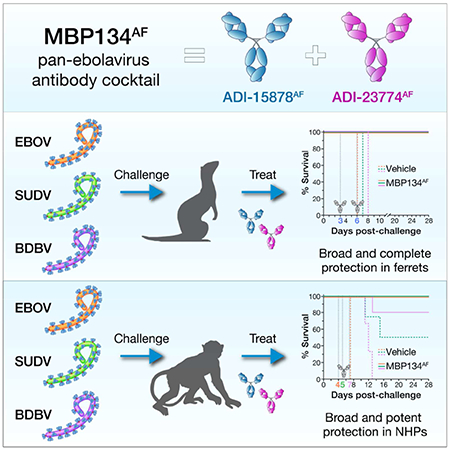
SUMMARY
Recent and ongoing outbreaks of Ebola virus disease (EVD) underscore the unpredictable nature of ebolavirus reemergence and the urgent need for antiviral treatments. Unfortunately, available experimental vaccines and immunotherapeutics are specific for a single member of the Ebolavirus genus, Ebola virus (EBOV), and ineffective against other ebolaviruses associated with EVD, including Sudan virus (SUDV) and Bundibugyo virus (BDBV). Here we show that MBP134AF, a pan-ebolavirus therapeutic comprising two broadly neutralizing human antibodies (bNAbs), affords unprecedented effectiveness and potency as a therapeutic countermeasure to antigenically diverse ebolaviruses. MBP134AF could fully protect ferrets against lethal EBOV, SUDV, and BDBV infection, and a single 25-mg/kg dose was sufficient to protect NHPs against all three viruses. The development of MBP134AF provides a successful model for the rapid discovery and translational advancement of immunotherapeutics targeting emerging infectious diseases.
Graphical Abstract
eTOC Blurb
Bornholdt et al. examine the therapeutic efficacy of MBP134AF a pan-ebolavirus cocktail comprising two human mAbs. MBP134AF reverted lethal disease in both ferret and nonhuman primates challenged with three divergent ebolaviruses. A single dose of MBP134AF administered post-infection was sufficient to protect non-human primates from ebolavirus disease.
INTRODUCTION
The 2013-2016 EBOV epidemic in Western Africa and the recent EBOV outbreaks in the Democratic Republic of Congo have established ebolaviruses as pathogens of global public health relevance. Of the five ebolaviruses known to infect humans, EBOV, SUDV, and BDBV have caused outbreaks with case-fatality rates up to 90% in the last decade (Burk et al., 2016). Although several therapeutic products are in clinical development for the treatment of Ebola virus disease (EVD), no medical countermeasures to SUDV or BDBV have progressed beyond proof-of-concept studies (Corti et al., 2016; Mire et al., 2013; Pascal et al., 2018; Qiu et al., 2014; Thi et al., 2016). To address this unmet public health need, we developed a two-antibody cocktail, MBP134AF, with demonstrable activity against all known ebolaviruses (companion report, Wec et al.), including the “pre-emergent” agent Bombali virus recently discovered in molossid bats in Sierra Leone (Goldstein et al., 2018). MBP134AF, comprising the human bNAbs ADI-15878AF and ADI-23774AF (companion report, Wec et al.), was selected after a systematic process including the assessment and/or optimization of multiple mAbs and their combinations for potency and breadth, Fc effector functions via glycan engineering, and in vivo efficacy in rodent models of EBOV and SUDV infection (companion report, Wec et al.). ADI-15878AF and ADI-23774AF both target unique, non-overlapping epitopes on the ebolavirus glycoprotein (GP), neutralize both the extracellular and endosomally cleaved forms of GP, and lack crossreactivity against the secreted GP isoform (sGP) that is abundant in the plasma of infected individuals (Bornholdt et al., 2016; Wec et al., 2017). The exceptional potency of MBP134AF against guinea pig-adapted EBOV and SUDV (companion report, Wec et al.) warranted continued evaluation in the ferret and NHP large-animal models of ebolavirus challenge to assess its clinical potential.
RESULTS
MBP134AF protects ferrets from lethal EBOV, SUDV and BDBV challenge
We determined MPB134AF’s protective efficacy against the wild-type Makona variant of EBOV (EBOV/Makona) (Mire et al., 2015), SUDV variant Gulu (SUDV/Gulu) and BDBV variant But-811250 (BDBV/But-811250) in the recently established ferret model, which does not require any viral adaptation and recapitulates key hallmarks of human EVD (Cross et al., 2016; Cross et al., 2018; Kozak et al., 2016). Ferrets challenged intranasally with a lethal dose of EBOV/Makona received two 15-mg doses of MBP134AF three days apart, with the treatment initiated on either day 2 (ferrets 1–4) or day 3 (ferrets 5–8) post infection (p.i.) (Figure 1A). MBP134AF fully protected from lethal challenge in both treatment groups and cleared viremia even in ferrets 5–8, which showed signs of active infection by reverse transcription polymerase chain reaction (RT-PCR) prior to treatment (Figures 1B and 1C). Similarly, administration of two 15-mg doses of MBP134AF on days 3 and 6 (ferrets 1–4) afforded complete protection in ferrets challenged with lethal doses of SUDV/Gulu (Figure 1D) or BDBV/But-811250 (Figure 1G) and inhibited viral replication (Figures 1E, 1F, 1H, and 1I). Because SUDV/Gulu and BDBV/But-811250 have been shown to be less virulent in ferrets than EBOV/Makona, with a relatively delayed time to peak viremia (Cross et al., 2016), we next evaluated MBP134AF in a lower dose-sparing treatment course of two 5-mg doses given on days 3 and 6 p.i. In this treatment group, ferrets 5–8 challenged with SUDV/Gulu displayed high levels of viremia and uniformly succumbed to disease by day 11 p.i. (Figures 1D-F). By contrast, ferrets 5–8 challenged with BDBV/But-811250 (characterized by the slowest onset of viremia (Cross et al., 2016)) were protected by the two 5-mg dose regimen, with reversion of viremia in some animals (Figures 1G-I). In summary, MBP134AF conveys full protection to ferrets challenged with ebolaviruses from three divergent species.
Figure 1. MBP134AF protects ferrets from lethal EBOV, SUDV and BDBV challenge.
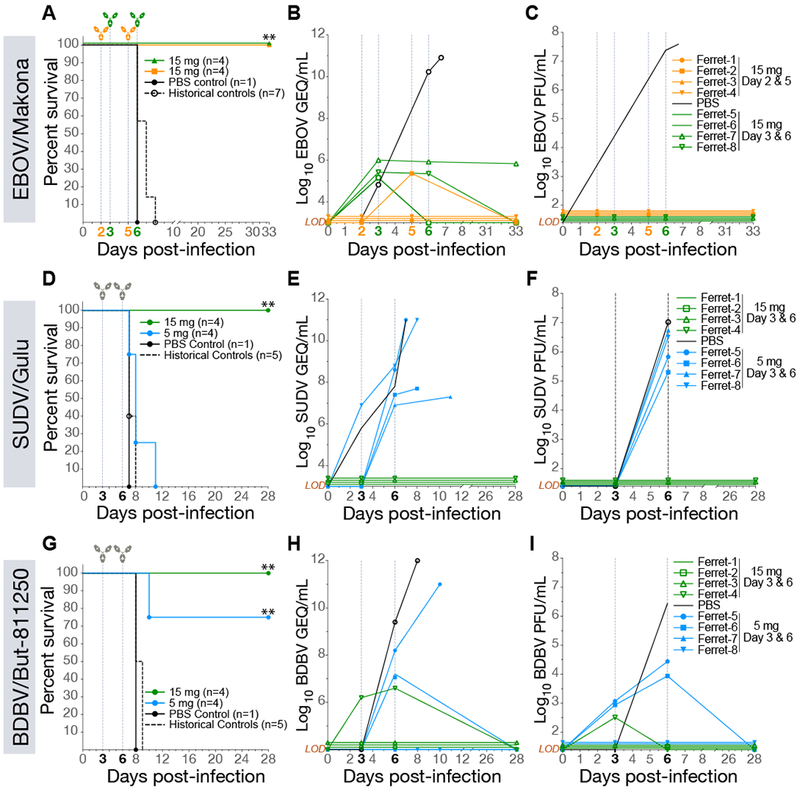
(A) Survival curves for ferrets challenged with EBOV/Makona and treated with 15 mg of MBP134AF on either Day 2 and 5 (orange) or Day 3 and 6 (green) p.i. **, P<0.01.
(B) Quantitative RT-PCR measuring average copies of EBOV/Makona genomic equivalents per mL of whole blood (GEQ/mL) from animals treated on day 2 and 5 (orange) and day 3 and 6 (green) p.i.
(C) Infectious EBOV/Makona measured via plaque assays (PFU/mL) present in the blood of animals treated on day 2 and 5 (orange) or day 3 and 6 (green).
(D) Survival curves for ferrets challenged with SUDV/Gulu and treated with 15-mg (green) or 5-mg (blue) doses of MBP134AF on day 3 and 6 post infection.
(E) The average GEQ/mL of SUDV/Gulu present in the blood of animals treated with 15-mg (green) or 5-mg doses (blue).
(F) SUDV/Gulu viremia present in the blood of animals treated with 15-mg (green) or 5-mg (blue) doses from panel D.
(G) Survival curves for ferrets challenged with BDBV/But-811250 and treated with 15-mg (green) or 5-mg (blue) doses of MBP134AF on day 3 and 6 post infection.
(H) The average GEQ/mL of BDBV/But-811250 from animals treated with two 15-mg (green) or 5-mg doses (blue).
(I) Viremia (PFU/mL) present in the blood of animals treated with two 15-mg (green) or 5-mg doses (blue) from panel G. LOD = limit of detection
A single 25 mg/kg dose of MBP134AF protects NHPs challenged with EBOV/Kikwit
We next evaluated the MBP134AF cocktail’s efficacy in the gold-standard non-human primate (NHP) model of Ebola virus challenge. Ten rhesus macaques were randomized into two treatment groups, NHPs 1–4 and NHPs 5–8, and a PBS control group of two animals, and then challenged intramuscularly (IM) with 1,000 plaque-forming units (PFUs) of the Kikwit variant of EBOV (EBOV/Kikwit). NHPs 1–4 received a single intravenous (IV) 25-mg/kg dose of MBP134AF on day 4 p.i., whereas NHPs 5–8 received a more conservative two-dose regimen of 50 mg/kg then 25 mg/kg on days 4 and 7 p.i., respectively. Remarkably, the single 25-mg/kg dose of MBP134AF completely reversed the onset of EVD and protected NHPs 1–4 from a lethal EBOV/Kikwit exposure (Figure 2A). All animals in this study were confirmed to have had an active EBOV/Kikwit infection via RT-PCR (107–1011 viral genome equivalents per mL (GEQ/mL)) and plaque assay (103–106 PFU/mL) prior to treatment on day 4 p.i. (Figures 2B and 2C). These high levels of viremia could nonetheless be reversed by MBP134AF treatment—viremia in animals from both treatment groups fell below the limit of detection in the plaque assay by day 7 p.i. and in the RT-PCR assay by day 14 p.i. (Figure 2B and 2C). Fever was detected in control animals and in three out of four animals in each treatment group at the time of the first MBP134AF dosing; however all treated animals returned to normal body temperature by day 10 p.i. Treated animals also maintained substantially lower clinical scores and reduced grade of thrombocytopenia compared to control NHPs (Figures 2D-2F). Two animals, NHP-3 and NHP-8, showed significant signs of EVD-induced liver injury prior to treatment, with elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, and a third animal, NHP-8, displayed significant increases in C-reactive protein (CRP) levels. These and other hallmarks of EVD were significantly reduced post-treatment with MBP134AF by day 10 p.i. (Figures 2D-2I, S1, and S2). Thus, the pan-ebolavirus MBP134AF cocktail could potently reverse the course of EVD and deliver complete therapeutic protection in NHPs following a lethal EBOV/Kikwit challenge with a single dose of only 25 mg/kg.
Figure 2. A single 25 mg/kg dose of MBP134AF protects rhesus macaques challenged with EBOV/Kikwit.

(A) Survival curves for NHPs challenged with EBOV/Kikwit and treated with a single 25-mg/kg dose of MBP134AF on day 4 (green) p.i or a more conservative two-dose regimen of 50 mg/kg on day 4 and 25 mg/kg on day 7 (orange) post infection. *, P<0.05.
(B) The average GEQ/mL of EBOV/Kikwit present in the blood of animals treated with a single dose of MBP134AF (green) or two doses of MBP134AF (orange). All detectable EBOV/Kikwit was eliminated 10 days post treatment.
(C) Infectious EBOV/Kikwit (PFU/mL) present in the blood of animals treated with either a single (green) or two-dose course of MBP134AF (orange). Infectious EBOV/Kikwit was no longer detectable by plaque assay by the next bleed of treated animals on day 7 post infection.
(D) Clinical scores of animals within the study cohort are shown. Aside from the control animals only NHP-1 and NHP-8 scored for partially or completely refusing their daily nutrition.
(E) Body temperatures taken during the course of the study show nine of the ten animals registered an elevated temperature by day 7 p.i. Animals receiving MBP134AF returned to baseline temperature by day 10 p.i.
(F) The platelet counts for the cohort show severe thrombocytopenia in the control animals post-infection. By contrast, animals receiving MBP134AF rapidly recovered and displayed little or no signs of thrombocytopenia. Data are represented as mean ± SD.
(G) The graphed CRP levels show NHP-3 and NHP-7 suffered from acute inflammation as a result of EVD prior to MBP134AF treatment.
(H) The ALT levels from each animal are shown, NHP-3 and NHP-8 demonstrated signs of advanced EVD that were alleviated post-treatment.
(I) AST levels for all the challenged animals showed initial signs of liver damage resultant of EVD. Samples from NHP-8 in particular show a significant spike in AST levels that returned to baseline post-treatment. Legend for graphs in top right-hand corner, LOD = limit of detection.
MBP134AF produced from CHOK1-AF cells is therapeutically equivalent to the plant produced MBP134AF
In the experiments described above, MBP134AF was produced using a Nicotiana benthamiana (tobacco) plant-based expression system (Marillonnet et al., 2005; Zeitlin et al., 2011). However, because the manufacturing infrastructure for Nicotiana-based products is still limited, we sought to transition MBP134AF to the well-established Chinese hamster ovary (CHO) cell production platform. Accordingly, we expressed MBP134AF in a GDP-fucose transporter SLC35C1-knockout cell line (CHOK1-AF), which maintains the afucosylated state of MBP134AF (Qiu et al., 2016). Comparative studies indicated that CHOK1-AF–produced MBP134AF is comparable or even surpasses its plant-produced counterpart in neutralization potential (data not shown), Fc effector functions relevant to this cocktail’s antiviral potency (Figure 3), and protective efficacy in guinea pigs (Figure 4). Therefore, the Nicotiana- and CHO-produced MBP134AF products are functionally equivalent. Accordingly, all remaining experiments described herein were performed with CHOK1-AF expressed MBP134AF, the manufacturing system being employed for its clinical development.
Figure 3. Induction of Fc effector function by plant- and CHOKI-produced ADI-23774AF and ADI-15878AF.
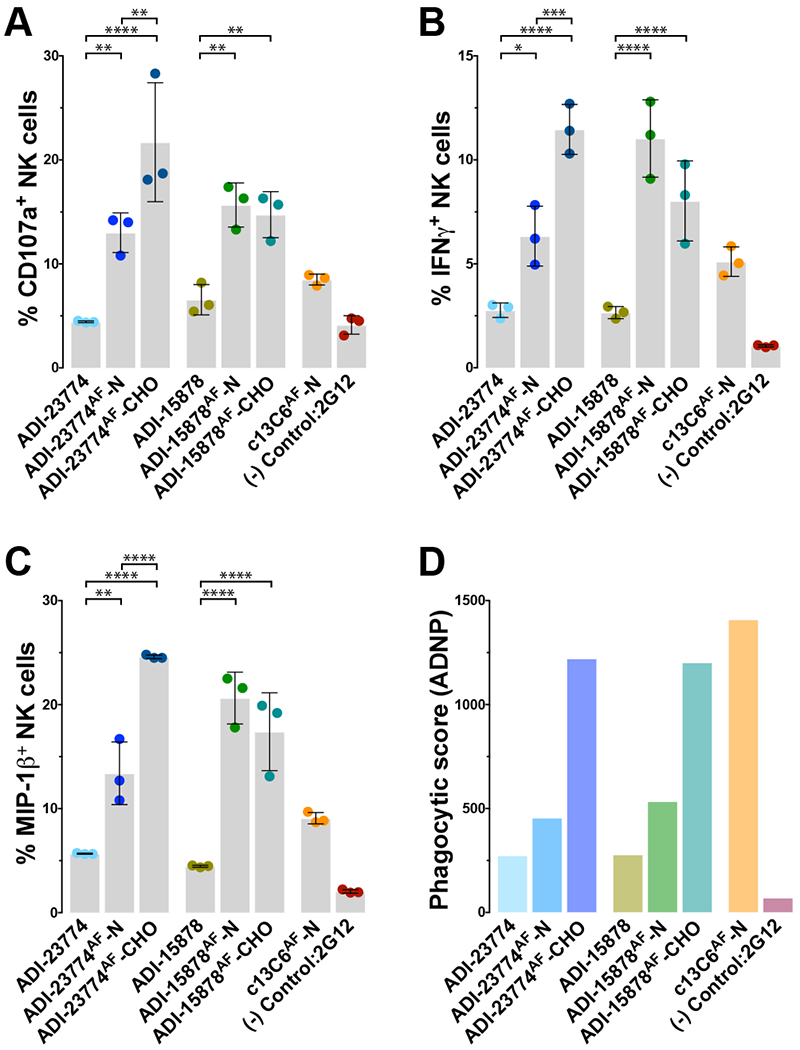
(A-C) Markers of antibody-dependent NK cell activation utilizing NK cells from seronegative donors are displayed. The levels of NK cell activation markers CD107a (A), intracellular cytokine IFNγ (B) and MIP-1β (C) are comparatively graphed for ADI-23774 and ADI-15878 produced from transiently expressed HEK293 cells as fucosylated mAbs or as afucosylated mAbs produced from either the plant (-N) or CHOK1-AF (-CHO) cell-based expression platforms. The graphs demonstrate a marked enhancement of Fc-mediated NK cell activation from the presence of an afucosylated glycan in the Fc region of either antibody. Further, both mAbs exceed the Fc effector function potency of c13C6-N, a component of the ZMapp cocktail used here as a positive control. Data are represented as mean ± SD.
(D) The phagocytic score or ability of each mAb to facilitate Fc-mediated antibody-dependent neutrophil activation/phagocytosis (ADNP) is shown. Expression in the CHOK1-AF platform greatly enhanced ADNP activity over the other expression platforms likely do to the more uniform heavy chain glycosylation propensity offered by the CHOK1-AF stable pools over transient expression of the antibodies within plants. The formula for calculating the phagocytic score is described in the materials and methods. The anti-human immunodeficiency virus GP120 mAb, 2G12, is utilized here as an irrelevant negative control mAb (Trkola et al., 1996). *, P<0.05. **, P<0.01. ***, P<0.001. ****, P<0.0001.
Figure 4. MBP134AF produced from CHOK1-AF cells is therapeutically equivalent to the plant produced MBP134AF.
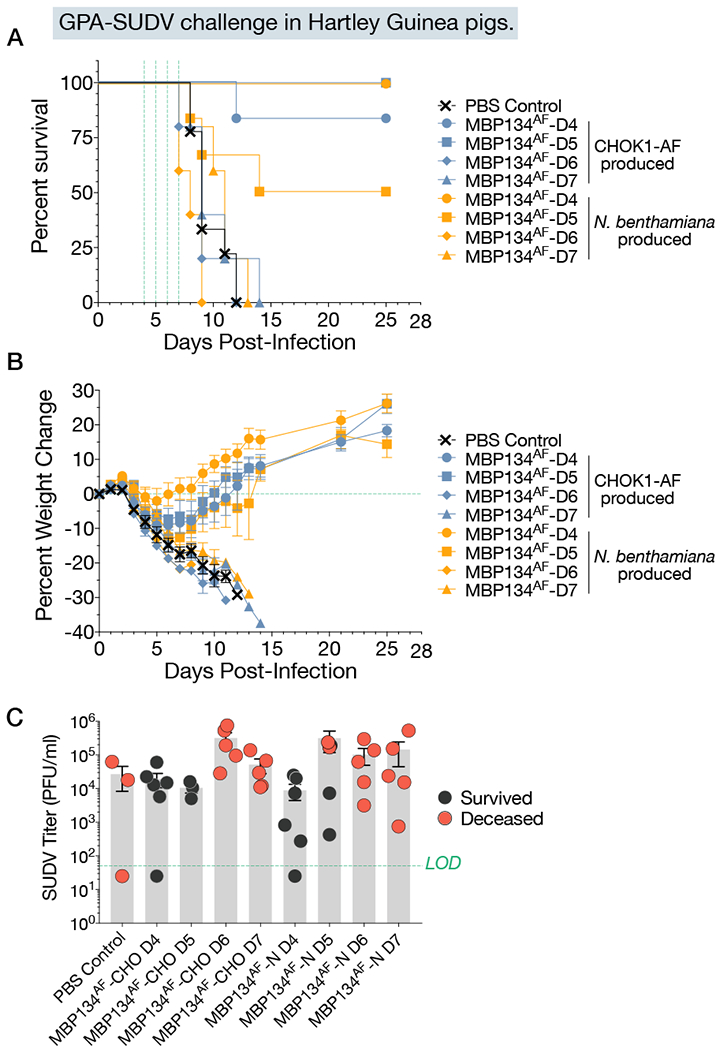
(A) Hartley guinea pigs were divided into eight treatment groups and given a 5-mg total dose of CHO or plant produced MBP134AF on either Day 4, 5, 6, or 7 post infection. Animals receiving MBP134AF treatment on Day 4 or 5 post infection showed either equivalent and/or significant levels of protection, while all animals treated on day 6 or 7 succumbed to SUDV/Boniface infection with no detectable delay to death when compared to the PBS-treated controls.
(B) Weight loss curves from animals treated with CHO or plant produced MBP134AF display equivalent weight change trends. Data are represented as mean ± SD.
(C) The viral titers (PFU/mL) in blood samples taken from each animal immediately prior to treatment are graphed. The data suggests MBP134AF produced from either platform equivalently protected animals with <105 PFU/mL of infectious virus, with reduced efficacy in animals that showed viral loads >105 PFU/mL. Data are represented as mean ± SD.
A single 25 mg/kg or 7.5 mg/kg dose of MBP134AF protects NHPs challenged with SUDV/Boniface
We tested MBP134AF in a blinded NHP study in which rhesus macaques were challenged IM with SUDV variant Boniface (SUDV/Boniface; 1,000 PFU). This model typically affords 50% lethality (Ellis et al., 1978) and unpublished data). Twelve animals were randomized into two treatment groups, NHPs 1–4 and NHPs 5–8, and one control group, PBS controls 1–4. On day 5 p.i., NHPs 1–4 and 5–8 received single 7.5-mg/kg and 25-mg/kg doses of MBP134AF, respectively. Both doses of MBP134AF provided full protection from SUDV disease and all MBP134AF-treated animals became viremia-negative by day 8 p.i. (Figure 5A-5C). MBP134AF-treated animals displayed little to no clinical signs of disease in contrast to the control animals—half of the latter succumbed to infection (Figure 5D). Importantly, all of the animals in the blinded MBP134AF cohort registered a fever prior to receiving MBP134AF on day 5 p.i. (Figure 5E). The two surviving control animals remained viremic past day 14 p.i. (Figures 5B and 5C), maintained elevated ALT, AST, and alkaline phosphatase (ALP) levels, and showed significant thrombocytopenia (Figures 5F-5I and S3) out to day 21 p.i.
Figure 5. A single 25 mg/kg or 7.5 mg/kg dose of MBP134AF protects rhesus macaques challenged with SUDV/Boniface.

(A) Survival curves for NHPs challenged with SUDV/Boniface receiving either PBS (black), a 25-mg/kg dose of MBP134AF (green) or a 7.5-mg/kg (purple) dose of MBP134AF on day 5 p.i.
(B) The average GEQ/mL of SUDV/Boniface present in the blood of animals receiving a 25-mg/kg dose of MBP134AF (green), a 7.5-mg/kg dose of MBP134AF (purple) or the PBS controls (black). In the MBP134AF treated groups detectable levels of SUDV/Boniface were eliminated by day 8 post infection (the next bleed post treatment).
(C) Infectious SUDV/Boniface (PFU/mL) present in the blood of animals receiving a 25-mg/kg dose of MBP134AF (green) or a 7.5-mg/kg dose of MBP134AF (purple) as well as in PBS controls (black). Infectious SUDV/Boniface was no longer detectable by plaque assay by the next bleed on day 8 p.i.
(D) All of the MBP134AF treated animals in the cohort had clinical scores on day 5 p.i. prior to receiving treatment. Animals dosed with MBP134AF no longer registered a clinical score by day 14 p.i., while the surviving controls continued scoring out to day 21 p.i.
(E) Body temperatures taken from the blinded cohort showed all the animals registered an elevated temperature by day 5 p.i., prior to receiving MBP134AF.
(F) The platelet counts for the cohort show declining counts p.i. with the MBP134AF treated animals rapidly recovering and the PBS controls displaying severe thrombocytopenia. Data are represented as mean ± SD.
(G) The ALT levels from each animal are shown; the control animals all display elevated levels from baseline post infection. In contrast, all of the animals receiving MBP134AF show little to no signs of EVD.
(H) AST levels for all the MBP134AF treated animals show little to no increase, while the control animals all show highly elevated AST levels by day 10 p.i.
(I) ALP levels are graphed for each animal in the cohort, no sign of EVD induced ALP levels are present in any of the MBP134AF treated animals. In contrast, all of the control animals that received PBS display elevated ALP levels resulting from EVD. Legend for graphs in top right-hand corner, LOD = limit of detection.
A single 25 mg/kg dose of MBP134AF protects cynomolgus macaques challenged with BDBV/But-811250
We next determined the protective efficacy of MBP134AF against lethal BDBV/But-811250 challenge in the cynomolgus macaque model of infection (Burk et al., 2016; Mire et al., 2013). We chose to treat animals at day 7 p.i. with MBP134AF because previous reports indicated that they were already viremic and showing signs of EVD at this time point (Mire et al., 2013). We reasoned that treatment under these post-exposure conditions would afford a rigorous evaluation of MBP134AF’s ability to reverse advanced EVD caused by BDBV.
Accordingly, a cohort of 9 animals was exposed to 1,000 PFU (IM) of BDBV/But-811250. Six randomly selected animals received a single 25-mg/kg IV infusion of MBP134AF on day 7 p.i. and three received PBS. This single dose of MBP134AF provided significant levels of protection (P value of 0.006 or 0.0108 if calculated including the historical controls (Mire et al., 2013)), with only one animal succumbing to infection. By contrast, uniform lethality was observed in the PBS control group (Figure 6A). Prior to MBP134AF treatment on day 7 p.i., animals registered elevated clinical scores and body temperatures, viremia as high as ~1011 GEQ/mL or 107 PFU/mL, and EVD-induced thrombocytopenia (Figure 6B-F). By the next blood collection point on day 10 p.i., five of the six animals that received MBP134AF had no detectable infectious BDBV in the blood, and clinical scores were reduced to basal levels by day 12 p.i., a complete reversion of infection and disease. The single treated animal that succumbed, NHP-5, did not have the highest viral load but showed acute liver injury prior to treatment, displaying the highest ALP, ALT, and AST levels of all the animals in the cohort on day 7 p.i. prior to receiving its dose of MBP134AF (Figure 6G-6I, S4, and S5). Given the recovery of two animals (NHP-4 and NHP-6) harboring higher viral loads prior to treatment, we postulate that NHP-5’s liver injury prior to treatment was too severe for it to recover despite receiving MBP134AF. In summary, MBP134AF demonstrates significant levels of protection and reversion of BDBV disease in cynomolgus macaques.
Figure 6. A single 25 mg/kg dose of MBP134AF protects cynomolgus macaques challenged with BDBV/But-811250.
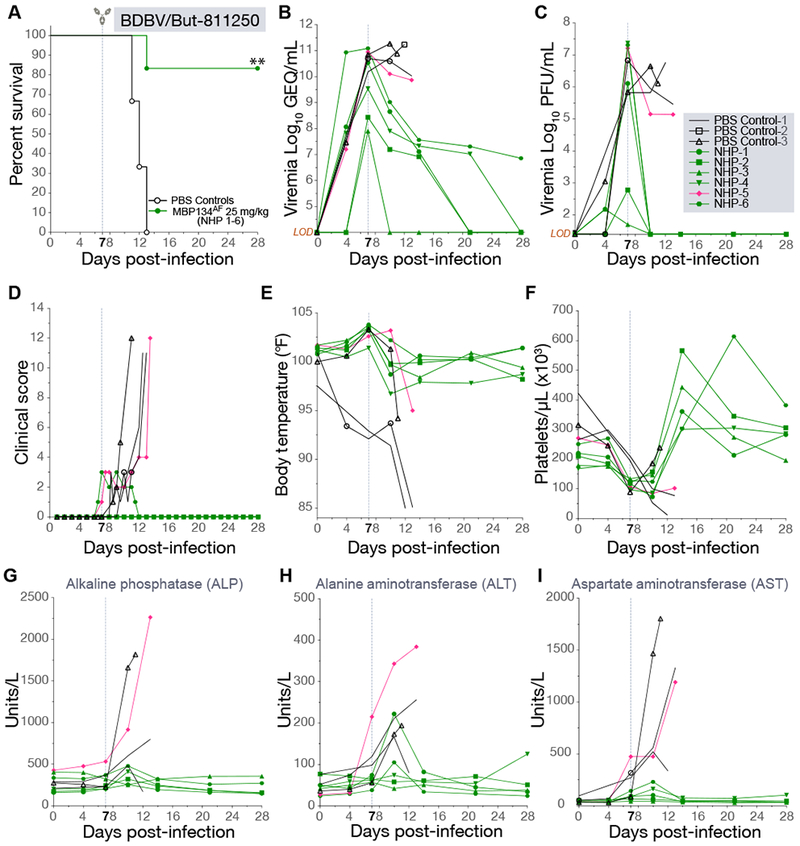
(A) Survival curves for NHPs challenged with BDBV/But-811250 and treated with a single 25-mg/kg dose of MBP134AF on day 7 post infection (green or pink) or PBS (black). **, P<0.01.
(B) The average GEQ/mL of BDBV/But-811250 present in the blood of animals treated with a single dose of MBP134AF (green or pink) or PBS (black). Serum samples taken from NHP-4 and NHP-6 on days 20 and 28 tested negative for viral genetic material.
(C) Infectious BDBV/But-811250 (PFU/mL) present in the blood of animals treated with MBP134AF (green or pink) or PBS (black). Infectious BDBV was no longer detectable by plaque assay by the next bleed on day 10 post infection in the surviving animals.
(D) Clinical scores show NHP-5 and NHP-6 registering scores from refusing nutrition, having a hunched posture and displaying petechiation over 10% of their bodies prior to receiving MBP134AF on day 7 p.i. While NHP-6 cleared clinical signs of infection by day 12 p.i., NHP-5 failed to recover and succumbed to infection on 13 p.i.
(E) Body temperatures from all the animals showed the majority registered an elevated temperature by day 7 p.i. prior to receiving MBP134AF.
(F) The platelet counts for the cohort show thrombocytopenia occurring in all the animals by day 7 p.i. prior to receiving MBP134AF. All the MBP134AF treated animals (excluding NHP-5, pink) cleared signs of thrombocytopenia by day 14 p.i., seven days post treatment.
(G) ALP levels are graphed for each animal in the cohort.
(H) ALT levels from each animal are shown. Notably NHP-5 (pink) displayed advanced signs of EVD prior to treatment.
(I) AST levels for the challenged animals show varying signs of liver damage resultant of EVD. Samples from NHP-5 (pink) in particular show a significant spike in AST levels suggesting a severe onset of disease beyond that of the other animals in the cohort, a possible explanation of NHP-5’s succumbing to EVD despite receiving the MBP134AF treatment course. Legend for graphs in top right-hand corner, LOD = limit of detection.
DISCUSSION
Prior to this work, the development of monoclonal antibody-based therapeutics has typically followed a “one bug, one drug” paradigm under the premise that mAbs with broad activity would not be as potent as those with clade-specific activity (Vigant et al., 2015). Here, we demonstrate that MBP134AF, a pan-ebolavirus immunotherapeutic comprising two bNAbs ADI-15878AF and ADI-23774AF could not only protect NHPs against every ebolavirus known to cause human disease outbreaks but could do so at an unparalleled single 25-mg/kg dose. Importantly, MBP134AF was effective against multiple ebolaviruses from different species, suggesting that it will retain activity in the face of both intra- and inter-species sequence divergence, a result of its targeting highly conserved epitopes in GP. Indeed, as shown in the companion paper (Wec et al.), MBP134AF recognizes and neutralizes entry by the newly identified Bombali virus glycoprotein. Further studies exploring 7.5 mg/kg or lower IV doses of MBP134AF could open the door to intramuscular or subcutaneous delivery via autoinjector, allowing for rapid and efficient drug administration to patients and reducing the burden on healthcare workers in the field and Ebola virus treatment units. The developmental path of MBP134AF presents a model for the rapid design of next-generation antiviral immunotherapeutics targeting World Health Organization-priority pathogens.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCES
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Larry Zeitlin. (Larry.Zeitlin@mappbio.com)
Experimental Model and Subject Details
Cell lines.
Vero female African grivet monkey kidney cells were obtained from American Type Culture Collection (ATCC) and maintained in high-glucose Dulbecco’s modified Eagle medium (DMEM; ThermoFisher, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 1% GlutaMAX (ThermoFisher), and 1% penicillin-streptomycin (ThermoFisher). Cells were maintained in a humidified 3713, 5% CO2 incubator. Suspension adapted HEK-293F (ThermoFisher) were maintained in serum free Freestyle HEK-293F expression medium (ThermoFisher). Cells were maintained in a humidified 37°C, 8% CO2, 125 RPM shaking incubator. CHOK1-AF female Cricetulus griseus ovarian SLC35C1-knockout stably expressing 23774AF or 15878AF (Mapp Biopharmaceuticals) were maintained in BalanCD Growth A medium containing MSX. Cells were maintained in a 37°C, 5% CO2 140 RPM shaking incubator.
Culture of primary human innate immune cells.
Primary human neutrophils and NK cells from deidentified donors were cultured and maintained in RPMI1640 supplemented with 10% fetal bovine serum, L-Glutamine, and penicillin/streptomycin at 37C in a humidified incubator at 5% CO2 for the duration of the assays.
Ethics statement for primary human innate immune cells.
Innate immune effector cells were isolated from fresh peripheral blood samples collected by the Ragon Institute or the MGH Blood bank from healthy human volunteers. All subjects signed informed consent and the study was approved by the MGH Institutional Review Board. Samples were collected from human adults older than 18 years of age, and were completely deidentified prior to use and thus, researchers were blinded to gender and age of donors.
Ferrets.
Female ferrets were purchased from Marshall Farms. Six to eight month old animals weighing between 0.75-1 kg, were housed 2-3 per cage per study were used for in vivo challenge studies as previously described (Cross et al., 2016).
Non-Human Primates.
Male and female rhesus macaques and cynomolgus monkeys were purchased from PrimGen. Animals aged 3-8 years old (4-7.8 kg) were randomly assigned to treatment groups, with even distribution of male and female macaques.
Guinea Pigs.
Female Hartley guinea pigs were purchased from Charles River Laboratories (Wilmington, MA). Animals between 5-6 weeks old, weighing 350-450 g, were randomly split into groups of 3-6 for in vivo challenge studies.
USAMRIID animal welfare, observation and euthanasia criterion.
Animal studies were conducted under IACUC-approved protocols in compliance with the Animal Welfare Act, PHS Policy, and other applicable federal statutes and regulations relating to animals and experiments involving animals. USAMRIID is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, lnternational (AAALAC) and adhere to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council. Physical exams were also completed on blood collection days to assess the overall physical condition of the animals and disease progression. Cage side observations were completed at least once daily throughout the course of the experiment to assess the general disposition of each animal and disease progression. Clinical scores for each animal were derived from cage side observations of awake animals and physical observations completed on anesthetized animals. Animals were assessed and given a cumulative score based on numerous behavioral and physical parameters, including responsiveness, petechial rash, temperature, weight change, gastrointestinal and renal output, anorexia, respiration, dehydration, and edema. Higher clinical scores indicate more severe signs of disease. Macaques determined to be moribund, in accordance with the USAMRIID Institutional Animal Care and Use Committee (IACUC) approved criteria, were promptly euthanized.
UTMB animal welfare, observation and euthanasia criterion.
All animal studies were conducted under a protocol approved by the University of Texas Medical Branch (UTMB) IACUC. IACUC approval of animal studies performed at UTMB are in compliance with the Animal Welfare Act, PHS Policy and other Federal statutes and regulations relating to animals and experiments involving animals. The facilities where this research was conducted are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and adhere to principles stated in the eighth edition of the Guide for the Care and Use of Laboratory Animals, National Research Council. All animals were monitored daily and scored for disease progression with an internal filovirus scoring protocol approved by the UTMB IACUC. The scoring changes measured from baseline included posture/activity level, attitude/behavior, food intake, respiration, and disease manifestations such as visible rash, hemorrhage, ecchymosis, or flushed skin. A score of > 9 indicated that an animal met criteria for euthanasia.
Authentic filoviruses.
The authentic filoviruses EBOV/Makona (Ebola virus/H.sapiens-tc/GIN/14/Makona-Gueckedou-C07) (Mire et al., 2015), EBOV/Kikwit (Ebola virus/H. sap-tc/COD/95/Kikwit-9510621) (Cross et al., 2016), BDBV (BDBV/H.sapiens-tc/UGA/07/But-811250) (Cross et al., 2016), SUDV/Gulu (Sudan virus/H.sapiens-tc/UGA/00/Gulu-200011676) (Thi et al. 2016), SUDV/Boneface (Sudan virus/H. sap-gp-tc/SDN/1976/Boneface-USAMRIID111808) (Ellis et al., 1978), Guinea pig-adapted SUDV/Boniface (SUDV-GPA) (Sudan virus/NML/C.porcellus-lab/SSD/1976/Nzara-Boneface-GP) (Wong et al, 2016) were used in this study.
METHOD DETAILS
Expression and purification of MBP134AF mAbs from ΔXTFT N. benthamiana plants.
The transient expression and purification of ADI-23774AF and ADI-15878AF from N. benthamiana tobacco plant expression platform was performed as previously described(Olinger et al., 2012).
Expression of ADI-23774AF and ADI-15878AF from CHOK1-AF cell lines.
CHOK1-AF cells stably expressing the ADI-23774Af and ADI-15878Af mAbs were generated as follows: a dual plasmid system containing expression cassettes for the heavy and light chains of the target mAb were co-transfected by chemical means into a modified CHOK1 host cell line. Stable selection was initiated by exchanging medium in the shake flask with growth medium containing MSX as a selection agent 24 hours post transfection. Upon recovery of the cells (~3 weeks), a Research Cell Bank was created for further development. Enriched stable pools were created from the original transfected pool using ClonePix2 technology (Molecular Devices). A vial from an enriched pool of CHOK1-AF cells expressing ADI-23774Af or ADI-15878Af was removed from cryostorage, thawed, and resuspended in 30 mL of BalanCD Growth A medium containing MSX in a 125 mL shake flask. The newly thawed culture was placed in a shaking CO2 incubator set at 5% CO2 and 140 RPM. Over the next 14 days the culture was expanded to a final volume of 13L in multiple 5L shake flasks (Thomson) for production. Upon completion of the final expansion the culture was maintained in batch-mode for 10 days, after which the supernatant was clarified via filtration and subsequently sterile filtered (0.2 μm) into a 20L bioprocess bag (Thermo Fisher) prior to protein A purification.
Purification of ADI-23774AF and ADI-15878AF.
MBP134AF mAbs, ADI-23774AF and ADI-15878AF, were purified using a GE MabSelect SuRe LX Protein A affinity chromatography column using an AKTA pure 150M3. The conditioned media were loaded onto the MabSelect column, washed with HyClone 1X PBS, then eluted using 0.1 M Acetic Acid containing 0.2 M L-Arginine, pH 2.9-3.0. Immediately post elution the mAbs were held at low pH for 3 hours as a viral inactivation step, then eluates were neutralized with 2 M Tris base to pH ~7. Neutralized eluate was then filtered through Sartorius Sartobind Q SingleSep Mini filters for endotoxin and host-cell DNA removal and diafiltered against a formulation buffer (20 mM Citrate, 10 mM Glycine, 8% Sucrose, pH 5.5) using a GE AKTA flux 6 TFF system equipped with a Sartorius Sartocon Slice Disposable PESU Cassette (MWCO of 50 kDa). After diafiltration, the mAbs were concentrated using a Millipore Labscale TFF system equipped with a Sartorius Vivaflow 200 Cassette (MWCO of 50 kDa) to concentration of 25-30 mg/mL. Polysorbate-80 was added to 0.01% after the target mAb concentration was reached. The MBP134AF mAb cocktail, consisting of ADI-23774AF and ADI-15878AF, was generated by mixing equal amounts, by protein mass, of the two mAbs.
UTMB ferret ebolavirus challenge studies.
Female ferrets were housed and challenged intranasally as previously described(Cross et al., 2016). Briefly, ferrets weighing between 0.75-1 kg were housed 2-3 per cage per study. Ferrets were anesthetized by intramuscular injection with a ketamine-acepromazine-xylazine cocktail prior to all procedures. Subjects were challenged intranasally with 1000 PFU of EBOV/Makona (n = 9), SUDV/Gulu (n = 9), and BDBV/But-811250 (n = 9), respectively. The treated ferrets received doses of MBP134AF via intraperitoneal (IP) injection on days 2 and 5 or days 3 and 6. Ferrets challenged with EBOV/Makona and treated on days 2 and 5 were bled on either day 0, 2, 5, and 33 or at time of euthanasia, while those treated on days 3 and 6 were bled on days 0, 3, 6, and 33 or upon being euthanatized. Animals challenged with SUDV/Gulu or BDBV/But-811250 were bled on days 0, 3, 6, and 28 or upon being euthanatized.
UTMB EBOV/Kikwit NHP challenge study.
Male and female rhesus macaques, ten in total, were challenged by intramuscular (IM) injection with 1,000 PFU of EBOV/Kikwit (actual dose: 988 PFU), isolate 199510621 from a 65-year-old female patient who had died on May 5th 1995. Animals (n=4/group) were treated IV with either a single 25 mg/kg dose on day 4 or a two-dose regimen of 50 mg/kg (on day 4) and 25 mg/kg (on day 7) of MBP134AF produced in ΔXTFT N. benthamiana plants. Control animals (n=2) received a pseudo IV treatment of PBS. Blood samples were collected from experimental macaques and control macaques on days 0, 4, 7, 10, 14, 21, and 28 p.i. The EBOV/Kikwit plaque assays and real-time qPCR analyses from whole blood and tissue samples were performed as previously described(Geisbert et al., 2009; Matassov et al., 2017).
USAMRIID SUDV/Boniface guinea pig challenge study.
Female Hartley guinea pigs (350-450 g) were randomly assigned to experimental groups and challenged IP with a target dose of 1000 PFU of guinea pig adapted SUDV(Wong et al., 2016) in 0.5 mL of MEM. Groups (n = 3-6) of guinea pigs were treated with 5mg of MBP134AF-N (plant expressed) or MBP134AF-CHO via IP injection on days 4 (n=6), 5 (n=3), 6 (n=5), or 7 (n=5) p.i. Control guinea pigs (n=6) were given an equal volume of vehicle on day 4 p.i. Blood was collected from each animal just prior to antibody delivery to determine viremia. Animals were observed for clinical signs of disease, survival, weight change and temperature change for 14 days, while survival was monitored for an additional 11 days.
USAMRIID SUDV/Boniface rhesus macaque study.
Male and female rhesus macaques (4-7.8 kg) were randomly assigned to treatment groups, with even distribution of male and female macaques, and study personnel remained blinded to all treatment groups. Macaques were inoculated via IM injection with a target dose of 1000 pfu (actual dose: 1750 pfu) of SUDV/Boniface. Experimental macaques were treated IV with a cocktail of CHOK1-AF produced MBP134AF. Control macaques (n=2) received an equal volume to weight ratio of vehicle IV on day 5 p.i. Blood samples were collected from experimental macaques and control macaques on days 0, 3, 5, 8, 11, 14, 21, and 28 p.i. Additional control animals (n=2, PBS Control-3 and PBS Control-4) from a separate experimental cohort run concurrently received an equal volume to weight ratio of vehicle IV on days 4 and 6 p.i. These animals were bled on days 0, 4, 6, 8, 11, 14, 21, and 28 p.i.
USAMRIID Hematology and serum biochemistry from the SUDV/Boniface NHP study.
Phlebotomy was performed while the animals were anesthetized, and blood was collected from the femoral vein using a venous blood collection system (Becton Dickinson). Hematological values for blood samples collected in tubes containing EDTA were determined using a Coulter® Ac-T diff™ analyzer (Beckman Coulter). Serum chemistry was analyzed using Piccolo® General Chemistry 13 reagent discs and a Piccolo Xpress® point-of-care blood analyzer (Abaxis).
USAMRIID SUDV/Boniface plaque assays.
Serial dilutions of serum were prepared in modified Eagle medium with Earle’s balanced salts and nonessential amino acids supplemented with 5% ΔFBS, 2mM L-glutamine and 1% gentamicin. Then 200 μl from each dilution was inoculated onto Vero E6 cell monolayers withinn 6-well plates. After adsorption for 1 hour at 37°C, 5% CO2, and 80% humidity, the cell monolayers were overlaid with a mixture of 1 part 1% agarose (Seakem) and 1 part 2X Eagle basal medium, 30mM HEPES buffer, and 5% ΔFBS. After incubation at 37Ό, 5% CO2, and 80% humidity for 8 days, a second overlay, supplemented with 5% neutral red, was then added. Plaques formed by infectious SUDV/Boniface virus were then counted 24 hours later and titers were expressed as PFU/mL.
USAMRIID Quantitative real-time PCR (qRT-PCR) assay.
SUDV/Boniface GEQ/mL in blood samples collected from macaques was determined by qRT-PCR as previously described(Olinger et al., 2012). Here, whole blood was mixed 1:3 with Trizol LS (Thermo Fisher) and RNA was extracted using the QIAamp Viral RNA Mini Kit in accordance with manufacturers guidelines. The qRT-PCR reactions were then completed using SUDV-specific primer/probe pairs on an Applied Biosystems 7500 Fast Dx System. Synthetic RNA, representative of the primer target region of the SUDV genome, was used to generate an eight-point standard curve. The GEQ/mL for each blood sample was then calculated by applying the cycle-threshold values of an individual sample to the synthetic RNA standard curve. The Qiagen QuantiFast Internal Control RT-PCR assay was used to monitor PCR inhibition and extraction integrity.
UTMB BDBV/But-811250 NHP challenge study.
Male and female cynomolgus monkeys at UTMB were challenged IM with target dose of 1,000 PFU of BDBV (200706291 2007 Uganda isolate, Vero E6 passage 2), actual dose was determined to be 938 PFU. One treatment group (n = 6) was treated with a single 25 mg/kg dose of MBP134AF (from CHOK1-AF) on day 7 post infection via IV infusion. Control animals (n = 3) received PBS as a vehicle control. All the animals were given physical examinations and blood was collected at the time of viral challenge; and on days 4, 7, 10, 14, 21, and 28 after challenge (or at time of euthanasia). The BDBV/But-811250 plaque assays and real-time qPCR analyses from whole blood and tissue samples were performed as previously described(Falzarano et al., 2011; Mire et al., 2013).
UTMB Hematology and serum biochemistry from EBOV/Kikwit and BDBV/But-811250 NHP challenge studies.
Total white blood cell counts, white blood cell differentials, red blood cell counts, platelet counts, hematocrit values, total hemoglobin concentrations, mean cell volumes, mean corpuscular volumes, and mean corpuscular hemoglobin concentrations were analyzed from blood collected in tubes containing EDTA using a laser based hematologic analyzer (Beckman Coulter). Serum samples were tested for concentrations of albumin, amylase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyltransferase (GGT), glucose, calcium, uric acid, total protein, blood urea nitrogen (BUN), creatinine (CRE), and C-reactive protein (CRP) by using a Piccolo point-of-care analyzer and Biochemistry Panel Plus analyzer discs (Abaxis).
Antibody-mediated activation of human NK cells.
Human NK cells were enriched from the peripheral blood of three different human donors by negative selection using RosetteSep negative selection kit (Stem Cell Technologies) followed by Ficoll separation. NK cells were rested overnight in the presence of 1ng/ml recombinant IL-15 (PeproTech). 3 ng/well of EBOV GP (IBT Bioservices) was coated on a Maxisorp ELISA plate (Nunc) at 4°C overnight, and plates were blocked with 5% BSA prior to addition of dilutions of antibodies (10pg/ml) in PBS for 2 hours at 37°C. Unbound antibodies were removed by washing wells 3X with PBS prior to addition of NK cells. The NK cells were added at 5 × 104 cells/well in the presence of brefeldin A (Sigma Aldrich), GolgiStop (BD), and anti-CD107a PE-Cy5 antibody (BD Biosciences clone H4A3) and incubated for 5 hours at 37°C. NK cells were stained with flow cytometry antibodies for the following surface markers: CD3 AlexaFluor700 (BD Biosciences clone UCHT1), CD56 PE-Cy7 (BD Biosciences clone B159), and CD16 APC-Cy7 (BD Biosciences clone 3G8), followed by intracellular staining for IFNγ (FITC, BD Biosciences clone B27) and MIP-Iβ (PE, BD Biosciences clone D21-1351) to detect the production of cytokines and chemokines. Cells were analyzed by flow cytometry on a BD LSR2 flow cytometer and data was analyzed using FlowJo software.
Antibody-dependent neutrophils phagocytosis.
EBOV GP recombinant protein (IBT Bioservices) was biotinylated and conjugated to streptavidin-coated Alexa488 beads (Life Technologies). EBOV GP-coated beads were incubated with antibodies diluted in culture medium to 5μg/ml for 2 hours at 37°C. Human white blood cells were isolated from peripheral blood by lysis of red blood cells using ammonium chloride potassium (ACK) lysis buffer. Cells were washed with PBS, and 5.0 × 104 cells/well were added to bead-antibody immune complexes, and incubated for 1 hour at 37°C. Cells were stained with the following antibodies to identify neutrophils: CD66b Pacific Blue (BioLegend clone G10F5), CD14 APC-Cy7 (BD Biosciences clone MφP9), and CD3 AlexaFluor700 (BD Biosciences clone UCHT1). Cells were fixed with 4% paraformaldehyde and were analyzed on a BD LSRII flow cytometer. Data was analyzed using FlowJo software, and a phagocytic score was determined using the following calculation: (% of AlexaFluor488+ cells)*(AlexaFluor488 geometric mean fluorescent intensity (MFI) of AlexaFluor488+ cells)/10,000.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analysis of survival curves was performed with the Mantel-Cox (log-rank) test. Statistical comparisons of NK cell activity were carried out by one-way ANOVA with Tukey’s correction for multiple comparisons testing. All analyses were carried out in GraphPad Prism.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| MBP134AF | this study, Adimab, LLC; Mapp Bio | RRID: AB_2750598 |
| Mouse anti-human CD107a (clone H4A3) | BD Biosciences | RRID: AB_396136 |
| Mouse anti-human CD3 (clone UCHT1) | BD Biosciences | RRID: AB_396952 |
| Mouse anti-human CD56 (clone B159) | BD Biosciences | RRID: AB_396853 |
| Mouse anti-human CD16 (clone 3G8) | BD Biosciences | RRID: AB_396864 |
| Mouse anti-human IFNγ (clone B27) | BD Biosciences | RRID: AB_398580 |
| Mouse anti-human MIP-1β (clone D21-1351) | BD Biosciences | RRID: AB_393549 |
| Mouse anti-human CD66b (clone G10F5) | BioLegend | RRID: AB_2563294 |
| Mouse anti-human CD14 (clone MφP9) | BD Biosciences | Cat # 561709 |
| Bacteria and Viruses | ||
| EBOV/Makona (Ebola virus/H.sapiens-tc/GIN/14/Makona-Gueckedou-C07) | (Mire et al. 2015); UTMB | N/A |
| EBOV/Kikwit (Ebola virus/H. sap-tc/COD/95/Kikwit-9510621) | (Cross et al., 2016); UTMB | N/A |
| BDBV (BDBV/H.sapiens-tc/UGA/07/But-811250) | (Cross et al., 2016); UTMB | N/A |
| SUDV/Gulu (Sudan virus/H.sapiens-tc/UGA/00/Gulu-200011676) | (Thi et al, 2016); UTMB | N/A |
| SUDV/Boneface (Sudan virus/H. sap-gp-tc/SDN/1976/Boneface-USAM RIID111808) | (Ellis et al., 1978); USAMRIID | N/A |
| Guinea pig-adapted SUDV/Boniface (SUDV-GPA) (Sudan virus/NML/C.porcellus-lab/SS D/1976/Nzara-Boneface-GP) | (Wong et al., 2016); USAMRI ID | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Soluble EBOVΔTM GP | IBT Bioservices | N/A |
| Brefeldin A | Sigma Aldrich | Cat# B7651 |
| GolgiStop | BD Biosciences | Cat# 554724 |
| Critical Commercial Assays | ||
| RosetteSep NK cell enrichment kit | Stem Cell Technologies | Cat# 15025 |
| Experimental Models: Cell Lines | ||
| HEK293-Freestyle | Thermo Fisher | R79007 |
| CHOK1-AF 15878AF | This study; Mapp Bio | N/A |
| CHOK1-AF 23774AF | This study; Mapp Bio | N/A |
| Vero C1008 cells | ATCC | CRL-1586 |
| (VERO 76, clone E6, Vero E6) | ||
| Primary human immune cells (NK cells) | MGH Blood Bank; Ragon Institute Clinical Core | N/A |
| Primary human immune cells (Neutrophil) | MGH Blood Bank; Ragon Institute Clinical Core | N/A |
| Experimental Models: Organisms/Strains | ||
| ΔXTFT N. benthamiana | (Olinger, G. et al. 2012) | N/A |
| Hartley guinea pigs: female | Charles River | N/A |
| NHP: rhesus macaque | PrimGen | N/A |
| NHP: cynomolgus macaques | PrimGen | N/A |
| Ferret: Female | Marshall Farms | N/A |
| Sequence-based reagents | ||
| EBOV (Makona and Kikwit): VP30 FWD: AGC ACG ATC ATC ATC CAG AG | This study | N/A |
| EBOV (Makona and Kikwit): VP30 REV: TAC AGT AGG AAC GCG CAC TT | This study | N/A |
| SUDV (Gulu): L FWD: TCA AAT ATT GCA ACC AAT GCT ATG | This study | N/A |
| SUDV (Gulu): L REV: GCA TGT AAC ATT GCG GAA TTA GG | This study | N/A |
| BDBV: VP35 intergenic FWD: CTG TTC CAC CAT CAC CAA AG | This study | N/A |
| BDBV: VP35 intergenic REV: GAT TCC GGA AGG AAG CAA TA | This study | N/A |
| Software and Algorithms | ||
| Prism | Graph Pad | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| ClonePix2 | Molecular Devices | https://www.moleculardevices.com/products/clone-screening-systems/mammalian-screening/clonepix-2-mammalian-colony-picker#gref |
| Coulter® Ac-T diff™ analyzer | Beckman Coulter | https://www.beckmancoulter.com/en/products/hematology/coulter-act-diff |
| Piccolo Xpress® point-of-care blood analyzer | Abaxis | https://www.abaxis.com/medical/piccolo-xpress |
Supplementary Material
HIGHLIGHTS.
The mAb cocktail MBP134AF protects ferrets from lethal EBOV, SUDV and BDBV challenge
A single 25 mg/kg dose of MBP134AF protects NHPs challenged with EBOV, SUDV and BDBV
Both CHOK1-AF cell-produced and plant produced MBP134AF are therapeutically effective
ACKNOWLEDGEMENTS
This project has been funded in part with federal funds from the Department of Defense under Contract No. HDTRA1-13-C-0018; the Department of Health and Human Services’ National Institutes of Health (U19AI109711, U19AI109762, AI1332204 and AI132256), the Public Health Agency of Canada (PHAC), and Office of the Assistant Secretary for Preparedness and Response, and Biomedical Advanced Research and Development Authority (BARDA), under Contract No. HHS0100201700023C. K.C. was additionally supported by an Irma T. Hirschl/Monique Weill-Caulier Research Award. Opinions, conclusions, interpretations, and recommendations are those of the authors and are not necessarily endorsed by the US. Army. The mention of trade names or commercial products does not constitute endorsement or recommendation for use by the Department of the Army or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
A.Z.W., Z.A.B., L.Z., L.M.W., and K.C. are co-inventors on a provisional patent application (No. 62/460,200) assigned to Mapp Biopharmaceutical Inc., Adimab LLC, and Albert Einstein College of Medicine Inc.) describing the development and use of MBP134 and variants thereof for anti-ebolavirus immunotherapy. A.Z.W., E.G., and L.M.W. are employees and shareholders in Adimab LLC. Z.A.B., D.M.A., N.B., O.B., D.H.K., M.H.P., J.V., K.J., and L.Z. are employees and shareholders in Mapp Biopharmaceutical Inc. K.J. and L.Z. are CEO and President of Mapp Biopharmaceutical Inc., respectively.
REFERENCES
- Bornholdt ZA, Turner HL, Murin CD, Li W, Sok D, Souders CA, Piper AE, Goff A, Shamblin JD, Wollen SE, et al. (2016). Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science 351, 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk R, Bollinger L, Johnson JC, Wada J, Radoshitzky SR, Palacios G, Bavari S, Jahrling PB, and Kuhn JH (2016). Neglected filoviruses. FEMS Microbiol Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, Ploquin A, Doria-Rose NA, Staupe RP, Bailey M, et al. (2016). Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. [DOI] [PubMed] [Google Scholar]
- Cross RW, Mire CE, Borisevich V, Geisbert JB, Fenton KA, and Geisbert TW (2016). The Domestic Ferret (Mustela putorius furo) as a Lethal Infection Model for 3 Species of Ebolavirus. J Infect Dis 214, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross RW, Speranza E, Borisevich V, Widen SG, Wood TG, Shim RS, Adams RD, Gerhardt DM, Bennett RS, Honko AN, et al. (2018). Comparative Transcriptomics in Ebola Makona-Infected Ferrets, Nonhuman Primates, and Humans. J Infect Dis 10.1093/infdis/jiy455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DS, Bowen ET, Simpson DI, and Stamford S (1978). Ebola virus: a comparison, at ultrastructural level, of the behaviour of the Sudan and Zaire strains in monkeys. Br J Exp Pathol 59, 584–593. [PMC free article] [PubMed] [Google Scholar]
- Falzarano D, Feldmann F, Grolla A, Leung A, Ebihara H, Strong JE, Marzi A, Takada A, Jones S, Gren J, et al. (2011). Single immunization with a monovalent vesicular stomatitis virus-based vaccine protects nonhuman primates against heterologous challenge with Bundibugyo ebolavirus. J Infect Dis 204 Suppl 3, S1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, and Feldmann H (2009). Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol 83, 7296–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein T, Anthony SJ, Gbakima A, Bird BH, Bangura J, Tremeau-Bravard A, Belaganahalli MN, Wells HL, Dhanota JK, Liang E, et al. (2018). The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat Microbiol 3, 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak R, He S, Kroeker A, de La Vega MA, Audet J, Wong G, Urfano C, Antonation K, Embury-Hyatt C, Kobinger GP, et al. (2016). Ferrets Infected with Bundibugyo Virus or Ebola Virus Recapitulate Important Aspects of Human Filovirus Disease. J Virol 90, 9209–9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, and Gleba Y (2005). Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol 23, 718–723. [DOI] [PubMed] [Google Scholar]
- Matassov D, Mire CE, Latham T, Geisbert JB, Xu R, Ota-Setlik A, Agans KN, Kobs DJ, Wendling MQS, Burnaugh A, et al. (2017). Single Dose Trivalent Vesiculovax Vaccine Protects Macaques from Lethal Ebolavirus and Marburgvirus Challenge. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire CE, Geisbert JB, Marzi A, Agans KN, Feldmann H, and Geisbert TW (2013). Vesicular stomatitis virus-based vaccines protect nonhuman primates against Bundibugyo ebolavirus. PLoS Negl Trop Dis 7, e2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire CE, Matassov D, Geisbert JB, Latham TE, Agans KN, Xu R, Ota-Setlik A, Egan MA, Fenton KA, Clarke DK, et al. (2015). Single-dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature 520, 688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinger GG Jr., Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, et al. (2012). Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci U S A 109, 18030–18035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal KE, Dudgeon D, Trefry JC, Anantpadma M, Sakurai Y, Murin CD, Turner HL, Fairhurst J, Torres M, Rafique A, et al. (2018). Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in non-human primates. J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Audet J, Lv M, He S, Wong G, Wei H, Luo L, Fernando L, Kroeker A, Fausther Bovendo H, et al. (2016). Two-mAb cocktail protects macaques against the Makona variant of Ebola virus. Sci Transl Med 8, 329ra333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, et al. (2014). Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi EP, Lee AC, Geisbert JB, Ursic-Bedoya R, Agans KN, Robbins M, Deer DJ, Fenton KA, Kondratowicz AS, MacLachlan I, et al. (2016). Rescue of non-human primates from advanced Sudan ebolavirus infection with lipid encapsulated siRNA. Nat Microbiol 1, 16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, and Katinger H (1996). Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol 70, 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigant F, Santos NC, and Lee B (2015). Broad-spectrum antivirals against viral fusion. Nat Rev Microbiol 13, 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wec AZ, Herbert AS, Murin CD, Nyakatura EK, Abelson DM, Fels JM, He S, James RM, de La Vega MA, Zhu W, et al. (2017). Antibodies from a Human Survivor Define Sites of Vulnerability for Broad Protection against Ebolaviruses. Cell 169, 878–890 e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G, He S, Wei H, Kroeker A, Audet J, Leung A, Cutts T, Graham J, Kobasa D, Embury-Hyatt C, et al. (2016). Development and Characterization of a Guinea Pig-Adapted Sudan Virus. J Virol 90, 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, Hiatt A, Ngo L, Steinkellner H, Whaley KJ, et al. (2011). Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci U S A 108, 20690–20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


