Abstract
Background
Chlorogenic acid (CGA), a dietary polyphenol derived from many plants, has been previously reported to exert neuroprotective properties. However, its pharmacological role in Parkinson’s disease (PD) and the underlying mechanisms are unclear.
Material/Methods
In the present study, we investigated the beneficial effects of CGA against the toxicity of 6-hydroxydopamine (6-OHDA) in animal and cellular models. One week after 6-OHDA administration, the behavioral activities of rats were determined by rotarod test and apomorphine-induced rotational test. The viability and apoptosis of SH-SY5Y cells following 6-OHDA exposure were determined by MTT assay and annexin V-FITC/PI double staining, respectively. The activities of antioxidant enzymes in the rat striatal tissues and SH-SY5Y cells were detected by ELISA.
Results
The results demonstrated that 6-OHDA-induced PD-like behavioral impairments of rats were significantly forestalled by CGA administration. The increased apoptosis and reduced activities of antioxidant enzymes in the striatum of 6-OHDA-lesioned rats were also attenuated by CGA. Moreover, in an in vitro experiment, the impaired viability and enhanced apoptosis of 6-OHDA-injured SH-SY5Y cells were significantly restored by CGA pretreatment. In addition, CGA also obstructed 6-OHDA-induced ROS production and endoplasmic reticulum (ER) stress in SH-SY5Y cells.
Conclusions
Taken together, these data show that CGA might be an effective neuroprotective compound that mitigates oxidative stress and ER stress in PD.
MeSH Keywords: Apoptosis, Chlorogenic Acid, Endoplasmic Reticulum Stress, Oxidative Stress, Parkinson Disease
Background
Parkinson’s disease (PD) is a chronic, progressively debilitating neurodegenerative diseases with a prevalence of about 4% to 5% at age 85 years worldwide [1]. PD is typically characterized by a massive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) region and the formation of Lewy bodies [2], eventually leading to classic movement disorders, including bradykinesia, tremor, and rigidity. As the pathogenesis of PD is complex, currently available therapeutic strategies only provide temporary relief of symptoms. Thus, identification of potent and effective neuroprotective agents with limited adverse effects is still of critical importance [3].
Recently, natural products have received great attention worldwide and are showing a bright future in the treatment of neurodegenerative diseases, including PD [4]. Chlorogenic acid (5-caffeoylquinic acid, CGA; Figure 1) is abundant in a variety of plant foods such as coffee beans and apples [5]. CGA has numerous pharmacological activities. It was reported that GCA provided protective effects against aluminium-induced cytotoxicity in primary hippocampal neuronal cells [6]. Kwon et al. also demonstrated that CGA could reduce 6-hydroxydopamine (6-OHDA)-induced toxicity in SH-SY5Y cells [7]. However, to date, the pharmacological effects and molecular mechanisms of CGA in PD, particularly in experimental PD animals, have been unclear.
Figure 1.
The chemical structure of chlorogenic acid (CGA).
6-OHDA, a hydroxylated analog of the natural neurotransmitter dopamine, is widely used as a neurotoxin to induce experimental PD both in vitro and in vivo [8]. In the present study, we investigated the beneficial effects of CGA in 6-OHDA-lesioned rats and 6-OHDA-injured SH-SY5Y cells, and explored the possible underlying molecular mechanisms.
Material and Methods
Animals and treatments
Fifty 6–8-week-old male Sprague-Dawley rats (weighing 240–260 g), purchased from Shanghai Laboratory Animal Center (Shanghai, China), were housed at 22±2°C with 60±10% humidity under an automatic 12 h/12 h light-dark cycle (the light was on from 08: 00 to 20: 00 h) throughout the acclimatization and experimental period. Food and water were always available. All animal care and experimental protocols were reviewed and approved by the Animal Use and Ethics Committee of Jining No. 1 People’s Hospital (Shandong, China). All efforts were made to minimize animal suffering [9].
The rats were randomized into 5 groups (n=10 rats/group). After 1 week of acclimatization, 40 rats were placed in a stereotaxic device (Stoelting; Wood Dale, IL, USA), and 10 μg of 6-OHDA-hydrobromide (Sigma, St. Louis, MO, USA) dissolved in 5 μl vehicle (0.9% saline with 0.1% ascorbic acid, pH 5.5) was stereotactically injected into the left striatum at a rate of 0.2 μl/min using a 5-μl Hamilton syringe. The 10 rats in the control group received the same volume of vehicle.
One hour after the 6-OHDA injection, 3 groups of 6-OHDA-treated rats were administrated 20, 40, or 60 mg/kg of CGA (Sigma) dissolved in 1 ml of PBS, daily by intraperitoneal injection for 7 consecutive days.
Behavioral testing
After 1 week, all rats were subjected to the following behavioral tests.
Rotarod test
The rotarod test was performed to evaluate impairment of motor coordination and balance [10]. In brief, before 6-OHDA injection, the rats were first acclimated to the rod (3 cm diameter). On the day of the test, the rats were placed on the rod and the rotation speed was started at 4 rpm and then gradually increased to 40 rpm within 5 min. Each rat was given 3 trials, and the length of time each rat stayed on the rod was recorded.
Apomorphine-induced rotational test
The apomorphine-induced rotational test was performed as previously described [11]. Briefly, after a subcutaneous injection of apomorphine hydrochloride (0.5 mg/kg, dissolved in saline; Sigma), full 360-degree rotations of rats were manually counted in a cylindrical container with a diameter of 33 cm and a height of 35 cm for 1 h. Finally, the net number of rotations was calculated as the positive scores (contralateral rotations) minus the negative scores (ipsilateral rotations).
All behavioral tests were performed in a double-blinded manner between 10 a.m. and 3 p.m.
Analysis of striatal dopamine concentration
After the behavioral tests, the rats of each group were immediately sacrificed by CO2 asphyxiation. The whole brain was immediately removed and the striatum was dissected on dry ice and homogenized in a tissue grinder. After centrifugation, the supernatant was stored at −80°C. The high-performance liquid chromatography (HPLC) assay was performed to measure the striatal dopamine concentration, which is a marker of dopaminergic synaptic function.
Cell culture and treatments
SH-SY5Y human neuroblastoma cells (passage ≤25), purchased from American Type Culture Collection (Rockville, MD, USA), were cultured in 75 cm2 polystyrene culture flasks and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin at 37°C in a humid environment (5% CO2, 95% air).
SH-SY5Y cells were treated with 200 μM 6-OHDA for 24 h to establish PD model in vitro. At 1 h prior to 6-OHDA stimulation, SH-SY5Y cells were pretreated with various concentrations of CGA (10, 50, and 100 μM). Control cells were only treated with PBS.
Cell viability analysis
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [12]. After the experimental treatments, 20 μl of MTT solution (5 mg/ml; Sigma) was added into each well and incubated at 37°C for an additional 4 h. The supernatants were then carefully aspirated, and the formazan crystals were dissolved in DMSO (Sigma). The absorbance at 570 nm was measured using a microplate reader (MultiskanEX, Lab systems, Helsinki, Finland).
Cell apoptosis analysis
Cell apoptosis was measured using flow cytometry with the Annexin V-FITC/PI double-staining kit (Invitrogen, Carlsbad, CA, USA). After the experimental treatments, the cells were resuspended in 1× binding buffer, and double-stained with 5 μl Annexin V-FITC and 5 μl PI at room temperature for 15 min. The apoptotic rate was then analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA).
Determination of intracellular ROS levels
Intracellular ROS levels were measured using a 2′,7′-dichlorofluorescein diacetate (DCFH-DA) fluorescent probe (Sigma). Briefly, after the experimental treatments, the cells were incubated with 10 μM DCFH-DA for 30 min at 37°C in the dark. After that, the fluorescence intensity was detected by flow cytometry.
Analysis of antioxidant enzymes
The activities of antioxidant enzymes, including SOD and GSH-Px, in the tissues and cells were analyzed using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Co., Nanjing, China) and quantified by a microplate reader.
Western blot analysis
The tissues and cells were washed twice with PBS, and lysed for 10 min in RIPA lysis buffer (Wuhan Goodbio Technology Co., Wuhan, China). Protein concentration was measured using the Bradford Protein Assay Kit (Beyotime, Haimen, China). Equal amounts of protein samples were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). After blocking with 5% nonfat milk in TBST, the membranes were probed overnight at 4°C with appropriate primary antibodies, followed by incubation with secondary antibodies for 1 h at room temperature. Protein bands were visualized using the enhanced chemiluminescence detection system (Amersham, Little Chalfont, UK). The results were normalized to GAPDH and quantified using ImageJ software (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
All statistical analysis was conducted using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). The data are expressed as the mean ± standard deviation (SD). Statistical evaluation of the data was performed using one-way analysis of variance (ANOVA) and Dunnett’s post hoc test. A probability value of P<0.05 was considered to be statistically significant.
Results
CGA reversed the motor deficits in 6-OHDA-lesioned rats
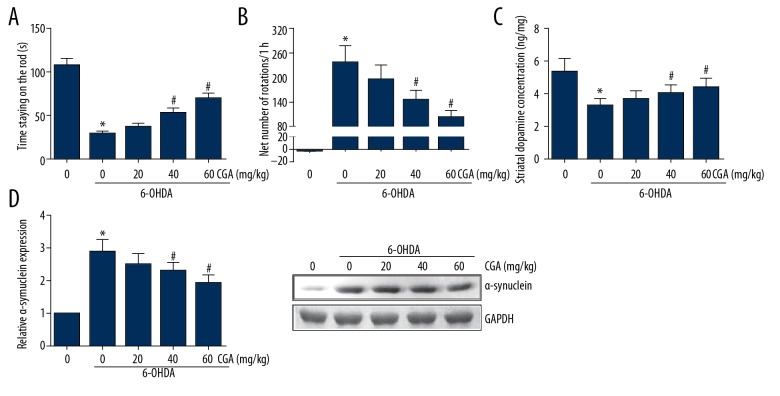
One week after 6-OHDA administration, compared with the rats in the control group, the 6-OHDA-lesioned rats developed more severe PD symptoms. The results of the rotarod test indicated that 6-OHDA-treated rats showed shorter times staying on the rod, and this reduction was partly improved by CGA administration (Figure 2A). In addition, as shown in Figure 2B, apomorphine led to a very significant contralateral turning in the 6-OHDA-treated rats. However, administration with CGA dose-dependently reduced the net number of rotations. These findings indicate that CGA prevented 6-OHDA-induced motor deficits. Moreover, we found that CGA administration markedly attenuated the 6-OHDA-induced decrease in striatal dopamine concentration (Figure 2C). The accumulation of α-synuclein in the striatum was also remarkably reduced by CGA administration (Figure 2D).
Figure 2.
CGA reversed the motor deficits in 6-OHDA-lesioned rats. (A) The length of time the rat stayed on the rod was recorded. (B) Net number of apomorphine-induced rotations was recorded. (C) HPLC analysis of dopamine concentration in the rat striatum. (D) Western blot analysis of α-synuclein expression levels in the rat striatum. Data are presented as mean ±SD. * P<0.05 vs. control group; # P<0.05 vs. 6-OHDA-treated group.
CGA enhanced the activities of antioxidant enzymes in the striatum of 6-OHDA-lesioned rats
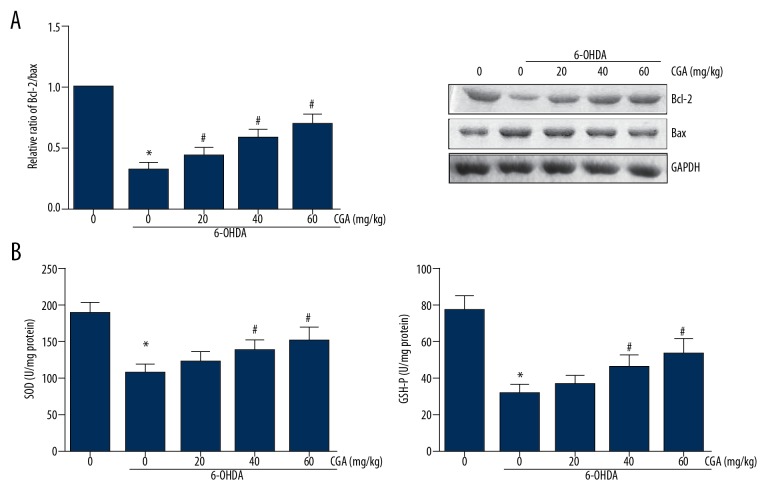
To determine the effects of CGA on 6-OHDA-induced apoptosis, we measured the expression levels of Bcl-2 and Bax in the striatum of rat brain by Western blotting, and the results demonstrated that 6-OHDA remarkably reduced the ratio of Bcl-2/Bax expression in the striatal tissues, and this reduction was restored by CGA administration (Figure 3A). In addition, compared with the rats in the control group, the 6-OHDA-lesioned rats exhibited significantly reduction of SOD and GSH-Px activities in the striatal tissues, whereas these effects were dose-dependently alleviated by administration of CGA (Figure 3B).
Figure 3.
CGA enhanced the activities of antioxidant enzymes in the striatum of 6-OHDA-lesioned rats. (A) Western blot analysis of Bcl-2 and Bax expression levels in the rat striatum. (B) The activities of SOD and GSH-Px in the rat striatum were detected by ELISA. Data are presented as mean ±SD. * P<0.05 vs. control group; # P<0.05 vs. 6-OHDA-treated group.
CGA restored the 6-OHDA-induced cytotoxicity in SH-SY5Y cells
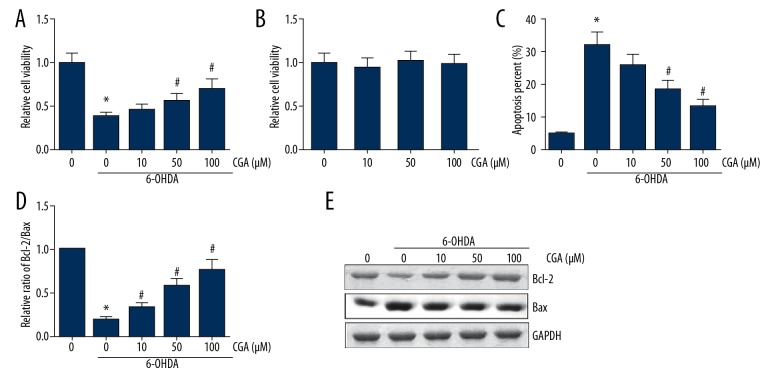
The neuroblastoma SH-SY5Y lineage is widely used in PD research [13]. As shown in Figure 4A, treatment with 200 μM 6-OHDA led to a nearly 60% reduction of cell viability in SH-SH5Y cells, and pretreatment with CGA dose-dependently alleviated the toxicity of 6-OHDA to cell viability. However, treatment with CGA alone at various doses did not exhibit obvious signs of cytotoxicity on SH-SY5Y cells (Figure 4B). The results of Annexin V-FITC/PI double-staining showed that CGA dose-dependently rescued SH-SY5Y cells from 6-OHDA-induced apoptosis (Figure 4C). Additionally, as shown in Figure 4D, 6-OHDA treatment decreased the Bcl-2/Bax ratio in SH-SY5Y cells, which was attenuated by CGA pretreatment in a dose-dependent manner.
Figure 4.
CGA restored the 6-OHDA-induced cytotoxicity in SH-SY5Y cells. (A, B) The viability of SH-SY5Y cells was determined by MTT assay. (C) The apoptosis of SH-SY5Y cells was determined by Annexin V-FITC/PI double-staining. (D) Western blot analysis of Bcl-2 and Bax expression levels in SH-SY5Y cells. Data are presented as mean ±SD. * P<0.05 vs. control cells; # P<0.05 vs. 6-OHDA-treated cells.
CGA alleviated ROS production and ER stress in 6-OHDA-treated SH-SY5Y cells
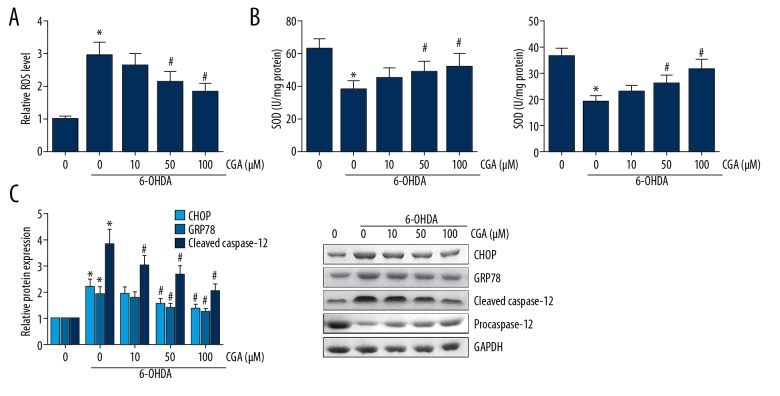
As demonstrated in Figure 5A, pretreatment with CGA significantly inhibited the 6-OHDA-induced production of ROS in SH-SY5Y cells. In addition, the activities of SOD and GSH-Px in 6-OHDA-treated SH-SY5Y cells were also remarkably enhanced upon CGA pretreatment (Figure 5B). The accumulation of ROS often induces ER stress. Western blot analysis showed that CGA pretreatment reduced the expression levels of ER stress markers, including CHOP and GRP94, in 6-OHDA-treated SH-SY5Y cells (Figure 5C). Caspase-12 is an ER-resident caspase, and we also verified that CGA significantly attenuated the 6-OHDA-induced procaspase-12 cleavage.
Figure 5.
CGA alleviated ROS production and ER stress in 6-OHDA-treated SH-SY5Y cells. (A) The ROS level in SH-SY5Y cells was determined by DCFH-DA staining. (B) The activities of SOD and GSH-Px in SH-SY5Y cells were detected by ELISA. (C) The expression levels of CHOP, GRP94, and cleaved caspase-12 in SH-SY5Y cells were detected by Western blot analysis. Data are presented as mean ±SD. * P<0.05 vs. control cells; # P<0.05 vs. 6-OHDA-treated cells.
Discussion
PD is common worldwide, and is becoming even more prevalent due to increased longevity; however, there is rare cure for PD at present. One primary symptom of PD patients is movement dysfunction. In the present study, we used the specific neurotoxin 6-OHDA for inducing a model of PD in rats, and through behavioral testing we found that CGA administration alleviated the movement deficits in the 6-OHDA-lesioned PD rat model. Neuronal apoptosis is also a key factor involved in the pathogenesis of PD. Exposure to 6-OHDA results in extensive apoptotic lesions in the SNpc of the rat brain [14]. Our results found that CGA administration increased the viability and suppressed the apoptosis of 6-OHDA-treated SH-SY5Y cells.
The neurotoxicity of 6-OHDA is related to the production of ROS [15]. Oxidative stress is the result of ROS accumulation, which is an important factor in the brain aging process. Dopaminergic neurons are vulnerable to oxidative products, and oxidative damage plays a critical role in the pathogenesis of PD [16]. The anti-oxidative role of CGA was previously reported in many earlier studies [17,18]. In the present study, increased oxidative stress and reduced activities of antioxidant enzymes were observed following 6-OHDA exposure, and these effects were dose-dependently prevented by CGA in animal and cellular models.
The endoplasmic reticulum (ER) plays a key role in protein synthesis and folding. ER stress induced by various stimuli often leads to the accumulation of unfolded or misfolded proteins [19]. Persistent oxidative stress commonly results in apoptosis via the induction of ER stress [20], and the activation of ER stress-mediated cell death is also implicated in PD [21]. Therefore, targeting ER stress may be a promising strategy for the treatment of PD. Caspase-12 is localized to the ER and mediates ER-specific apoptosis [22]. The present study also found that the 6-OHDA-induced activation of ER stress markers and procaspase-12 cleavage were significantly attenuated by CGA pretreatment, indicating that CGA exerts its beneficial role partly through inhibition of ER stress.
Conclusions
The present study demonstrates for the first time that CGA exerts beneficial effects on 6-OHDA-induced neurotoxicity, partly through reducing oxidative stress and ER stress. These findings indicate that CGA might have therapeutic effects for PD. However, the real function of CGA in PD patients needs further investigation.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Giugni JC, Okun MS. Treatment of advanced Parkinson’s disease. Curr Opin Neurol. 2014;27:450–60. doi: 10.1097/WCO.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 3.Strecker K, Schwarz J. Parkinson’s disease: Emerging pharmacotherapy. Expert Opin Emerg Drugs. 2008;13:573–91. doi: 10.1517/14728210802596906. [DOI] [PubMed] [Google Scholar]
- 4.Solanki I, Parihar P, Parihar MS. Neurodegenerative diseases: From available treatments to prospective herbal therapy. Neurochem Int. 2016;95:100–8. doi: 10.1016/j.neuint.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Nardini M, Cirillo E, Natella F, Scaccini C. Absorption of phenolic acids in humans after coffee consumption. J Agric Food Chem. 2002;50:5735–41. doi: 10.1021/jf0257547. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Fan X, Yuan S, et al. Chlorogenic acid protects against aluminium-induced cytotoxicity through chelation and antioxidant actions in primary hippocampal neuronal cells. Food Funct. 2017;8:2924–34. doi: 10.1039/c7fo00659d. [DOI] [PubMed] [Google Scholar]
- 7.Kwon SH, Ma SX, Hong SI, et al. Eucommia ulmoides Oliv. bark. attenuates 6-hydroxydopamine-induced neuronal cell death through inhibition of oxidative stress in SH-SY5Y cells. J Ethnopharmacol. 2014;152:173–82. doi: 10.1016/j.jep.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/845618. 845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. J Gene Med. 2010;12:561–63. doi: 10.1002/jgm.1473. [DOI] [PubMed] [Google Scholar]
- 10.Monville C, Torres EM, Dunnett SB. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods. 2006;158:219–23. doi: 10.1016/j.jneumeth.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Borlongan CV, Randall TS, Cahill DW, Sanberg PR. Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res. 1995;676:231–34. doi: 10.1016/0006-8993(95)00150-o. [DOI] [PubMed] [Google Scholar]
- 12.Sladowski D, Steer SJ, Clothier RH, Balls M. An improved MTT assay. J Immunol Methods. 1993;157:203–7. doi: 10.1016/0022-1759(93)90088-o. [DOI] [PubMed] [Google Scholar]
- 13.Xicoy H, Wieringa B, Martens GJ. The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener. 2017;12:10. doi: 10.1186/s13024-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res. 2005;162:1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, et al. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: Potential implication in relation to the pathogenesis of Parkinson’s disease. J Neurochem. 2000;74:1605–12. doi: 10.1046/j.1471-4159.2000.0741605.x. [DOI] [PubMed] [Google Scholar]
- 16.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36–28. [DOI] [PubMed] [Google Scholar]
- 17.Ali N, Rashid S, Nafees S, et al. Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: An experimental approach. Chem Biol Interact. 2017;272:80–91. doi: 10.1016/j.cbi.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Song J, Guo D, Bi H. Chlorogenic acid attenuates hydrogen peroxideinduced oxidative stress in lens epithelial cells. Int J Mol Med. 2018;41:765–72. doi: 10.3892/ijmm.2017.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 20.Bhandary B, Marahatta A, Kim HR, Chae HJ. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14:434–56. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu EJ, Harding HP, Angelastro JM, et al. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22:10690–98. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]