Abstract
Background
RAW264.7 cells are induced by lipopolysaccharide (LPS) as a rheumatoid arthritis (RA) model. The present study investigated the effect of cimifugin on the proliferation, migration, chemotaxis, and release of inflammation-related factors and inflammation-related signaling pathways of LPS-induced RAW264.7 cells.
Material/Methods
MTS assay was used to determine the proliferation of RAW264.7 cells. Transwell assay was employed to examine the migration and chemotaxis of the cells. ELISA was performed to measure the contents of chemotactic factors and inflammatory factors in cell culture supernatants. Western blotting was carried out to detect the expression of factors related with MAPKs and NF-κB signaling pathways.
Results
Cimifugin (0–100 mg/L) had no cytotoxicity for RAW264.7 cells. LPS stimulation induced morphological differentiation of RAW264.7 cells, but intervention by cimifugin inhibited the activation effect by LPS by about 50%. Cimifugin (100 mg/L) decreased the migration and chemotaxis of RAW264.7 cells to 1/3 of that in control cells by decreasing the release of migration- and chemotaxis-associated factors by at least 30%. Cimifugin (100 mg/L) suppressed the release of inflammatory factors from RAW264.7 cells to less than 60% of that in the LPS group. In addition, cimifugin (100 mg/L) inhibited the activities of MAPKs and NF-κB signaling pathways.
Conclusions
The present study demonstrates that cimifugin reduces the migration and chemotaxis of RAW264.7 cells and inhibits the release of inflammatory factors and activation of related signaling pathways induced by LPS. Cimifugin may have potential pharmacological effects against RA.
MeSH Keywords: Cytokines, Felty Syndrome, MAP Kinase Signaling System, NF-kappa B
Background
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized mainly by synovitis in middle-aged and elderly populations [1]. The features of RA include synovial hyperplasia and chronic erosive inflammation and autoimmune disorders, and the disease often affects the joints [2,3]. About 1% of the world’s population is affected by RA [4], and the prevalence rate of the disease is about 0.32–0.36% in China [5].
Anti-inflammatory therapy has become an important direction in the basic and clinical treatments for RA [6]. The pathogenesis of RA is closely related to 2 important signaling cascades that are involved in inflammatory response: the mitogen-activated protein kinase (MAPK) signal transduction pathway [7,8] and the nuclear factor-kappa B (NF-κB) signal transduction pathway [9]. The decrease of MAPK and NF-κB pathway activity can alleviate inflammatory responses, and new therapies and drugs have been developed against these signaling pathways [10,11].
Saposhnikoviae Radix, the dry roots of the plant Saposhnikovia divaricata (Turcz) schischk prepared at its vegetative growth period, are widely used in East Asia for antipyretic, analgesic, and anti-inflammatory purposes [12,13]. Chromones are among the main active components of Saposhnikoviae Radix [12]. Prim-o-glucosylcimifugin content is relatively high among all chromones [14], and it is demonstrated to have anti-inflammatory and analgesic effects [15]. Prim-o-glucosylcimifugin can be easily converted into its aglycone, cimifugin, in vivo [16,17]. Cimifugin suppresses allergic inflammation via regulating tight junctions [18]; however, it is still unclear whether cimifugin is an active component that exerts anti-inflammatory effects in vivo. In addition, it is not reported whether cimifugin affects the inflammatory factors and signaling pathways that are related with RA. In the present study we investigated the effect of cimifugin on the proliferation, migration, chemotaxis, release of inflammation-related factors, and inflammation-related signaling pathways of RAW264.7 cells using lipopolysaccharide (LPS) as the inducing reagent. In addition, the present study aimed to provide a theoretical basis for the prevention and treatment of RA.
Material and Methods
Cells
RAW264.7 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in DMEM high-glucose medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum at 37°C and 5% CO2. For detection, the medium was concentrated by centrifugation at 1000×g and 4°C for 10 min.
MTS assay
RAW264.7 cells (2×105/well) were seeded onto 96-well plates in triplicate. After being cultured in the presence of 100 mg/L, 50 mg/L, 25 mg/L, 12.5 mg/L, 6.25 mg/L, or 0 mg/L cimifugin (A1271; Bellancom Chemistry, Beijing, China) for 72 h, the cells were subjected to viability test using the CellTiter 9 AQueous One Solution Cell Proliferation Assay kit (CTB169; Promega, Fitchburg, WI, USA) following the manufacturer’s manual. Each test was performed in triplicate.
Determination of nitric oxide (NO) content
The content of NO was determined by use of an NO detection kit (S0021, Beyotime, Shanghai, China) according to the manufacturer’s manual and following a previously published method [19]. Each test was performed in triplicate.
Transwell assay
The QCM Laminin Migration Assay kit (ECM220, 5.0μm; Merck Millipore, Merck KGaA, Darmstadt, Germany) was used for Transwell assay. After being treated with 100 mg/L (high), 50 mg/L (medium) and 25 mg/L (low) cimifugin for 48 h, RAW264.7 cells were seeded onto the upper Transwell chamber at a density of 2×105/well in 24-well plates. Because cell migration could be driven by serum, it was generally necessary to culture the cells without sera for a period of time to synchronize the cells. Therefore, the cells were cultured in serum-free DMEM high-glucose medium for 12 h to eliminate the influence of serum. To test migration ability, 500 μL DMEM high-glucose medium supplemented with 10% fetal bovine serum was added into the lower Transwell chamber. To study chemotaxis, 500 μL serum-free DMEM high-glucose medium containing 1 μg/mL LPS (Sigma-Aldrich, St. Louis, MO, USA) was added into the lower Transwell chamber. Then, the culture plates were incubated at 37°C and 5% CO2 for 24 h. Afterwards, the medium in the chamber was discarded, and the chamber was washed with phosphate-buffered saline twice to eliminate remaining medium. The chamber was fixed with methanol for 30 min, and the cells in the upper chamber were wiped off. After the chamber was air-dried, it was stained for 15 min in 0.1% crystal violet solution, which was subsequently washed away. Following air-drying, the chamber was observed under a microscope. Five fields were randomly chosen for each chamber, and images were taken at a magnification of 200×. The number of cells that crossed the membrane was counted and used for the evaluation of migration and chemotaxis of cells. Each test was performed in triplicate.
Enzyme-linked immunosorbent assay (ELISA)
RAW264.7 cells were divided into a control group (no LPS was added), LPS group (treated with 1 μg/mL LPS for 24 h), high-dose group (1 μg/mL LPS+100 mg/L cimifugin for 24 h), medium-dose group (1 μg/mL LPS+50 mg/L cimifugin for 24 h), and low-dose group (1 μg/mL LPS+25 mg/L cimifugin for 24 h). Cell culture supernatants (100 μL/well) were added into ELISA plates for the determination of the concentrations of IL-6 (EMC004.96; Neobioscience Technology Company, Shenzhen, China), TNF-α (EMC102a.96; Neobioscience Technology Company, Shenzhen, China), IL-1β (EMC001b.96; Neobioscience Technology Company, Shenzhen, China), MIP-2 (EMC122.96; Neobioscience Technology Company, Shenzhen, China), MCP-1 (EMC113.96; Neobioscience Technology Company, Shenzhen, China), and IL-8 (EMC104.96; Neobioscience Technology Company, Shenzhen, China) following the respective manuals. Each sample was tested in triplicate.
Western blotting
RAW264.7 cells were divided into a control group (no LPS was added), LPS group (treated with 1 μg/mL LPS for 24 h), high-dose group (1 μg/mL LPS+100 mg/L cimifugin for 24 h), medium-dose group (1 μg/mL LPS+50 mg/L cimifugin for 24 h), and low-dose group (1 μg/mL LPS+25 mg/L cimifugin for 24 h). Total proteins were extracted using the E.Z.N.A Total DNA/RNA/Protein kit (Omega Bio-Tek Inc., Norcross, GA, USA) following the manufacturer’s manual. The protein concentration was determined by using the bicinchoninic acid (BCA) protein concentration determination kit (P0010; Beyotime Biotechnology, Shanghai, China). Protein samples were then mixed with 5× sodium dodecyl sulfate loading buffer before denaturation in a boiling water bath for 5 min. Afterwards, the samples (20 μg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked with 5% skimmed milk at room temperature for 1 h. Then, the membranes were incubated with rabbit anti-human P65 polyclonal primary antibody (ZS-372; ZSGB-BIO, Beijing, China), rabbit anti-human P50 polyclonal primary antibody (ZS-1140; ZSGB-BIO, Beijing, China), mouse anti-human p-IκB-α monoclonal antibody (ZS-8404; ZSGB-BIO, Beijing, China), rabbit anti-human P-P65 polyclonal primary antibody (sc-33020; Santa Cruz, Dallas, TX, USA), rabbit anti-human P-P50 polyclonal primary antibody (sc-33022; Santa Cruz, Dallas, TX, USA), rabbit anti-human IκB-α polyclonal primary antibody (sc-371; ZSGB-BIO, Beijing, China), rabbit anti-human ERK1/2 polyclonal primary antibody (ab115799; Abcam, Cambridge, UK), rabbit anti-human P-ERK1/2 monoclonal primary antibody (#4370; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-human P38 monoclonal primary antibody (#9212; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-human P-P38 (#4511; Cell Signaling Technology, Danvers, MA, USA), and mouse anti-human GAPDH monoclonal antibody (TA-08; ZSGB-BIO, Beijing, China) at 4°C overnight. After extensive washing with phosphate-buffered saline with Tween 20 5 times for 5 min each time, the membranes were incubated with goat anti-rabbit IgG secondary antibody (ZDR-5306; ZSGB-BIO, Beijing, China) or goat anti-mouse IgG (ZDR-5307; ZSGB-BIO, Beijing, China) for 1 h at room temperature before washing with phosphate-buffered saline with Tween 20 for 5 times for 5 min each time. Then, the membrane was developed using an enhanced chemiluminescence detection kit (Sigma-Aldrich, St. Louis, MO, USA) for imaging. Image lab v3.0 software (Bio-Rad, Hercules, CA, USA) was used to acquire and analyze imaging signals. The relative expression of target proteins was calculated against GAPDH. Each test was performed in triplicate.
Statistical analysis
The results were analyzed using SPSS 18.0 statistical software (IBM, Armonk, NY, USA). The data are expressed as means ±SEM. Data were tested for normality. In case of homogeneity of variance, multigroup measurement data were analyzed using one-way ANOVA and Dunnett test. In case of heterogeneity of variance, multigroup measurement data were analyzed using Kruskal-Wallis test followed by Tamhane’s T2 or Dunnett’s T3 method. The 2 groups of data were examined by Mann-Whitney method. P<0.05 indicated statistically significant differences.
Results
Cimifugin (0–100 mg/L) has no cytotoxicity for RAW264.7 cells
To test the effect of cimifugin on the viability of RAW264.7 cells, MTS assay was performed. The data showed that the viability of RAW264.7 cells treated with 100 mg/L, 50 mg/L, 25 mg/L, 12.5 mg/L, 6.25 mg/L, or 0 mg/L cimifugin for 72 h were not different from each other (P>0.05) (Figure 1). The results suggest that cimifugin (0–100 mg/L) has no cytotoxicity for RAW264.7 cells.
Figure 1.
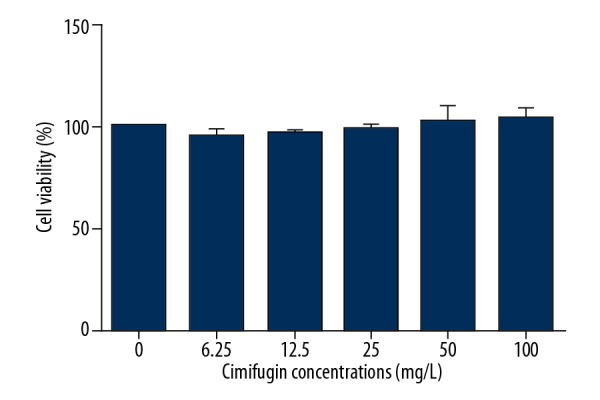
Effect of cimifugin on the viability of RAW264.7 cells. MTS assay was performed to determine cell viability after treatment with 100 mg/L, 50 mg/L, 25 mg/L, 12.5 mg/L, 6.25 mg/L, or 0 mg/L cimifugin for 72 h.
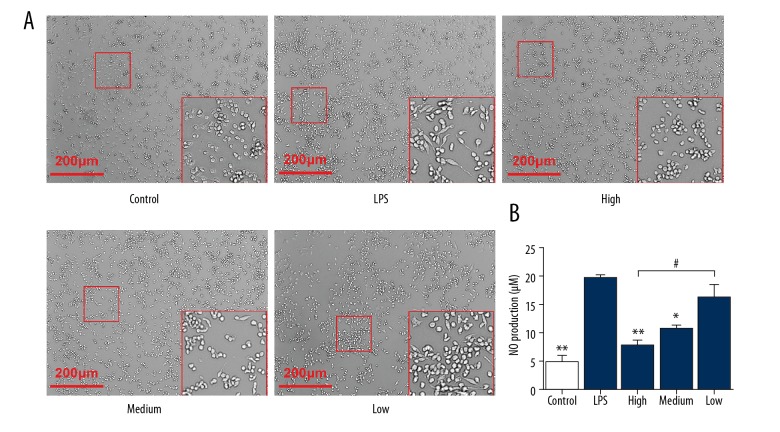
LPS stimulation induces morphological differentiation of RAW264.7 cells, but intervention by cimifugin inhibits the activation effect by LPS
To examine the effect of cimifugin on RAW264.7 cells stimulated by LPS, the cells were observed under a microscope and NO content in cell culture supernatant was determined. Microscopy showed that some of the cells gradually became spindle-shaped after treatment with LPS, while treatment with cimifugin inhibited the morphological differentiation of the cells (Figure 2A). Detection of NO content in supernatant of cell culture showed that LPS stimulation significantly upregulated the release of NO to a level 4 times that in the control group (P<0.05). By contrast, treatment with 100 mg/L or 50 mg/L cimifugin reduced the release of NO to 40–50% that in the LPS group (P<0.05) (Figure 2B). The results indicate that LPS stimulation induces morphological differentiation of RAW264.7 cells, but intervention by cimifugin inhibits the activation effect by LPS.
Figure 2.
Effect of cimifugin on (A) morphology and (B) NO secretion of RAW264.7 cells. RAW264.7 cells were divided into a control group (no LPS was added), LPS group (treated with 1 μg/mL LPS for 24 h), high-dose group (1 μg/mL LPS+100 mg/L cimifugin for 24 h), medium-dose group (1 μg/mL LPS+50 mg/L cimifugin for 24 h), and low-dose group (1 μg/mL LPS+25 mg/L cimifugin for 24 h). Microscopy was used to observe cell morphology. NO content in cell supernatant was determined as described in Material and Methods. * P<0.05 and ** P<0.01 compared with LPS group. # P<0.05 compared among indicated groups.
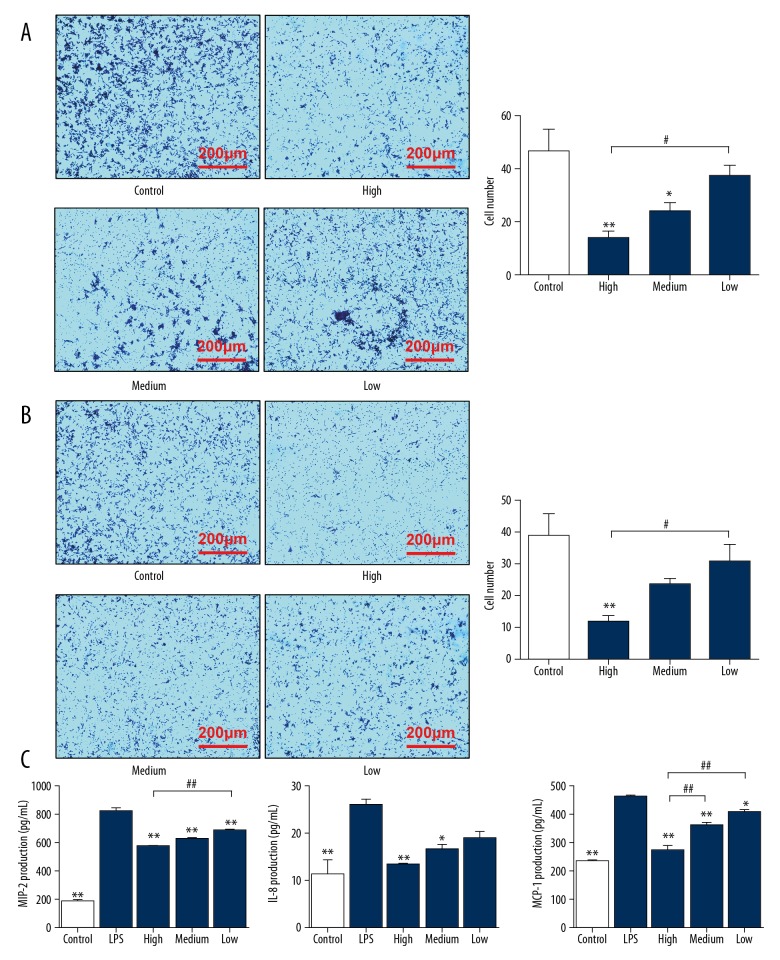
Cimifugin decreases the migration and chemotaxis of RAW264.7 cells by inhibiting the release of migration- and chemotaxis-associated factors
To study how cimifugin affects the migration and chemotaxis of RAW264.7 cells induced by fetal bovine serum or LPS, Transwell assay was carried out. The data showed that cells induced by serum crossed the membrane successfully, but 100 mg/L and 50 mg/L cimifugin significantly reduced the number of cells that crossed the membrane to 33% and 50% of that in the control group, respectively (P<0.05) (Figure 3A). Similarly, cells induced by LPS crossed the membrane successfully, but 100 mg/L cimifugin significantly reduced the number of cells that crossed the membrane to 28% of that in the control group (P<0.05) (Figure 3B). To determine the concentrations of migration- and chemotaxis-associated factors in cell supernatants, ELISA was employed. The data showed that the concentrations of migration- and chemotaxis-associated cytokines, MIP-2, IL-8 and MCP-1, were at least doubled after LPS stimulation (P<0.05). In contrast, treatment with 100 mg/L and 50 mg/L cimifugin reduced their concentrations in cell supernatants to 50–70% of that in the LPS group (P<0.05) (Figure 3C). The results suggest that cimifugin decreases the migration and chemotaxis of RAW264.7 cells by inhibiting the release of migration- and chemotaxis-associated factors.
Figure 3.
Effect of cimifugin on the migration and chemotaxis of RAW264.7 cells. RAW264.7 cells were divided into a control group (no LPS was added), LPS group (treated with 1 μg/mL LPS for 24 h), high-dose group (1 μg/mL LPS+100 mg/L cimifugin for 24 h), medium-dose group (1 μg/mL LPS+50 mg/L cimifugin for 24 h), and low-dose group (1 μg/mL LPS+25 mg/L cimifugin for 24 h). (A, B) Effect of cimifugin on the migration and chemotaxis of RAW264.7 cells after treatment with (A) fetal bovine serum or (B) LPS. Transwell assay was performed to determine the migration of cells. * P<0.05 and ** P<0.01 compared with control group. # P<0.05 compared among indicated groups. (C) Effect of cimifugin on the concentrations of migration- and chemotaxis-associated factors in cell culture supernatants. ELISA was used to determine the concentrations of the factors. * P<0.05 and ** P<0.01 compared with LPS group. # P<0.05 and ## P<0.01 compared among indicated groups.
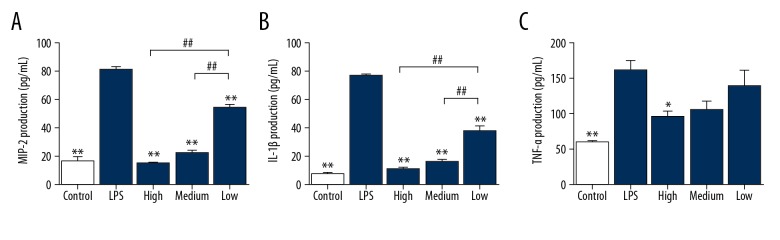
Cimifugin suppresses the release of inflammatory factors from RAW264.7 cells
To measure the contents of inflammatory factors in cell culture supernatants, ELISA was used. The data showed that the concentrations of IL-6, IL-1β, and TNF-α in the supernatants of RAW264.7 cells stimulated with LPS were more than 3 times that in the control group (P<0.05). However, treatment with 100 mg/L, 50 mg/L, and 25 mg/L cimifugin decreased the release of IL-6 and IL-1β to less than 20% of that in the LPS group (P<0.05), and treatment with 100 mg/L cimifugin reduced the concentration of TNF-α to 60% of that in LPS group (P<0.05) (Figure 4). The results indicate that cimifugin suppresses the release of inflammatory factors from RAW264.7 cells.
Figure 4.
Effect of cimifugin on the contents of inflammatory factors in RAW264.7 cell culture supernatants. RAW264.7 cells were divided into a control group (no LPS was added), LPS group (treated with 1 μg/mL LPS for 24 h), high-dose group (1 μg/mL LPS+100 mg/L cimifugin for 24 h), medium-dose group (1 μg/mL LPS+50 mg/L cimifugin for 24 h), and low-dose group (1 μg/mL LPS+25 mg/L cimifugin for 24 h). (A) IL-6, (B) IL-1β, and (C) TNF-α concentrations were determined by ELISA. * P<0.05 and ** P<0.01 compared with LPS group. ## P<0.01 compared among indicated groups.
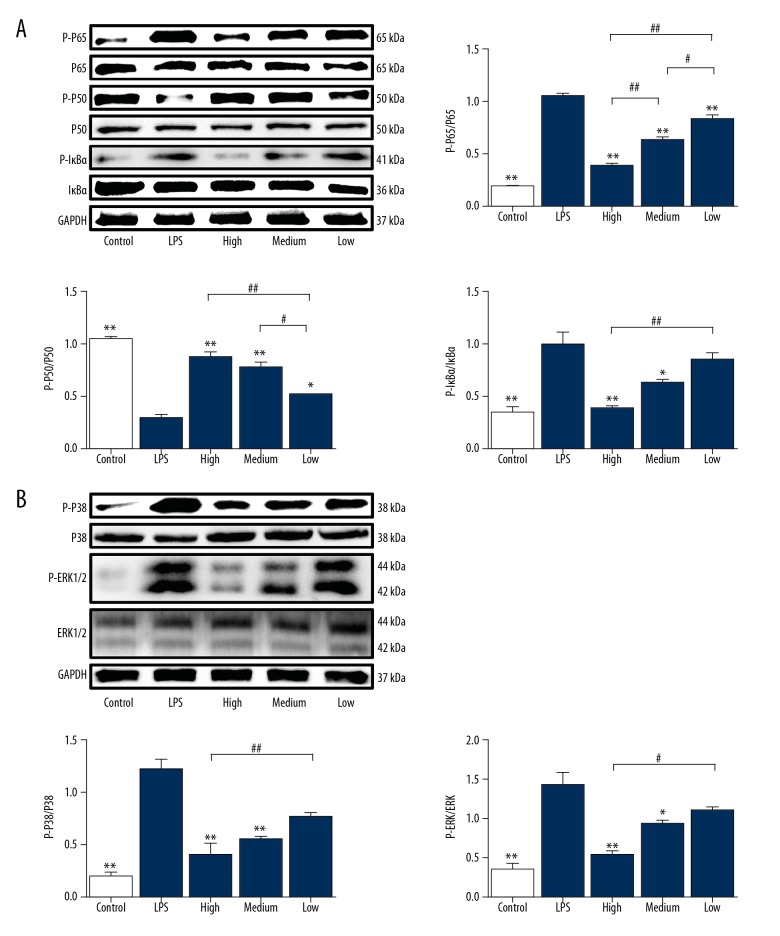
Cimifugin inhibits the activities of MAPKs and NF-κB signaling pathways
To understand the molecular mechanism by which cimifugin inhibits the release of cytokines from RAW264.7 cells induced by LPS, Western blotting was utilized. The data showed that relative expression of P-P65, P-IκBα, P-ERK1/2, and P-P38 in RAW264.7 cells stimulated by LPS was 3 times that of the control group, while the expression of P-P50 phosphorylated protein was 1/3 that of the control group (P<0.05) (Figure 5A, 5B). In contrast, treatment with 100 mg/L or 50 mg/L cimifugin reduced the expression of P-P65, P-IκBα, P-ERK1/2, and P-P38 in RAW264.7 cells to 40% of that in the LPS group (P<0.05), but at least doubled the expression of P-P50 phosphorylated protein compared with LPS group (P<0.05) (Figure 5A, 5B). The results suggest that cimifugin inhibits the activities of MAPKs and NF-κB signaling pathways.
Figure 5.
Effect of cimifugin on the activities of (A) MAPKs and (B) NF-κB signaling pathways. (A) Expression of P-P65, P65, P-P50, P50, P-IκBα, and IκBα proteins in MAPKs signaling pathway. (B) Expression of P-P38, P38, P-ERK1/2, and ERK1/2 proteins in NF-κB signaling pathway. Western blotting was used to measure protein expression. * P<0.05 and ** P<0.01 compared with LPS group. # P<0.05 and ## P<0.01 compared among indicated groups.
Discussion
Prim-o-glucosylcimifugin has anti-inflammatory effects by regulating MAPK and NF-κB signaling pathways [20]. Cimifugin, which is the aglycone of prim-o-glucosylcimifugin, is shown to inhibit allergic inflammation [18]. In the present study, we first investigated the cytotoxicity of cimifugin on RAW264.7 cells. Many drugs or compounds have certain inhibitory effects on the proliferation of macrophages, and down-regulate relevant signaling pathways or reduce the release of inflammatory factors [21,22]. However, cimifugin (0–100 mg/L) showed no differences in proliferation compared with the control group, suggesting that cimifugin at doses smaller than 100 mg/L does not inhibit the proliferation of RAW264.7 cells.
RAW264.7 cells induced by LPS is a classical cell model for inflammation research [23,24]. The stimulation of LPS can induce M1-like macrophages, and the biomarkers activated by M1 include the upregulation of TNF-α and iNOS [25]. In addition, the process of inflammation is accompanied by the release of a series of cytokines [26]. Our results show that the shapes of RAW264.7 cells changed to spindle shapes, and the release of NO is increased, suggesting that the RAW264.7 cell model of inflammation was successfully constructed. However, the number of RAW264.7 cells with differentiated shapes was decreased after treatment with cimifugin. In addition, the release of NO in the supernatants of RAW264.7 cells after treatment with high- and medium-dose cimifugin was inhibited, suggesting that cimifugin inhibits the differentiation of RAW264.7 cells and may have anti-inflammation effects.
Subsequently, we used fetal bovine serum and LPS to induce RAW264.7 cells [27,28], and tested the effect of cimifugin on the migration and chemotaxis of RAW264.7 cells. The results show that treatment with high- or medium-dose cimifugin reduced the number of cells after inducing by fetal bovine serum. In addition, treatment with high-dose cimifugin reduced the number of cells after inducing by LPS. This suggests that cimifugin reduces the migration and chemotaxis of RAW264.7 cells. The contents of migration- and chemotaxis-associated factors MIP-2, MCP-1, and IL-8 [29–32] are reduced by cimifugin. This suggests that intervention by cimifugin reduces the accumulation of macrophages at inflammatory sites and alleviates inflammatory symptoms.
Release of inflammatory factors and activation of inflammatory signaling pathways after stimulation by LPS are important in anti-RA inflammatory drug research. In RA and RA model animals, proinflammatory cytokines TNF-α, IL-1β, and IL-6 are highly expressed at lesion sites [33]. Cytokines play important roles in the development of RA, and are important factors in the destruction of joints and articular cartilages in patients with RA [34]. Our results in the present study show that NO release is reduced by treatment with cimifugin. In addition, the release of IL-6 and IL-1β is also decreased with increased dosage of cimifugin, while the release of TNF-α is only decreased after treatment by high-dose cimifugin. These results suggest that cimifugin has inhibitory effects on the release of inflammatory factors.
MAPK and NF-κB signaling pathways are closely related with the pathogenesis of RA and inflammatory responses, and can be activated by LPS induction. Stimulation by LPS can activate the NF-κB signaling pathway [35,36]. Our results demonstrate that intervention by cimifugin reduces the expression of phosphorylated P65 and IκBα proteins and elevates the expression of phosphorylated P50 protein. These results suggest that cimifugin inhibits inflammatory responses induced by LPS via the reduction of NF-κB signaling pathway activity.
Induction by LPS activates the phosphorylation of MAPKs in RAW264.7 cells [37–39] and increases the expression of inflammatory factors and chemotactic factors [40–42]. Studies show that NF-κB also interacts with the MAPK signaling pathway [43,44]. In the present study, we found that cimifugin significantly reduced the expression of phosphorylated P38 and ERK proteins, indicating that cimifugin also blocks the ERK and P38 MAPK signaling pathway, and reduces inflammatory responses of RAW264.7 cells stimulated by LPS.
Interestingly, the present study shows that high- or medium-dose cimifugin has effects on migration and chemotaxis, release of inflammatory factors, and activation of inflammatory signaling pathways. However, the effect of low-dose cimifugin is relatively weak. We speculate that the reason may be that the pharmacological action of cimifugin has its own focus, or that the pharmacological action of cimifugin is dose-dependent.
In summary, the present study demonstrates that cimifugin inhibits NF-κB and MAPK signaling pathways, reduces the release of inflammatory factors and chemotactic factors from RAW264.7 cells induced by LPS, weakens the migration and chemotaxis of RAW264.7 cells, and decreases the differentiation of RAW264.7 cells, thereby exerting anti-inflammatory effects.
Conclusions
Taken together, our results show that cimifugin inhibits the NF-κB and MAPK signaling pathways, reduces the release of inflammatory factors and chemotactic factors from RAW264.7 cells induced by LPS, weakens the migration and chemotaxis of RAW264.7 cells, and decreases the differentiation of RAW264.7 cells, thereby exerting anti-inflammatory effects. Cimifugin may have potential pharmacological effects against RA.
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 81760112)
Conflict of interest
None.
References
- 1.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:297–310. doi: 10.1016/j.rdc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen J, Aletaha D. The burden of rheumatoid arthritis and access to treatment: A medical overview. Eur J Health Econ. 2008;8(Suppl 2):S39–47. doi: 10.1007/s10198-007-0087-9. [DOI] [PubMed] [Google Scholar]
- 3.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 4.Klein K, Ospelt C, Gay S. Epigenetic contributions in the development of rheumatoid arthritis. Arthritis Res Ther. 2012;14:227. doi: 10.1186/ar4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang N, Wigley R, Zeng Q. [Rheumatic diseases in China]. Zhonghua Nei Ke Za Zhi. 1995;34:79–83. [in Chinese] [PubMed] [Google Scholar]
- 6.Lee JY, Choi JK, Jeong NH, et al. Anti-inflammatory effects of ursolic acid-3-acetate on human synovial fibroblasts and a murine model of rheumatoid arthritis. Int Immunopharmacol. 2017;49:118–25. doi: 10.1016/j.intimp.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Li ZZ, Tan JP, Wang LL, Li QH. Andrographolide benefits rheumatoid arthritis via inhibiting MAPK pathways. Inflammation. 2017;40:1599–605. doi: 10.1007/s10753-017-0600-y. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Sun C, Tao R, et al. Pyrroloquinoline quinone decelerates rheumatoid arthritis progression by inhibiting inflammatory responses and joint destruction via modulating NF-kappaB and MAPK pathways. Inflammation. 2016;39:248–56. doi: 10.1007/s10753-015-0245-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Wang LS, Zhou YH. Elevated microRNA-125b promotes inflammation in rheumatoid arthritis by activation of NF-kappaB pathway. Biomed Pharmacother. 2017;93:1151–57. doi: 10.1016/j.biopha.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 10.Aravilli RK, Vikram SL, Kohila V. Phytochemicals as potential antidotes for targeting NF-kappaB in rheumatoid arthritis. 3 Biotech. 2017;7:253. doi: 10.1007/s13205-017-0888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alivernini S, Tolusso B, Petricca L, et al. Synovial features of patients with rheumatoid arthritis and psoriatic arthritis in clinical and ultrasound remission differ under anti-TNF therapy: A clue to interpret different chances of relapse after clinical remission? Ann Rheum Dis. 2017;76:1228–36. doi: 10.1136/annrheumdis-2016-210424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B, Yang X, Yang X, Zhang L. [Chemical constituents of roots of Saposhnikovia divaricata]. Zhongguo Zhong Yao Za Zhi. 2010;35:1569–72. doi: 10.4268/cjcmm20101214. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZQ, Tian YJ, Zhang J. [Studies on the antioxidative activity of polysaccharides from radix Saposhnikoviae]. Zhong Yao Cai. 2008;31:268–72. [in Chinese] [PubMed] [Google Scholar]
- 14.Kim MK, Yang DH, Jung M, et al. Simultaneous determination of chromones and coumarins in Radix Saposhnikoviae by high performance liquid chromatography with diode array and tandem mass detectors. J Chromatogr A. 2011;1218:6319–30. doi: 10.1016/j.chroma.2011.06.103. [DOI] [PubMed] [Google Scholar]
- 15.Wu LQ, Li Y, Li YY, et al. Antinociceptive effects of prim-O-glucosylcimifugin in inflammatory nociception via reducing spinal COX-2. Biomol Ther (Seoul) 2016;24:418–25. doi: 10.4062/biomolther.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao XL, Liu L, Di LQ, et al. [Studies on effects of calycosin-7-O-beta-D-glucoside on prim-O-glucosylcimifugin and cimifugin in vivo pharmacokinetics]. Zhongguo Zhong Yao Za Zhi. 2014;39:4669–74. [in Chinese] [PubMed] [Google Scholar]
- 17.Li Y, Zhao L, Zhang H, et al. Comparative pharmacokinetics of prim-O-glucosylcimifugin and cimifugin by liquid chromatography-mass spectrometry after oral administration of Radix Saposhnikoviae extract, cimifugin monomer solution and prim-O-glucosylcimifugin monomer solution to rats. Biomed Chromatogr. 2012;26:1234–40. doi: 10.1002/bmc.2684. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Jiang X, Yu X, et al. Cimifugin suppresses allergic inflammation by reducing epithelial derived initiative key factors via regulating tight junctions. J Cell Mol Med. 2017;21:2926–36. doi: 10.1111/jcmm.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Q, Wang T, Wang HY. Ginsenoside Rb2 enhances the anti-inflammatory effect of omega-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression. Acta Pharmacol Sin. 2017;38:192–200. doi: 10.1038/aps.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen N, Wu Q, Chi G, et al. Prime-O-glucosylcimifugin attenuates lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2013;16:139–47. doi: 10.1016/j.intimp.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan L, Hu X, Wu Q, et al. CQMUH-011, a novel adamantane sulfonamide compound, inhibits lipopolysaccharide- and D-galactosamine-induced fulminant hepatic failure in mice. Int Immunopharmacol. 2017;47:231–43. doi: 10.1016/j.intimp.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Ayed Y, Sghaier RM, Laouini D, Bacha H. Evaluation of anti-proliferative and anti-inflammatory activities of Pelagia noctiluca venom in Lipopolysaccharide/Interferon-gamma stimulated RAW264.7 macrophages. Biomed Pharmacother. 2016;84:1986–91. doi: 10.1016/j.biopha.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Zhang X, Ling P, et al. Immunomodulatory effects of xanthan gum in LPS-stimulated RAW 264.7 macrophages. Carbohydr Polym. 2017;169:65–74. doi: 10.1016/j.carbpol.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Ma F, Liu F, Ding L, et al. Anti-inflammatory effects of curcumin are associated with down regulating microRNA-155 in LPS-treated macrophages and mice. Pharm Biol. 2017;55:1263–73. doi: 10.1080/13880209.2017.1297838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safavian D, Leung CH, Kapus A, et al. Hemorrhagic shock/resuscitation reduces the M2 phenotype of alveolar macrophages: A potential mechanism contributing to increased LPS-induced lung injury. Shock. 2018 doi: 10.1097/SHK.0000000000001135. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Amanzada A, Moriconi F, Mansuroglu T, et al. Induction of chemokines and cytokines before neutrophils and macrophage recruitment in different regions of rat liver after TAA administration. Lab Invest. 2014;94:235–47. doi: 10.1038/labinvest.2013.134. [DOI] [PubMed] [Google Scholar]
- 27.Yang YH, Li DL, Bi XY, et al. Acetylcholine inhibits LPS-induced MMP-9 production and cell migration via the alpha7 nAChR-JAK2/STAT3 pathway in RAW264.7 cells. Cell Physiol Biochem. 2015;36:2025–38. doi: 10.1159/000430170. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Tong Q, Ye J, et al. Nogo-B facilitates LPS-mediated immune responses by up-regulation of TLR4-signaling in macrophage RAW264.7. Cell Physiol Biochem. 2017;41:274–85. doi: 10.1159/000456094. [DOI] [PubMed] [Google Scholar]
- 29.Wang ZW, Wang JJ, Zhang JZ, et al. Thrombolysis of deep vein thrombosis and inhibiting chemotaxis of macrophage by MCP-1 blockage. Eur Rev Med Pharmacol Sci. 2017;21:1695–701. [PubMed] [Google Scholar]
- 30.Hol J, Wilhelmsen L, Haraldsen G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. J Leukoc Biol. 2010;87:501–8. doi: 10.1189/jlb.0809532. [DOI] [PubMed] [Google Scholar]
- 31.Kadioglu A, Andrew PW. Susceptibility and resistance to pneumococcal disease in mice. Brief Funct Genomic Proteomic. 2005;4:241–47. doi: 10.1093/bfgp/4.3.241. [DOI] [PubMed] [Google Scholar]
- 32.Gerber J, Pohl K, Sander V, et al. Rifampin followed by ceftriaxone for experimental meningitis decreases lipoteichoic acid concentrations in cerebrospinal fluid and reduces neuronal damage in comparison to ceftriaxone alone. Antimicrob Agents Chemother. 2003;47:1313–17. doi: 10.1128/AAC.47.4.1313-1317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 34.Choi EM, Hwang JK. Effects of methanolic extract and fractions from Litsea cubeba bark on the production of inflammatory mediators in RAW264.7 cells. Fitoterapia. 2004;75:141–48. doi: 10.1016/j.fitote.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Gil A, Maria Aguilera C, Gil-Campos M, Canete R. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Br J Nutr. 2007;98(Suppl 1):S121–26. doi: 10.1017/S0007114507838050. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 37.Winkler C, Ferdous F, Dimmick M, Scott T. Lipopolysaccharide induced Interleukin-6 production is mediated through activation of ERK 1/2, p38 MAPK, MEK, and NFkappaB in chicken thrombocytes. Dev Comp Immunol. 2017;73:124–30. doi: 10.1016/j.dci.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Pang M, Yuan Y, Wang D, et al. Recombinant CC16 protein inhibits the production of pro-inflammatory cytokines via NF-kappaB and p38 MAPK pathways in LPS-activated RAW264.7 macrophages. Acta Biochim Biophys Sin (Shanghai) 2017;49:435–43. doi: 10.1093/abbs/gmx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JY, Lim MS, Kim SI, et al. Quercetin-3-O-beta-D-glucuronide suppresses lipopolysaccharide-induced JNK and ERK phosphorylation in LPS-challenged RAW264.7 cells. Biomol Ther (Seoul) 2016;24:610–15. doi: 10.4062/biomolther.2016.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–92. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 41.Roget K, Ben-Addi A, Mambole-Dema A, et al. IkappaB kinase 2 regulates TPL-2 activation of extracellular signal-regulated kinases 1 and 2 by direct phosphorylation of TPL-2 serine 400. Mol Cell Biol. 2012;32:4684–90. doi: 10.1128/MCB.01065-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol Pharmacol. 1999;55:481–88. [PubMed] [Google Scholar]
- 43.Robinson MJ, Beinke S, Kouroumalis A, et al. Phosphorylation of TPL-2 on serine 400 is essential for lipopolysaccharide activation of extracellular signal-regulated kinase in macrophages. Mol Cell Biol. 2007;27:7355–64. doi: 10.1128/MCB.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nick JA, Avdi NJ, Young SK, et al. Selective activation and functional significance of p38alpha mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J Clin Invest. 1999;103:851–58. doi: 10.1172/JCI5257. [DOI] [PMC free article] [PubMed] [Google Scholar]