ABSTRACT
Rottlerin as a natural agent, which is isolated from Mallotus philippinensis, has been identified to play a critical role in tumor inhibition. However, the molecular mechanism of rottlerin-mediated anti-tumor activity is still ambiguous. It has been reported that EZH2 exhibits oncogenic functions in a variety of human cancers. Therefore, inhibition of EZH2 could be a promising strategy for the treatment of human cancers. In this study, we aim to explore whether rottlerin could inhibit tumorigenesis via suppression of EZH2 in prostate cancer cells. Multiple approaches such as FACS, Transwell invasion assay, RT-PCR, Western blotting, and transfection were performed to determine our aim. We found that rottlerin treatment led to inhibition of cell growth, migration and invasion, but induction of apoptosis in prostate cancer cells. Importantly, we defined that rottlerin decreased the expression of EZH2 and H3K27me3 in prostate cancer cells. Moreover, overexpression of EZH2 abrogated the rottlerin-induced inhibition of cell growth, migration, and invasion in prostate cancer cells. Consistently, down-regulation of EZH2 enhanced rottlerin-triggered anti-tumor function. Collectively, our work demonstrated that rottlerin exerted its tumor suppressive function via inhibition of EZH2 expression in prostate cancer cells. Our findings indicated that rottlerin might be a potential therapeutic compound for treating patients with prostate cancer.
KEYWORDS: Rottlerin, EZH2, prostate cancer, cell growth, cell invasion
Introduction
In the United States, the occurrence rate of prostate cancer is almost 1 in 5 new diagnosis and the estimated deaths of prostate cancer is 9%, secondary to the lung cancer [1]. Multiple treatments have been used for patients including pain medications, bisphosphonates, rank ligand inhibitors, hormonal treatment, chemotherapy, radiopharmaceuticals, immunotherapy and focused radiation [2]. Although these advanced measurements and techniques have improved the survival rate, the treatment outcome of the disease is still unsatisfactory. Thus it is urgent to identify novel therapeutic agents to benefit the patients with prostate cancer.
It has been known that great deals of anticancer drugs are pure natural products or natural product derivatives or natural product mimics [3]. Recently, rottlerin, known as mallotoxin, is isolated from the plant Mallotus phillippinensis [4]. Increasing evidence showed that rottlerin exerted its anti-cancer role in multiple cancer via inhibition of cell proliferation, cell metastasis, cell invasion, but promotion of cell apoptosis and autophagy [5]. For instance, rottlerin has been identified as an inhibitor of PKCδ (protein kinase C δ), while PKCδ accelerated the tumorigenesis in many human cancers [6]. Interestingly, studies identified that rottlerin improved apoptosis and autophagy through PKCδ-mediated pathway [7]. While rottlerin increased DR5 (death receptor 5) expression via PKCδ-independent signaling pathway in human tumor cells [8]. Lim et al. reported that rottlerin induced pro-apoptotic endoplasmic reticulum stress via PKCδ-independent pathway in human colon cancer cells [9]. One last review showed that rottlerin could bind to ERK and mTOR directly and dysregulated cap-dependent protein translation via mTORC1/eIF4E axis and by inhibition of eIF2 in breast and skin cancer cell lines [10]. Importantly, one study indicated that rottlerin inhibited the expression and phosphorylation of LRP6 (low density lipoprotein receptor-related protein-6), and depressed Wnt/β-catenin and mTORC1 pathways, and thus led to promotion of cell apoptosis and inhibition of cell growth in prostate and breast cancer [11]. Kumar et al. demonstrated that rottlerin promoted autophagy and apoptosis via PI3K/Akt/mTOR pathway in prostate cancer [12]. Despite of the studies of rottlerin in tumorigenesis, further investigations are essential to be performed to explore the molecular mechanism of tumor suppression by rottlerin.
EZH2 (enhancer of zeste homolog 2) is a catalytic component of PRC2, which methylates lysine 27 of histone H3 to promote transcription regulation [13,14]. Unsurprisingly, increased studies demonstrated the critical role of EZH2 in cancer progression [14,15]. For example, one study indicated the critical role of EZH2 in promoting cell growth and transcriptional inhibition in prostate cancer [13]. The similar results have been found in breast cancer, bladder cancer, endometrial cancer and melanoma cancer, and demonstrated the correlation of EZH2 overexpression with the aggressive and advanced disease in above cancers [16–18]. Therefore, inactivation of EZH2 could be a promising approach to benefit the cancer patients.
In this study, we investigated whether rottlerin could be a potential inhibitor of EZH2 in prostate cancer. Our results confirmed the tumor suppressive role of rottlerin via suppression of EZH2 in prostate cancer by a series of methods including cell growth assay, FACS, wound healing assay and Transwell invasion analysis. Taken together, the results identified that rottlerin exerted its anti-tumor function via inactivation of EZH2 in prostate cancer, which revealed rottlerin could be a useful agent for prostate cancer patients.
Results
Rottlerin inhibited cell proliferation
It has been reported that rottlerin suppressed cell growth in prostate CSCs (cancer stem cells) [12]. To determine whether rottlerin could inhibit cell proliferation in prostate cancer cells, we performed CTG assay in PC3 and DU145 cells after different concentration of rottlerin treatment for 48h and 72h, respectively. Our results showed that rottlerin inhibited cell proliferation in dose-dependent manners in both prostate cancer cell lines (Figure 1(a)). Specifically, 3 μM and 5 μM rottlerin treatments led to 70% and 90% of cell inhibition, respectively, at 72 hours in PC3 cells, and the cell growth inhibition was 60% and 75% in DU145 with the 3 μM and 5 μM rottlerin treatments. Then we conducted the following study using the 3 μM and 5 μM rottlerin. Rottlerin significantly reduced colony numbers in both PC3 and DU145 cell lines, which indicated that rottlerin suppressed cell proliferation in prostate cells (Figure 1(b)).
Figure 1.
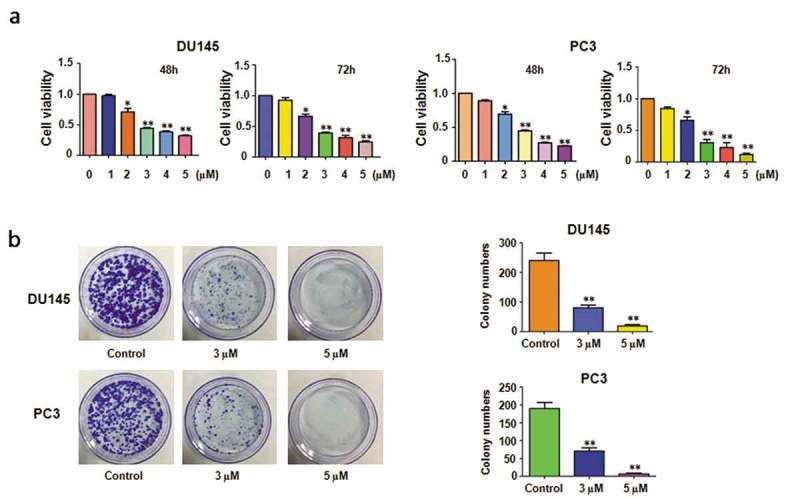
Effect of rottlerin on cell growth. (a) CTG assay was exerted to measure cell proliferation in prostate cancer cells with different concentration of rottlerin treatment for 48 hours and 72 hours. *P < 0.05, **P < 0.01, compared to the control (DMSO treatment). (b) The colony activity was detected by Colony formation analysis in prostate cancer cells treated with rottlerin.
Rottlerin promoted apoptosis
Rottlerin treatment triggered cell apoptosis in prostate CSCs [12]. Thus we further investigated whether rottlerin-mediated cell growth inhibition was subjected to the apoptosis in prostate cancer cells. To this end, PI-FITC-annexin assay was exerted to measure cell apoptosis in both PC3 and DU145 cells with different concentration of rottlerin treatment for 48 hours. Our results revealed that rottlerin markedly induced cell apoptotic death in both prostate cancer cell lines (Figure 2(a)). The apoptotic cells increased from 5% to 26% in DU145 cells, and from 5% to 13% in PC3 cells (Figure 2(a)). Our findings demonstrated that the inhibition of cell growth partly due to the induction of cell apoptosis by rottlerin in prostate cancer cells.
Figure 2.
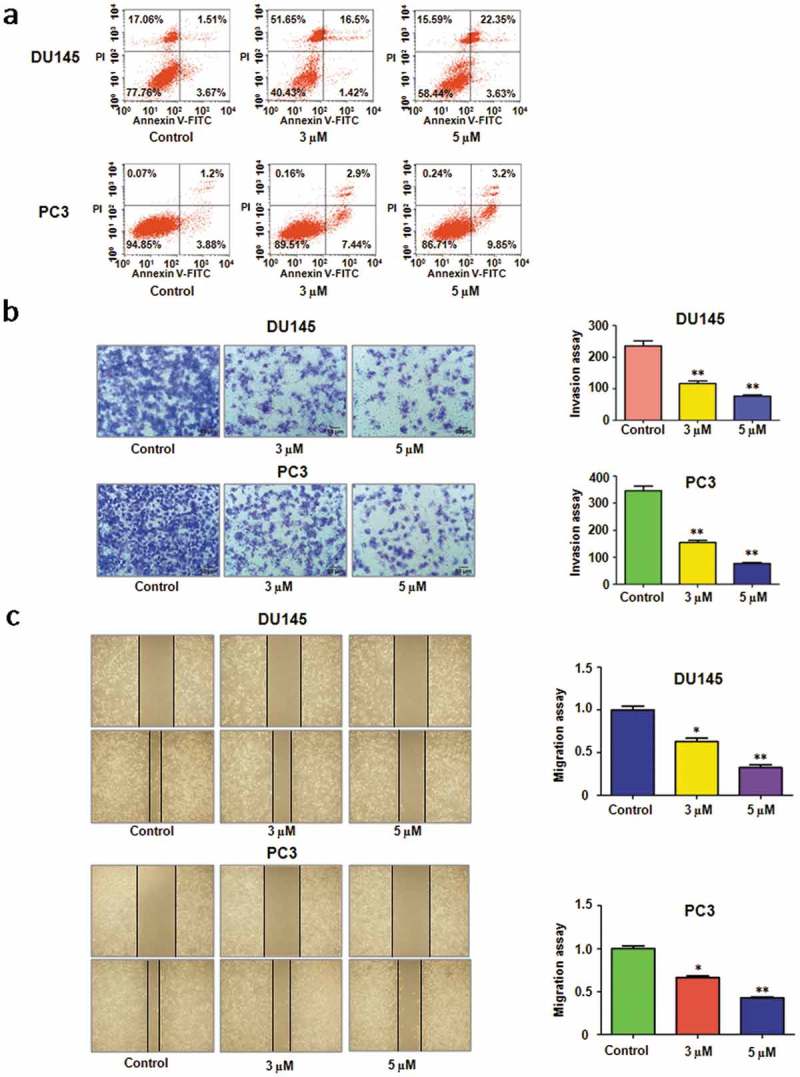
Effect of rottlerin on cell apoptosis, migration and invasion. (a) Cell apoptosis was analyzed by Flow cytometry in rottlerin-treated prostate cancer cells. (b) Left panel, The cell invasive ability was detected by Transwell chambers assay in PC3 and DU145 cells. Right panel, Quantitative results are illustrated for left panel. **P < 0.01 vs control. (c) Left panel, The wound healing assay was performed to measure the inhibitory of rottlerin in prostate cancer cells. Right panel, Quantitative results are illustrated for left panel. *P < 0.05, **P < 0.01 vs control.
Rottlerin retarded cell migration and invasion
To determine whether rottlerin inhibited cell invasive activity, invasion assay was applied in prostate cancer cells treated with rottlerin for 20 hours. Our results showed that the cell numbers of penetration through the Matrigel-coated membrane were reduced in a dose-dependent manner, which suggested that rottlerin inhibited cell invasion in prostate cancer cells (Figure 2(b)). Furthermore, we exerted wound healing assay to detect the role of rottlerin in cell migration. The results identified that rottlerin treatments significantly suppressed the migratory activity in a dose-dependent manner in PC3 and DU145 cells (Figure 2(c)).
Rottlerin suppressed EZH2 expression
Emerging evidence has shown that EZH2 worked as an oncogene in prostate cancer cells. Therefore, inhibition of EZH2 could be an efficient therapy to treat prostate cancer. We measured whether rottlerin could decrease the expression of EZH2 in PC3 and DU145 cells. The real-time PCR analysis data demonstrated that the mRNA level of EZH2 was decreased in both prostate cancer cells after rottlerin treatment (Figure 3(a)). At the same time, we detected the protein level of EZH2 in PC3 and DU145 cells, and we found that the expression of EZH2 was reduced after rottlerin treatment (Figure 3(b,c)). Since H3K27me3 (tri-methylation of histone H3 at Lys 27) is one key target of EZH2, we also measured the expression of H3K27me3 in cells after rottlerin treatment [19]. We found that rottlerin inhibited the expression of H3K27me3 in prostate cancer cells (Figure 3(b,c)). In summary, our findings demonstrated that the mRNA and protein levels of EZH2 were reduced by rottlerin treatment in prostate cancer cells.
Figure 3.
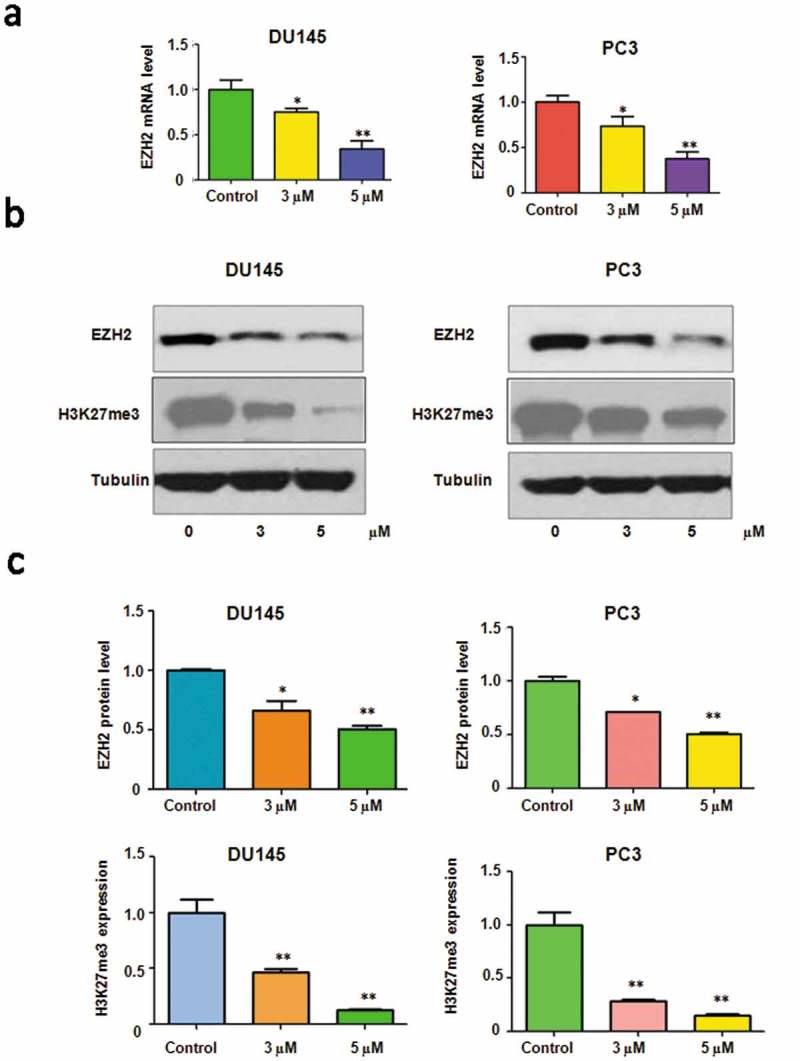
Rottlerin inhibited EZH2 expression at RNA and protein levels. (a) EZH2 mRNA level was measured by RT-PCR in prostate cancer cells with rottlerin treatment. *P < 0.05, **P < 0.01 vs control (DMSO treatment). (b) Top panels, Western blotting analysis were conducted to determine the expression of EZH2 in PC3 and DU145 cells. Bottom panels, Quantitative results are illustrated for Top panel. * P < 0.05, **P < 0.01 vs control (DMSO treatment).
Over-expression of EZH2 rescued rottlerin-induced cell growth inhibition
To dissect the function of EZH2 in rottlerin-mediated cell proliferation, EZH2 cDNA or empty vector was transfected into prostate cancer cells followed by rottlerin treatment for 48 hours. The results showed that overexpression of EZH2 promoted cell proliferation in both prostate cancer cell lines (Figure 4(a)). More importantly, the overexpression of EZH2 rescued the cell growth inhibition caused by the rottlerin in PC3 and DU145 cells. These findings suggested that rottlerin inhibited cell growth partly due to the down-regulation of EZH2 in prostate cancer cells.
Figure 4.
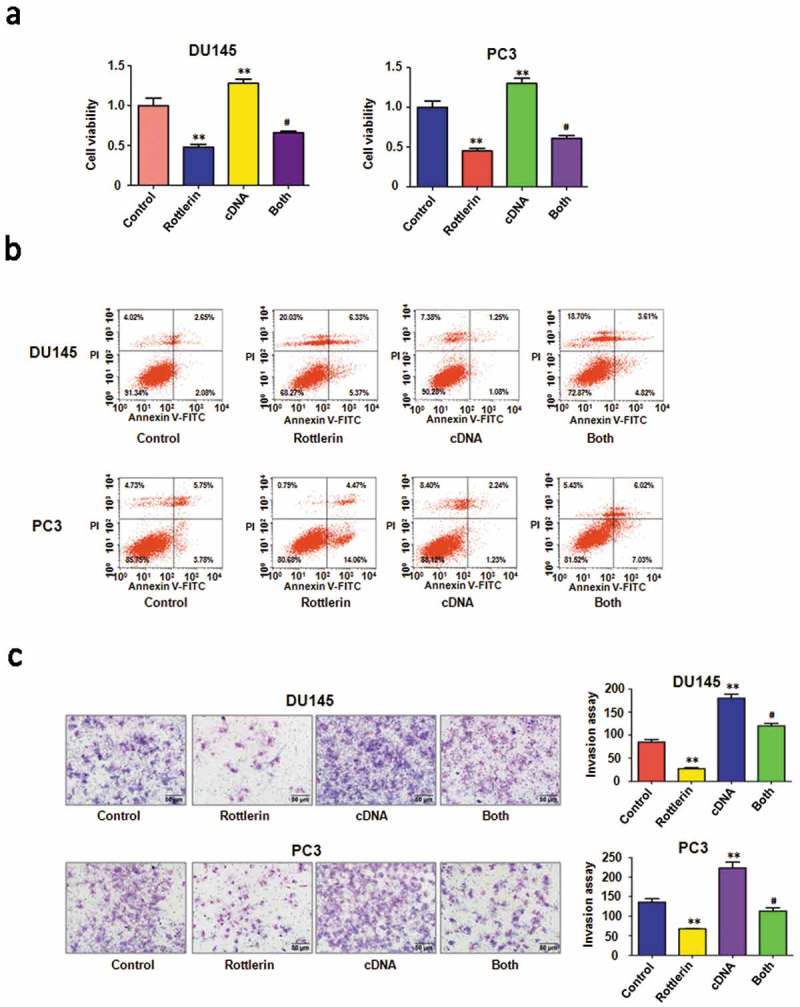
The effect of EZH2 overexpression on cell growth, apoptosis and invasion. (a) CellTiter-Glo luminescence assay was used to describe the effect of EZH2 overexpression in combination with rottlerin treatment on prostate cancer cell proliferation. Control: pcDNA3.0; cDNA: EZH2 cDNA; Both: EZH2 cDNA+ rottlerin. **P < 0.01, compared with control; #P < 0.05 compared with rottlerin treatment alone or EZH2 cDNA transfection alone. (b) The cell apoptosis was conducted by FACS in PC3 and DU145 cells after EZH2 cDNA transfection and rottlerin treatment. (c) Left panel, Invasion assay was performed in prostate cancer cells after EZH2 cDNA transfection and rottlerin treatment. Right panel, Quantitative results are illustrated for left panel **P < 0.01.
Over-expression of EZH2 rescued rottlerin-induced apoptosis and motility inhibition
Next, we observed that EZH2 overexpression decreased apoptosis in prostate cancer cells (Figure 4(b)). Importantly, the up-regulation of EZH2 abrogated rottlerin-induced apoptosis in both PC3 and DU145 cells (Figure 4(b)). Obviously, the overexpression of EZH2 promoted the cell invasive activity in prostate cancer cells (Figure 4(c)). The combination of EZH2 transfection and rottlerin treatment increased the number of invasive cells compared with the rottlerin treated alone in PC3 and DU145 cells (Figure 4(c)). Consistently, the wound healing assay indicated that overexpression of EZH2 induced more numbers of migratory cells across the wound (Figure 5(a)). EZH2 overexpression promoted cell migration and rescued rottlerin-mediated migratory inhibition in both prostate cells (Figure 5(a)). Furthermore, the western blot assay exhibited that the overexpression of EZH2 antagonized the rottlerin-mediated suppression of EZH2 in prostate cancer cells (Figure 5(b,c)). Altogether, rottlerin exerted its tumor suppressive function in part via down-regulation of EZH2 in prostate cancer cells.
Figure 5.
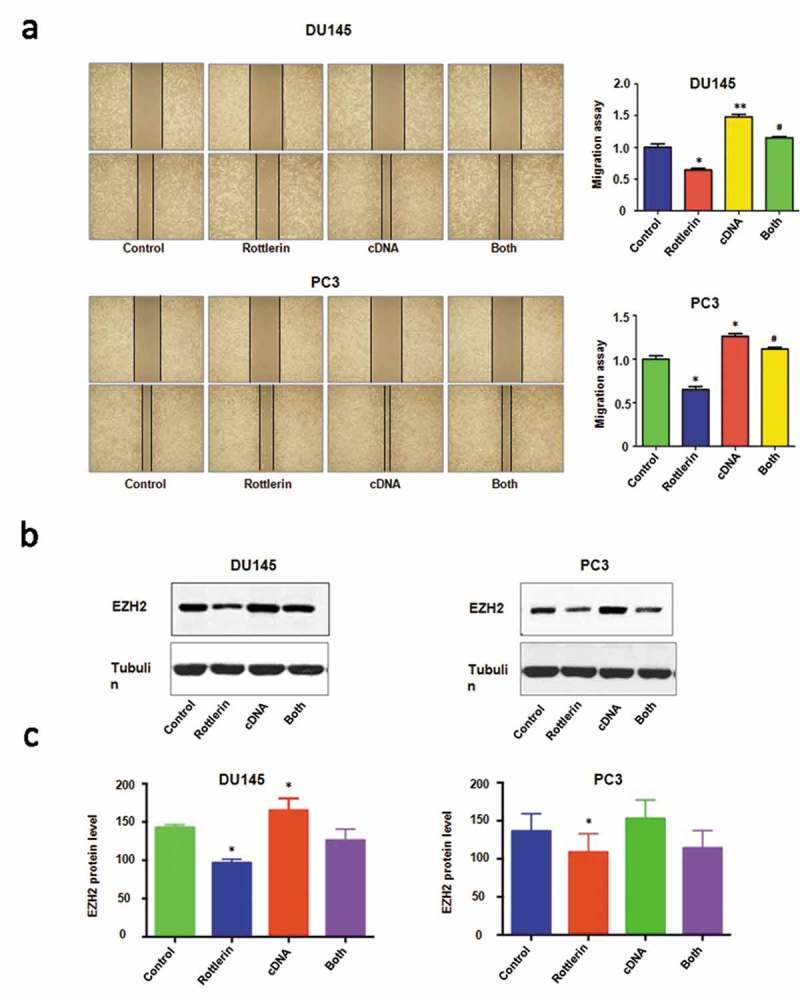
The effect of EZH2 overexpression on cell migration. (a) Left panel, The cell migratory activity was analyzed by wound healing assay with EZH2 cDNA transfection and rottlerin treatment. Control: pcDNA3.0 cDNA: EZH2 cDNA; Both: EZH2 cDNA+ rottlerin. Right panel, Quantitative results are illustrated for left panel. *P < 0.05, compared with control; #P < 0.05 compared with rottlerin treatment alone or EZH2 cDNA transfection alone. (b) The expression of EZH2 was performed by western blotting in prostate cancer cells with EZH2 cDNA transfection and rottlerin treatment. (c) Quantitative results are illustrated for top panel. *P < 0.05, compared with control; #P < 0.05, compared with rottlerin treatment alone or EZH2 cDNA transfection alone.
Down-regulation of EZH2 by its siRNA promoted rottlerin-induced anti-tumor activity
To further explore the role of EZH2 in rottlerin-mediated tumor suppressive activity, we depleted EZH2 by its siRNA in prostate cancer cells. We found that EZH2 siRNA inhibited cell proliferation in both prostate cancer cells (Figure 6(a)). Notably, cell growth was significantly inhibited by rottlerin combined with EZH2 siRNA transfection (Figure 6(a)). Moreover, depletion of EZH2 triggered cell apoptosis in PC3 and DU145 cells (Figure 6(b)). EZH2 siRNA resulted in more apoptotic cells induced by rottlerin compared with rottlerin alone or EZH2 siRNA transfection alone in prostate cancer cells (Figure 6(b)). Furthermore, we identified that down-expression of EZH2 suppressed invasion and migration in both PC3 and DU145 cell lines (Figures 6(c) and 7(a)). The rottlerin treatment combination with depletion of EZH2 suppressed cell migration and invasion to a greater degree compared with rottlerin treatment alone (Figures 6(c) and 7(a)). Mechanically, the Western blotting analysis demonstrated that EZH2 siRNA transfection downregulated EZH2 expression in both prostate cancer cells (Figure 7(b,c)). Depletion of EZH2 promoted rottlerin-induced EZH2 downregulation in prostate cancer cells (Figure 7(b,c)).
Figure 6.
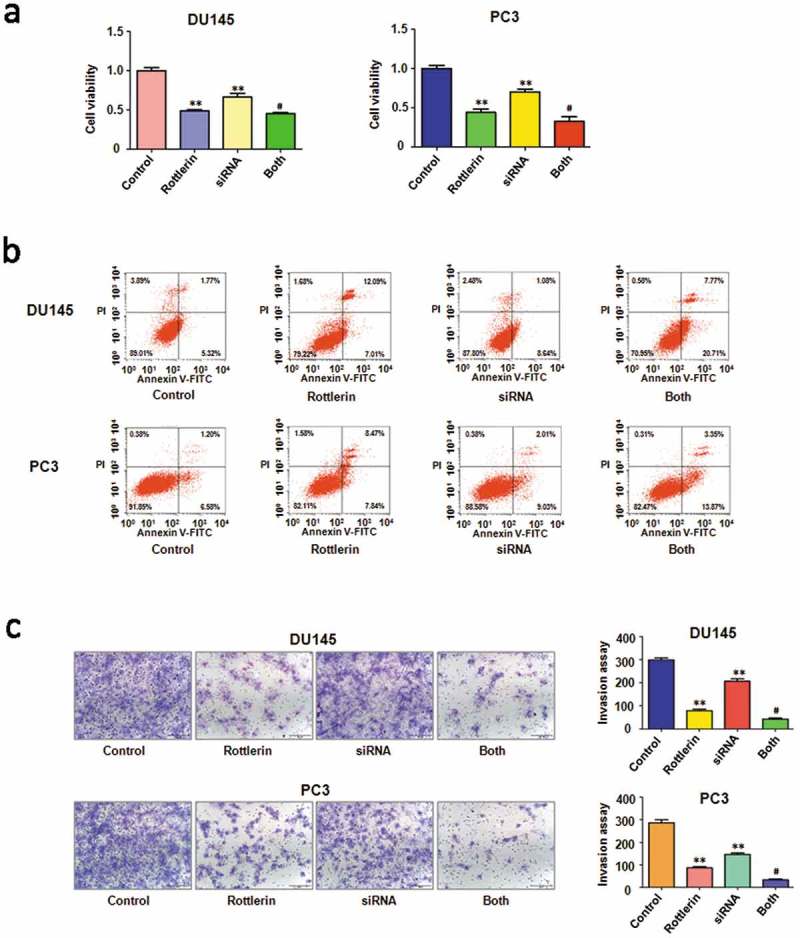
The effect of EZH2 downregulation on cell growth, apoptosis and invasion (a) CellTiter-Glo luminescence assay was applied for detecting the effect of EZH2 siRNA in combination with rottlerin treatment on prostate cancer cell growth. **P < 0.01, compared with control; #P < 0.05 compared with rottlerin treatment or EZH2 siRNA transfection. Control: siRNA control; SiRNA: EZH2 siRNA; Both: rottlerin + EZH2 siRNA. (b) Flow cytometry was used to describe in PC3 and DU145 cells. (c) Left panel, Invasion assay was exerted in prostate cancer cells after EZH2 siRNA transfection and rottlerin treatment. Right panel, quantitative results are illustrated for left panel. **P < 0.01, vs control; #P < 0.05 vs rottlerin treatment or EZH2 siRNA transfection.
Figure 7.
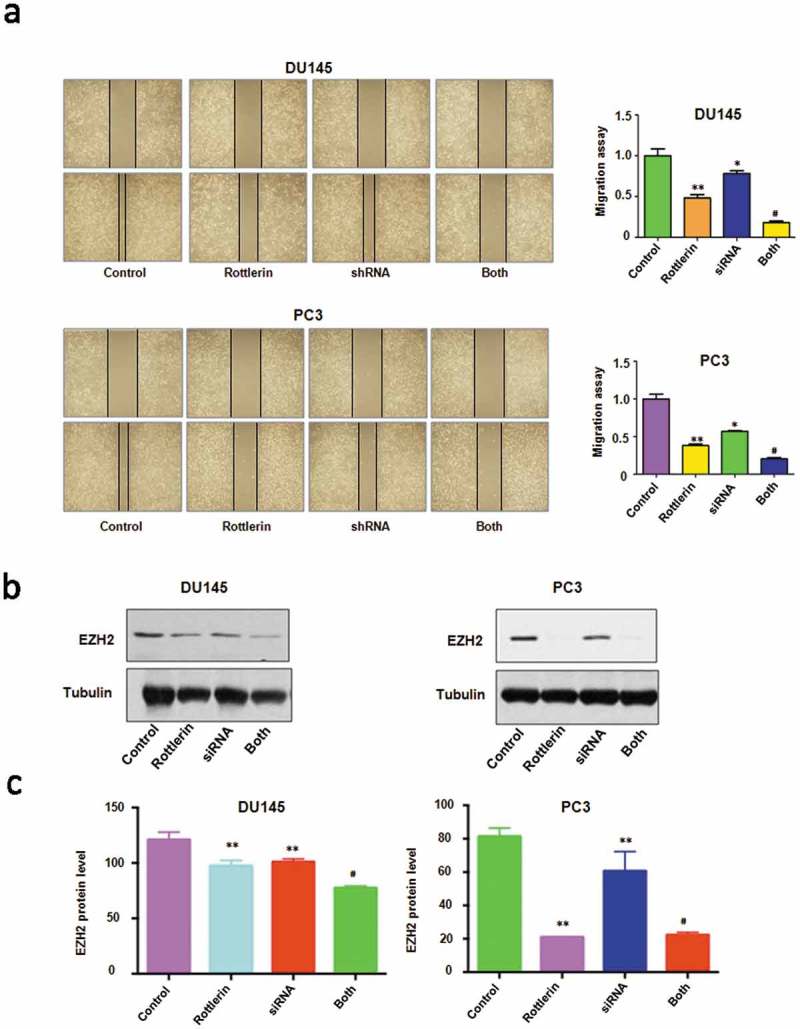
The effect of EZH2 downregulation on cell migration. (a) Left panel, The wound healing assay was exerted to investigate the cell migration in prostate cancer cells after EZH2 siRNA transfection and rottlerin treatment. Right panel, quantitative results are illustrated for left panel. Control: siRNA control; SiRNA: EZH2 siRNA; Both: rottlerin + EZH2 siRNA. *P < 0.05, **P < 0.01, vs control; #P < 0.05 vs rottlerin treatment or EZH2 siRNA transfection. (b) The expression of EZH2 was tested by western blotting in prostate cancer cells with EZH2 siRNA transfection and rottlerin treatment. (c) Quantitative results are illustrated for top panel. *P < 0.05, compared with control; #P < 0.05, compared with rottlerin treatment alone or EZH2 siRNA transfection alone.
Discussion
Emerging evidence has implicated the oncogenic role of EZH2 in the development and progression of a variety of cancers including breast cancer, bladder cancer, endometrial cancer, and melanoma [16–18]. The overexpression of EZH2 has been associated with aggressiveness and advanced disease in each of these cancer types [16–18]. Previous observation had shown the close association between EZH2 overexpression and worse progression of prostate cancer [13]. The overexpression of EZH2 resulted in the development of myeloproliferative disorder in mice [20]. Moreover, the tissue microarray analysis identified that the expression of EZH2 was strong in breast cancer cells compared with normal breast epithelia [21]. Additionally, the overexpression of EZH2 also promoted cell growth, migration, and invasion in breast cancer cells [21,22]. Chen et al. reported that the overexpression of EZH2 has poor outcome in patients with colorectal cancer [23]. One study identified that the activation of EZH2 inspired by E2F1 promoted progression and aggression in bladder cancer [24]. Similarly, we demonstrated the overexpression of EZH2 increased cell migration and invasion in prostate cancer cells. While the depletion of EZH2 retarded cell migratory and invasive activity in prostate cancer cells. Our findings suggest that EZH2 is an oncoprotein in prostate cancer and targeting EZH2 could be a useful approach for the treatment of prostate cancer patients.
Recently, a great amount of evidence showed that rottlerin exhibited its anti-tumor activity in human cancers. For instance, rottlerin suppressed LRP6 protein level and led to the inhibition of mTORC1 and Wnt/β-catenin signaling in breast cancer cells [11]. Basu et al found that rottlerin induced caspase-2 via ubiquitin proteasome-mediated pathway in HeLa cells and ovarian cancer cells [25]. Another study indicated that rottlerin stimulated cell apoptosis via interaction with proteins of the Bcl-2 family in pancreatic cancer cells [26]. Interestingly, rottlerin exerted its anti-cancer therapy via up-regualtion of DDX3 in hepatocellular carcinoma [27]. Huang et al discovered that rottlerin targeted multiple cell signaling pathways such as Akt, Notch, and Shh (sonic hedgehog) pathways, leading to the cell growth inhibition and apoptosis promotion in pancreatic cancer cells [28]. One study identified rottlerin promoted apoptosis through upregulation of NAG-1 (non- steroidal anti-in ammatory drug activated gene-1) via ERK and p38 MAPK dependent mechanism in colon carcinoma cells [29]. Importantly, rottlerin also induced autophagy via inhibition of mTOR or through PKCδ- independent manner in several cancer cells [7,12,30–32]. Furthermore, rottlerin inhibited migration of follicular thyroid carcinoma cells by PKCδ-independent destabilization of the focal adhesion complex [33]. Yin et al found that rottlerin exerted its anti-tumor activity through inhibition of Skp2 (s-phase kinase associated protein 2) in breast cancer cells and pancreatic cancer cells [34,35]. Another study showed that rottlerin suppressed cell proliferation and invasion via inhibition of Cdc20 (cell divison cycle protein 20) in glioma cells [36]. Zhao et al. identified that rottlerin decreased the expression of TAZ (transcriptional coactivator with PDZ-binding motif) in non-small cell lung cancer [37]. Similarly, another study rottlerin inhibited cell growth via down-regulation of Notch-1 pathway in nasopharyngeal carcinoma cells [38]. Keep in line with the notion, we found that rottlerin suppressed cell growth, migration and invasion via down-regulation of EZH2 in prostate cancer cells. It has been known that EZH2 catalyzes H3K27me3 to regulate gene expression via epigenetic machinery [19,39]. Indeed, we found the down-regulation of H3K27me3 in cells after rottlerin treatment. EZH1 has been reported to be physically present in PRC2 complex as an H3K27 methyltransferase [40]. Interestingly, we did not observe the change of EZH1 level in rottlerin-treated cells (data not shown), indicating that rottlerin mainly targets EZH2 expression in prostate cancer cells. It is worth noting that the down-regulation of EZH2 might be the secondary effect of rottlerin. The massive cell cycle or DNA damage by rottlerin could lead to EZH2 downregulation. Additionally, rottlerin could target the upstream genes of EZH2 and govern the EZH2 level. Therefore, deeper investigation is required to explore the detailed mechanism of rottlerin-mediated EZH2 downregulation.
Considering the role of EZH2 in tumorigenesis, inactivation of EZH2 could be benefit for patients with prostate cancer. Many studies have discovered several inhibitors of EZH2. For instance, DZNep was the first inhibitor widely used to inhibit the activity of PRC2 component including EZH2 in experimental work [41]. Another inhibitor, EPZ005687 was reported to bind to wild-type EZH2 and inhibited its role in human protein methylation [42]. The small-molecule EZH2 inhibitor, GSK126, inhibited both wild-type and mutant EZH2 with higher efficiency, might provide a promising treatment for EZH2 mutant lymphoma [43,44]. UNC1999 was synthesized as the first orally bioavailable inhibitor that was highly selective for both wild-type EZH2 and EZH2 Y641 mutant [45]. EPZ-6438 was subsequently developed with improved pharmacokinetic properties including good oral bioavailability [46]. Since these chemical inhibitors exhibited side effects, it is urgent to discover natural agents with non-toxic nature to inactivate EZH2 in human cancer. Factually, several natural compounds have been reported as EZH2 inhibitor including curcumin [47], Salinomycin [48], Flavokawain A (FKA) [49], butylidenephthalide [50]. In this study, rottlerin inhibited the expression of EZH2 in prostate cancer cells. Rottlerin could be a new inhibitor of EZH2 in prostate cancer cells. Since the rottlerin is non-toxic inhibitor, the inhibition of EZH2 by rottlerin could be more a safer strategy to treat patients with prostate cancer.
Methods and materials
Cell culture and reagents
Human DU145 and PC3 cells were cultured in DMEM medium with 10% fetal bovine serum and 1% penicillin and streptomycin in a 5% CO2 atmosphere at 37 °C. Primary antibody for EZH2 (ab228697) was purchased from Abcam. Anti-H3K27me3 antibody was bought from Cell Signaling Technology Company (Beverly, MA, USA). All secondary antibodies were obtained from Thermo Scientific. Lipofectamine 2000 was purchased from Invitrogen. Monoclonal anti-tubulin and rottlerin (CAS number R5648, ≥85% rottlerin) were obtained from Sigma-Aldrich (St. Louis, MO). Rottlerin was dissolved in DMSO to make a 10 mM stock solution. CTG (Cell Titer-Glo Luminescent) was purchased from Promega (Madison, WI). Matrigel and Transwell inserts were purchased from BD Biosciences. Cells were treated with 0.1% DMSO as the control group [34,35].
Cell viability assay
Exponentially growing cells were seeded at 6 × 103 cells/well in 96-well plates for 24 hours and then treated with various concentration of rottlerin. After 48h and 72h, 20 μl CTG solution was added to each well and cell viability was measured by the microplate. Each value was normalized to cells treated with DMSO [34].
Colony formation assay
PC3 and DU145 cells were seeded in 6 cm2 dishes at 1 × 103 cells/well with 3 μM and 5 μM concentrations of rottlerin. After two weeks, 4 % paraformaldehyde was added to fix the cells and crystal violet was used to stain the colonies.
Cell apoptosis analysis
PC3 and DU145 were cultured in 6-well plate overnight with different concentration of rottlerin. After 48h, cells were collected and washed with PBS, and the cells were resuspended in 500 μl binding buffer with 5 μl propidium iodide (PI) and 5 μl FITC-conjugated anti-Annexin V antibody. Subsequently apoptosis was detected by a FACScalibur flow cytometer (BD, USA).
Cell invasion assay
To determine the invasive ability of prostate cells, the Transwell assays were performed in PC3 and DU145 cells. Cells transfected with EZH2 siRNA or EZH2 cDNA were treated with rottlerin or not and were cultured in 200 μl serum-free medium in the up camber with Matrigel. The complete medium was added in the under chamber with the same concentration of rottlerin. The cells were incubated for 20h at 37 °C with 5% CO2 and then the membrane of the chamber was stained with Giemsa and photographed with Box-Type Fluorescence imaging Device (FSX100, OLYMPUS).
Cell scratch assay
PC3 and DU145 cells were seeded in 6-well plate. The prostate cells were transfected with EZH2 siRNA. After cells were converged almost 100%, then scratched the cells with a sterile 20 μl pipette tip. Washed the cells with PBS and added the medium with different concentration of rottlerin. The scratch was photographed with a microscope at 0 h and 20 h, respectively.
Transfection
Cells were cultured into 6-well plates for overnight and transfected with EZH2 cDNA or siRNA or empty vector using lipofectamine 2000 following the instruction’s protocol. After 6 h, the medium was changed by fresh DMEM. Then 48h later, the cells were collected to further analysis as described under the results sections.
Quantitative real-time reverse transcription-pcr analysis
The total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA) and reversed-transcribed into cDNA by RevertAid First Strand cDNA Synthesis Kit. PCR were performed using Power SYBR Green PCR Master Mix and the results were calculated by 2-ΔΔCt method. The primers used in the PCR reaction are: EZH2, forward primer and reverse primer (5′-GTG GAG AGA TTA TTT CTC AAG ATG-3′) and reverse primer (5′-CCG ACA TAC TTC AGG GCA TCA GCC–3′). GAPDH, forward primer (5′-ACC CAG AAG ACT GTG GAT GG-3′) and reverse primer (5′-CAG TGA GCT TCC CGT TCA G-3′).
Western blotting analysis
The cells were collected and washed by PBS and lysed by protein lysis buffer in ice. After centrifuged, the concentration of the supernatant protein level was detected by BCA Protein Assay kit (Thermo Scienti c, MA). Same amount of protein samples were separated by electrophoresis in Sodium Dodecyl Sulfonate (SDS) polyacrylamide gel and then transferred onto a Polyvinylidene Fluoride (PVDF) membrane, and then incubated with primary antibody at 4°C overnight. The membrane was washed for three times with TBST and incubated with second antibody for one hour in room temperature. Then the expression of protein was detected by electro-chemiluminescence (ECL) assay [34].
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA). ANOVA was performed to evaluate statistical significance. Results were presented as means ± SD. P < 0.05 was considered as statistically significant.
Funding Statement
This work was supported by the National Natural Science Foundation of China [81572936 and 81773186].
Acknowledgments
This work was supported by grant from National Natural Science Foundation of China (NSFC number 81572936 and 81773186) and the priority academic program development of Jiangsu higher education institutions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Leslie SW, Siref LE. Cancer, Prostate. Treasure Island (FL): StatPearls; 2018. [Google Scholar]
- [3].Ge JJ, Wang LT, Chen P, et al. Two new tetracyclic triterpenoids from the barks of Melia azedarach. J Asian Nat Prod Res. 2016;18:20–25. [DOI] [PubMed] [Google Scholar]
- [4].Hasima N, Ozpolat B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014;5:e1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maioli E, Torricelli C, Valacchi G. Rottlerin and cancer: novel evidence and mechanisms. ScientificWorldJournal. 2012;2012:350826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Koo KH, Jeong WJ, Cho YH, et al. K-Ras stabilization by estrogen via PKCdelta is involved in endometrial tumorigenesis. Oncotarget. 2015;6:21328–21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Song KS, Kim JS, Yun EJ, et al. Rottlerin induces autophagy and apoptotic cell death through a PKC-delta-independent pathway in HT1080 human fibrosarcoma cells: the protective role of autophagy in apoptosis. Autophagy. 2008;4:650–658. [DOI] [PubMed] [Google Scholar]
- [8].Lim JH, Park JW, Choi KS, et al. Rottlerin induces apoptosis via death receptor 5 (DR5) upregulation through CHOP-dependent and PKC delta-independent mechanism in human malignant tumor cells. Carcinogenesis. 2009;30:729–736. [DOI] [PubMed] [Google Scholar]
- [9].Lim JH, Park JW, Kim SH, et al. Rottlerin induces pro-apoptotic endoplasmic reticulum stress through the protein kinase C-delta-independent pathway in human colon cancer cells. Apoptosis. 2008;13:1378–1385. [DOI] [PubMed] [Google Scholar]
- [10].Maioli E, Daveri E, Maellaro E, et al. Non-conventional rottlerin anticancer properties. Arch Biochem Biophys. 2018;645:50–53. [DOI] [PubMed] [Google Scholar]
- [11].Lu W, Lin C, Li Y. Rottlerin induces Wnt co-receptor LRP6 degradation and suppresses both Wnt/beta-catenin and mTORC1 signaling in prostate and breast cancer cells. Cell Signal. 2014;26:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014;343:179–189. [DOI] [PubMed] [Google Scholar]
- [13].Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. [DOI] [PubMed] [Google Scholar]
- [14].Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat Med. 2016;22:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gan L, Yang Y, Li Q, et al. Epigenetic regulation of cancer progression by EZH2: from biological insights to therapeutic potential. Biomark Res. 2018;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bracken AP, Pasini D, Capra M, et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. Embo J. 2003;22:5323–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bachmann IM, Halvorsen OJ, Collett K, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–273. [DOI] [PubMed] [Google Scholar]
- [18].Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yan KS, Lin CY, Liao TW, et al. EZH2 in cancer progression and potential application in cancer therapy: a friend or foe?. Int J Mol Sci. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Herrera-Merchan A, Arranz L, Ligos JM, et al. Ectopic expression of the histone methyltransferase Ezh2 in haematopoietic stem cells causes myeloproliferative disease. Nat Commun. 2012;3:623. [DOI] [PubMed] [Google Scholar]
- [21].Kleer CG, Cao Q, Varambally S, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA. 2003;100:11606–11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chien YC, Liu LC, Ye HY, et al. EZH2 promotes migration and invasion of triple-negative breast cancer cells via regulating TIMP2-MMP-2/-9 pathway. Am J Cancer Res. 2018;8:422–434. [PMC free article] [PubMed] [Google Scholar]
- [23].Chen Z, Yang P, Li W, et al. Expression of EZH2 is associated with poor outcome in colorectal cancer. Oncol Lett. 2018;15:2953–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee SR, Roh YG, Kim SK, et al. Activation of EZH2 and SUZ12 regulated by E2F1 predicts the disease progression and aggressive characteristics of bladder cancer. Clin Cancer Res. 2015;21:5391–5403. [DOI] [PubMed] [Google Scholar]
- [25].Basu A, Adkins B, Basu C. Down-regulation of caspase-2 by rottlerin via protein kinase C-delta-independent pathway. Cancer Res. 2008;68:2795–2802. [DOI] [PubMed] [Google Scholar]
- [26].Ohno I, Eibl G, Odinokova I, et al. Rottlerin stimulates apoptosis in pancreatic cancer cells through interactions with proteins of the Bcl-2 family. Am J Physiol Gastrointest Liver Physiol. 2010;298:G63–G73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Z, Shen GH, Xie JM, et al. Rottlerin upregulates DDX3 expression in hepatocellular carcinoma. Biochem Biophys Res Commun. 2018;495:1503–1509. [DOI] [PubMed] [Google Scholar]
- [28].Huang M, Tang SN, Upadhyay G, et al. Rottlerin suppresses growth of human pancreatic tumors in nude mice, and pancreatic cancer cells isolated from Kras(G12D) mice. Cancer Lett. 2014;353:32–40. [DOI] [PubMed] [Google Scholar]
- [29].Lim JH, Woo SM, Min KJ, et al. Rottlerin induces apoptosis of HT29 colon carcinoma cells through NAG-1 upregulation via an ERK and p38 MAPK-dependent and PKC delta-independent mechanism. Chem Biol Interact. 2012;197:1–7. [DOI] [PubMed] [Google Scholar]
- [30].Torricelli C, Daveri E, Salvadori S, et al. Phosphorylation-independent mTORC1 inhibition by the autophagy inducer Rottlerin. Cancer Lett. 2015;360:17–27. [DOI] [PubMed] [Google Scholar]
- [31].Singh BN, Kumar D, Shankar S, et al. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem Pharmacol. 2012;84:1154–1163. [DOI] [PubMed] [Google Scholar]
- [32].Kumar D, Shankar S, Srivastava RK. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol Cancer. 2013;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lin CJ, Lin CY, Chen Y, et al. Rottlerin inhibits migration of follicular thyroid carcinoma cells by PKCdelta-independent destabilization of the focal adhesion complex. J Cell Biochem. 2010;110:428–437. [DOI] [PubMed] [Google Scholar]
- [34].Yin X, Zhang Y, Su J, et al. Rottlerin exerts its anti-tumor activity through inhibition of Skp2 in breast cancer cells. Oncotarget. 2016;7:66512–66524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Su J, Wang L, Yin X, et al. Rottlerin exhibits anti-cancer effect through inactivation of S phase kinase-associated protein 2 in pancreatic cancer cells. Am J Cancer Res. 2016;6:2178–2191. [PMC free article] [PubMed] [Google Scholar]
- [36].Wang L, Hou Y, Yin X, et al. Rottlerin inhibits cell growth and invasion via down-regulation of Cdc20 in glioma cells. Oncotarget. 2016;7:69770–69782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao Z, Zheng N, Wang L, et al. Rottlerin exhibits antitumor activity via down-regulation of TAZ in non-small cell lung cancer. Oncotarget. 2017;8:7827–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hou Y, Feng S, Wang L, et al. Inhibition of Notch-1 pathway is involved in rottlerin-induced tumor suppressive function in nasopharyngeal carcinoma cells. Oncotarget. 2017;8:62120–62130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu B, Konze KD, Jin J, et al. Targeting EZH2 and PRC2 dependence as novel anticancer therapy. Exp Hematol. 2015;43:698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shen X, Liu Y, Hsu YJ, et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tan J, Yang X, Zhuang L, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Song X, Zhang L, Gao T, et al. Selective inhibition of EZH2 by ZLD10A blocks H3K27 methylation and kills mutant lymphoma cells proliferation. Biomed Pharmacother. 2016;81:288–294. [DOI] [PubMed] [Google Scholar]
- [43].Verma SK, Tian X, LaFrance LV, et al. Identification of potent, selective, cell-active inhibitors of the histone lysine methyltransferase EZH2. ACS Med Chem Lett. 2012;3:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. [DOI] [PubMed] [Google Scholar]
- [45].Konze KD, Ma A, Li F, et al. An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem Biol. 2013;8:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Knutson SK, Warholic NM, Wigle TJ, et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci USA. 2013;110:7922–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ide H, Lu Y, Noguchi T, et al. Modulation of AKR1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018;109:1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang R, Li Y, Hou Y, et al. The PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal transition in gemcitabine resistance hepatoma cells. Oncotarget. 2015;6:7000–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li X, Yokoyama NN, Zhang S, et al. Flavokawain A induces deNEDDylation and Skp2 degradation leading to inhibition of tumorigenesis and cancer progression in the TRAMP transgenic mouse model. Oncotarget. 2015;6:41809–41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Huang MH, Lin SZ, Lin PC, et al. Brain tumor senescence might be mediated by downregulation of S-phase kinase-associated protein 2 via butylidenephthalide leading to decreased cell viability. Tumour Biol. 2014;35:4875–4884. [DOI] [PubMed] [Google Scholar]


