Abstract
Genetic and pharmacological manipulation of endocannabinoid (eCB) signaling has previously been shown to have an important role on the rewarding properties of drugs of abuse, including cocaine. Recently, fatty acid binding proteins (FABPs) have been proposed as intracellular transporters of the endocannabinoid anandamide (AEA) as well as other bioactive lipids to their catabolic enzyme, fatty acid amide hydrolase (FAAH). The role of these transporters in modulating the brains reward system has yet to be investigated. This study examined the effects of genetic deletion of FABP 5/7 on cocaine preference, as assessed by the Conditioned Place Preference (CPP) paradigm. Male and female wild type (WT) and FABP 5/7 KO mice showed similar acquisition of cocaine CPP, with no differences found in overall locomotor activity. In addition, while male and female WT mice showed stress-induced CPP for cocaine, male and female FABP 5/7 KO mice failed to show a stress-induced preference for the cocaine-paired chamber. Additionally, serum corticosterone levels were analyzed to explore any potential differences in stress response that may be responsi-ble for the lack of stress-induced preference for cocaine. Serum samples were obtained in animals under basal conditions as well as following a 30-min tube restraint stress. Male and female FABP 5/7 KO mice showed reduced corticosterone levels under stress compared to their WT counter-parts. The reduction in corticosterone response under stress may mediate that lack of a stress-induced preference for cocaine in the FABP 5/7 KO mice. Thus, the role of FABPs may play an important role in drug-seeking behavior under stressful conditions.
Keywords: addiction, cocaine, conditioned place preference, endocannabinoid
1 |. INTRODUCTION
Cocaine is a very potent drug of abuse that is capable of producing long-term deleterious effects in the central nervous system (CNS) (Buchta & Riegel, 2015). Cocaine elicits its rewarding effects by blocking the reuptake of monoamines, including the neurotransmitter dopamine (DA) (Howell & Kimmel, 2008). This in turn causes elevations in synaptic DA levels in areas like the nucleus accumbens (NAc), which is a key brain area involved in driving reward-related behavior (Kalivas & Duffy, 1990). Despite a large amount of work exploring cocaine addiction, many fundamental questions still remain (Moreira, Jupp, Belin, & Dalley, 2015). Early work has shown the endocannabinoid (eCB) system plays a key role in modulating the brains reward system, as it’s ubiquity in the CNS is capable of regulating multiple neurotransmitter systems (Parsons & Hurd, 2015). eCBs are retrograde messengers that activate cannabinoid receptors on presynaptic neurons, causing inhibition of neurotransmitter release (Lu & Mackie, 2016). Cannabinoid type-1 (CB1) receptors are widely distributed throughout the CNS, including in the reward-related mesolimbic and mesocortical pathways (Johnson & Lovinger, 2016), and are activated by the eCBs 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide/ AEA). Previous literature has shown that eCB signaling is capable of modulating DA neuronal activity in the ventral tegmental area (VTA) (Haj-Dahmane & Shen, 2010). Specifically, CB1 activation on presynaptic GABAergic neurons suppresses neurotransmitter release and enhances the activity of the dopamine neurons (Covey, Mateo, Sulzer, Cheer, & Lovinger, 2017). eCB transmission appears to have differential effects on the acquisition, maintenance, and relapse to cocaine in self-administration paradigms; changes in eCB tone seem to play a larger role in relapse to cocaine than either acquisition or maintenance (Arnold, 2005). While exogenous cannabinoids appear to change responsiveness to cocaine in some ways, cocaine is also capable of modifying brain eCB tone. Wang, Treadway, Covey, Cheer, and Lupica (2017) found that cocaine is capable of mobilizing 2-AG content in the VTA to increase the frequency of DA transients in the NAc. Moreover, eCB signaling has also been heavily explored in the context of stress-associated behaviors (Sartim, Moreira, & Joca, 2017). Namely, eCB tone in the amygdala has been demonstrated to play a pivotal role in regu-lating the hypothalamic–pituitary–adrenal (HPA), whose activation is largely responsible for the stress response (Hill et al., 2010). Therefore, the manipulation of eCB tone may also play an important role in stress-induced relapse to drugs of abuse like cocaine.
Fatty acid binding proteins (FABPs) have been shown to mediate the intracellular transport of AEA to fatty acid amide hydrolase (FAAH) (Kaczocha, Glaser, & Deutsch, 2009). Among the various FABPs isoforms, FABPs 5 (epidermal-type) and 7 (brain-type) are highly expressed in the CNS, and are potential targets for the modulation of AEA levels due to their tissue specificity (Berger et al., 2012). Genetic deletion of FABP 5 and 7 has been shown to increase AEA levels in the brain and elicits analgesic and anti-inflammatory effects (Kaczocha, 2015). However, it is unknown whether FABP manipulation affects the rewarding effects of drugs of abuse like cocaine. The current study seeks to address how global brain expression of FABPs 5 and 7 influence cocaine-seeking behavior as well as stress behavior.
2 |. METHODS
2.1 |. Animals
Male and female C57BL/6J (wild type, WT, n 531) and FABP 5/7 double knockouts (KO, n 5 25) (20–23 weeks of age) were used; KOs were gener-ated as previously described (Matsumata et al., 2012). Mice were single housed in temperature controlled conditions (228C) on a reverse light cycle (9:00–21:00), with all experiments being conducted between the hours of 09:00 and 16:00. Food and water were provided ad libitum. The proce-dures for this study conform to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the protocol was approved by the University Institutional Animal Care and Use Committee at the University of Buffalo.
2.2 |. Cocaine conditioned place preference
Cocaine conditioned place preference (CPP) was performed using commercially available equipment (Coulbourn Instruments, Allentown, PA) as pre-viously described (Thanos et al., 2016). Briefly, animals were subjected to one day of a 15 min preconditioning session to evaluate any baseline pref-erence for one of the two CPP test chambers before undergoing conditioning. The biased method was used and cocaine conditioning took place in the less-preferred chamber. The present study used chambers depicting either black and white striped walls with laminated gray flooring or with 1 inch polka dots on a white background with perforated stainless steel flooring. Alternating cocaine or vehicle administrations were performed (at 10 mg/kg, i.p.) on conditioning days (8 consecutive days with duration of 30 min). Preference was denoted as significantly higher time spent in the drug-paired chamber compared to the vehicle-paired chamber.
2.3 |. Extinction and reinstatement
Following test day, all mice underwent extinction which was carried out by daily 15 min sessions of free access to the apparatus without further drug exposure (Figure 1 timeline). Preference was considered extinguished when time spent in the cocaine-paired chamber did not significantly dif-fer from the vehicle-paired chamber. After extinction criteria had been reached, stress-induced reinstatement was carried out using the conical tube restraint procedure described by McGill et al. (2006). Mice were enclosed for 30 min in 50 mL propylene tubes with holes drilled for ventilation. They were then immediately placed into the central corridor of the testing apparatus for another test of chamber preference.
FIGURE 1.
Cocaine CPP experimental timeline. During conditioning, subjects received treatment on Days 2, 4, 6, and 8. All subjects received vehicle injections on Days 3, 5, 7, and 9
2.4 |. CPP locomotor activity
Locomotor activity was simultaneously assessed during the CPP by locomotor sensors mounted in each chamber. Total activity was denoted as the beam break sum for each subject and was averaged per day (cocaine or vehicle).
2.5 |. Corticosterone analysis
About 48 hr following stress-reinstatement, half of the mice from each group were physically restrained for 30 min in a Falcone tube to produce peak corticosterone levels (McGill et al., 2006) while the other half were left undisturbed in the home cage. Serum was obtained via cardiac punc-ture, allowed to clot for 30–45 min and spun at 3,000 RPM at 48C for 15 min. Serum was stored at 2808C until the day of analysis with an IBL Mouse/Rat Corticosteroid ELISA Kit (IBL-America, Minneapolis, MN). All samples were randomized and assayed in triplicate. Final values were obtained by averaging the results of triplicate samples.
2.6 |. Statistical analysis
Tests for preference, extinction, and reinstatement were carried out using Two-way ANOVAs with the factors of genotype and sex used to examine the effects of cocaine on test day preference, each reinstatement day, as well as for locomotor activity analysis. Tests yielding p < .05 were consid-ered statistically significant for all comparisons. Statistics and graphs were generated using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA) and Statistica 10.0 (TIBCO Inc., Palo Alto, CA).
3 |. RESULTS
3.1 |. Cocaine CPP
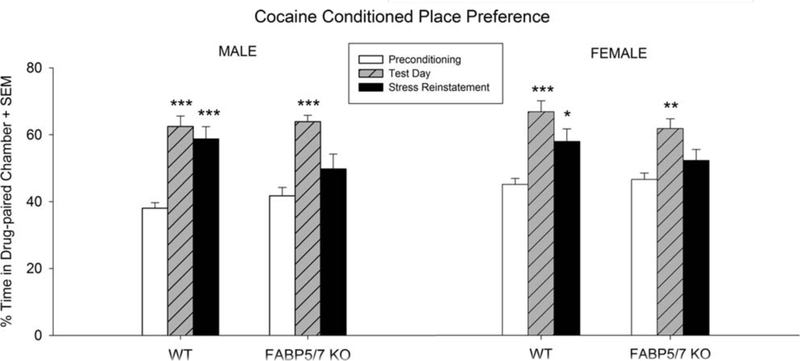
One-way ANOVAs were conducted within each sex and genotype to assess preference for cocaine-paired chamber (Figure 2). Male [F(2,42) 5 20.122; p < .001] and female [F(2,45) 512.892; p < .001] WT mice both showed a significant preference for the cocaine-paired chamber on test day compared to preconditioning. In addition, both male and female WT mice displayed a stress-induced preference for cocaine compared to pre-conditioning (p < .05 and p < .001, respectively).
FIGURE 2.
Cocaine CPP in male and female WT and FABP 5/7 KO mice. Mean percent of time spent in drug-paired chamber on precondi-tioning day, test day, and stress-induced reinstatement day. Stress-induced reinstatement consisted of placing in conical tubes for 30 min and retesting their preference for the cocaine-paired chamber. All groups showed a significant preference for the cocaine-paired chamber on test day. Male and female WT mice showed a stress-induced reinstatement of preference for the drug-paired chamber while male and female FABP 5/7 KO mice did not. Male WT n = 15, Female WT n = 16, Male KO n = 18, Female KO n = 7. *p < .05, **p < .01, ***p < .001
Male [F(2,51) 512.851; p < .001] and female [F(2,18) 5 7.861; p < .01] FABP 5/7 KO mice also showed a significant preference for the cocaine-paired chamber on test day compared to preconditioning. Unlike their WT counterparts, both male and female FABP 5/7 KO mice failed to show a stress-induced preference for the cocaine-paired.
3.2 |. CPP conditioning locomotor activity
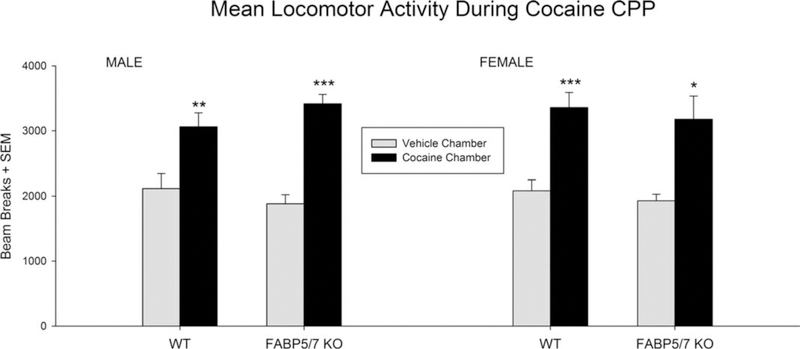
A three-way repeated measures ANOVA was conducted with the factors of Genotype [WT, FABP 5/7 KO], Sex [male, female], and Treatment [saline, cocaine] to assess locomotor activity across the eight conditioning days of CPP (Figure 3). Only a main effect for Treatment was found [F(1,47) 595.1446; p < .001] where higher locomotor activity was observed in mice following cocaine treatment. No genotype or sex differences were found.
FIGURE 3.
Locomotor activity throughout place conditioning. All four groups displayed greater mean locomotor activity on days when administered 10 mg/kg cocaine intraperitoneally compared to saline. Male WT n = 15, female WT n = 16, Male KO n = 18, Female KO n = 7. *p < .05, **p < .01, ***p < .001
3.3 |. Corticosterone analysis
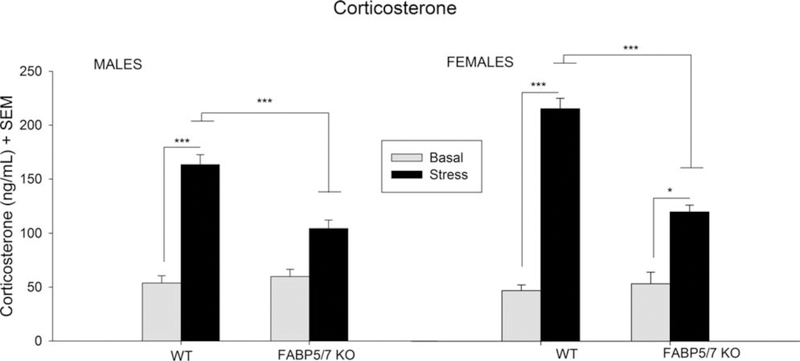
A three-way ANOVA was conducted with the factors of Genotype [WT, FABP 5/7 KO], Sex [male, female] and condition [nonstress, stress] to examine corticosterone levels (Figure 4). Significant main effects were found for Genotype [F(1,34) 524.8516; p < .001], Condition [F(1,34) 5 189.4089; p < .001], Sex 3 Condition [F(1,34) 5 6.2405; p < .001], and Genotype 3 Condition [F(1,34) 541.1386; p < .001]. Post hoc tests using Tukeys HSD showed WT male and female mice to have higher corticosterone levels following stress (p < .001). Similarly, the female FABP 5/7 KO mice also displayed corticosterone levels following stress (p < .05). However, corticosterone levels in male FABP 5/7 KO mice under stress did not differ from their nonstress counterparts. Both male and female FABP 5/7 KO groups showed reduced corticosterone levels under stress compared to their WT counterparts (p < .001).
FIGURE 4.
Corticosterone levels in male and female WT and FABP 5/7 KO mice under basal and stressed conditions. No changes in corticosterone were observed under basal conditions. All groups showed significantly higher corticosterone levels under stress apart from the male FABP 5/7 KO mice. Male and female FABP 5/7 KO mice show reduced corticosterone levels under stress compared to WT counterparts (p < .001). n 57 for male and female WT (for both stress and nonstress), n 54 for male and female FABP5/7 KO (for both stress and nonstress). *p < .05, ***p < .001
4 |. DISCUSSION
Here, we demonstrated that male and female mice with genetic deletion of FABP 5 and 7 did not show a stress-induced reinstatement for cocaine, which was seen in the WT cohorts. No differences in cocaine place preferences were seen between WT and FABP 5/7 KO groups, indicating that dual KO of the FABP 5 and 7 genes did not play a role in the acquisition for cocaine place preference. Therefore, genetic deletion of FABPs 5 and 7 seems to have a greater impact on stress behavior associated with cocaine-seeking behavior.
Numerous studies have demonstrated the importance of CB1 activity in regulating stress-related behavior and tonic suppression of the hypo-thalamus–pituitary–adrenal axis. Patel, Roelke, Rademacher, Cullinan, and Hillard (2004) have shown that antagonist of the CB1 receptor enhances the effects of restraint stress-induced activation of the HPA axis activation. Similarly, amplification of eCB signaling or the application of agonist of CB1 receptors was able to dampen or block restraint stress activation of the HPA axis, thereby reducing corticosterone release (Patel, Roelke, Rade-macher, & Hillard, 2005). In particular, eCB system involvement in the basolateral amygdala (BLA) been shown to be crucial to the tonic suppression of the HPA axis under basal conditions (Hill et al., 2010).
Within the BLA, activation of CB1 mediates the activity of glutamate neurons, thereby preventing the activation of the HPA axis. When a stres-sor is present, FAAH activity increases thereby lowering AEA and initiation the HPA axis in response (Hill et al., 2010). Studies have demonstrated that administration of FAAH inhibitors are capable of reducing anxiety and depression and have shown to significantly decrease corticosterone release in the response of stress (Hill et al., 2009; McLaughlin, Hill, & Gorzalka, 2014). The deletion of the FABP 5/7 genes have previously been shown to significantly increase endogenous AEA levels in the brain, similar to FAAH inhibition (Kaczocha, 2015). The dampen response to stress seen in the corticosterone analysis could be due to elevated AEA potentiating CB1 activation in the BLA. This dampening of the HPA axis activation may also explain the lack of stress-reinstatement found in the FABP 5/7 KOs. However, given that the same restraint stressor used prior to blood collection was also used during stress-induced reinstatement for cocaine CPP, the differences seen in corticosterone levels may be the results of dif-ferences in habituation to the stressor.
A study examining the role of corticosterone in stress-induced relapse demonstrated that footshock stress was unable to induce a stress-induced reinstatement in rats following an adrenalectomy (Erb, Shaham, & Stewart, 1998). However, this was reversed with the replacement of cor-ticosterone (Erb et al., 1998). This suggest that a proper corticosterone response is necessary to induce a stress-reinstatement in cocaine seeking animals. Accordingly, FABP 5/7 KOs may lack a stress-induced reinstatement due to the diminished corticosterone response.
In summary, FABPs appear to play an important role in modulating stress-induced effects on cocaine preference. Further work is needed to explore the extent to which FABPs can mediate the rewarding properties of other drugs of abuse as well as the precise mechanism by which this occurs.
ACKNOWLEDGMENT
This research was funded by the NY Research Foundation (RIAQ0940) and the NIH (DA035923, DA035949 and DA045863).
Funding information
NY Research Foundation, Award/Grant number: RIAQ0940; NIH, Award/Grant numbers: (DA035923, DA035949, DA045863
REFERENCES
- Arnold JC (2005). The role of endocannabinoid transmission in cocaine addiction. Pharmacology Biochemistry and Behavior, 81(2), 396–406. [DOI] [PubMed] [Google Scholar]
- Berger WT, Ralph BP, Kaczocha M, Sun J, Balius TE, Rizzo RC, … Deutsch DG (2012). Targeting fatty acid binding protein (FABP) ananda-mide transporters—A novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS One, 7(12), e50968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta WC, & Riegel AC (2015). Chronic cocaine disrupts mesocortical learning mechanisms. Brain Research, 1628 (Part A), 88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Mateo Y, Sulzer D, Cheer JF, & Lovinger DM (2017). Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacol-ogy, 124, 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, & Stewart J (1998). The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. Journal of Neuroscience, 18(14), 5529–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, & Shen RY (2010). Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A path-way in midbrain dopamine neurons. The Journal of Physiology, 588(14), 2589–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, & Gorzalka BB (2009). Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology, 34(13), 2733–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, & Bains JS (2010). Functional interactions between stress and the endocannabinoid system: From synaptic signaling to behavioral output. Journal of Neuroscience, 30(45), 14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, & Kimmel HL (2008). Monoamine transporters and psychostimulant addiction. Biochemical Pharmacology, 75(1), 196–217. [DOI] [PubMed] [Google Scholar]
- Johnson KA, & Lovinger DM (2016). Presynaptic G protein-coupled receptors: Gatekeepers of addiction? Frontiers in Cellular Neuroscience, 10, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M (2015). Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mech-anisms. Molecular Pain, 11, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, & Deutsch DG (2009). Identification of intracellular carriers for the endocannabinoid anandamide. Proceedings of National Academic Sciences United States of America, 106(15), 6375–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, & Duffy P (1990). Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse, 5(1), 48–58. [DOI] [PubMed] [Google Scholar]
- Lu HC, & Mackie K (2016). An introduction to the endogenous cannabinoid system. Biological Psychiatry, 79 (7), 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumata M, Sakayori N, Maekawa M, Owada Y, Yoshikawa T, & Osumi N (2012). The effects of Fabp7 and Fabp5 on postnatal hippocampal neurogenesis in the mouse. Stem Cells (Dayton, Ohio), 30(7), 1532–1543. [DOI] [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, & Zoghbi HY (2006). Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proceedings of National Academic Sciences United States of America, 103(48), 18267–18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, & Gorzalka BB (2014). A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emo-tional behavior. Neuroscience & Biobehavioral Reviews, 42, 116–131. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Jupp B, Belin D, & Dalley JW (2015). Endocannabinoids and striatal function: Implications for addiction-related behaviours. Behaviou-ral Pharmacology, 26(1–2), 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, & Hurd YL (2015). Endocannabinoid signalling in reward and addiction. Nature Reviews Neuroscience, 16(10), 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Roelke CT, Rademacher DJ, Cullinan WE, & Hillard CJ (2004). Endocannabinoid signaling negatively modulates stress-induced acti-vation of the hypothalamic-pituitary-adrenal axis. Endocrinology, 145(12), 5431–5438. [DOI] [PubMed] [Google Scholar]
- Patel SA, Roelke CT, Rademacher DJ, & Hillard CJ (2005). Inhibition of restraint stress-induced neural and behavioural activation by endoge-nous cannabinoid signalling. The European Journal of Neuroscience, 21(4), 1057–1069. [DOI] [PubMed] [Google Scholar]
- Sartim AG, Moreira FA, & Joca SR (2017). Involvement of CB1 and TRPV1 receptors located in the ventral medial prefrontal cortex in the modu-lation of stress coping behavior. Neuroscience, 340, 126–134. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Clavin BH, Hamilton J, O’rourke JR, Maher T, Koumas C, … Kaczocha M (2016). Examination of the addictive and behavioral properties of fatty acid binding protein inhibitor SBFI26. Frontiers in Psychiatry, 7, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Treadway T, Covey DP, Cheer JF, & Lupica CR (2015). Cocaine-induced endocannabinoid mobilization in the ventral tegmental area. Cell Report, 12(12), 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]