Abstract
To clarify the physiological and psychological effects of deep breathing, the effects of extreme prolongation of expiration breathing (Okinaga) were investigated using electroencephalogram (EEG) and electrocardiogram (ECG). Participants were five male Okinaga practitioners in their 50s and 60s. Participants performed Okinaga for 31 minutes while continuous EEG and ECG measurements were taken. After 16 minutes of Okinaga, and until the end of the session, the percentages of theta and alpha 2 waves were significantly higher than at baseline. After 20 minutes, and until the end of the session, the percentage of beta waves was significantly lower than at baseline. The high frequency component of heart rate variability was significantly lower after 12 minutes of Okinaga and lasted until 23 minutes. The low frequency/high frequency ratio was significantly lower after 18 minutes of Okinaga and until the end of the session. Okinaga produced relaxation, suggesting that deep breathing may relieve anxiety. However, study limitations include potential ambiguity in the interpretation of the low frequency/high frequency ratio, the small sample, and the fact that EEG was measured only on the forehead.
Key words: EEG, prolonged expiratory breathing, sympathetic nervous activity, parasympathetic nervous activity, anxiety
Introduction
Deep or slow breathing using the diaphragm is effective both psychologically and physiologically in relieving stress and anxiety.1-3 I recently reported that breathing using both 6-second expiration and 4-second inspiration promotes dominance of parasympathetic nervous function.4
To obtain an insight into the effects of deep breathing at the neurophysiological level, electroencephalogram (EEG) measurements can be used to study theta, alpha, and beta brain oscillations. Changes in these bands reflect the current state of consciousness. A previous randomized controlled trial of the effect of deep breathing showed that when a breathing frequency of 0.1 Hz was maintained for 5, 7, or 9 minutes, frontal theta power increased significantly after both 5 and 9 minutes, but there was no difference from control for 7 minutes.1 There is a negative correlation between breathing frequency and the mean power of theta, alpha, and beta bands.5 Previous studies on the effects of deep breathing for 5 to 20 minutes have shown an increase in alpha power at 5 minutes that remains unchanged until the end of the 20 minutes, but an increase in theta power at both 15 and 20 minutes.6,7
There is little research on the association between EEG changes and deep breathing. However, previous studies on mindfulness are relevant, as deep breathing is an element of many types of mindfulness meditation.3 Findings on EEG changes during mindfulness meditation are inconsistent, perhaps owing to the range of meditation techniques. An increase in frontal theta power during meditation is often reported.1,6-8 Several studies suggest that alpha power is associated with the frontal brain region,8-10 although alpha waves also appear in the occipital region.9 Although some studies have reported a decrease in beta waves associated with deep breathing,1 others have refuted this finding.11,12
The aim of this study was to clarify the physiological effects of deep breathing in individuals able to perform extreme prolongation of expiration breathing, known as Okinaga. Okinaga is a technique that was practiced by the covert special operations agents known as “ninjas,” who operated in feudal Japan. Some individuals today practice the technique of Okinaga. I measured EEG and autonomic nervous function during Okinaga practice in five participants who had mastered Okinaga. The aim was to clarify the effects of deep breathing on the mind and body, and to inform the application of the technique to anxiety disorders and depression.
Materials and Methods
Participant recruitment and ethical considerations
Recruitment focused on individuals who practiced Okinaga. Five males in their 50s and 60s volunteered to participate. This study was conducted with the approval of the ethics committee of Mie University Graduate School of Medicine, Japan. All participants provided informed consent.
Study setting
The study was conducted in a quiet room with a temperature of 22°C and illuminance of 300 lux. Participantswere seated on a soft chair with their both arms placed on a table. All participants were tested individually.
Study design
Participants performed normal breathing for 10 minutes to provide EEG and ECG data, and then performed Okinaga for 31 minutes. The Okinaga breathing frequency was about one breath per minute. During the Okinaga, EEG and ECG measurements were taken, as described below. Participants were asked to keep their eyes closed during the study.
EEG measurement
A handheld high-powered EEG was used (BrainPro Light FM828T, Futek Electronics Co., Ltd., Yokohama, Kanagawa); sensor bands with two electrodes were attached to the participant’s forehead. The explorator electrode was Ep2 and the reference electrode was Ep1. EEG data were recorded for 3-minute periods. The percentages of the theta (4-6 Hz), alpha 1 (7-8 Hz), alpha 2 (9-11 Hz), alpha 3 (12-13 Hz), and beta (14-30 Hz) waves were calculated. Data of 20 μV or more per second at 3.0 Hz were excluded as artifacts.
EEG was measured before starting Okinaga (baseline) and then measured at 0– 3 minutes, 4–7 minutes, 8–11 minutes, 12–15 minutes, 16–19 minutes, 20–23 minutes, 24–27 minutes, and 28–31 minutes after the start of Okinaga.
ECG measurement
ECG was measured using a CheckMyHeart recorder (TRYTECH Co., Ltd., Tokyo). Measurements were taken at 5- minute periods. ECG frequency data were analyzed using a power spectrum analysis of according to the principle of maximum entropy, and the low frequency (LF; 0.04 Hz to 0.15 Hz) and high frequency (HF; 0.15 Hz to 0.4 Hz) components of heart rate variability (HRV) were calculated. The HF and LF/HF values were used as indices of parasympathetic nervous activity and sympathovagal balance, respectively. ECG was measured at baseline and then at 0–5 minutes, 6–11 minutes, 12–17 minutes, 18– 23 minutes, and 24–29 minutes after the start of Okinaga.
Statistical analysis
The mean percentage of each wave for the EEG, HF, and LF/HF values at each time point was tested using the Bonferroni/Dunn test. This tests for significant differences between all pairs; P<0.05 was considered to indicate significance.
Results
Changes in the percentage of the theta, alpha 2, and beta waves in this study are shown in Figure 1. There was no change in alpha 1 or alpha 3 waves. Theta and alpha 2 waves were significantly higher 16 minutes after the start of Okinaga compared with the values before the start of Okinaga (baseline); these higher values were retained until the end of the session. Theta and alpha 2 values were also significantly higher than values at 0–3 minutes and 4–7 minutes after the start of Okinaga. There was no significant difference among the values in the period 16 minutes from the start of Okinaga to the end of the session. Between 20 minutes after the start of Okinaga and the end of the session, beta values were significantly lower than at baseline, and were significantly lower than values at 0–3 minutes and 4–7 minutes after the start of Okinaga.
Figure 1.
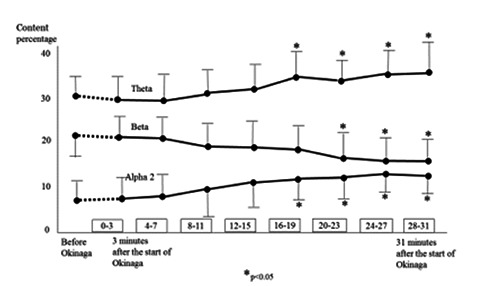
Changes in content percentages of theta, alpha 2, and beta waves over the time course of Okinaga.
Changes in LF/HF values are shown in Figure 2. These were significantly lower at 18–23 minutes and 24–29 minutes after the start of Okinaga than at baseline and at 0–5 minutes and 6–11 minutes after the start of Okinaga.
Figure 2.
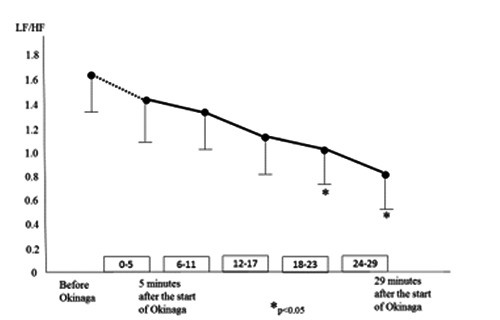
Changes in the LF/HF ratio over the time-course of Okinaga.
The HF values are shown in Figure 3. These were significantly lower at 12–17 minutes and 18–23 minutes after the start of Okinaga than at baseline and at 0–5 minutes after the start of Okinaga.
Figure 3.
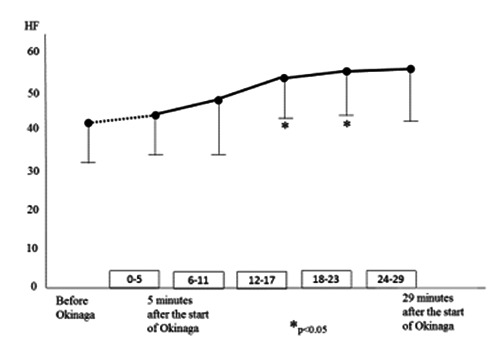
Changes in HF value over the time course of Okinaga.
Discussion
Okinaga increased theta and alpha 2 waves and decreased beta waves as shown in the EEG measurements. In addition, Okinaga resulted in parasympathetic dominance. These changes were seen from about 15 minutes after starting Okinaga.
Changes in each EEG band provide information about an individual’s current state of consciousness. Although there are no studies investigating the influence of the type of deep breathing used in Okinaga, the effect of deep breathing at various frequencies has been reported. The present study showed an increase in theta waves consistent with EEG findings for 3 or 4 breaths per minute,6,7 or 0.1 Hz breath.1 It is also noteworthy that theta waves increased during the Okinaga session. The increase in frontal theta power could be interpreted as indicating greater focused attention.13-15 One previous study showed that frontal theta power and anxiety were negatively correlated during deep breathing.16
An increase in theta activity may be interpreted as simply fatigue or a transition into sleep. It is difficult to determine by EEG alone whether an increase in theta waves reflects a deep state of meditation or stage 1 sleep. During meditation, theta activity continues even after the eyes are opened, and it can be indicated by an alert state in participants.17,18Although in this study EEG measurements were not taken after participants opened their eyes, it is unlikely that the theta activity indicated sleep because all participants confirmed that they were not sleeping and reported that their sensations remained acute. Several previous studies reported that localized frontal midline theta waves increased during meditation, indicating concentrated cognitive engagement,19-21 which is consistent with the findings of this study. Although modern applications of meditation have emphasized the hypoarousing and relaxing effects, meditation should be considered to have significance as a state of relaxed alertness that must guard against both excessive hyperarousal (restlessness) and excessive hypoarousal (drowsiness, sleep).21
In this study, alpha 2 waves increased with the duration of breathing. There are several reports that alpha power increases during meditation,8-10 but no studies have examined the effect of deep breathing on different types of alpha waves (i.e., alpha 1, 2, and 3). Although alpha waves initially appear in the occipital region,9 there are also reports that they can increase in the frontal region; this has been interpreted as indicating relaxation.8-10,22 The increase of alpha 2 in this study is considered to be consistent with these reports. The lower alpha band reflects vigilance and attention and the upper alpha band reflects taskspecific processes, such as perceptual and cognitive processing.23 In this study, an increase was observed in the mid-alpha band; the association of such an increase with attention and cognition requires further examination.
In this study, the beta waves were significantly lower 20 minutes after the start of Okinaga. Previous research has shown that 0.1 Hz deep breathing for 5, 7, or 9 minutes is associated with a reduction in beta power reported in the whole brain, including the frontal region.1 This decrease in beta power has been interpreted as a decrease in anxiety24 and is consistent with parasympathetic nervous system dominance.25 One study found that 10 minutes of HRV biofeedback was associated with a decrease in beta power.23 However, other reports have suggested that deep breathing does not decrease beta power.11,12
HRV is a measure of beat-to-beat temporal changes in heart rate and provides indirect insight into autonomic nervous system tone. HRV analysis is a reliable noninvasive technique to quantify cardiovascular autonomic regulatory responses and mechanisms.26 The Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology have recommended several time-domain measures for descriptive statistical methods.26 These HRV indices are markers to assess sympathetic and parasympathetic tone. HF power is almost entirely mediated by parasympathetic nervous system activity, whereas LF power reflects the mixed modulation of parasympathetic and sympathetic activities.26 The ratio of LF power to HF power (LF/HF) reflects the ratio between sympathetic nervous system activity and parasympathetic nervous system activity.26 A shift of dominance from sympathetic to parasympathetic nerve activity reduces anxiety and leads to relaxation.24 In this study, parasympathetic nervous activity became dominant as the the Okinaga session progressed. It has been suggested that the HRV LF/HF value does not accurately measure sympathovagal balance and that its correspondence with psychological and physiological conditions is not unique.27,28 Although there are ongoing efforts to resolve the interpretive ambiguity of LF/HF,29 this issue requires more recognition and future research.
In this study, no significant findings were observed until 12 minutes after starting Okinaga. The lack of significance may relate to the small sample size; however, it may also indicate that participants require time to adapt to very prolonged expiration. It is possible that long expiration may create stress owing to oxygen deficiency in the initial period of Okinaga.
Deep breathing may be beneficial for alleviating anxiety. Research on deep breathing is insufficient, although many studies on mindfulness suggest that deep breathing is an important factor. Although this study investigated Okinaga, a special skill developed by ninjas in ancient Japan, the findings clarify the effects of deep breathing more generally. Studies on meditation have emphasized its hypoarousing and relaxing effects, but the arousing and alertness-promoting effects of meditation have been relatively ignored.18,21 As ninjas regularly faced dangerous, lifethreatening situations, vigilance to their surroundings was essential. It seems reasonable to argue that Okinaga leads to relaxed alertness, but further examination of its effects is necessary. Moreover, there are some limitations of the present study, such as the small sample size, and the fact that methodological issue of EEG measurements was only taken from the forehead. Further research in this field is required.
Conclusions
This study investigated a breathing method known as Okinaga, in which expiration may reach one breath per minute. The effects of Okinaga deep breathing on brain waves and autonomic nervous system function were examined. Theta and alpha 2 waves increased and beta waves decreased as Okinaga progressed. Parasympathetic dominance was observed as Okinaga progressed. These findings suggest that deep breathing is relaxing and relieves anxiety. Future research should build upon the methodology used here to advance our understanding of the effects of deep breathing.
Acknowledgments
The author thank Catherine Deeprose, PhD, and Diane Williams, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. He also thanks Yuji Yamada (Professor, Faculty of Humanities, Law & Economics, Mie University) for assistance with recruitment of participants, Jinichi Kawakami (Specially-appointed Professor, Community-University Research Cooperation Center, Mie University) who gave advice on Okinaga, and Makoto Hisamatsu (Speciallyappointed Professor, Community-University Research Cooperation Center, Mie University) who provided an electroencephalograph for use in this study.
Funding Statement
Funding: none.
References
- 1.Cheng KS, Han RPS, Lee PF. Neurophysiological study on the effect of various short durations of deep breathing: A randomized controlled trial. Respir Physiol Neurobiol 2018;249:23-31. [DOI] [PubMed] [Google Scholar]
- 2.Paul G, Elam B, Verhulst SJ. A longitudinal study of students’ perceptions of using deep breathing medication to reduce testing stresses. Teach Learn Med 2007;19:287-92. [DOI] [PubMed] [Google Scholar]
- 3.Brown RP, Gerberg PL. Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression: part IIclinical application and guidelines. J Altern Complement Med 2005;11:711-7. [DOI] [PubMed] [Google Scholar]
- 4.Komori T. The relaxation effect of prolonged expiratory breathing. Mental Illness 2018;10:7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busek P, Kemlink D. The influence of the respiratory cycle on the EEG. Physiol Res 2005;54:327-33. [PubMed] [Google Scholar]
- 6.Fumoto M, Sato-Suzuki I, Seki Y, et al. Appearance of high-frequency alpha band with disappearance of lowfrequency alpha band in EEG is produced during voluntary abdominal breathing in an eyes-closed condition. Neurosci Res 2004;59:307-17. [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Fumoto M, Nakatani Y, et al. Activation of the anterior prefrontal cortex and serotonergic system is associated with improvements in mood and EEG changes induced by Zen meditation practice in novices. Int J Psychophysiol 2011;80:10-111. [DOI] [PubMed] [Google Scholar]
- 8.Lee DJ, Kulubya E, Goldin P, et al. Review of the neural oscillations underlying meditation. Front Neurosci 2018;12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DelosAngeles D, Williams G, Burston J, et al. Electroencephalographic correlates of states of concentrztive meditation. Int J Psychophysiol 2001;110, 27-39. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Murata T, Hamada T, et al. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int J Psychophysiol 2005;55:199-207. [DOI] [PubMed] [Google Scholar]
- 11.Stancák A, Pfeffer D, Hrudová L, et al. Electroencephalographic correlates of paced breathing. Neuroreport 1993;4:723-6. [DOI] [PubMed] [Google Scholar]
- 12.Gaurav S, Meenakshi S, Jayshri G, Ramanjan S. Effect of alterations in breathing pattern on EEG activity in normal human subjects. Int J Curr Res Med Sci 2016;2:38-45. [Google Scholar]
- 13.Aftanas LI, Golocherikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: highresolution EEG investigation of meditation. Neurosci Lett 2001;310:57-60. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima K, Sato H. Relationship between frontal midline theta activity in EEG and concentration. J Hum Elgol (Tokyo) 1993;22:63-7. [PubMed] [Google Scholar]
- 15.Park JR, Yagyu T, Saito N, et al. Dynamics of brain electric field during recall of Salpuri dance performance. Percept Mot Skills 2002;9:955-62. [DOI] [PubMed] [Google Scholar]
- 16.Inanaga K. Frontal midline theta rhythm and mental activity. Psychiatry Clin Neurosci 1998;52:555-66. [DOI] [PubMed] [Google Scholar]
- 17.Britton BB, Lindahl JR, Cahn BR, et al. Awakening is not a metaphor: the effects of Buddhist meditation practices on basic wakefulness. Ann N Y Acad Sci 2014;1307:64-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fell J, Axmacher N, Haupt S. From alpha to gamma: electrophysiological correlates of meditation-related states of consciousness. Med Hypotheses 2010;75:218-24. [DOI] [PubMed] [Google Scholar]
- 19.Baijal S, Srinivasan N. Theta activity and meditative states: spectral changes during concentrative meditation. Cogn Process. 2010;11:31-8. [DOI] [PubMed] [Google Scholar]
- 20.Valadez EA, Simons RF. The power of frontal midline theta and post-error slowing to predict performance recovery: evidence for compensatory mechanisms. Psychophysiology 2018;55:13010. [DOI] [PubMed] [Google Scholar]
- 21.Britton WB, Lindahl JR, Cahn BR, et al. Awakening is not a metaphor: the effects of Buddhist meditation practices on basic wakefulness. Ann N Y Acad Sci 2014;1307:64-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prinsloo GE, Rauch HGL, Karpul D, Derman WE. The effect of a single session of short duration heart rate variability biofeedback on EEG: a pilot study. Appl Psychophysiol Biofeedback 2013;38:45-56. [DOI] [PubMed] [Google Scholar]
- 23.Vignaud P, Donde C, Sadki T, et al. Neural effects of mindfulness-based interventions on patients with major depressive disorder: a systemic review. Neurosci Biobehav Rev 2018;88:98-105. [DOI] [PubMed] [Google Scholar]
- 24.Pavlenko VB, Chernyi SV, Goubkina DG. EEG correlates of anxiety and emotional stability in adult healthy subjects. Neurophysiol 2009;41:337-45. [Google Scholar]
- 25.Miu AC, Heilman RM, Miclea M. Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Auton Neurosci Basic Clin 2009;145:99-103 [DOI] [PubMed] [Google Scholar]
- 26.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043-65. [PubMed] [Google Scholar]
- 27.Wu JJ, Cui Y, Yang YS. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain- derived neurotrophic factor. Complement Ther Med 2014;22:456-62. [DOI] [PubMed] [Google Scholar]
- 28.Billman GE. The LF/HF ratio does not accurately measure cardiac sympathovagal balance. Front Physiol 2013;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Rosenberg W, Chanwimalueang T, Adjei T, et al. Resolving Ambiguities in the LF/HF Ratio: LF-HF Scatter Plots for the Categorization of Mental and Physical Stress from HRV. Front Physiol 2017;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]


