were approved by the authors’ Institutional Review Board (University of California, Los Angeles IRB# 11–002552).
Participants (M age = 66 years, SD = 9.04; range 45–87 years) were predominantly White (85%), married (77%), and well-educated (59% with 4-year college degree or more). The majority underwent radical prostatectomy (71%), 32% received radiation, had a mean Gleason score of 6.0 (SD = 1.45), and 78% of the sample received their diagnosis within 3 years of study entry.
2.2. Measures
2.2.1. Benefit finding
The 17-item Benefit Finding Scale (BFS)2 was used to measure the degree to which patients found benefit from their experience of prostate cancer. Each of the 17 items begins with the stem “Having had prostate cancer ..,” and participants are asked to rate the degree to which they have found benefit within various life domains, such as stronger interpersonal relationships, more developed sense of purpose in life, and better acceptance of hardship. The response scale ranged from Not at all (1) to Extremely (5). An average score was computed across items in which higher scores indicate greater BF. The BFS is widely used in cancer populations and has demonstrated sound psychometric properties, including convergent and discriminant validity and test-retest reliability.2 The internal consistency of the BFS is .95 in the current sample.
2.2.2. Positive affect and negative affect
The Positive and Negative Affect Schedule (PANAS)22 was used to assess PA and NA in this study. The PANAS includes items measuring both negative (eg, afraid, hostile, nervous) and positive (eg, happy, joyful, delighted) affective states using a list of 20 adjectives. Each scale consists of 10 items. Respondents are asked to recall their experience during the past few weeks and rate the degree to which they felt each emotion, from Very slightly or Not at all (1) to Extremely (5). Mean scores across all PA and NA items were calculated for each participant. The PANAS has demonstrated psychometric properties including high internal consistency, construct validity, and test-retest reliability.22 In this study, internal consistency coefficients were .87 and .89 for PA and NA, respectively.
Diurnal cortisol was assessed from saliva collected at home using Salivette collection tubes (Sarstedt, Inc.). Participants collected saliva upon awakening (morning), 8 hours post-awakening (afternoon), and at bedtime (evening) for 3 consecutive days. They were instructed not to eat, drink, or brush their teeth for at least 20 minutes before sampling. Each day, participants self-reported compliance with collection instructions via telephone or text. Average sample collection times were as follows: waking: 6:17 am (SD = 1:01); 8 hours post-waking: 2:42 pm (SD = 1:40); bedtime: 11:34 pm (SD = 1:45). Participants refrigerated samples until returning them via express mail. Salivettes were stored in a −20°C freezer until analyzed. Concentrations of salivary free cortisol were measured in duplicate using a commercially available chemiluminescence-immunoassay at the TUD Biopsychology Laboratory in Dresden, Germany. Assay sensitivity was measured to be 0.015 μg/dL. The lower detection limit is 0.41 nmol/L, and inter-assay and intra-assay coefficients of variance are <10%.
2.3. Statistical analysis
Relationships of relevant demographic, biobehavioral, and disease-specific variables with cortisol were examined to identify possible covariates.14 Only age and body mass index (BMI) were related to cortisol and so were included as covariates in all the analyses.
Two cortisol indices were used in this study: diurnal slope and area under the curve with respect to ground (AUCg). For diurnal slope, hierarchical linear modeling (HLM) was used to estimate the slope of the diurnal change in cortisol levels and examine the relationship between BF and cortisol slope and the mediation of PA and NA. HLM provides analysis of change over time on an individual basis (ie, cortisol levels across the day). Data were analyzed using HLM 7.0 statistical software program, SSI Inc. Cortisol data were log-transformed to control for skewness. Cortisol observation times were entered as Level 1 variable in the analyses, so the intercept provided an estimate of waking cortisol level. Greater slope values reflect more rapid declines in cortisol levels, whereas smaller values reflect flatter declines. Cortisol intercept and slope and all the predictors (Level 2 variables) were allowed to vary randomly. Regression coefficients were estimated using restricted maximum-likelihood estimation.
For mediation, the association between BF (predictor) and PA/NA (mediators) was estimated by multiple regression models, controlling for covariates. Then, a 2-step approach using HLM was adopted in this study. PA and NA were examined in separate models. This approach helps understand the independent function of PA (or NA). First, we estimated BF’s effect on cortisol slope (direct path). Second, PA (or NA) was included simultaneously with BF as predictors of cortisol slope (indirect path). For multilevel model mediation, confidence intervals (CI) for indirect effects were estimated using the Monte Carlo method with 20 000 repetitions.23 All level 2 predictors were mean centered.
Linear regressions were conducted to examine direct effects of BF and tests of mediation in models predicting cortisol daily output (AUCg).
3. RESULTS
3.1. Descriptive and correlation analyses
Primary study variables (ie, BF, PA, and NA) were not correlated with age or BMI, except for the significant negative correlation between NA and age (r = −.28, P = .02). PA and NA were moderately negatively correlated (r = −.40, P < .001). Neither PA (r = .22, P = .07) nor NA (r = −.17, P = .25) were significantly correlated with BF.
3.2. Mediation model: Cortisol slope
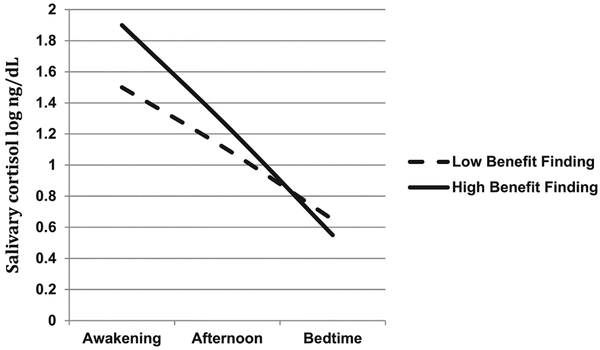
Controlling for age and BMI, BF significantly predicted steeper cortisol slope (b = −.12, SE = .06, t = −2.22, P = .03; Table 1, Model 1). Diurnal cortisol patterns at relatively high (+1 SD) and low (−1 SD) levels of BF are depicted in Figure 1.
TABLE 1.
Multilevel models of diurnal cortisol
| Model 1 Direct Path | Model 2 Indirect Path | |||
|---|---|---|---|---|
| Estimate (SE) | P | Estimate (SE) | P | |
| Awakening cortisol (intercept) | 1.701 (0.096) | <.001 | 1.701 (0.089) | <.001 |
| Age | −0.004 (0.012) | .737 | −0.011 (0.012) | .367 |
| BMI | −0.017 (0.022) | .437 | −0.012 (0.020) | .542 |
| Benefit finding | 0.215 (0.117) | .073 | 0.100 (0.114) | .384 |
| Positive affect | 0.098 (0.143) | .497 | ||
| Diurnal cortisol slope | −0.545 (0.045) | <.001 | −0.545 (0.044) | <0.001 |
| Age | 0.006 (0.006) | .282 | 0.013 (0.006) | .036 |
| BMI | 0.015 (0.010) | .136 | 0.014 (0.010) | .149 |
| Benefit finding | −0.123 (0.056) | .029 | −0.059 (0.056) | .289 |
| Positive affect | −0.177 (0.070) | .012 | ||
Abbreviation: BMI, body mass index.
FIGURE 1.
Diurnal cortisol patterns at high (1 SD above the mean) and low (1 SD below the mean) levels of benefit finding
Regression and HLM analyses were used to test whether the association between BF and diurnal cortisol slopes could be explained by an indirect effect through PA. Multiple regression analysis controlling for covariates showed that BF significantly predicted PA levels (B = .20, SE = .09, P = .03). We next tested the indirect effect of BF via PA on cortisol slope. To evaluate this, we simultaneously entered BF and PA into the specified HLM model to predict cortisol slope. As shown in Model 2 of Table 1 (Indirect Path), PA was a significant predictor of steeper cortisol slope (B = −.18, SE = .07, p = .012), whereas BF was not significant. The effect of BF reduced considerably after entering PA. The effect of BF on cortisol slope is fully mediated by PA—that is, the pathway by which BF influences cortisol slope is through its potential influence on PA. PA was associated with a steeper cortisol slope, indicating that those individuals who had higher levels of PA experienced a greater cortisol decline throughout the day. Monte Carlo estimation analyses showed the indirect effect of BF on cortisol slope via PA was significant (95% CI [−0.087, −0.001]).
In the alternative model, PA was replaced by NA. BF did not significantly predict NA (B = −.15, SE = .09, P = .09). In the HLM model, NA was not significantly associated with cortisol slope (b = .13, SE = .07, P = .07). Because the NA model did not fit the requirements for mediation, we did not test the indirect effect.
3.3. Total cortisol output
AUCg was neither related to BF nor PA in the regression models. Therefore, the indirect effect was not tested.
4. DISCUSSION
Our results indicate that survivors who derive more benefit from their experience of prostate cancer demonstrate steeper diurnal cortisol slope across the day. Consistent with our hypotheses, the results showed PA, and not NA, mediated the association between BF and diurnal cortisol slope. PA is a likely psychological pathway by which BF might serve to regulate HPA activity.
Cortisol levels, influenced by HPA activity, generally peak in the morning and then decrease over the course of the day.24 Alterations in this diurnal pattern have been linked to physiological and functional outcomes including increased inflammation, disease-related functional disability, tumor progression, and cancer survival.25–27 Our results imply that BF has a role in physical health, in addition to psychological wellbeing.
The findings suggest that finding benefit from cancer may be an adaptive effort in adjustment to cancer. However, its exact role in the adaptation process has been debated. BF has been characterized as both a system of appraisal and as a strategy for coping. As a process of favorable cognitive appraisal, BF is thought to enhance notions of the self and overall coping efficacy, which protects people from the detrimental effects of cancer stress.28 As a process of coping,29 BF might serve to facilitate meaning-making and positive construal of from difficult occurrences.5 When successful, such coping efforts might contribute to positive transformation4 and psychological thriving.11
To the degree that adaptive coping is supported, BF will facilitate PA.3,10 This pathway can be further elaborated by the connection between BF and positive appraisal, coping resources, sense of mastery, and interpersonal resources.2,7 It might be that BF, and associated PA, is associated with health via increasing personal resources in coping, interpersonal support, and self-efficacy.30 Such enhancement in personal resources promotes psychological preparedness for future stressors or “enhanced allostasis” whereby BF promotes physical reactivity, recovery, and habituation.10 For example, a person who strengthens social support and affirms personal goals during cancer may be less vulnerable to body image concerns following treatment, resulting in more regulated HPA axis activity across time.
Support for the PA pathway supports Folkman’s model of meaning-focused coping.3 To the extent that BF is a process of meaning- focused coping, the generation of positive emotions regulates stress by enhancing coping resources and potentially providing motivation for future coping efforts. This is especially relevant for stressors with no imminent resolution.
Notably, effects were found only for cortisol slope but not daily cortisol production. These indices capture independent, though related, components of HPA axis function31 (correlation in our study was r = .33, P < .05). Higher daily cortisol volume, reflecting cumulative hormone burden, tends to be linked to recent and ongoing stress; while diurnal pattern tends to better reflect the HPA capacity to respond to stress and so is associated with perception of challenge and controllability of the event.14,24,32 This interpretation would be consistent with the view that BF reflects a process of coping, or response, to cancer-related stressors. It is possible that the participants in this study did not perceive consistently intense threat, but rather were engaged in a process of response to unresolved cancer-related sequelae, such as relationship changes, goal adjustment, and sexual and urinary symptoms. Men with higher BF might perceive stressors as controllable challenges.
As shown in Figure 1, with high BF morning output is higher (compared with low BF), evening secretion is similar. The boost hypothesis33 suggests that cortisol morning rise implies an active coping effort, in which the body is mobilizing its resources to confront the anticipated challenges. Furthermore, cortisol morning peak is positively associated with greater social support and more coping activities and negatively associated with fatigue, exhaustion, and hopelessness.34,35 Taken together, this supports the notion that BF is associated with perceiving stress as a challenge and bolstering personal coping resources.
Understanding BF may be particularly useful in the context of coping with the consequences of prostate cancer treatment. Treatment side effects typically include declines in sexual and urinary function, social role changes, and existential concerns,1 with more rapid recovery of function in the 12 to 24 months following treatment. The limited research on BF in men with prostate cancer36,37 suggests that BF is associated with greater use of active coping and social support seeking. BF in the initial recovery period following treatment might work to facilitate adaptive coping and reduce physiological stress.
It is worth noting that age became a significant predictor of cortisol slope when PA was added to the model (see Table 1). It may be a case of so-called “inconsistent mediation”,38 in which the total effect of age on cortisol slope was not significant because the relationships of age to PA and PA to cortisol are in opposite directions. This would imply that older men have flatter slopes, controlling for the effect of PA.
4.1. Study limitations
These results should be considered in light of several limitations. First, given the cross-sectional nature of the study, the results show associations among BF, PA, and cortisol slope rather than causal links. Our study calls upon theory to conceptualize PA as the mediator of BF’s relationship to physiology.3 However, alternate relationships are also possible. We tested BF as a mediator of PA’s effect on cortisol slope as an alternative hypothesis, and the results did not support BF as a mediator. Future research should extend the results by using a prospective design to better distinguish the time course of these relationships.
Second, the current sample was composed of a homogenous sample of men. It is critical to replicate findings in more diverse samples. Moreover, while our study focuses on prostate cancer survivors, the generalizability to other types of cancer and survivor groups (eg, women, young adults) merits exploration. Future studies should also consider other potential mediators that might be associated with the effect of BF on health, coping resources,39 self-efficacy, and interpersonal resources.30 Also, the possibility of response bias and recall accuracy associated of self-report measures should be considered. Finally, this study relied on a relatively small sample; however, power analyses suggest adequate power would be achieved with the addition of 2 participants. Future studies will need to replicate findings in larger samples.
4.2. Clinical implications
Our findings extend previous studies in women with breast cancer15,16 by examining in BF in a sample of men with prostate cancer and pointing to PA as a potential mechanism of BF. Given the potential for physical benefit of BF, our study has implications for the development of interventions designed to enhance the adjustment in cancer survivors, especially men with prostate cancer. Beyond targeting reduction of distress, interventions that facilitate BF and focus on enhancement of PA have the potential to benefit physical health. This will require assessment and recognition of the potential for BF and the ability to facilitate the growth appropriately across the survivorship trajectory.
Several clinical interventions have demonstrated enhanced BF in cancer survivors, including cognitive-behavioral stress management2 and mindfulness-based cancer recovery.40 Moreover, interventions that encourage cancer survivors to disclose their feelings and stories can help them engage in a meaning-making process in which they explore the personal impact of cancer, and reappraise their lives in a way that leads to the discovery of positive outcomes. Clinical approaches that allow patients to identify “benefits found” (versus direct imperatives to find benefit) will likely prove most efficacious. That is, growth is originated from within the person.4 Existential or client-centered approaches that value self-exploration, intrinsic motivation, and activation of positive emotion may prove useful in facilitating the recognition of growth.
ACKNOWLEDGEMENTS
This research was supported by funds from the UCLA Cousins Center for Psychoneuroimmunology, and support from NIH: SC1-CA187494.
Funding information
National Cancer Institute, Grant/Award Number: SC1-CA187494; UCLA Cousins Center for Psychoneuroimmunology
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- References: 1.Bloch S, Love A, Macvean M, Duchesne G, Couper J, Kissane D. Psychological adjustment of men with prostate cancer: a review of the literature. BioPsychoSocial Medicine. 2007;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 2.Antoni MH, Lehman JM, Kilbourn KM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early- stage breast cancer. Health Psychol. 2001;20(1):20–32. [DOI] [PubMed] [Google Scholar]
- References: 3.Folkman S The case for positive emotions in the stress process. Anxiety Stress Coping. 2008;21(1):3–14. [DOI] [PubMed] [Google Scholar]
- References: 4.Tedeschi RG, Calhoun LG. Posttraumatic growth: conceptual foundations and empirical evidence. Psychological Inquiry. 2004;15:1–18. [Google Scholar]
- References: 5.Davis CG, Nolen-Hoeksema S, Larson J. Making sense of loss and benefiting from the experience: two construals of meaning. J Pers Soc Psychol. 1998;75:561. [DOI] [PubMed] [Google Scholar]
- References: 6.Carver CS, Antoni MH. Finding benefit in breast cancer during the year after diagnosis predicts better adjustment 5 to 8 years after diagnosis. Health Psychol. 2004;23(6):595–598. [DOI] [PubMed] [Google Scholar]
- References: 7.Helgeson VS, Reynolds KA, Tomich PL. A meta-analytic review of benefit finding and growth. J Consult Clin Psychol. 2006;74(5):797–816. [DOI] [PubMed] [Google Scholar]
- References: 8.Sawyer A, Ayers S, Field AP. Posttraumatic growth and adjustment among individuals with cancer or Hiv/Aids: a meta-analysis. Clin Psychol Rev. 2010;30(4):436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 9.Wilson B, Morris BA, Chambers S. A structural equation model of posttraumatic growth after prostate cancer. Psychooncology. 2014;23(11): 1212–1219. [DOI] [PubMed] [Google Scholar]
- References: 10.Bower JE, Low CA, Moskowitz JT, Sepah S, Epel E. Benefit finding and physical health: positive psychological changes and enhanced allostasis. Social and Personality Psychology Compass. 2008;2(1):223–244. [Google Scholar]
- References: 11.Epel ES, McEwen BS, Ickovics JR. Embodying psychological thriving: physical thriving in response to stress. Journal of Social Issues. 1998;54(2):301–322. [Google Scholar]
- References: 12.Carrico AW, Ironson G, Antoni MH, et al. A path model of the effects of spirituality on depressive symptoms and 24-hurinary-free cortisol in Hiv-positive persons. J Psychosom Res. 2006;61:51–58. [DOI] [PubMed] [Google Scholar]
- References: 13.Moskowitz JT, Epel ES. Benefit finding and diurnal cortisol slope in maternal caregivers: a moderating role for positive emotion. The Journal of Positive Psychology. 2006;1(2):83–91. [Google Scholar]
- References: 14.Saxbe DE. A field (researcher’s) guide to cortisol: tracking Hpa axis functioning in everyday life. Health Psychol Rev. 2008;2(2):163–190. [Google Scholar]
- References: 15.Cruess DG, Antoni MH, McGregor BA, et al. Cognitive-behavioral stress management reduces serum cortisol by enhancing benefit finding among women being treated for early stage breast cancer. Psychosom Med. 2000;62(3):304–308. [DOI] [PubMed] [Google Scholar]
- References: 16.Diaz M, Aldridge-Gerry A, Spiegel D. Posttraumatic growth and diurnal cortisol slope among women with metastatic breast cancer. Psychoneuroendocrinology. 2014;44:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 17.McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive–behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56(1):1–8. [DOI] [PubMed] [Google Scholar]
- References: 18.Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci U S A. 2005;102:6508–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 19.Watson D, Tellegen A. Toward a consensual structure of mood. Psychol Bull. 1985;98(2):219–235. [DOI] [PubMed] [Google Scholar]
- References: 20.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. [DOI] [PubMed] [Google Scholar]
- References: 21.Giese-Davis J, Wilhelm FH, Conrad A, et al. Depression and stress reactivity in metastatic breast cancer. Psychosom Med. 2006;68(5):675–683. [DOI] [PubMed] [Google Scholar]
- References: 22.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS Scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- References: 23.Selig JP, Preacher KJ. Monte Carlo method for assessing mediation: an interactive tool for creating confidence intervals for indirect effects [Computer Software]. In 2008.
- References: 24.Pruessner JC, Wolf OT, Hellhammer DH, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adreno-cortical activity. Life Sci. 1997;61(26):2539–2549. [DOI] [PubMed] [Google Scholar]
- References: 25.Schrepf A, Thaker PH, Goodheart MJ, et al. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology. 2015;53: 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 26.Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30:S163–S170. [DOI] [PubMed] [Google Scholar]
- References: 27.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26(29):4820–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 28.Taylor SE. Adjustment to threatening events. a theory of cognitive adaptation. American Psychologist. 1983;38(11):1161–1173. [Google Scholar]
- References: 29.Affleck G, Tennen H. Construing benefits from adversity: adaptational significance and dispositional underpinnings. J Pers. 1996;64(4): 899–922. [DOI] [PubMed] [Google Scholar]
- References: 30.Fredickson B The role of positive emotions in positive psychology. American Psychologist. 2001;56(3):218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 31.Khoury JE, Gonzalez A, Levitan RD, et al. Summary cortisol reactivity indicators: interrelations and meaning. Neurobiology of Stress. 2015;2:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 32.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. [DOI] [PubMed] [Google Scholar]
- References: 33.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci. 2006;103(45):17058–17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 34.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–278. [DOI] [PubMed] [Google Scholar]
- References: 35.Sjögren E, Leanderson P, Kristenson M. Diurnal saliva cortisol levels and relations to psychosocial factors in a population sample of middle-aged Swedish men and women. Int J Behav Med. 2006;13(3): 193–200. [DOI] [PubMed] [Google Scholar]
- References: 36.Thornton AA, Perez MA. Posttraumatic growth in prostate cancer survivors and their partners. Psychooncology. 2006;15(4):285–296. [DOI] [PubMed] [Google Scholar]
- References: 37.Kinsinger DP, Penedo FJ, Antoni MH, et al. Psychosocial and sociodemographic correlates of benefit-finding in men treated for localized prostate cancer. Psychooncology. 2006;15(11):954–961. [DOI] [PubMed] [Google Scholar]
- References: 38.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58(1):593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References: 39.Sears SR, Stanton AL, Danoff-Burg S. The Yellow Brick Road and the Emerald City: benefit finding, positive reappraisal coping, and posttraumatic growth in women with early-stage breast cancer. Health Psychol. 2003;22(5):487–497. [DOI] [PubMed] [Google Scholar]
- References: 40.Carlson LE, Tamagawa R, Stephen J, et al. Randomized-controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy among distressed breast cancer survivors (mindset): long-term follow-up results. Psychooncology. 2016;25:750–759. [DOI] [PubMed] [Google Scholar]