B cells are increasingly recognized as a therapeutic target in autoimmune diseases. B cell-depleting therapy using rituximab, a monoclonal antibody to CD20, is approved in rheumatoid arthritis and ANCA-associated vasculitis, and is frequently used off-label in lupus-nephritis 1,2. The rationale for targeting B cells has remained controversial in MS, particularly since major animal models of MS are independent of B cells 3,4. Nevertheless, rituximab, and its almost fully humanized successor, ocrelizumab, were tried in MS patients and appear to be exceedingly potent in suppressing signs of inflammation in the CNS and disability progression 5–7. Anti-CD20 therapy does not target plasma cells and, accordingly, the amount of immunoglobulin in patients treated with rituximab was not decreased. Thus, it is considered unlikely that rituximab acts by reducing autoantibody levels. Rather, several reports in experimental models and in humans suggest that B cells might play an important role as antigen-presenting cells for auto-antigens in MS 4. Steady state survival and maintenance of B cells is regulated by several means, including a network of B cell growth factors like BAFF and APRIL 8.
Although Sardinians have one of the highest rates of centenarians in the world, they also have the highest incidence rates of MS. To date, the genetic factors associated with MS in Sardinia have not been fully defined. This issue of The New England Journal of Medicine includes a report that has identified a novel insertion-deletion variant of TNFSF13B (encoding BAFF), which results in higher BAFF levels due to resistance of TNFSF13B mRNA degradation by microRNA. This variant results in enhanced humoral immunity and is associated with an increased risk of developing MS or SLE. The increased incidence of this variant of TNFSF13B in the Sardinian population appears to be the result of a selection process. The authors speculate that carriers of the variant have an increased fitness in host defense against malaria, which used to be endemic in Sardinia until the 1950ies. These findings are in line with studies that have found that BAFF plasma levels increase during acute malarial disease and that BAFF-over-expressing mice are protected from lethal malaria infections 9.
Examples of selection processes due to advantages in fitness of mutants causing monogenetic diseases are well appreciated: A mutation in the HBB locus leads to sickle cell anemia, but protects against disease burden in malaria 10. APC resistance due to a mutation in the F5 gene leads to thrombosis, but is protective in certain forms of sepsis 11. However, more recently, certain SNPs associated with complex genetic diseases were also demonstrated to be maintained in the genetic pool of distinct populations due to selective pressure: Besides BAFF (see present report), the adaptor protein SH2B3, which is a key negative regulator of cytokine signaling, may be an example for this phenomenon. A variant of SH2B3 (missense mutation), which is associated with increased risk of developing celiac disease, leads at the same time to increased responses of the pattern recognition receptor NOD2 upon stimulation with muramyl dipeptide and thus improved host defense against certain bacteria 12.
The BAFF system is complex. It has three receptors, BAFF-R, BCMA, and TACI. Soluble trimeric BAFF binds to BAFF-R, which is expressed on B cells. Trimeric APRIL, an alternative ligand, binds to BCMA, which is expressed on plasma cells. TACI, which is expressed on B cells and in particular on “innate-like” B1 cells, is only engaged by higher-order oligomers of BAFF and APRIL. While in general, BAFF and APRIL promote the expansion and differentiation of B cells and plasma cells, respectively, signaling through TACI can have complex outcomes. Unexpectedly, mice deficient in TACI exhibit increased B cell proliferation and autoimmunity 13,14. Therefore, effects of blocking BAFF are difficult to predict. Indeed, a large phase II trial with a blocking agent to BAFF and APRIL (atacicept) had to be halted in MS, since patients in the treatment arm developed twice as many relapses compared to placebo 15. This was unexpected as atacicept appears to be beneficial in rheumatoid arthritis and SLE 16,17. However, since one of the BAFF receptors (TACI) conveys inhibitory (pro-apoptotic) signals into B cells, adverse net effects might be inherent to this interventional strategy. An intense biomarker program accompanied that study and it turned out that high levels of BAFF mRNA were associated with a shorter time to first relapse, suggesting that BAFF expression is under a negative feedback control.
It will be a challenge for the future to assess whether the insertion-deletion variant of TNFSF13B can be used to stratify patients for a specific therapy. While the data of the present study clearly point into this direction, the discriminatory power of this solitary SNP may not be sufficient for clinical decision making. However, stratification of patients according to the variant of TNFSF13B may be useful for clinical trials assessing B cell directed therapies.
figure:
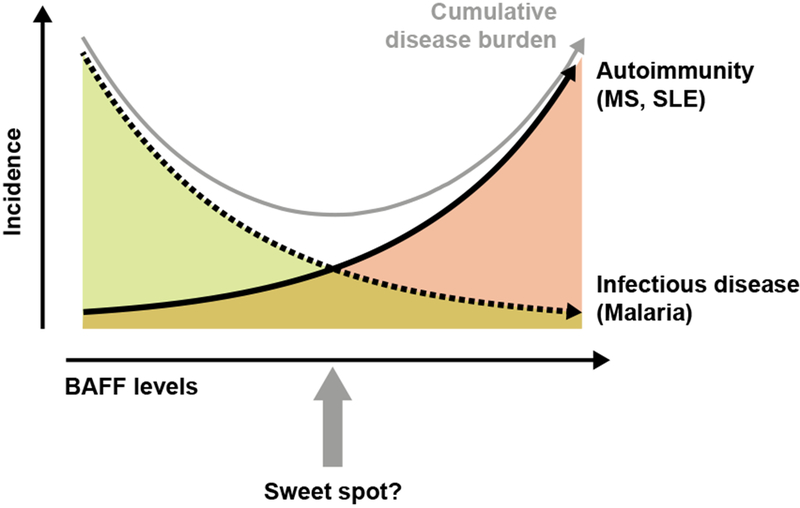
When disease incidence in a population is plotted against levels of BAFF, the area under the curve represents the disease burden of a given disease entity as a function of BAFF levels. In this issue of The New England Journal of Medicine, Cucca and colleagues provide evidence for an association of increased BAFF levels with an increased susceptibility for MS and SLE in the Sardinian population and show that the genetic variants of BAFF that lead to increased BAFF levels were selected because they result in a greater protection from Malaria infection. Increased levels of BAFF would result in a decrease in the disease burden due to Malaria but an increase in the disease burden due to autoimmune diseases. Thus, there might be an “optimum” BAFF level (sweet spot), at which the cumulative primitive function of both incidence curves reaches a minimum (gray curve).
References
- 1.Sanz I Systemic lupus erythematosus: Extent and patterns of off-label use of rituximab for SLE. Nat Rev Rheumatol 2016;12(12):700–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhelmus S, Bajema IM, Bertsias GK, et al. Lupus nephritis management guidelines compared. Nephrol Dial Transplant 2016;31(6):904–13. [DOI] [PubMed] [Google Scholar]
- 3.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol 2003;171(1):462–8. [DOI] [PubMed] [Google Scholar]
- 4.Molnarfi N, Schulze-Topphoff U, Weber MS, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med 2013;210(13):2921–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358(7):676–88. [DOI] [PubMed] [Google Scholar]
- 6.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 2016; [DOI] [PubMed] [Google Scholar]
- 7.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med 2016; [DOI] [PubMed] [Google Scholar]
- 8.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009;9(7):491–502. [DOI] [PubMed] [Google Scholar]
- 9.Liu XQ, Stacey KJ, Horne-Debets JM, et al. Malaria infection alters the expression of B-cell activating factor resulting in diminished memory antibody responses and survival. Eur J Immunol 2012;42(12):3291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aidoo M, Terlouw DJ, Kolczak MS, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 2002;359(9314):1311–2. [DOI] [PubMed] [Google Scholar]
- 11.Kerlin BA, Yan SB, Isermann BH, et al. Survival advantage associated with heterozygous factor V Leiden mutation in patients with severe sepsis and in mouse endotoxemia. Blood 2003;102(9):3085–92. [DOI] [PubMed] [Google Scholar]
- 12.Zhernakova A, Elbers CC, Ferwerda B, et al. Evolutionary and functional analysis of celiac risk loci reveals SH2B3 as a protective factor against bacterial infection. Am J Hum Genet 2010;86(6):970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan M, Wang H, Chan B, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol 2001;2(7):638–43. [DOI] [PubMed] [Google Scholar]
- 14.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity 2003;18(2):279–88. [DOI] [PubMed] [Google Scholar]
- 15.Kappos L, Hartung H-P, Freedman MS, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol 2014;13(4):353–63. [DOI] [PubMed] [Google Scholar]
- 16.Tak PP, Thurlings RM, Rossier C, et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum 2008;58(1):61–72. [DOI] [PubMed] [Google Scholar]
- 17.Isenberg D, Gordon C, Licu D, Copt S, Rossi CP, Wofsy D. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis 2015;74(11):2006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


