ABSTRACT
Background
Non-invasive ventilation (NIV) is a valuable treatment in the management of acute hypercapnic respiratory failure. NIV is not without risks. One such adverse effect is the development of pressure ulcers over the nasal bridge which have an incidence of up to 20% of patients requiring NIV in this setting. The role of medical devices in the development of hospital acquired pressure ulcers has been increasingly recognised with 10-35% of all hospital acquired ulcers attributed to medical devices. Guidelines on acute NIV use suggest good skin care strategies. However, data on the magnitude of the problem of nasal bridge pressure ulceration and the effect of proactive preventative steps remains scant.
Method
A quality improvement project was designed to reduce the incidence of nasal bridge pressure ulcers during acute NIV. Hydrocolloid dressings were placed over the nasal bridge in all patients requiring NIV between 30th October 2015 and the 29th October 2016. Tissue viability was assessed daily with new pressure ulceration defined as grade 2 or above. Rates of nasal bridge pressure ulcers were compared to all patients requiring NIV in the 12-month period prior to intervention.
Results
In Group 1, there were 161 admissions and 9 grade 2 pressure ulcers from 666 NIV bed-days. In Group 2 there were 134 admissions and 0 pressure ulcers from 718 NIV bed-days. There was a statistically significant reduction in grade 2 pressure ulceration rates (p= 0.0013) in Group 2 compared to Group 1.
Conclusion
Application of an early prophylactic pressure-relieving hydrocolloid nasal dressing reduces the risk of developing grade 2 pressure ulcers in patients in patients requiring acute NIV.
Keywords: Hydrocolloid, non-invasive ventilation, patient safety, pressure ulcer, wound care
INTRODUCTION
Non-invasive ventilation (NIV) is a valuable treatment for acute hypercapnic respiratory failure. Use of ward based NIV is increasing, with approximately 9000 episodes yearly within the UK1. Exacerbations of COPD remain the most common indication2 with hypercapnic respiratory failure complicating up to 20% of acute admissions3. NIV has been shown to reduce mortality4 and avoids the need for intubation thereby avoiding associated complications such as ventilator associated pneumonia. The use of NIV is not without risks. These range from relatively minor complications to more clinically significant effects, such as a heightened risk of aspiration, and untoward haemodynamic effects.6 The impact of the device itself on the skin and the predisposition to skin breakdown in this context is now appreciated to be another clinically significant untoward effect of NIV.6
The role of medical devices in the development of hospital acquired pressure ulcers has been increasingly recognised over recent years. A variety of medical devices have been shown to increase the risk with patients 2.4 times more likely to develop a pressure ulcer if any medical device is used7. 10-35% of hospital acquired pressure ulcers are directly related to medical devices7,8.
Nasal bridge pressure ulcers related to the use of NIV masks occur in 5-20% cases5,6,9. The development of pressure lesions can result in intolerance to NIV and potentially treatment failure. Patient comfort and enhanced compliance are key factors in determining NIV outcome. Lesions develop as a result of pressure exerted by the mask which can approach pressures of 70mmHg10. In the presence of shear forces, such as that generated between inspiratory and expiratory phases of ventilation, pressures of as low as 30mmHg may be sufficient to result in tissue damage within a few hours11.
Current guidelines regarding the management of NIV suggest ensuring best mask fit along with pressure relieving strategies. This includes regular breaks from the mask, alternating between two interface types or barrier dressings9, however data regarding this is lacking. Our objective was to examine the effect of a proactive approach to reducing grade 2 or above nasal bridge pressure ulcers in patients requiring acute NIV. We aimed to reduce the incidence of pressure ulcers by using a hydrocolloid dressing placed over the nasal bridge throughout the episode of NIV.
Fig 1.
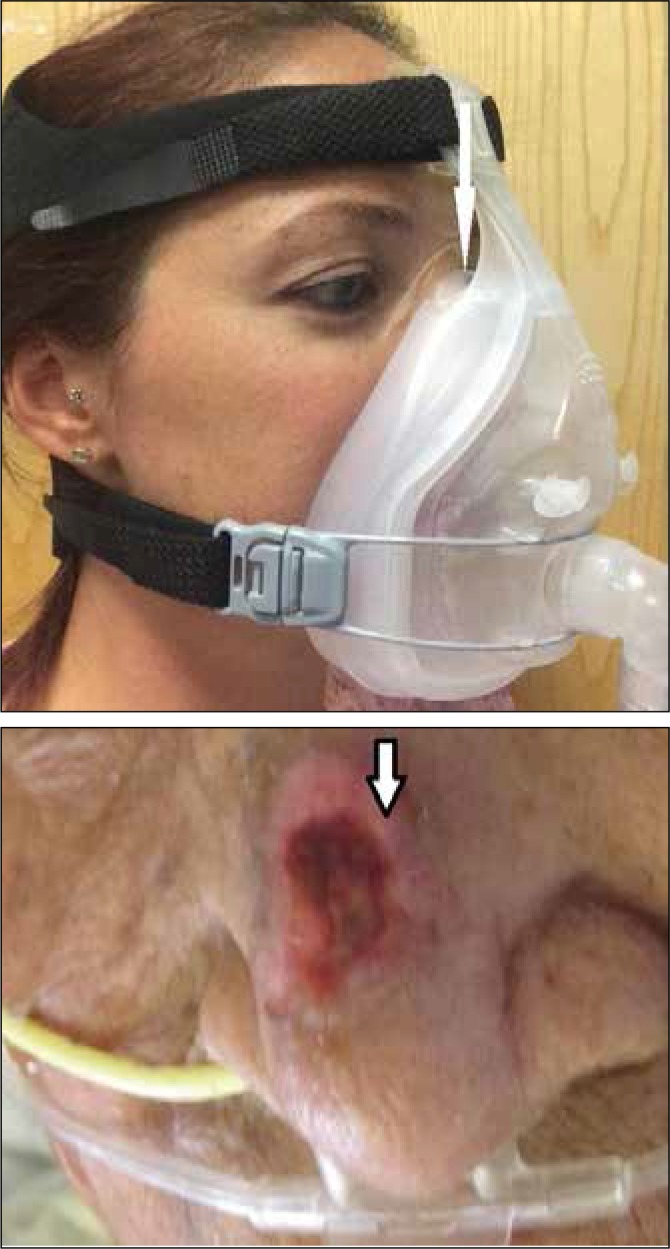
Top – A typical full face mask used for acute non-invasive ventilation: the nasal bridge is the most prominent bony structure in contact with the mask cushion (white arrow); Bottom – Grade 2 Nasal bridge pressure ulcer (bordered arrow)
Fig 2.
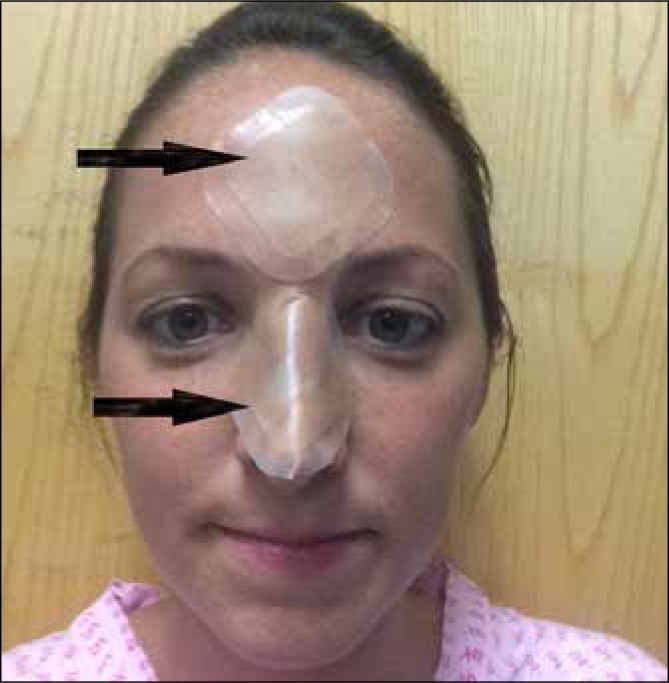
Application of hydrocolloid dressings to prevent nasal bridge ulceration (black arrows)
METHODS
Aim: We designed a quality improvement project to assess the effect of a proactive preventative approach towards nasal bridge pressure ulceration through the prophylactic use of a hydrocolloid dressing on the incidence of nasal bridge pressure ulcers in patients requiring acute non-invasive ventilation. This study is a report of a Quality Improvement Project (QIP) performed as a systematic, data-guided activity designed to bring about immediate improvements in health delivery. This QIP was registered on the audit database managed by the Clinical Standards Committee of the Heart of England NHS Foundation Trust, Birmingham, B9 5SS, UK. Data was collected from the continuous audit of all NIV admissions registered with the audit database of the Heart of England NHS Foundation Trust (audit registration number: 2399). Rates of development of nasal bridge pressure ulcers were compared to all patients requiring NIV in the 12-month period prior to intervention.
Subjects and intervention: We aimed to do a pre and post observational study following the introduction of the proactive preventative approach. Consecutive patients admitted to our dedicated physiotherapy-led, respiratory ward based NIV unit requiring NIV for acute hypercapnic respiratory failure between 30th October 2014 to -30th October 2015 were included. NIV was delivered in a ward-based setting using standard non-invasive ventilators in spontaneous-timed (ST) and volume-assured Pressure support modes via a FreeMotion RT040 (Fisher Paykel) oronasal mask sized according to manufacturer instructions. NIV settings were managed according to local protocols based on British Thoracic Society guidelines for the use of acute NIV.
Group 1 included all patients commencing NIV between 30th October 2014 and 29th October 2015, who received usual care. Group 2 included all patients commencing NIV between 30th October 2015 and 29th October 2016. Group 2 received hydrocolloid dressings (BeneHold Bordered Hydrocolloid dressing 5cm x 5cm [Aspen Medical]) which were positioned in a diamond formation over the centre of the forehead with a further dressing positioned as a diamond over the nasal bridge. The NIV mask was placed over this. All other care, including NIV pressure changes and breaks off NIV, was given according to local protocols which remained unchanged between the time periods.
Data collection: Data regarding age, sex, admission diagnosis, co-morbidity, length of NIV use, IPAP, EPAP, and nasal bridge tissue viability grading was recorded. The nasal bridge was formally inspected daily by a nurse trained in skin and pressure ulcer grading. Inspection involved removing the hydrocolloid dressing and assessment using hospital guidelines adapted from NPUAP/EPUAP pressure ulcer classification system. A pressure ulcer was diagnosed when criteria for grade 2 pressure change (partial thickness skin loss involving epidermis, dermis or both) was observed. If there was no evidence of pressure change or grade 1 change only, a new hydrocolloid dressing was placed and NIV continued via oronasal mask.
Statistical analysis: Chi squared and Fisher exact tests were used for analysis of incidence of grade 2 pressure ulcers between groups and other categorical data. Mann Whitney U test was used to analyse all other variables.
RESULTS
A total of 295 patients were included, 161 in Group 1 (pre) and 134 in Group 2 (post). 1 patient in Group 1 had incomplete records regarding co-morbidities and was excluded from analysis of this parameter, but all other categories were complete and the patient was therefore included in the study. Demographics including sex and age, and diagnosis or reason for commencement of NIV did not differ significantly between groups (Table 1).
Table 1.
Patient demographics and primary clinical indication for NIV
| Group 1 (161 episodes NIV) | Group 2 (134 episodes NIV) | P value | |
|---|---|---|---|
| Male | 70 (43.5%) | 46 (34.3%) | 0.109 |
| Age (mean years) | 69.7 | 69.2 | 0.610 |
| Diagnosis | |||
| COPD | 129 (80.1%) | 110 (82.1%) | 0.668 |
| Obesity | 11 (6.8%) | 13 (9.7%) | 0.369 |
| Musculoskeletal | 6 (3.7%) | (2.2%) | 0.459 |
| Other | 15 (9.3%) | 8 (6.0%) | 0.286 |
Pressure ulcer incidence: Pressure ulcer development differed significantly (p=0.001) between groups. For Group 1, 9 out of 161 episodes of acute NIV resulted in a grade 2 nasal bridge pressure ulcer during 666 NIV bed days. As for Group 2, none of 134 episodes of acute NIV resulted in a grade 2 nasal bridge pressure ulcer during 718 NIV bed days.
IPAP and EPAP used were not significantly difference between groups (IPAP p=0.110, Group 1 mean 19.8 [median 20, IQR 16-24], Group 2 mean 19.0 [median 18, IQR 16-22], EPAP p=0.100, Group 1 mean 6.1 [median 6, IQR 5-7], Group 2 mean 5.68 [median 6, IQR 5-6]).
Co-morbidity: Co-morbidities considered to have an association with an increased risk of pressure ulcers were not significantly difference between groups (Table 2).
Table 2.
Comorbidity in patient groups
| Co-morbidity | Pre | Post | P value |
|---|---|---|---|
| Diabetes | 52 | 36 | 0.293 |
| Vascular disease | 61 | 47 | 0.589 |
| Chronic kidney disease | 30 | 19 | 0.295 |
| Chronic dermatological | 2 | 0 | 0.502 |
Adverse effects: There were no local adverse effects (eg rash, contact dermatitis) related to dressings.
DISCUSSION
The use of prophylactic hydrocolloid dressings placed over the bridge of the nose effectively removed the risk of grade 2 nasal bridge pressure ulcers. Previous studies have shown the incidence of nasal bridge pressure ulcers during the use of acute NIV to be between 5-20%5,6,9. The incidence in our pre-intervention group was 6%. There were no ulcers evident in the intervention group.
The development of pressure ulcers related to NIV is due to a combination of pressure effects and shear forces exerted by the presence of the mask, pressure changes during different phases of ventilation, and mask strap tension6,10,11. The use of oronasal masks and increasing time spent on NIV increase the risk of pressure ulcers forming, as do patient factors including age, sensory impairment, chronic skin conditions, and hypotension amongst others.5
Previous studies into reducing NIV related pressure ulcers have examined the effect of dispersing pressure effects by changing the interface from an oronasal mask to a full face or helmet mask with a significant reduction in the incidence of pressure ulcers13. With regard to ventilation there is no evidence that any one interface is superior. Laboratory modelling suggested an increase in the internal volume of the interface may increase dead space and CO2 rebreathing14, however this has not been borne out in vivo15. Despite this, oronasal masks remain the most popular interface with a Europe wide survey showing them to be first choice in 70% of cases. Reasons given by respondents for their choice include reduced air leaks, patient comfort and cost13.
Three previous studies examining the effect of dressings in reducing nasal bridge pressure ulcers were identified. Weng et al report a significant reduction in grade 1 nasal bridge pressure ulcers with both Tegasorb and Tegaderm dressings when compared to no intervention17. Callaghan et al support this finding, using Granuflex compared to usual care18. Evaluation of a protective solution by Pena-Otero et al found a trend towards a protective benefit with use of a solution of hyperoxygenated fatty acids but no improvement with either an adhesive thin polyurethane dressing or an adhesive foam dressing19. All of these studies were limited by small sample sizes with the largest containing only 40 patients per group.
There is a larger body of evidence that considers more traditionally recognised pressure ulcers or ‘bedsores’ rather than ulcers related to medical devices. Preventative measures including turning regimes, pressure redistributing devices e.g. appropriate mattresses, and optimisation of nutritional status are now well known. Pooled analysis of RCTs of preventative dressings within this field demonstrate an overall 79% risk reduction in the incidence of new pressure ulcers with use of dressings20, although it was noted that the studies included in this analysis had a high risk of bias.
Our study is the assessment of a real-life quality improvement project. It is therefore limited in that its style it is a quasi-experimental ‘before-and-after’ study, lacking randomisation or blinding. There are a number of potentially confounding factors regarding the risk of developing pressure ulcers that were not systematically assessed, namely nutritional status and use of certain medications such as steroids. It does, however, provide real world data and is therefore easily transferrable to practice.
CONCLUSION
The current evidence base regarding both the incidence of nasal bridge pressure ulcers and the effect of preventative strategies is limited. We have demonstrated a strategy to reduce the incidence of grade 2 pressure ulcers associated with NIV, thereby reducing avoidable harm to patients and improving quality and safety of their care. We would therefore advocate the use of hydrocolloid dressings to prevent NIV related nasal bridge pressure ulcers.
Footnotes
Provenance: externally peer-reviewed.
UMJ is an open access publication of the Ulster Medical Society (http://www.ums.ac.uk).
REFERENCES
- 1.Juniper M, Ellis G, Smith NC, Protopapa KL, Mason M. NCEPOD reports 2017. London:: National Confidential Enquiry into Patient Outcome and Death; 2017.; 2017. Inspiring change: a review of the quality of care provided to patients receiving acute non-invasive ventilation. [Internet] Available from: http://www.ncepod.org.uk/2017niv.html. [Accessed September 2018] [Google Scholar]
- 2.Davies M. London:: 2013. British Thoracic Society. NIV Adult 2013 (national audit period 1 February – 31 March 2013) [Internet] Available from: https://www.brit-thoracic.org.uk/document-library/audit-and-quality-improvement/audit-reports/bts-adult-niv-audit-report-2013/ [Accessed September 2018] [Google Scholar]
- 3.Roberts CM, Stone RA, Buckingham RJ, Pursey N, Lowe D, National Chronic Obstructive Pulmonary Disease Resources and Outcomes Project implementation group. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66((1)):43–8. doi: 10.1136/thx.2010.153114. [DOI] [PubMed] [Google Scholar]
- 4.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicenter randomised controlled trial. Lancet. 2000;355((9219)):1931–5. doi: 10.1016/s0140-6736(00)02323-0. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguti WP, Moderno EV, Yamashita SY, Gomes TG, Maida AL, Kondo CS, et al. Treatment-related risk factors for development of skin breakdown in subjects with acute respiratory failure undergoing non-invasive ventilation or CPAP. Respir Care. 2014;59((10)):1530–6. doi: 10.4187/respcare.02942. [DOI] [PubMed] [Google Scholar]
- 6.Gay P. Complications of Noninvasive Ventilation in Acute Care. Respir Care. 2009;54((2)):246–58. [PubMed] [Google Scholar]
- 7.Black JM, Cuddigan JE, Walko MA, Didier LA, Lander MJ, Kelpe MR. Medical device-related pressure ulcers in hospitalized patients. Int Wound J. 2010;7((5)):358–65. doi: 10.1111/j.1742-481X.2010.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008–2009 International Pressure Ulcer Prevalence Survey and a 3-year, acute care, unit-specific analysis. Ostomy Wound Manage. 2009;55((11)):39–45. [PubMed] [Google Scholar]
- 9.Davidson AC, Banham S, Elliott M, Kennedy D, Gelder C, Glossop A, et al. BTS/ICS guidelines for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71((Suppl 2)):S1–S35. doi: 10.1136/thoraxjnl-2015-208209. [DOI] [PubMed] [Google Scholar]
- 10.Munckton K, Ho KM, Dobb GJ, Das-Gupta M, Webb SA. The pressure effects of facemasks during noninvasive ventilation: a volunteer study. Anaesthesia. 2007;62((11)):1126–31. doi: 10.1111/j.1365-2044.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- 11.Dellweg D, Hochrainer D, Klauke M, Kerl J, Eiger G, Kohler D. Determinants of skin contact pressure formation during non-invasive ventilation. J Biomech. 2010;43((4)):652–7. doi: 10.1016/j.jbiomech.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Worsley PR, Prudden G, Gower G, Bader DL. Investigating the effects of strap tension during non-invasive ventilation mask application:a combined biomechanical and biomarker approach. Med Devices. 2016;9:409–17. doi: 10.2147/MDER.S121712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schallom M, Cracchiolo L, Falker A, Foster J, Hager J, Morehouse T, et al. Pressure ulcer incidence in patients wearing nasal-oral versus full-face noninvasive ventilation masks. Am J Crit Care. 2015;24((4)):349–56. doi: 10.4037/ajcc2015386. [DOI] [PubMed] [Google Scholar]
- 14.Schettino GP, Chatmongkolchart S, Hess DR, Kacmarek R. Position of exhalation port and mask design affect CO2 rebreathing during noninvasive positive pressure ventilation. Crit Care Med. 2003;31((8)):2178–82. doi: 10.1097/01.CCM.0000081309.71887.E9. [DOI] [PubMed] [Google Scholar]
- 15.Fraticelli AT, Lellouche F, L’Her E, Taille S, Mancebo J, Brochard L. Physiological effects of different interfaces during noninvasive ventilation for acute respiratory failure. Crit Care Med. 2009;37((3)):939–45. doi: 10.1097/CCM.0b013e31819b575f. [DOI] [PubMed] [Google Scholar]
- 16.Crimi C, Noto A, Princi P, Esquinas A, Nava S. A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36((2)):362–9. doi: 10.1183/09031936.00123509. [DOI] [PubMed] [Google Scholar]
- 17.Weng M. The effect of protective treatment in reducing pressure ulcers for non-invasive ventilation patients. Intensive Crit Care Nurs. 2008;24((5)):295–9. doi: 10.1016/j.iccn.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Callaghan S, Trapp M. Evaluating two dressings for the prevention of nasal bridge pressure sores. Prof Nurs. 1998;13((6)):361–4. [PubMed] [Google Scholar]
- 19.Otero DP, Domniguez DV, Fernandez LH, Magarino AS, Gonzalez VJ, Klepzing JV, et al. Preventing facial pressure ulcers in patients under non-invasive mechanical ventilation: a randomised control trial. J Wound Care. 2017;26((3)):128–36. doi: 10.12968/jowc.2017.26.3.128. [DOI] [PubMed] [Google Scholar]
- 20.Moore ZE, Webster J. Dressings and topical agents for preventing pressure ulcers. Cochrane Database Syst Rev. 2013;((8)):CD009362. doi: 10.1002/14651858.CD009362.pub2. [DOI] [PubMed] [Google Scholar]


