Abstract
Background:
Pre-exposure prophylaxis (PrEP) uptake has been slow among African American men who have sex with men (AAMSM) in the United States. We used an agent-based model (ABM) to simulate race-specific PrEP coverage to estimate their impact on racial disparities in HIV incidence among MSM in Atlanta, Georgia.
Methods:
An ABM was constructed to simulate HIV transmission in a dynamic network of 10,000 MSM over ten years, beginning in 2015. We modeled a base scenario with estimated PrEP coverage of 2.5% among AAMSM and 5.0% among white MSM (WMSM). We then compared HIV incidence over ten years and calculated a disparity ratio of AAMSM to WMSM incidence rates across varying PrEP scale-up scenarios, with equal and unequal coverage among AAMSM and WMSM.
Results:
Assuming current coverage remains constant, the model predicts HIV incidence rates of 2.95 and 1.76 per 100 person-years among AAMSM and WMSM respectively, with a disparity ratio of 1.68. If PrEP coverage were to increase six-fold without addressing inequities in PrEP uptake, the model predicts incidences of 2.65 and 1.34, corresponding to a mean decrease of 10.4% and 24.0% in HIV incidence, respectively. This stronger benefit for WMSM increased the disparity ratio to 1.98. Equal PrEP coverage among AAMSM and WMSM resulted in lower incidence rates overall with lower disparity ratios.
Conclusion:
Lower uptake among AAMSM relative to WMSM may limit the population-level impact of PrEP use among AAMSM, which may ultimately culminate in wider racial disparities in HIV incidence among MSM.
INTRODUCTION
There are stark and persistent racial disparities in HIV incidence among gay, bisexual, and other men who have sex with men in the United States (US).1 The lifetime risk of an HIV diagnosis in the US is 1 in 2 for African American MSM (AAMSM) compared to 1 in 11 for white MSM (WMSM).2 Several explanations for these disparities have been proposed, including both social (e.g., race-based assortative mixing in sexual partnerships) and complex structural factors (e.g., racism, mass incarceration, inadequate access to care and low rates of health insurance coverage).3 Despite efforts to address the multifactorial causes of these disparities, incidence has decreased among WMSM in the US since 2008, while no significant changes have been observed in AAMSM.4
Once daily pre-exposure prophylaxis (PrEP), formulated as a single tablet containing tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC), is an acceptable and effective biomedical HIV prevention tool among MSM.5–7 PrEP use has increased among MSM in the US since 2012,8,9 but has been slow due to a number of factors, including financial costs,10 lack of perceived appropriateness based on perceived risk for HIV acquisition,11 concerns regarding behavioral risk compensation and increasing rates of sexually transmitted infections (STIs) among PrEP-using MSM,12 and stigma.13 Previous modeling studies have shown that expanded PrEP coverage could provide population-level benefits.14,15 PrEP uptake has been uneven across racial subgroups of MSM,16–18 which may contribute to our nation’s persistent and widening racial disparities in HIV incidence.1,2 Previous studies have shown that there is little or no difference in willingness to use PrEP between AAMSM and WMSM, but PrEP use is much lower among AAMSM (2.5% nationwide in 2014) compared to WMSM (5.3% nationwide in 2014).19 A growing body of literature has begun to document racial/ethnic inequities in PrEP utilization, particularly among AAMSM, and have theorized the role these inequities play in furthering racial/ethnic disparities in HIV incidence.20–22
Employing an agent-based model (ABM), we simulated the impact of varying levels of PrEP use among MSM, with and without explicit racial differences in adherence and retention in care, on racial disparities in HIV incidence among a population of MSM. We use the city of Atlanta, Georgia as a case study, given the marked racial disparities in HIV prevalence and incidence in the city.23,24 Using a base implementation scenario with current estimated race-specific PrEP coverage levels (2.5% among AAMSM, 5.0% among WMSM) with explicit racial differences in adherence and retention in care,19,25 we compare relative decreases in HIV incidence in each subgroup as well as a disparity ratio of AAMSM to WMSM incidences. We hypothesized that continued low PrEP coverage among AAMSM relative to WMSM, along with no improvements to any downstream aspects of the PrEP continuum (i.e., retention in PrEP care and PrEP adherence) would decrease HIV incidence among both groups but exacerbate the existing disparities in HIV incidence, while increasing coverage among AAMSM to a level equal to or higher than that among WMSM, along with improvements to retention in PrEP care and PrEP adherence among AAMSM, would reduce these disparities.
METHODS
Model Setting
Agent-based modeling (ABM) is an individual-based micro-simulation approach to understanding how micro-level interactions generate and influence macro-level phenomenon at the population-level.26 The ABM simulated HIV transmission between 2015 and 2024 within a population representing all adult AAMSM and WMSM in Atlanta, Georgia. Detailed information regarding parameter values, calibration, key model assumptions, and data sources are shown in the supplemental appendix. Each individual agent’s behaviors and characteristics were informed by local data where possible, as well as by estimates from the existing literature as needed. The ABM modeled a dynamic population of adults aged 18 to 64 years in steady state, where individuals left the population at death or due to aging out age 65. Demographic, behavioral, and clinical characteristics for new incoming individuals were drawn stochastically from the same distribution used to create the base population at model initiation (Table 1).
Table 1.
Summary of key model parameters
| Domain | Overall | AAMSM | WMSM | Source |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Population size (n) | 10,000 | 5,120 | 48,880 | 40 |
| Age distribution (%) | 40 | |||
| 18 to 24 years | 18.0 | 15.1 | ||
| 25 to 34 years | 22.6 | 28.2 | ||
| 35 to 44 years | 18.4 | 21.0 | ||
| 45 to 54 years | 16.9 | 17.3 | ||
| 55 to 64 years | 14.3 | 11.6 | ||
| 65 to 74 years | 9.9 | 6.7 | ||
| Sexual Behaviors | ||||
| Number of sex partners per year (%) | 41 | |||
| Median (Interquartile Range) | 5 (3–9) | 7 (4–12) | ||
| Duration of sexual partnerships (%) | 28 | |||
| <1 month | 28.1 | 27.3 | ||
| 1 to 3 months | 20.9 | 14.7 | ||
| 4to 12 months | 28.1 | 32.0 | ||
| > 13 months | 23.0 | 26.1 | ||
| Probability of condom use | ||||
| 0 prior encounters with partner | 0.688 | 0.528 | 28 | |
| 1 prior encounter with partner | 0.629 | 0.483 | ||
| 2 to 9 prior encounters with partner | 0.578 | 0.444 | ||
| ≥10 prior encounters with partner | 0.198 | 0.152 | ||
| Probability of HIV transmission (per act)27 | ||||
| Condomless insertive anal intercourse | 0.0011 | |||
| Condomless receptive anal intercourse | 0.0138 | |||
| Pre-Exposure Prophylaxis Use | ||||
| Retention in clinical care, 6 months (%) | 38.8 | 35.8 | 43 | |
| Optimal adherence (≥4 pills per week) (%) | 50.8 | 51.4 | 43 | |
| Reduction in risk of HIV acquisition (%) | 29 | |||
| Optimal adherence | 96.0 | |||
| Suboptimal adherence | 76.0 |
Sexual Behavior and Sexual Networking
As the model progressed through discrete time-steps (each representing one calendar month), individuals formed and dissolved sexual partnerships with other individuals, engaged in sexual acts, and acquired HIV infection within serodiscordant partnerships. The formation and dissolution of these dyadic partnerships generated dynamic sexual networks within the model. Each individual was assigned a target annual number of sex partners each year, as well as a static sexual role class to determine their anal intercourse behaviors within a sexual dyad (exclusively insertive, exclusively receptive, and versatile). Individuals engaged in a specific number of sex acts with their partners based on an assigned target annual number of sex acts.
HIV Transmission, Treatment, and Progression
The base per-act probabilities of HIV transmission for condomless insertive and receptive anal intercourse were based on estimates from prior literature.27 These per-act probabilities of HIV transmission were scaled based on several dyad-specific factors, including whether the HIV-infected agent had achieved viral load suppression and whether the HIV-uninfected agent was using oral PrEP and, if so, their level of adherence to PrEP. To increase computational efficiency, condom-protected acts were not explicitly modeled and assumed to carry a negligible risk of HIV transmission. The probability of condom use decreased as a function of the number of prior contacts with a sex partner.28 Upon seroconversion, HIV-infected agents probabilistically moved through the treatment cascade from diagnosis to initiation and persistence on highly active antiretroviral treatment (HAART) and achievement of viral load suppression. Parameters for all stages of the treatment cascade were race-stratified, reflecting lower rates of diagnosis, linkage and retention in care, and viral load suppression among AAMSM relative to WMSM.3
Upon initiation of HAART, individuals were assigned an adherence classification, where it is assumed that individuals with optimal adherence to HAART (i.e., those who take 90% or more of all doses) will have an undetectable viral load at a threshold of less than 200 copies/mL. Given the potential for HIV treatment interruption and discontinuation, it is assumed that some agents on HAART may discontinue therapy for any length of time, with race-stratified monthly probabilities of HAART discontinuation. Those who discontinue HAART at any time may re-initiate at the same rates of those who are newly diagnosed.
A base probability of progression to AIDS was assigned to all HIV-infected agents. HIV-infected agents with suboptimal adherence to HAART (i.e., those who take less than 90% of all doses and without viral load suppression) had identical probabilities of progression to AIDS as all other HIV-infected agents (regardless of their diagnosis status). HIV-infected agents with optimal adherence to HAART were assumed to be less likely to progress to AIDS and experienced a scalar reduction in the probability of progression.
Oral Pre-Exposure Prophylaxis (PrEP) Use and Continuum of Care
In the model, individuals were considered eligible to initiate PrEP if they were HIV-uninfected and had engaged in condomless anal intercourse in the past year. All individuals who met these criteria had an equal probability of initiating PrEP until the target coverage level was achieved. Parameters pertaining to PrEP users were based on the assumption that a patient will receive a 90-day prescription at each visit, and that a patient is considered retained in care if they had a clinical care visit in the past six months. Individuals were categorized as out of care, and therefore, no longer using PrEP, if they had not had a visit within six months. At initiation, individuals were categorized with a static level of adherence as optimal (defined as taking four or more doses per week) or suboptimal (defined as taking two or three doses per week). Those with optimal adherence experienced a 96% reduction in the per-act probabilities of HIV acquisition, with those with partial adherence had a 76% reduction.29 Any individual who was not retained in care but continued to engage in condomless anal intercourse was eligible to re-initiate PrEP after twelve months with the same probability as any other eligible individual. Individuals who used PrEP but were not retained in care were immediately replaced with a new individual on PrEP to maintain the target coverage level.
Model Scenarios
The main analyses compared HIV incidence among AAMSM and WMSM across PrEP implementation scenarios with varying scenarios of equal and unequal race-specific coverage to a base implementation scenario. The base implementation scenario was based on current estimates of race-specific PrEP coverage among AAMSM (2.5%) and WMSM (5.0%), with no racial differences in adherence and retention in care. Each scenario began in January 2015 with a set constant coverage level of PrEP use. The model was calibrated to reproduce race-specific estimates of HIV incidence derived from a prospective cohort of MSM in Atlanta.23
In the first set of scale-up scenarios, PrEP coverage among AAMSM and WMSM are increased by the same factor (i.e., two- and six-fold). These simulations represent scenarios where no explicit intervention is implemented to improve the rate of PrEP initiation among AAMSM relative to WMSM (that is, where WMSM are twice as likely to initiate PrEP than AAMSM). In the second set of scale-up scenarios, PrEP coverage is increased to equal levels among AAMSM and WMSM (i.e., to 5%, 10%, and 30%). These simulations represent scenarios where explicit interventions are implemented to improve the rate of PrEP initiation among AAMSM relative to WMSM such that AAMSM and WMSM are equally likely to initiate PrEP.
Outcome Measures
The primary comparison measure was the disparity ratio of the HIV incidence among AAMSM to the HIV incidence among WMSM, where a disparity ratio of 1 indicates equivalent HIV incidence rates among AAMSM and WMSM. For each scale-up scenario, the HIV incidence for the overall population is provided in addition to separate race-specific HIV incidence values in order to reflect the effect of each scenario on HIV transmission in the overall population of MSM as well as within each racial subgroup. In addition, the percent reduction in HIV incidence overall as well as for each subgroup was calculated relative to the base implementation scenario. All estimates are presented as median values with 95% simulation intervals (SIs).
RESULTS
The ABM simulated HIV transmission among 10,000 MSM in the city of Atlanta over ten years (51.2% AAMSM; 48.8% WMSM). In the base implementation scenario, with 2.5% of AAMSM and 5% of WMSM using PrEP, incidence for the overall population was 2.37 infections per 100 person-years (95% SI: 2.26, 2.48), with an incidence of 2.95 per 100 person-years among AAMSM (95% SI: 2.80, 3.10) and 1.76 per 100 person-years among WMSM (95% SI: 1.63, 1.88) and a disparity ratio of 1.68 (95% SI: 1.55, 1.82).
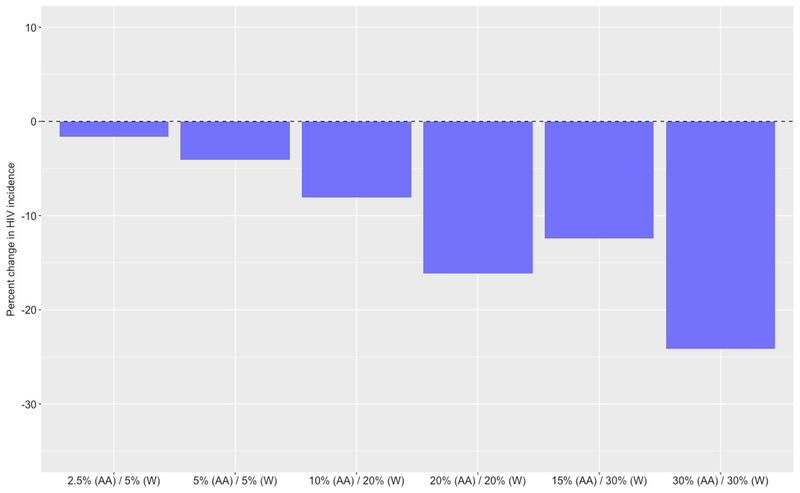
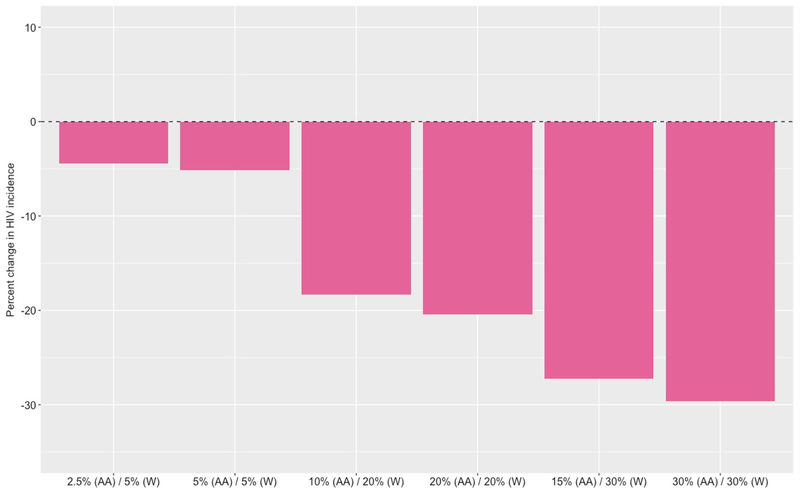
Based on these trends, if both coverage levels were to immediately increase to 5% among AAMSM and 10% among WMSM (i.e., increase two-fold without any additional intervention to increase the rate of initiation among AAMSM), the model predicts a median overall incidence of 2.15 infections per 100 person-years (95% SI: 2.04, 2.25), with a median incidence of 2.77 per 100 person-years (95% SI: 2.04, 2.25) among AAMSM and 1.50 per 100 person-years (95% SI: 1.39, 1.60) among WMSM (Table 2). This corresponds to a median decrease of 9.3% in overall HIV incidence, including a 6.0% decrease among AAMSM (Figure 1) and 14.9% decrease among WMSM (Figure 2). However, despite these decreases in HIV incidence, the disparity ratio increases by a median of 10.2% to 1.85 (95% SI: 1.71, 2.01).
Table 2.
Incidence rates and associated disparity ratio among African American men who have sex with men (AAMSM) and white men who have sex with men (WMSM) in Atlanta, Georgia (2015 to 2024) with increased PrEP coverage, where no intervention is implemented to increase the rate of PrEP initiation among AAMSM.
| Pre-Exposure Prophylaxis Coverage | Incidence Rate (per 100 person-years) | Disparity Ratio (Median [95% SI]) | ||
|---|---|---|---|---|
| African American MSM | White MSM | African American MSM (Median [95% SI]) | White MSM (Median [95% SI]) | |
| 2.5% | 5.0% | 2.95 (2.80, 3.10) | 1.76 (1.63, 1.88) | 1.68 (1.55, 1.82) |
| 10.0% | 20.0% | 2.77 (2.63, 2.91) | 1.50 (1.39, 1.60) | 1.85 (1.71, 2.01) |
| 15.0% | 30.0% | 2.65 (2.52, 2.79) | 1.34 (1.23, 1.44) | 1.98 (1.83, 2.17) |
Figure 1.
Percent reduction in HIV incidence among African American men who have sex with men (AAMSM) in Atlanta, Georgia (2015 to 2024) with increased pre-exposure prophylaxis (PrEP) coverage, compared to a base case scenario where PrEP is unavailable
Figure 2.
Percent reduction in HIV incidence among White men who have sex with men (WMSM) in Atlanta, Georgia (2015 to 2024) with increased pre-exposure prophylaxis (PrEP) coverage, compared to a base case scenario where PrEP is unavailable
If both coverage levels were to increase further to 15% among AAMSM and 30% among WMSM (i.e., increase six-fold without any additional intervention to increase the rate of initiation among AAMSM), the model predicts a median overall incidence of 2.01 infections per 100 person-years (95% SI: 1.91, 2.10), with a median incidence of 2.65 per 100 person-years (95% SI: 2.52, 2.79) among AAMSM and 1.34 (95% SI: 1.23, 1.44) among WMSM. This corresponds to a median decrease of 15.2% in overall HIV incidence, among a 10.4% decrease among AAMSM and 24.0% decrease among WMSM. However, despite these decreases in HIV incidence, the disparity ratio increases by a median of 18.3% to 1.98 (95 SI: 1.83, 2.17).
Intervening to increase the rate of PrEP initiation among AAMSM can reduce also reduce HIV incidence (Table 3). If both coverage levels were set to 20%, for example, the model predicts a median overall incidence of 2.01 (95% SI: 1.91, 2.11), representing a median decrease of 15.2%. The median incidence among AAMSM decreases by 14.2% to 2.53 per 100 person-years (95% SI: 2.40, 2.68), while the median incidence among WMSM decreases by 16.7% to 1.46 (95% SI: 1.35, 1.58) (Figure 2). In addition to producing significant decreases in HIV incidence, equalizing coverage exacerbates existing disparities to a lesser extent. In this case, the disparity ratio increases by a median of 3.1% to 1.73 (95% SI: 1.59, 1.90), rather than increasing by 10.2% to 1.85 (95% SI. 1.71, 2.01) when WMSM were twice as likely to initiate PrEP as AAMSM.
Table 3.
Incidence rates and associated disparity ratio among African American men who have sex with men (AAMSM) and white men who have sex with men (WMSM) in Atlanta, Georgia (2015 to 2024) with increased PrEP coverage, where a hypothetical intervention is implemented to increase the rate of PrEP initiation among AAMSM.
| Pre-Exposure Prophylaxis Coverage | Incidence Rate (per 100 person-years) | Disparity Ratio (Median [95% SI]) | ||
|---|---|---|---|---|
| African American MSM | White MSM | African American MSM (Median [95% SI]) | White MSM (Median [95% SI]) | |
| 5.0% | 5.0% | 2.89 (2.75, 3.02) | 1.74 (1.60, 1.88) | 1.66 (1.53, 1.81) |
| 20.0% | 20.0% | 2.53 (2.40, 2.68) | 1.46 (1.35, 1.58) | 1.73 (1.59, 1.90) |
| 30.0% | 30.0% | 2.28 (2.14, 4.44) | 1.29 (1.19, 1.41) | 1.77 (1.61, 1.94) |
DISCUSSION
To our knowledge, this study is among the first to assess the potential impact of PrEP scale-up on racial disparities in HIV incidence among AAMSM and WMSM. While studies have modeled the potential effect of increased use of PrEP among MSM and suggested that expanded use may lead to reductions in HIV transmission,14,15 to date, no studies have evaluated how observed and continued racial inequities in access to PrEP impact racial disparities in HIV incidence. Despite generating significant decreases in the cumulative number of new HIV infections over a ten-year period among both AAMSM and WMSM, increasing PrEP use alone was insufficient in narrowing racial disparities in HIV incidence between these two groups.
Among MSM in Atlanta, large increases in coverage of PrEP among AAMSM and WMSM are needed to generate decreases in HIV incidence in line with the goals of the National HIV/AIDS Strategy.30 These results demonstrate that increasing PrEP coverage without addressing racial inequities in access to PrEP may exacerbate the existing disparities in HIV incidence among AAMSM. Significant increases in PrEP use are needed among AAMSM, but PrEP alone cannot narrow these disparities. Similar conclusions were reached by Jenness and colleagues (2017) who used an agent-based model of MSM to predict the impact of intervening at each point along the PrEP continuum among AAMSM, suggesting that improvements in PrEP uptake among AAMSM could further reduce HIV incidence.20 However, the exacerbation of these disparities in HIV incidence cannot be prevented entirely by increasing PrEP uptake among AAMSM to levels equal to those among WMSM, as it is likely that the impact of PrEP among AAMSM is impacted by other aspects of PrEP continuum of care that may also have important impacts on HIV incidence, such as adherence and retention in PrEP care.20
Other HIV prevention and access to care interventions, along with improvements to the HIV treatment cascade, are also needed. AAMSM report high willingness to use PrEP,19 but multilevel interventions are needed to improve access to and initiation of PrEP among this population. Structural interventions, such as those that expand insurance coverage, may alleviate cost-related barriers to PrEP initiation and retention,31 while community-level interventions, such as educational campaigns that address common concerns and misconceptions regarding PrEP use, may alleviate stigma-related barriers.32 Provider-level interventions may also help to ensure that AAMSM are able to disclose their same-sex sexual behavior and have their need for PrEP assessed in line with existing clinical recommendations.33 Although fewer AAMSM have behavioral indications for PrEP,19 current clinical recommendations suggest that clinicians “consider the epidemiologic context of the sexual practices reported by the patient” when prescribing PrEP34 As such, the high incidence and prevalence of HIV infection among AAMSM in many settings in the US warrants expanded use of PrEP despite less frequent engagement in condomless anal intercourse. However, despite high levels of interest in using PrEP and offering PrEP free of charge to all interested individuals, Rolle and colleagues (2017) found that few young AAMSM in Atlanta initiated PrEP.21 As such, more intensive interventions, such as the client-centered care coordination counseling (C4) approach used in HPTN 073, a PrEP demonstration project among AAMSM in three U.S. cities, may be needed,35 as the low uptake of PrEP among AAMSM cannot be explained by lack of access alone.
Limitations
These findings are subject to several limitations. First, local data were used to parameterize the model where possible, but as in many individual-base models, the input parameters were derived from a number of different sources, which may introduce bias and adversely affect the representativeness of the model as well as the generalizability of the simulation outputs.36 However, given that the vast majority of parameters were derived from studies involving MSM in Atlanta, we are confident that the magnitude of this bias, if present, is relatively minimal. In addition, while the current model uses a network formation process based on a negative binomial distribution of the target number of sexual partners per year, other tools for creating these networks are available (e.g., exponential random graph models [ERGMs]),37 with their own strengths and limitations. Despite differences between our approach and other available tools, our findings and conclusions regarding the impact of racial inequities in PrEP use and persistence on racial disparities are similar to an ERGM-based model that aimed to predict the impact of intervening at each point along the PrEP continuum among AAMSM.20 Second, in all scenarios, PrEP was targeted to individuals in the model who engaged in condomless anal intercourse in the past twelve months. We acknowledge that there are other indications for PrEP use based on current recommendations that were not included in the model, including recent diagnoses with STIs.34 As such, the proportion of MSM who are eligible to initiate PrEP use may be underestimated in the base scenario. In addition, the ABM does not model STI transmission. Because having an active untreated STI increases risk of HIV acquisition and transmission,38 the model may underestimate individual susceptibility to HIV infection or probability of HIV transmission. Future models should incorporate STI transmission alongside HIV transmission to further understand the relative contributions of STIs to HIV transmission. We did not model breakthrough resistant infections among PrEP users who were partially adherent to the medication, as these have been shown to be rare.39 Future models should incorporate parameters to simulate the development and impact of drug resistance to better understand its contributions to HIV transmission in contexts where PrEP is used.
CONCLUSION
Lower levels of use limit the population-level impact of PrEP among AAMSM, leading to higher than current HIV incidence disparities but with lower than currently observed incidence rates among both subgroups. Novel interventions are needed to improve PrEP uptake among AAMSM to avoid further exacerbation of these existing disparities.
Supplementary Material
Acknowledgements:
We would like to thank Jesse L. Yedinak, MPA for her research and administrative assistance.
Sources of Funding and Conflicts of Interest: This work was supported by the National Institute on Drug Abuse (DP2 DA040236) and the National Institute on Mental Health (R21 MH109360). Mark N. Lurie, PhD is also supported by grants from the National Institute on Mental Health (R01 MH106600) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R24 HD077976). Amy S. Nunn, ScD and Philip A. Chan, MD, MS are supported in part by grants from the National Institute on Mental Health (R01 MH114657 and R34 MH109371). The funders had no role in the study analysis, decision to published, or preparation of this manuscript. The authors report no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2016. Atlanta, Georgia: National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention;2017. [Google Scholar]
- 2.Hess KL, Hu X, Lansky A, Mermin J, Hall HI. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27(4):238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: A meta-analysis. Lancet. 2012;380(9839):341–348. [DOI] [PubMed] [Google Scholar]
- 4.Hall HI, Song R, Tang T, et al. HIV trends in the United States: Diagnoses and estimated incidence. JMIR Public Health Surveill. 2017;3(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61(10):1601–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng P, Su S, Fairley CK, et al. A global estimate of the acceptability of pre-exposure prophylaxis for HIV among men who have sex with men: a systematic review and meta-analysis. AIDS and Behavior. 2017; [DOI] [PubMed] [Google Scholar]
- 8.Siegler AJ, Mouhanna F, Mera Giler R, et al. Distribution of active PrEP prescriptions and the PrEP-to-need ratio, US, Q2 2017. Conference on Retroviruses and Opportunistic Infections; 2018; Boston, Massachusetts. [Google Scholar]
- 9.Mera Giler R, Magnuson D, Trevor H, Bush S, Rawlings K, McCallister S. Changs in Truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States: 2012–2016. Paris, France; 2017; International AIDS Society Conference on HIV Science. [Google Scholar]
- 10.Patel RR, Singh S, Farag C, et al. Out-of-pocket costs impede PrEP use among young MSM in the private healthcare system. Conference on Retroviruses and Opportunistic Infections; 2018; Boston, Massachusetts. [Google Scholar]
- 11.Rendina HJ, Whitfield TH, Grov C, Starks TJ, Parsons JT. Distinguishing hypothetical willingness from behavioral intentions to initiate HIV pre-exposure prophylaxis (PrEP): Findings from a large cohort of gay and bisexual men in the US. Soc Sci Med. 2017;172:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt M, Murphy DA. Individual versus community-level risk compensation following preexposure prophylaxis of HIV. Am J Public Health. 2017;107(10):1568–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golub SA. PrEP stigma: Implicit and explicit drivers of disparity. Curr HIV/AIDS Rep. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenness SM, Goodreau SM, Rosenberg E, et al. Impact of the Centers for Disease Control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J Infect Dis. 2016;214(12):1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeVasseur MT, Goldstein ND, Tabb LP, Olivieri-Mui BL, Welles SL. The effect of PrEP on HIV incidence among men who have sex with men in the context of condom use, treatment as prevention, and seroadaptive practices. J Acquir Immune Defic Syndr. 2018;77(1):31–40. [DOI] [PubMed] [Google Scholar]
- 16.Scott H, Hirozawa A, Nordell M, et al. Disparities in PrEP uptake among primary care patients screened for HIV/STIs in SF. Conference on Retroviruses and Opportunistic Infections; 2018; Boston, Massachusetts. [Google Scholar]
- 17.Mayer KH, Grasso C, Levine K, et al. Increasing PrEP uptake, persistent disparities in at-risk patients in a Boston center. Conference on Retroviruses and Opportunistic Infections; 2018; Boston, Massachusetts. [Google Scholar]
- 18.Smith DK, Van Handel M, Grey JA. By race/ethnicity, Blacks have highest number needing PrEP in the United States, 2015. Conference on Retroviruses and Opportunistic Infections; 2018; Boston, Massachusetts. [Google Scholar]
- 19.Hoots BE, Finlayson T, Nerlander L, et al. Willingness to take, use of, and indications for pre-exposure prophylaxis among men who have sex with men—20 US cities, 2014. Clin Infect Dis. 2016;63(5):672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenness S, Maloney K, Smith DK, et al. The PrEP care continuum and HIV racial disparities among men who have sex with men. Conference on Retroviruses and Opportunistic Infections; 2018; Boston, Massachusetts. [Google Scholar]
- 21.Rolle C-P, Rosenberg ES, Siegler AJ, et al. Challenges in translating PrEP interest into uptake in an observational study of young black MSM. J Acquir Immune Defic Syndr. 2017;76(3):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley CF, Kahle E, Siegler A, et al. Applying a PrEP continuum of care for men who have sex with men in Atlanta, Georgia. Clin Infect Dis. 2015;61(10):1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan PS, Rosenberg ES, Sanchez TH, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: A prospective observational cohort study. Ann Epidemiol. 2015;25(6):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg ES, Sullivan PS, Kelley CF, et al. Race and age disparities in HIV incidence and prevalence among MSM in Atlanta, Georgia. Conference on Retroviruses and Opportunistic Infections; 2014; Boston, Massachusetts. [Google Scholar]
- 25.Rolle CPM, Onwubiko U, Jo J, Sheth AN, Kelley CF, Holland DP. PrEP implementation and persistance in a county health department in Atlanta, GA. Conference on Retroviruses and Opportunistic Infections; 2018; Boston, Massachusetts. [Google Scholar]
- 26.Marshall BD, Galea S. Formalizing the role of agent-based modeling in causal inference and epidemiology. Am J Epidemiol. 2014;181(2):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: A systematic review. AIDS. 2014;28(10):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberger JG, Reece M, Schick V, et al. Condom use during most recent anal intercourse event among a US sample of men who have sex with men. J Sex Med. 2012;9(4):1037–1047. [DOI] [PubMed] [Google Scholar]
- 29.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125–151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. Washington, DC: United States Department of Health and Human Services;2015. [Google Scholar]
- 31.Patel RR, Mena L, Nunn A, et al. Impact of insurance coverage on utilization of pre-exposure prophylaxis for HIV prevention. PLoS One. 2017;12(5):e0178737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cahill S, Taylor SW, Elsesser SA, Mena L, Hickson D, Mayer KH. Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in black compared to white gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts. AIDS Care. 2017;29(11):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stupiansky NW, Liau A, Rosenberger J, et al. Young men’s disclosure of same sex behaviors to healthcare providers and the impact on health: Results from a US national sample of young men who have sex with men. AIDS Patient Care STDs. 2017;31(8):342–347. [DOI] [PubMed] [Google Scholar]
- 34.United States Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States: A Clinical Practice Guideline. Atlanta, Georgia: Centers for Disease Control and Prevention;2014. [Google Scholar]
- 35.Wheeler DP, Fields S, Nelson LE, et al. HPTN 073: PrEP uptake and use by black men who have sex with men in 3 US cities. Conference on Retroviruses and Opportunistic Infections; 2016; Boston, Massachusetts. [Google Scholar]
- 36.Murray EJ, Robins JM, Seage GR, Freedberg KA, Hernán MA. A comparison of agent-based models and the parametric g-formula for causal inference. Am J Epidemiol. 2017;186(2):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter DR, Handcock MS, Butts CT, Goodreau SM, Morris M. ergm: A package to fit, simulate and diagnose exponential-family models for networks. J Stat Softw. 2008;24(3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward H, Rönn M. The contribution of STIs to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh UM, Mellors JW. Should we fear resistance from tenofovir/emtricitabine PrEP? Curr Opin HIV AIDS. 2016;11(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.United States Census Bureau. Community Facts - City of Atlanta, Georgia. [Web]. 2010; https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF. Accessed March 11, 2017.
- 41.Chan PA, Rose J, Maher J, et al. A latent class analysis of risk factors for acquiring HIV among men who have sex with men: Implications for implementing pre-exposure prophylaxis programs. AIDS Patient Care STDs. 2015;29(11):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wall KM, Stephenson R, Sullivan PS. Frequency of sexual activity with most recent male partner among young, Internet-using men who have sex with men in the United States. J Homosex. 2013;60(10):1520–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre‐exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.