Abstract
Background
Radix Tetrastigma Hemsleyani Flavone (RTHF) has detoxification and anti-inflammation activity and is widely used. Here, we report that RTHF inhibits cell proliferation and induces apoptosis in cutaneous squamous cell carcinoma A431 cells and is a potential strategy for cancer therapy.
Material/Methods
A431 cells were cultured in different concentrations of RTHF. The inhibition of cell proliferation was assessed by MTT assay, cell apoptosis was shown through FCM, and cell invasion was assessed by Transwell methods. Enzyme proteasome assay was used to detect the activity of proteasome and DUB. Expression of apoptosis-related and ubiquitin proteasome pathway-associated proteins were assessed by PCR and Western blot.
Results
RTHF obviously suppressed the proliferation and induced apoptosis of A431 cells in a dose-dependent manner. Transwell assay showed that RTHF inhibited the cell metastasis significantly. Enzyme proteasome assay show that the RTHF treatment of activity of proteasome and DUB was significantly lower than in control. RTHF increased the expression of Bax and inhibited Bcl-2, pro-caspase3, and pro-caspase9 activity. The expression of USP14, UCHL5, and POH1 decreased and ub-prs increased significantly in the treatment group.
Conclusions
Our study reveals that RTHF-mediated inhibition of DUBs and proteasome may provide a potential strategy for cancer therapy.
MeSH Keywords: Apoptosis; Carcinoma, Squamous Cell; Proteasome Inhibitors
Background
Cutaneous squamous cell carcinoma (cSCC) is the second most common cause of carcinoma in non-melanoma skin [1]. Nowadays, its incidence is continuously increasing due to the aging population and increased ultraviolet exposure [2]. Despite many advances in cancer treatment, there is not always a lasting response, and many drugs have serious adverse effects that limit sustained clinical benefit, so there is a need to assess new therapeutic methods and strategies.
Radix Tetrastigma Hemsleyani Flavone (RTHF) is derived from the root of the Chinese herb Radix Tetrastigma Hemsleyani (RTH), which has been shown to possess detoxification, anti-inflammation, and anti-cancer activities. In vitro, RTHF causes anti-proliferation and apoptosis effects in multiple cancer cell models [3–6]. In vivo, it can inhibit tumor growth in mouse models [7]. However, the mechanism of RTHF is largely unknown. Here, we found that RTHF inhibits proliferation and induces apoptosis of cutaneous squamous cell carcinoma A431 cells. The possible anti-cancer mechanism is proteasome activation.
Material and Methods
Reagents
Radix Tetrastigma Hemsleyani Flavone (RTHF) was synthesized in the Key Laboratory Research and Development of New Drugs of Traditional Chinese Medicine of Zhejiang Province. Our experiments used the following: FBS and RPMI 1640 (HyClone), MTT (Beyotime), Annexin V-FITC Apoptosis Detection Kit (Keygen), Transwell chamber (Corning, USA), Matrigel (BD Biosciences USA), Proteasome-Glo Chymotrypsin-like Cell-Based Assay kit, DUB-Glo Protease Assay kit (Promega, USA), human 19S proteasome (Boston Biochem), and the antibodies to Bax, Bcl-2, Caspase-3/9, ub-prs, USP14, UCHL5, and POH1 (CST).
Cell line
A431 cells were obtained from Zhejiang Province Academy of Medical Science. Cells were maintained in RPMI-1640 at 37°C with 5% CO2.
Cell viability assay
MTT assay was used to determine A431 cell viability in media containing RTHF for 48 h. We exposed cells to 20 ml MTT for 4 h before culture termination. Finally, the absorbance was read at 570 nm, with cell viability of control as 100%.
Apoptosis assay
A431 cells were cultured in different concentrations of RTHF. Treated and control cells were harvested and resuspended in binding buffer followed by Annexin V-FITC, and PI was analyzed by flow cytometry.
Cell invasion assay
A431 cell invasion assay was determined by Transwell experiments, in which 2×104 cells/well in the upper chamber were added to Matrigel in serum-free medium and cultured with various concentrations of RTHF. FBS was used as a chemoattractant in the lower chamber medium. After 12 h, we removed the cells in the upper chamber and the invasive cells were stained with crystal violet.
Proteasome activity assay
The treated A431 cells were detected with a microplate reader (3001, Thermo, USA). The proteasome activity was determined according to the instructions of the Proteasome-Glo Chymotrypsin-like Cell-Based Assay kit.
DUB activity assay
Treated A431 cells were placed in ice-cold DUB buffer, centrifuged at 20 000 g for 10 min, and recorded with a microplate reader (3001, Thermo, USA). The DUB activity was detected according to the instructions of the DUB-Glo Protease Assay kit.
Real-time PCR assay
To determine mRNA expression, total RNA extracted from each sample was used for reverse transcript reaction using the QuantiTect Reverse Transcription kit (Qiagen). The SYBR Green PCR Kit was used for real-time PCR reaction. Gene transcript was normalized using the Ct method, with the control as 1.
Western blot assay
Whole-cell lysates were prepared in RIPA buffer. Protein lysates from cultured cells were fractionated by SDS-PAGE and electrically transferred onto a PVDF membrane. After transfer, primary antibodies and secondary horseradish peroxidase-conjugated antibodies were used to detect the designated proteins. The target proteins were reacted to the ECL detection reagents.
Statistics
All values are presented as the mean ±SD. Statistical differences were determined by t test or one-way ANOVA, with P<0.05 considered significant.
Results
RTHF inhibits the proliferation of A431 cells
The growth inhibition effects of RTHF on A431 cells were detected with MTT assay. As shown in Figure 1, RTHF dramatically inhibited A431 cell growth in a dose-dependent manner (P<0.05). The proliferative inhibition effect of the control group was 0%.
Figure 1.
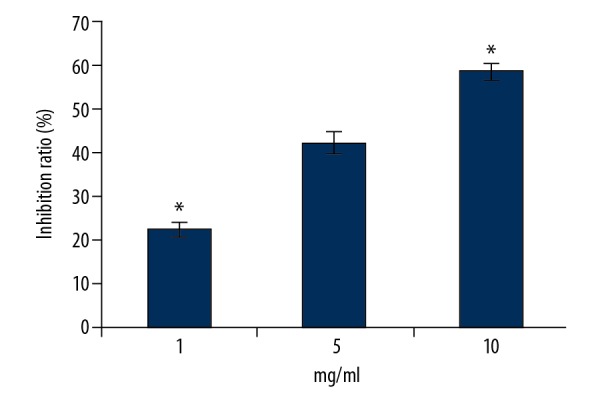
Proliferative inhibition effect of RTHF on A431 cells. * P<0.05 vs. control group.
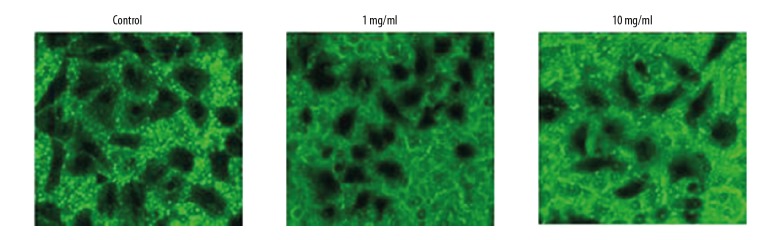
RTHF induced apoptosis of A431 cells
A431 cells were incubated with RTHF for 48 h and cell apoptosis was determined by flow cytometry. As shown in Figure 2, cell apoptosis increased significantly (p<0.05).
Figure 2.
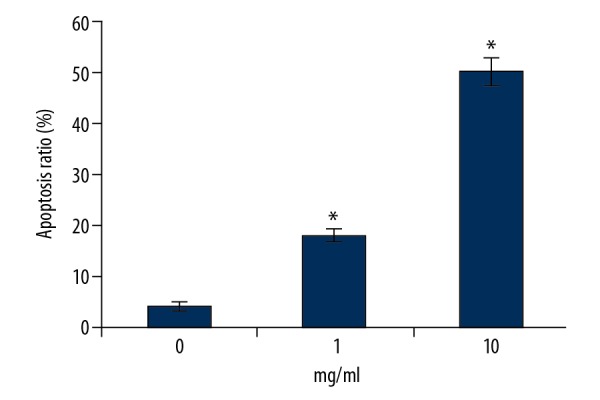
Apoptotic promotion effect of RTHF on A431 cells. * P<0.05 vs. control group.
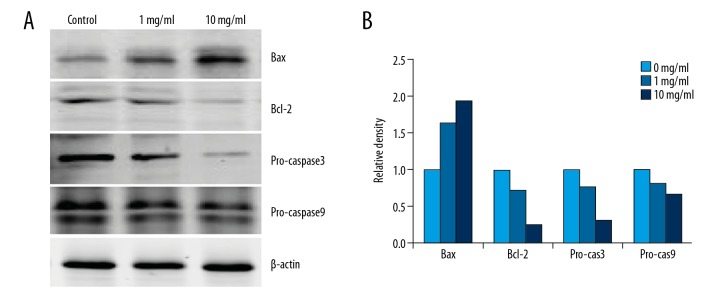
RTHF influenced apoptosis-related proteins
Apoptosis-related proteins expression of Bax, Bcl-2, pro-caspase3, and pro-caspase9 were analyzed by Western blotting. Bax was significantly upregulated and Bcl-2 and pro-caspase3/9 were downregulated after treatment with RTHF (Figure 3).
Figure 3.
(A) The protein expression of Bax, Bcl-2, and pro-caspase3/9 measured by Western blotting. (B) Proteins were subsequently quantified by densitometric analysis with the control as one-fold.
RTHF inhibits invasion of A431 cells
Cell invasion was analyzed by the Transwell culture system. As shown in Figure 4, after culturing with RTHF for 12 h, there was a significant decrease in the cell invasion ability of squamous cell carcinoma A431 cells (P<0.05).
Figure 4.
Effect of RTHF on cell invasion in A431 cells.
RTHF inhibited the proteasome activity of A431 cells
We detected the effects of RTHF on proteasome CT-like peptidase activity in A431 cells. After incubation for 48 h, RTHF dramatically inhibited the CT-like activity (p<0.05) (Figure 5).
Figure 5.
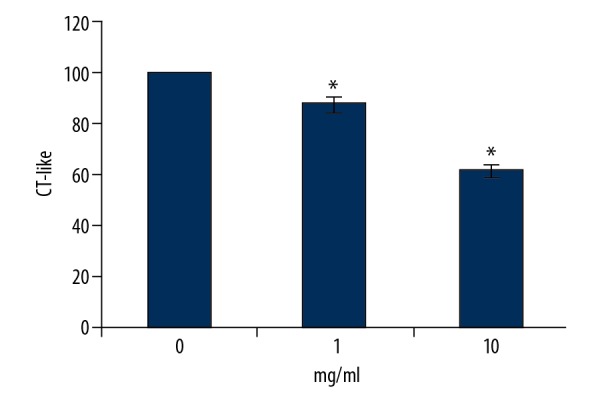
The CT-like activity of A431 cells cultured with RTHF. * P<0.05 vs. control group.
RTHF inhibited the DUB activity of A431 cells
The activities of DUB from cell lysates were detected. As shown in Figure 6, RTHF caused a dose-dependent decrease of DUB activity in A431 cancer cells (p<0.05).
Figure 6.
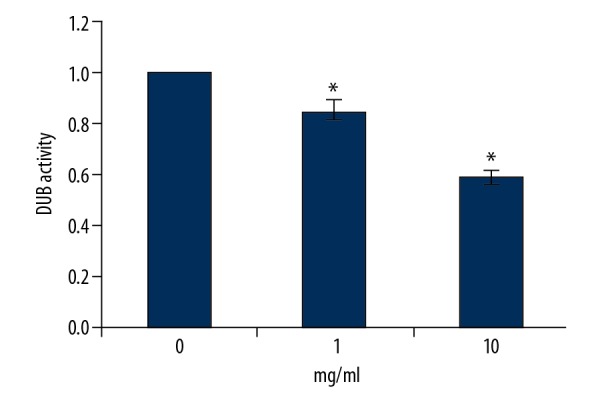
The DUB activity of A431 cells cultured with RTHF. * P<0.05 vs. control group.
RTHF influenced the ubiquitin proteasome pathway-associated proteins expression, as shown by PCR
We investigated the effect of RTHF on ubiquitin proteasome pathway proteins in A431 cells by real-time PCR assay. As shown in Figure 7, the level of ub-prs increased and levels of USP14, UCHL5, and POH1 decreased after RTHF treatment.
Figure 7.
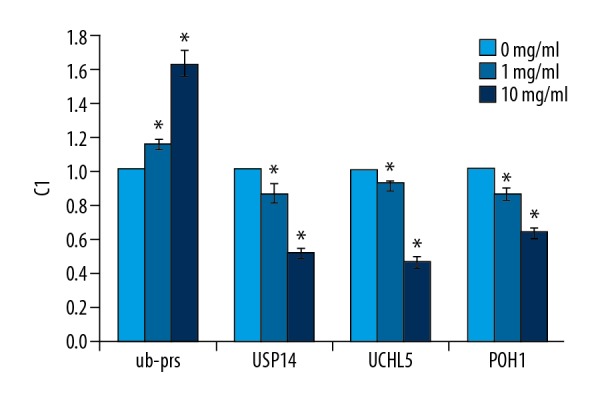
Validation of differentially expressed genes by real-time PCR. * P<0.05 vs. control group
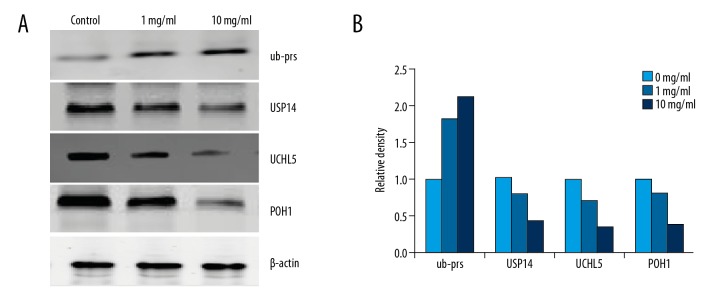
RTHF influenced ubiquitin proteasome pathway-associated proteins expression, as shown by Western blot
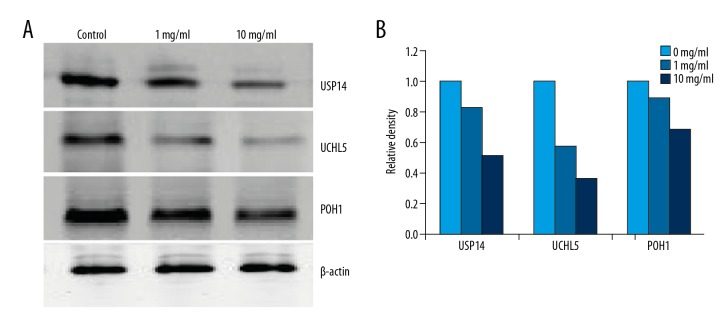
We investigated RTHF on ubiquitin proteasome pathway proteins in A431 cells by Western blot assay. As shown in Figure 8, RTHF increased the level of ub-prs but decreased levels of USP14, UCHL5, and POH1 in a concentration-dependent manner. In addition, we investigated the effect of RTHF in 19S RP by Western blot. As shown in Figure 9, expression of USP14, UCHL5, and POH1 were significantly decreased in a dose-dependent manner compared to control.
Figure 8.
(A) Effect of RTHF on the activities of ub-prs, USP14, UCHL5, and POH1 in A431 cells. (B) Proteins were subsequently quantified by densitometric analysis with control being one-fold.
Figure 9.
(A) Effect of RTHF on the activities of USP14, UCHL5, and POH1 in 19S RP. (B) Proteins were subsequently quantified by densitometric analysis with that of control being one-fold.
Discussion
Recently, several herbal medicines have been investigated for their potential as major sources of pharmaceutical agents with various activities [8–10]. Flavonoids are effective anti-cancer agents without toxicity to normal cells and tissues, making them ideal in cancer therapy [11]. Radix Tetrastigma Hemsleyani Flavone (RTHF) has a surprisingly wide range of beneficial properties, including anti-inflammation, anti-cancer, and improving immune response activities. In this study, we showed that RTHF significantly inhibited the growth and induced cell apoptosis of A431 cells.
The ubiquitin proteasome pathway (UPS) is the major pathway that controls the expression of proteins in cell cycle, proliferation, and apoptosis [12]. It plays an important part in the regulation of numerous cellular functions [13,14]. In numerous cancer types, UPS over-expression is often associated with chemo-resistance and poor prognosis [15–18]. In fact, the proteasome has chymotrypsin-like, trypsin-like, and peptidyl-glutamyl peptide-hydrolyzing (PHGH)-like activity. In this study, we showed the ability of RTHF to significantly decrease the chymotrypsin-like activity. The addition of ubiquitin molecules to targeted proteins can be reversed, called deubiquitination, and is performed by proteases termed deubiquitinases (DUBs) [19]. Recent findings indicate several DUBs are considered to be oncogenes [20–22]. Several inhibitors of DUB activity have been reported to increase the expression of poly-Ub proteins and enhance cancer cell apoptosis, indicating that DUB inhibitors are potential anti-cancer agents [23,24]. In this report, we showed that RTHF significantly inhibits the activity of DUB compared to control.
The 26S proteasome is comprised of a 20S core catalytic component (20S proteasome) capped at one or both ends by a 19S regulatory component. Recent research has revealed the functions of 19S RP include binding ubiquitinated proteins, recycling ubiquitin, and unfolding proteins, and is considered a novel anti-cancer drug target [25,26]. In humans, 19S RP is associated with 3 deubiquitinases (DUBs), USP14/Ubp6, and UCHL5/Uch37, which belong to the ubiquitin-specific proteases (USP) and ubiquitin C-terminal hydrolases (UCH) families, respectively. POH1 is a Zn21-dependent protease of the JAMM family [27,28]. USP14 has been shown to be related to cancer progression. Several studies have shown that the expression USP14 is negatively correlated with patient survival in some carcinomas, such as colorectal, liver, and lung cancer [29]. Recent observations show that USP14 may be associated with metastasis. A recent study reported that UCHL5 can promote cell growth, in part by altering the expression of apoptotic mediators. UCHL5 is a subunit of 19S RP and has a role by suppressing or promoting the degradation of specific proteasome substrates in eukaryotic cells [30,31]. Knockdown of UCHL5 results in alterations in the mitochondrial apoptosis pathway and reduced cell survival in non-small cell lung carcinoma [32]. POH1 is essential for viability, and POH1 knockdown inhibits proteasome activity and reduces cell survival [33]. In addition, POH1 over-expression promotes resistance to several anti-cancer agents currently in clinical use. Indeed, it has been reported that knockdown of both UCHL5 and USP14 but (not either one alone) leads to defects in UPS and impairs proteasome activity [30]. RTHF significantly decreases USP14, UCHL5, and POH1 expression in cutaneous squamous cell carcinoma A431 cells.
Conclusions
We found that RTHF significant mediates inhibition of growth and induction of apoptosis in A431 cells, and showed that inhibition of DUBs might be one of the major mechanisms by which RTHF inhibits tumor cells.
Footnotes
Source of support: This work was supported by the Postdoctoral Science Foundation of Zhejiang, China (No. zj20180153), the Postdoctoral Science Foundation of China (No. 2018M642494), the Chinese Medicine Science and Technology Project of Zhejiang (No. 2019ZA095), the Natural Science Foundation of Zhejiang, China (No. LQ15H290006, LQ16H290001), and the National Natural Science Foundation of China (No. 81173271, 81174313, 81541084)
Conflict of interests
None.
References
- 1.Capalbo C, Belardinilli F, Filetti M, et al. Effective treatment of a platinum-resistant cutaneous squamous cell carcinoma case by EGFR pathway inhibition. Mol Clin Oncol. 2018;9(1):30–34. doi: 10.3892/mco.2018.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green AC, Olsen CM. Cutaneous squamous cell carcinoma: An epidemiological review. Br J Dermatol. 2017;177:373–81. doi: 10.1111/bjd.15324. [DOI] [PubMed] [Google Scholar]
- 3.Zhong LR, Zheng JX, Sun QQ, et al. Radix Tetrastigma hemsleyani flavone inhibits proliferation, and invasion of human lung carcinoma A549 cells. Oncotargets Ther. 2016;9:635–41. doi: 10.2147/OTT.S92707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding G, Zheng J, Wei K, et al. Toxicological effects of the extract of Tetrastigma hemsleyanum Diels et. Gflg on hepatocellular carcinoma cell line HepG2 and primary rat hepatocytes in vitro. J Zhejiang Prev Med. 2005;17(9):1–5. [Google Scholar]
- 5.Zhong L, Chen X, Wei K. Radix Tetrastigma Hemsleyani Flavone induces apoptosis in human lung carcinoma A549 cells by modulating the MAPKs pathway. Asian Pac J Cancer Prev. 2013;14(10):5983–87. doi: 10.7314/apjcp.2013.14.10.5983. [DOI] [PubMed] [Google Scholar]
- 6.Xu C, Wu P, Meng J, et al. Inhibitory effect on proliferation of K562 cell line by extract from Tetrastigma hemsleyanum diels et. Gilg. Chin J Health Lab Tech. 2010;20(11):2801–3. [Google Scholar]
- 7.Lin S, Zhong LR, Wei KM. [Apoptosis-inducing effect of ethylacetate extracts of sanyeqing (Tetrastigma hemsleyanum) on colorectal cancer Cell HT29 subcutaneous transplanted tumor]. Chinese Journal of Traditional Medical Science and Technology. 2016;23(5):542–45. [in Chinese] [Google Scholar]
- 8.Mishra BB, Tiwari VK. Natural products: An evolving role in future drug discovery. Eur J Med Chem. 2011;46:4769–807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 9.Mondal S, Bandyopadhyay S, Ghosh MK, et al. Natural products: Promising resources for cancer drug discovery. Anticancer Agents Med Chem. 2012;12:49–75. doi: 10.2174/187152012798764697. [DOI] [PubMed] [Google Scholar]
- 10.De Luca V, Salim V, Atsumi SM, et al. Mining the biodiversity of plants: A revolution in the making. Science. 2012;336:1658–61. doi: 10.1126/science.1217410. [DOI] [PubMed] [Google Scholar]
- 11.Le Marchand L. Cancer preventive effects of flavonoids – a review. Biomed Pharmacother. 2002;56(6):296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 12.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 13.Reinstein E, Ciechanover A. Narrative review: Protein degradation and human diseases: The ubiquitin connection. Ann Intern Med. 2006;145:676–84. doi: 10.7326/0003-4819-145-9-200611070-00010. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Dou QP. The ubiquitin-proteasome system as a prospective molecular target for cancer treatment and prevention. Curr Protein Pept Sci. 2010;11:459–70. doi: 10.2174/138920310791824057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christian PA, Fiandalo MV, Schwarze SR. Possible role of death receptor-mediated apoptosis by the E3 ubiquitin ligases Siah2 and POSH. Mol Cancer. 2011;10:57. doi: 10.1186/1476-4598-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–44. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- 17.Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–89. doi: 10.1200/JCO.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama KI, Nakayama K. Ubiquitin ligases: Cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Park KC, Chung SS, et al. Deubiquitinating enzymes as cellular regulators. J Biochem. 2003;134:9–18. doi: 10.1093/jb/mvg107. [DOI] [PubMed] [Google Scholar]
- 20.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–97. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain S, Zhang Y, Galardy PJ. UBs and cancer: The role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 2009;8:1688–97. doi: 10.4161/cc.8.11.8739. [DOI] [PubMed] [Google Scholar]
- 22.Sacco JJ, Coulson JM, Clague MJ, et al. Emerging roles of deubiquitinases in cancer – associated pathways. IUBMB Life. 2010;62:140–57. doi: 10.1002/iub.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Xia H, Kim M, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–34. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Arcy P, Brnjic S, Olofsson MH, et al. Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med. 2011;17:1636–40. doi: 10.1038/nm.2536. [DOI] [PubMed] [Google Scholar]
- 25.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Arcy P, Linder S. Proteasome deubiquitinases as novel targets for cancer therapy. Int J Biochem Cell Biol. 2012;44:1729–38. doi: 10.1016/j.biocel.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Fraile JM, Quesada V, Rodriguez D, et al. Deubiquitinases in cancer: New functions and therapeutic options. Oncogene. 2012;31:2373–88. doi: 10.1038/onc.2011.443. [DOI] [PubMed] [Google Scholar]
- 28.Chen D, Cui QC, Yang H, et al. Disulfiram, a clinically used antialcoholism drug and copper- binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–33. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 29.Shinji S, Naito Z, Ishiwata S, et al. Ubiquitin-specific protease 14 expression in colorectal cancer is associated with liver and lymph node metastases. Oncol Rep. 2006;15:539–43. [PubMed] [Google Scholar]
- 30.Koulich E, Li X, DeMartino GN. Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell. 2008;19:1072–82. doi: 10.1091/mbc.E07-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson AD, Zhang NY, Xu P, et al. The lysine 48 and lysine 63 ubiquitin conjugates are processed differently by the 26s proteasome. J Biol Chem. 2009;284:35485–94. doi: 10.1074/jbc.M109.052928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Niu X, Li Z, et al. Effect of ubiquitin carboxy-terminal hydrolase37 on apoptotic in A549 cells. Cell Biochem Funct. 2011;29:142–48. doi: 10.1002/cbf.1734. [DOI] [PubMed] [Google Scholar]
- 33.Gallery M, Blank JL, Lin Y, et al. The JAMM motif of human deubiquitinase Poh1 is essential for cell viability. Mol Cancer Ther. 2007;6:262–68. doi: 10.1158/1535-7163.MCT-06-0542. [DOI] [PubMed] [Google Scholar]