Abstract
The glenohumeral joint, the most mobile joint in the body of hominoids, is involved in the locomotion of all extant primates apart from humans. Over the last few decades, our knowledge of how variation in its morphological characteristics relates to different locomotor behaviors within extant primates has greatly improved, including features of the proximal humerus and the glenoid cavity of the scapula, as well as the muscles that function to move the joint (the rotator cuff muscles). The glenohumeral joint is a region with a strong morphofunctional signal, and hence, its study can shed light on the locomotor behaviors of crucial ancestral nodes in the evolutionary history of hominoids (e.g., the last common ancestor between humans and chimpanzees). Hominoids, in particular, are distinct in showing round and relatively big proximal humeri with lowered tubercles and flattened and oval glenoid cavities, morphology suited to engage in a wide range of motions, which enables the use of locomotor behaviors such as suspension. The comparison with extant taxa has enabled more informed functional interpretations of morphology in extinct primates, including hominoids, from the Early Miocene through to the emergence of hominins. Here, I review our current understanding of glenohumeral joint functional morphology and its evolution throughout the Miocene and Pleistocene, as well as highlighting the areas where a deeper study of this joint is still needed.
Keywords: evolutionary morphology, glenohumeral morphology, hominins, hominoids, locomotion, Miocene apes
1. INTRODUCTION
The evolution of primate locomotion and, especially, ape locomotion is fundamental to the understanding of human origins. Its importance resides on a central question of the discipline: How and when did we start walking on two feet? Bipedalism is a defining feature of being humans, and the study of the evolution of primate locomotor behaviors, with an emphasis on how morphological variation throughout the skeleton may relate to function, is key to answering such question. Theories on the origin of bipedalism, which try to elucidate the locomotor behavior exhibited by the last common ancestor (LCA) between humans and chimpanzees, range from characterizing the LCA as gorilla and chimpanzee‐like (thus knuckle‐walking; e.g., Richmond & Strait, 2000); as largely arboreal and similar to orangutans today (e.g., Thorpe, Holder, & Crompton, 2007); or engaging in a more heterogeneous and generalized pool of arboreal behaviors (climbing, clambering, bridging) as seen in the living arboreal primates (e.g., Arias‐Martorell, Potau, Bello‐Hellegouarch, & Pérez‐Pérez, 2015c). It is unfortunate that fossil remains throughout primate and, in particular, ape evolutionary history are scarce, and thus, we lack the evidence to fully support any one theory.
Among nonhuman hominoids, the forelimb is critical to a diversity of locomotor behaviors, ranging from terrestrial knuckle‐walking to suspension to ricochetal brachiation, but is largely removed from locomotion in humans. Within the forelimb, the glenohumeral joint—the articulation between the scapula's glenoid fossa and the proximal humerus—is the primary joint involved in arm movement and the most mobile joint in the body in hominoids (including Gorilla, Pan, Pongo, Homo, and the hylobatid family). As such, it has been the focus of morphological and biomechanical studies for decades and its major external morphological features have been functionally associated with the use of certain locomotor behaviors in primates (Arias‐Martorell, Alba, Potau, Bello‐Hellegouarch, & Pérez‐Pérez, 2015b; Arias‐Martorell, Tallman, Potau, Bello‐Hellegouarch, & Pérez‐Pérez, 2015a; Arias‐Martorell et al., 2015c; Kagaya, 2007; Larson, 1993, 1995, 2007a; Rose, 1989).
Within hominoids, the high degree of glenohumeral mobility has been widely linked to the evolution of an upright body posture, also known as orthogrady, which involved the displacement of the scapula onto the back of a mediolaterally wide and anteroposteriorly shallow thorax (e.g., Andrews & Groves, 1976; Gebo, 1996, 2010; Keith, 1903, 1923; Ward, 2015). The scapular displacement resulted in a more mobile and less stable glenohumeral joint, which may move in all directions and may combine all possible movements, from flexion and extension to abduction and adduction, and axial rotation (Larson, 1993). It is important that in hominoids (as well as in the groups of nonhominoids that use suspensory behaviors), the study of the glenohumeral joint is particularly useful to explore the presence of below‐branch positional behaviors and assess their dependence on suspensory locomotion (Arias‐Martorell et al., 2015a,b,c; Larson, 1993, 1995). However, despite decades of research, there still remains several unanswered questions, misconceptions, and debate about, for example, the functional morphology of the hominoid glenohumeral joint, particularly with regard to the role of soft tissue, specifically the cartilage surrounding the glenoid cavity (i.e., glenoid labrum; Arias‐Martorell et al., 2015c; Patton & Thiboudieau, 2010), the adaptive role played by the morphology of the joint with regard to the locomotor behaviors exhibited by the primates, the timing and acquisition path of such morphology, and intraspecific morphological differences between species of primates, in particular hominoids, with varied frequency on the use of the same locomotor behaviors.
The aim of this article is, then, to offer first a comprehensive review of the morphofunctional aspects of the glenohumeral joint and their relationship to the different locomotor behaviors exhibited by primates, with special emphasis on the ape clade, including Miocene hominoids and hominins, and then discuss future questions and aspects of the research that still need undertaking. In detail, I will first provide a general review of glenohumeral morphology and function, including soft tissues, giving an overall assessment of the morphological and functional diversity across primates and how they generally relate to the main locomotor modes found within the order. The review will then discuss in‐depth key morphofunctional characters of the proximal humerus and the glenoid cavity focusing on hominoids and using other primate groups to highlight differences in morphology related to opposing locomotor behaviors (e.g., suspension vs. quadrupedalism). In particular, as these traits are also commonly used to infer locomotor behaviors in the past, I will address this topic in the following sections dealing with the locomotion and evolutionary history of Miocene hominoids and hominins. The final section will be, as mentioned, a reflection on areas of future studies and possible directions on the analysis of the glenohumeral joint.
2. THE GLENOHUMERAL JOINT: OVERALL MORPHOLOGY AND FUNCTION
The glenohumeral joint describes the articulation between the proximal humerus and glenoid cavity of the scapula (Figure 1). Important features include the rotator cuff muscles, which originate in the scapular blade (or fossae) and attach at the greater and lesser tubercles of the proximal humerus and provide stability and movement to the joint (Figure 1). There are four rotator cuff muscles: the subscapularis muscle, originating in the subscapular fossa of the scapula and inserting in the lesser tubercle; the supraspinatus muscle, originating in the supraspinous fossa of the scapula and attaching in the superior aspect of the greater tubercle; the infraspinatus muscle, originating in the infraspinous fossa and attaching in the lateral aspect of the greater tubercle; and the teres minor muscle, originating in the axial border of the infraspinous fossa and inserting distally to the infraspinatus insertion, also in the lateral aspect of the lesser tubercle (Figure 1; Testut & Latarjet, 1975; Rouviére & Delmas, 2002).
Figure 1.
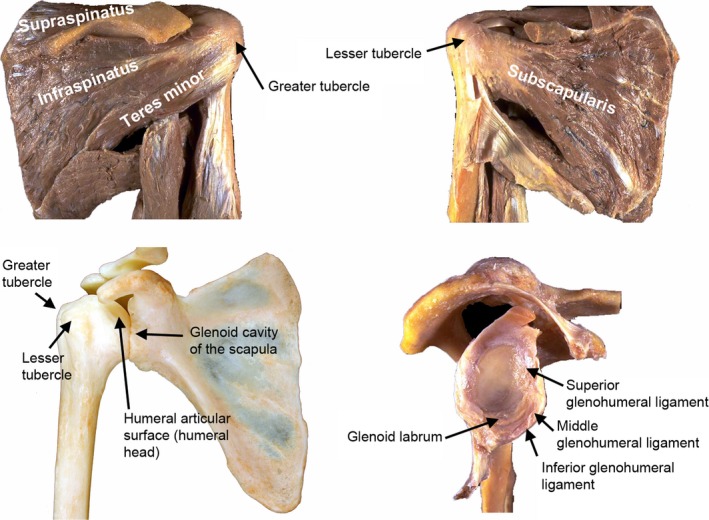
The rotator cuff muscles (supraspinatus, infraspinatus, teres minor, subscapularis) shown on a dissected human shoulder (top of the image). Osteology of the glenohumeral joint (bottom left). Soft tissue surrounding the glenoid cavity of the scapula, including the glenoid labrum and the remains of the capsule and the ligaments (superior, middle and inferior; bottom right). Pictures courtesy of JM Potau and A. Meri
The glenohumeral joint has a flexible capsule that includes the glenohumeral ligaments (superior, medial, and inferior), which also aid to the stability of the joint. Finally, the glenoid labrum (the cartilage surrounding the glenoid cavity of the scapula) is the main cartilaginous structure with a potentially functional aspect, which is to extend the contact surface area of the glenoid, adding stability (Terry & Chopp, 2000; Figure 1). The morphology of the glenohumeral joint of primates is primarily the result of a compromise between mobility and stability. Primates engaging in quadrupedalism as their primary locomotor behavior favor more stable glenohumeral joints, whereas more acrobatic primates—particularly, hominoids—favor less stable glenohumeral joints, which result in increased mobility (Arias‐Martorell et al., 2015a; Larson, 1995; Rose, 1989), even though there have been some opposing views to that statement. Chan (2007) conducted a study on glenohumeral mobility and another on overall shoulder mobility in hominoids and monkeys (Chan, 2008) and concluded that in fact, hominoids had less mobile shoulders than monkeys. However, the study (2008) was conducted on sedated animals, which accounts for passive circumduction and not awake flexibility ranges, which might very well be extremely different and ultimately rendering hominoid shoulders more mobile. This study aligns with the latter standpoint (which is supported by the majority of research conducted on the subject; e.g., Arias‐Martorell et al., 2015a; Inman, Saunders, & Abbot, 1944; Larson, 1993; Veeger & van der Helm, 2007) and also follows the widespread convention that more mobility at the glenohumeral joint implies less stability, and on the contrary, less mobility is brought about by favoring more stability.
As such, terrestrial quadrupedal primates have the most stable glenohumeral joints, thus the least mobile. The proximal humerus and glenoid cavity are more proportionate in size, such that the glenoid cavity is larger and concave. This morphology allows for much of the proximal humerus to articulate with the glenoid during its full‐range motion. The proximal humerus is flattened in its cranial aspect, and its overall shape narrow and elongated relative to the more spherical shape of high‐mobility joints (seen in hominoids, e.g., see below). Furthermore, the tubercles are relatively large and protruding above the articular surface. Together, these features restrict mobility at the joint because of the lateral position of the scapula on the narrow thorax (Larson, 1988, 1993, 1995; Nakatsukasa, 1994; Preuschoft et al., 2010; Rose, 1989; Figure 2).
Figure 2.
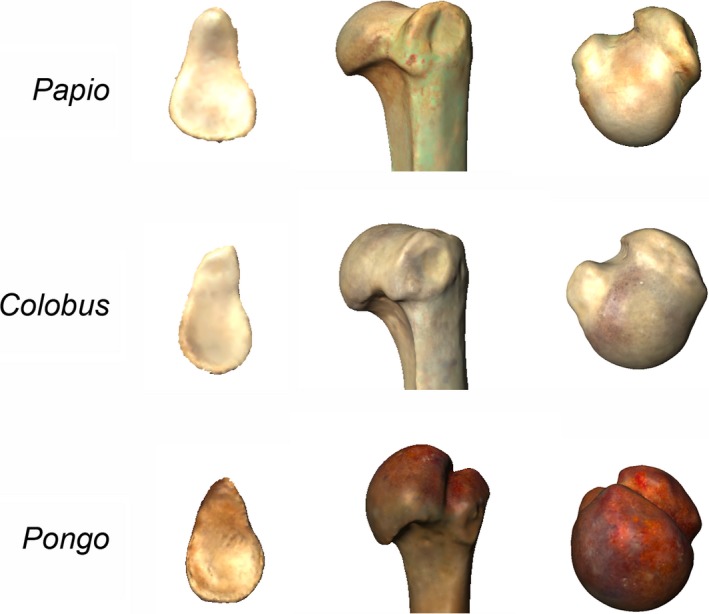
3D renderings of proximal humeri and glenoid cavities of a Papio (baboon), a Colobus (colobus monkey) and a Pongo (orangutan), showing the three main morphologies of the glenohumeral joint related to locomotion: terrestrial quadrupedal, with protruding humeral tubercles and a pear‐shaped glenoid cavity; arboreal quadrupedal, with a rounder humeral head and less protruding tubercles than the terrestrial quadrupeds; and suspensors, with a well‐rounded, globular humeral head and oval glenoid cavity
Arboreal quadrupedal monkeys display a similar morphology to terrestrial quadrupedal monkeys, although their morphology reflects stability as well as increased mobility. In particular, the globularity of the articular surface is greater, especially in its medial part, and the tubercles do not protrude as far from the humeral head (Larson, 1993, 1995; Rose, 1989). This configuration determines two functional regions within the articular surface, one in which the joint is fully flexed (protracted) and one in which the joint is fully extended (Larson, 1993). In extended positions, the region with which the glenoid cavity articulates with the humeral head is nearly spherical in outline, turning it into an almost a ball‐and‐socket joint (in that particular region), enabling relatively free mobility for feeding activities (reaching) and manipulative capabilities (as seen in some capuchin monkeys; Larson, 1993; Rose, 1989).
For the glenoid cavity, both terrestrial and arboreal quadrupedal primates exhibit a pear‐shaped morphology, produced by a ventral projection of the cranial margin of the facet into a more or less elongated lip structure, producing a high craniocaudal degree of curvature. For example, Old World Monkeys (cercopithecoids) tend to have slightly to strongly craniocaudally curved glenoid cavities, which favors flexion/extension movements at the glenohumeral joint over rotatory movements (MacLatchy, Gebo, Kityo, & Pilbeam, 2000; Figure 2). This morphology is typical of most quadrupedal animals and is probably a primitive condition (MacLatchy et al., 2000; Roberts, 1974).
Primates using below‐branch locomotor behaviors (suspension) have the least stable and most mobile glenohumeral joints. They typically show protruding and large, globular humeral articular surfaces, with relatively small tubercles lying well below the superior aspect of the humeral head, which increases the mobility and the motion range of the joint (Larson, 1993, 1995; Rose, 1989; Figure 2), to the extent that only 25%–30% of the humeral head is in contact with the glenoid cavity at any given time (Terry & Chopp, 2000) in this group of primates. A major functional feature of the glenohumeral joint of suspensory primates is the degree to which the tubercles of the proximal humerus are rotated to allocate for additional articular surface in the transverse plane (Corruccini & Ciochon, 1976; Fleagle & Simons, 1982; Larson, 1993; Rose, 1989), which results in an extensive, inflated articular surface that protrudes well above the superior aspect of the greater tubercle and it is directed medially (relative to the transverse axis of the elbow).
The glenoid cavity of suspensory primates exhibits an oval shape, which seems related to rapid limb motion with high acceleration increment when coupled with other elements such as elongated limbs, narrow scapulae and proximal concentration of musculature (Roberts, 1974). These primates also exhibit a moderate craniocaudal curvature, which allows a wide range of rotational shoulder movements (MacLatchy et al., 2000). It is in this group—which includes the suspensory hominoids—a small pool of Old and New World monkeys (e.g., Presbytis—Sumatran surili and Ateles—South American spider monkey) and also humans where the stabilization of the glenohumeral joint becomes a challenge. These primates achieve a substantial degree of abduction–adduction and axial rotation of the glenohumeral joint even when the joint is in a fully flexed position (Larson, 1993; Rose, 1989), which turns this into the most mobile joint of their body (Patton and Thiboudieau, 2010). Hence, these primates have to relay in additional sources for joint stabilization, both passive and active, which are reviewed in the following section.
2.1. Ligaments and musculature: function
The stabilization of the glenohumeral joint is achieved through a combination of passive—ligamentous and cartilaginous—and active structures—muscles. The role of the passive structures is not yet thoroughly understood, but the active structures, the rotator cuff muscles of the scapula that surround the joint with tendinous insertions, have been thoroughly studied (e.g., Ashton & Oxnard, 1963; Patton & Thiboudieau, 2010). Most data derive from electromyographical analyses, which have extensively recorded the activity and recruitment patterns of the rotator cuff muscles in primates (e.g., Inman et al., 1944; Jungers & Stern, 1981; Larson, 1993; Larson & Stern, 1986, 1987, 1989, 1992, 2013; Tuttle & Basmajian, 1978a,b).
During quadrupedal locomotion, either terrestrial or arboreal, Larson and Stern (1989) describe the supraspinatus muscle as being active during arm elevation, silent during swing phase, and active again during support phase. Other studies by the same authors demonstrate that the pattern is recurrent for baboons and macaques and suggest that this pattern could be common to all quadrupedal primates (Larson & Stern, 1989, 1992). The infraspinatus is, together with the supraspinatus, the main contributor to stabilization during the support phase of the quadrupedal gait. This muscle shows exclusive recruiting (along with the supraspinatus) during the swing phase of the gait (Larson & Stern, 2013). The infraspinatus is also involved in preventing humeral displacement during the support phase of quadrupedal walking (and knuckle‐walking, which I mention here as it is a modified form of quadrupedalism; Larson & Stern, 1987). More terrestrial primates seem to have more laterally facing insertions for the infraspinatus (Larson, 1995). In quadrupedal walking, there is a low level but high variability of recruitment of the teres minor muscle, which has prompted Larson and Stern (2013) to suggest that it is involved in finer rotatory adjustments of the glenohumeral joint rather than a key participant of the motion. At last, the subscapularis shows the same pattern of recruitment during quadrupedal walking than that of teres minor, thus seeming to also be involved in fine repositioning of the glenohumeral joint during gait (Larson & Stern, 2013). In African hominoids, it is especially important during the support phase of knuckle‐walking as well, when there is need to internally rotate the humerus to compensate for the shearing stresses caused at the glenohumeral joint due to the dorsal positioning of the scapula.
During suspensory locomotion, the supraspinatus acts as an abductor with the dual role of resisting humeral dislocation while assisting the deltoid in providing abductory power (Inman et al., 1944; Larson, 1993; Larson & Stern, 1986; Preuschoft, 1973; Preuschoft et al., 2010; Tuttle & Basmajian, 1978a,b). However, due to the protruding humeral head in primates using suspensory locomotion, the lever arm of the supraspinatus is reduced, posing a disadvantage that is solved by increasing the overall size of the supraspinatus itself (Fleagle & Simons, 1982; Larson & Stern, 1989, 1992; but see below for a more nuanced discussion on this topic). The infraspinatus acts as an abductor and lateral rotator, contributing to abduction through the middle phase of arm‐rising, as a primary synergist to the deltoid (Larson, 1993; Larson & Stern, 1986). The involvement in abduction seems to be related to the proximolateral oriented insertion of the muscle in the greater tubercle in hominoids. During locomotion, the infraspinatus is involved in bimanual and unimanual hanging and during the support phase of arm‐swinging, playing a specific role as a transarticular stress resistor by stabilizing the joint (Larson, 1993, 2013; Larson & Stern, 1986). During the support phase of arm‐swinging/suspensory behaviors, the teres minor is recruited as an adductor (tensile‐stress resistor) along with the infraspinatus—and there is barely any activity from the other rotator cuff muscles at this point (Larson & Stern, 2013). The teres minor also acts along with the teres major as a propulsor, and along with the caudal deltoid as an abductor during hoisting, also with a component of lateral rotation (Larson, 1993; Larson & Stern, 1986). The teres minor is differently recruited within suspensory hominoids, with great hominoids showing a small teres minor activity burst at the end of the swing phase, to tuck the elbow in, and lesser hominoids showing an early recruitment of the muscle during arm elevation, at hand release (Larson & Stern, 2013). Finally, the subscapularis primary role as medial rotator of the arm in primates—motion that can be combined with abduction or adduction—makes it extremely important during the support or “pull‐up” phase of climbing on a vertical trunk in hominoids (Larson & Stern, 1986, 1987). This medial rotatory function is also engaged in the support phase of true brachiation (Bello‐Hellegouarch et al., 2012; Larson, 1988). The subscapularis is divided in three portions, upper, middle and lower. During arm‐swinging, the lower and middle subscapularis are active during the first half of the medial rotational swing after hand release in arm‐swinging, while the upper part is silent. After that, re‐elevation of the humerus (abduction or elevation portion of the swing phase) is conducted by other rotator cuff components (Larson & Stern, 2013).
3. THE PROXIMAL HUMERUS: MORPHOLOGY AND FUNCTION IN DEPTH
Previous research has shown via 3D geometric morphometrics that there are two key aspects of the proximal humerus morphology that strongly reflect functional differences across different primate locomotor behaviors: the size and shape of articular surface and distribution of the insertions of the rotator cuff muscles on the humeral tubercles. Both of these aspects of morphology appear to more strongly reflect function rather than phylogeny (Arias‐Martorell et al., 2015a). This section will focus more deeply on these characters in hominoids, mentioning outgroups of New World and Old World monkeys either because of similarities with hominoids, or to highlight their differences.
3.1. Articular surface
The major morphofunctional characteristics of the articular surface are the degree of globularity and the shape of the perimeter, which are largely indicative of the range of motion of the joint (Harrison, 1989; Larson, 1993; Rafferty & Ruff, 1994; Rose, 1989; Ruff & Runestad, 1992; Figure 3).
Figure 3.
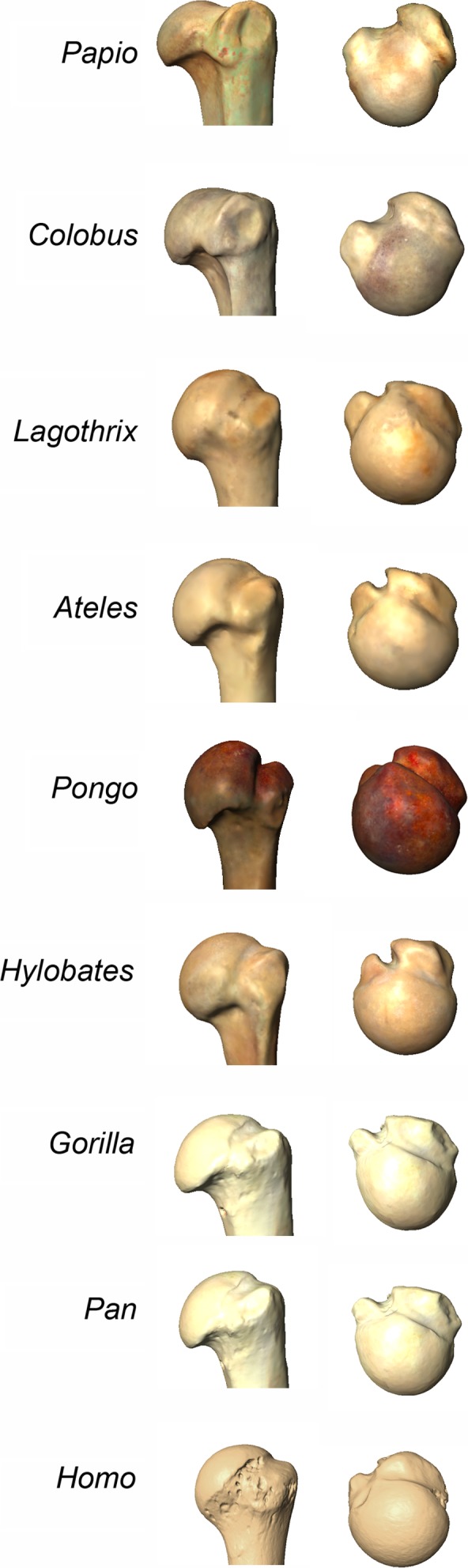
Comparative proximal humeral morphologies of all the groups mentioned, from the terrestrial quadrupedal Papio (baboon), the brachiator Hylobates (gibbon), to the terrestrial knuckle‐walkers Pan and Gorilla (chimpanzee and gorilla)
Primates that engage in quadrupedal locomotion (e.g., Colobus—colobus monkey, Papio—baboon) tend to show overall similar morphologies, with broad, oval‐shaped, and flat humeral heads that extended between the tubercles. However, differences can be observed regarding whether they are arboreal or terrestrial quadrupeds. Arboreal quadrupeds (e.g., Colobus and Cebus—capuchins) tend to exhibit slightly more rounded and inflated articular surfaces, whereas terrestrial quadrupeds (e.g., Papio) show flatter, most oval, and clearly extended between the tubercles articular surfaces. In general, quadrupedal monkeys (whether arboreal or terrestrial) offer a large articular surface of contact with the glenoid on the superior aspect of the humerus, which allows the joint to effectively transmit forces during the weight‐bearing phase of the gait (Larson, 1993; Preuschoft et al., 2010; Rafferty & Ruff, 1994; Rose, 1989; Figure 3).
Lagothrix (woolly monkeys), even though being primarily quadrupedal, exhibit an interesting intermediate morphology between fully suspensory primates and quadrupedal primates (Arias‐Martorell et al., 2015a). This group uses suspensory locomotion during only 11% of the time while traveling (Cant, Youlatos, & Rose, 2001, 2003; Kagaya, 2007), but their articular surface is still fairly globular and rounded, clearly differing from the protruding and extremely globular articular morphology of suspensory primates (see below), and, at the same time, clearly departed from the flattened and smaller articular surfaces of quadrupedal monkeys. The outline of the surface is oval‐shaped corresponding to its broader, more pronograde‐like locomotor repertoire (Cant et al., 2001, 2003; Kagaya, 2007; Figure 3).
In primates that engage in suspensory locomotion, there is a direct relationship between proximal humeral shape and amount of suspension they engage in (Arias‐Martorell et al., 2015a). Especially hylobatids (gibbons and siamangs), as they rely on brachiation as main locomotion mode (up to an 80% of the time; Fleagle, 1976; Hunt, 1991a; Michilsens, Vereecke, D'Août, & Aerts, 2009, 2010) and are the only group of primates that engage in its extreme form, ricochetal brachiation. Morphologically, then, hylobatids exhibit the most globular articular surfaces with circular perimeters, with proximal humeri well‐suited to perform a wide range of movements in the anteroposterior plane, mainly achieved by a lateral progression of the lesser tubercle (Figure 3). That allows the articular surface to expand in that direction, indicating a positive selection toward high‐mobility rates and wide‐range circumduction capabilities at the shoulder joint in this group (Arias‐Martorell et al., 2015a; Rafferty & Ruff, 1994; Ruff & Runestad, 1992).
Pongo (orangutans), who engage in varied forms of suspension (uni‐ or bimanual arm hanging, arm‐swinging and brachiation) show the most globularity on the proximal aspect of the articular surface (rather than its medial aspect), which is also expanded medially (toward the bicipital groove) instead of laterally as seen in hylobatids (Arias‐Martorell et al., 2015a; Figure 3). It is interesting that Pongo and the New World monkey Ateles (spider monkey) share the same proximal humeral morphology. This display of homoplasy between the two could be brought about by the varied use of locomotion of these two primates (from all forms of below‐branch locomotion to quadrupedalism, especially in Ateles), as well as a match in the amount of suspension they engage in without fully relying in this form of locomotion. One of the few proximal humeral shape‐focused studies available (Kagaya, 2007) found Ateles to be more similar to hylobatids, however, this study did not include Pongo in their comparative sample. Lack of proper comparative sample is an issue that will be discussed in subsequent sections.
Gorilla (gorillas) and Pan (chimpanzees) present a glenohumeral joint seemingly preserving all the traits necessary to engage in arboreal and suspensory locomotion (Arias‐Martorell et al., 2015a; Hunt, 1991b; Larson & Stern, 1987), despite their main locomotor behavior being a modified form of quadrupedalism known as knuckle‐walking (where they use of the back of the middle phalanges to make contact with the ground). Their humeral articular surface is still relatively big, globular and rounded, showing adaptations to a great range of motion. There appears to be a flattening of the central aspect of the joint (Figure 3), which instead of being a result of engaging in knuckle‐walking and of the compressive forces that could be acting at the joint during such activity, seems to be more advantageous for the functional demands of dealing with transarticular stresses during suspension for an effective diffusion of loads (Preuschoft, 1973; Preuschoft et al., 2010). This hypothesis, however, is at this time only valid for Pan troglodytes (common chimpanzee), species which we know only carry a 20% of their total body mass in their arms during knuckle‐walking (Kimura, 1985; Preuschoft, 1973, 2004). Data on forelimb weight‐bearing for Gorilla is needed to confirm or contest such hypothesis in this group.
Even though the proximal humeral morphology of Pan and Gorilla does not look exactly the same, more detailed studies focusing on their differences as well as finer studies on intraspecific variation of Pan and Gorilla species (for example, between Pan troglodytes and Pan paniscus (bonobos), and between Gorilla gorilla (lowland gorilla) and Gorilla beringei (mountain gorilla) and its relationship to differential locomotor behaviors, as studies conducted on other regions such as the hands or feet suggest exist; Dunn, Tocheri, Orr, & Jungers, 2014; Knigge, Tocheri, Orr, & McNulty, 2015; Tocheri et al., 2011) would have to be carried out to distill the differences between their glenohumeral morphology).
At last, modern humans, while still exhibiting rounded and protruding articular surfaces, show a morphological departure from the suspensory hominoids in that they display mediolaterally longer articular surfaces with an expansion in its medial aspect (Figure 3). The medial increase of articular surface could be related to functional demands of external rotation, which is important in retarding the contact between the greater tubercle and the acromion during the elevation of the arm in humans (Basmajian & De Luca, 1985; Inman et al., 1944). The mediolaterally longer heads seem to be related to the neutral (pendant) position of the lowered arm in humans, as the glenoid cavity mainly articulates with this region when the arm is downwards (Arias‐Martorell, Potau, Bello‐Hellegouarch, Pastor, & Pérez‐Pérez, 2012; Arias‐Martorell et al., 2015c). Small and precise movements to reposition the elbow and hands during manipulative activities could also occur in this area, favoring an enlarged surface for the glenoid to glide on.
3.2. Rotator cuff insertions
The muscles of the glenohumeral joint insert in the rotator cuff in two locations: the greater tubercle (supraspinatus, infraspinatus and teres minor) and the lesser tubercle (subscapularis). The positioning of the insertion sites on the greater tubercle in different hominoids seemingly responds to the major stresses that apply to the joint, that is, compressive, shearing or tensile (Arias‐Martorell et al., 2015a; Figure 4).
Figure 4.
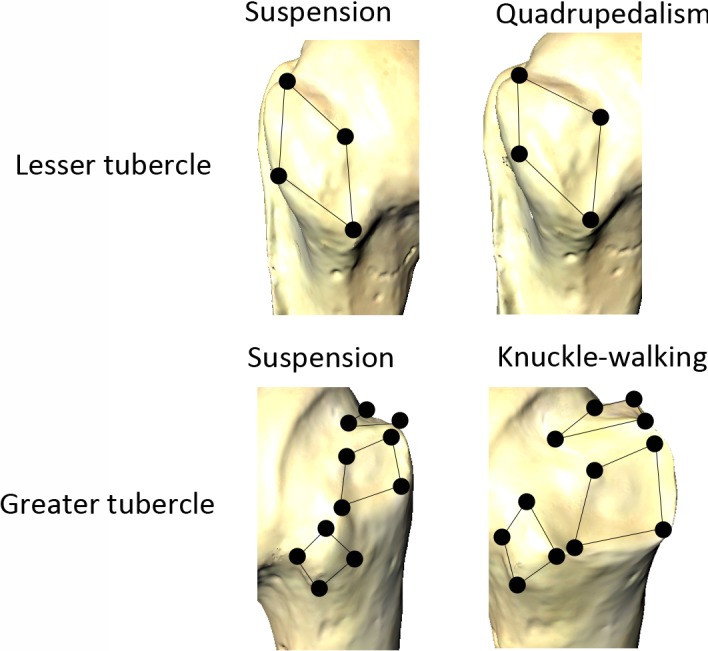
Distinct morphologies of the rotator cuff muscles attachments to the lesser and greater tubercles with respect to the locomotion used by the primates. Note the specific distribution of the greater tubercle insertions of the knuckle‐walking primates (Gorilla and Pan; Modified after Arias‐Martorell et al., 2015a)
As is the case with the articular surface, groups that rely or engage in relatively high amounts of suspensory locomotion exhibit certain similar characteristics. Hylobatids, Pongo, and Ateles exhibit the same pattern for the insertions on the greater tubercle: a line‐up in a proximodistal direction, mainly achieved by a lateral displacement of the teres minor insertion (Arias‐Martorell et al., 2015a; Figure 4). This pattern might be advantageous to secure the joint against tensile stresses: the teres minor muscle is activated in Pongo in overhead humeral adduction (Larson & Stern, 1986; Tuttle & Basmajian, 1978a,b), and the significant amount of lateral displacement of the teres minor insertion of highly suspension‐dependent taxa with respect to the articular may indicate a higher activity pattern for this muscle (Arias‐Martorell et al., 2015a).
Another feature of the rotator cuff insertions relating to the use of suspension in primates is the orientation of the infraspinatus insertion site. In hominoids in general, a higher degree of cranial/superior orientation of the muscle's facet seems to be related to the function of the infraspinatus muscle as the main stabilizer of the glenohumeral joint against forces pushing the humerus head away or along the glenoid cavity, mainly during pendant suspension and the support phase of arm‐swinging/brachiation (Larson, 1995; Larson & Stern, 1986; Roberts, 1974). Hylobatids display the least cranially orientated facet (Arias‐Martorell et al., 2015a; Larson, 1995), which is consistent with lesser apes being less dependent on the infraspinatus as abductor and lateral rotator during arm‐rising due to their low degree of humeral torsion (Larson, 1988, 1995) when compared to other hominoids and Ateles, which shows an orientation of the insertion similar to Pongo.
It is interesting to note that humans, who do not use any significant amount of suspension in their daily lives in general, also exhibit the above‐described pattern of the greater tubercle insertion. However, the glenohumeral joint of humans is indeed subjected to tensile stresses on the pendant limb. Differing from the suspensory primates, the greater tubercle of humans is overall smaller, with reduced insertion sites for the rotator cuff muscles, which possibly indicates a decrease of reliance on the active stabilizers of the glenohumeral joint due to a reduction in size of the rotator cuff, as there is no need to power a locomotor mode that would have this joint carry the total body weight of an adult (Arias‐Martorell et al., 2012). At the same time, this would have allowed for fast and precise manipulation movements. In particular, the supraspinatus muscle is fairly reduced in size in humans (Bello‐Hellegouarch, Potau, Arias‐Martorell, Pastor, & Pérez‐Pérez, 2013; Larson, 1995, 2007a; Potau et al., 2009; Roberts, 1974), which brings about a separation between the rotator cuff insertion areas and the humeral head. This happens for both the supraspinatus and the teres minor insertions and it might contribute to the increase of the leverage of both muscles to compensate for their relatively smaller size (Basmajian & De Luca, 1985; Inman et al., 1944; Potau, Bardina, & Ciurana, 2007; Potau et al., 2009; Roberts, 1974).
The main difference in the morphology of the greater tubercle insertions is seen in the knuckle‐walking Gorilla and Pan: contrary to all other hominoids, they exhibit a triangular disposition of the insertions, achieved by a lateral displacement of the infraspinatus insertion, and a closeness of the proximal and distal ends of the teres minor and supraspinatus, respectively (Figure 4). This may be advantageous to secure such mobile joint against the shearing stresses occurring during knuckle‐walking. Gorilla and Pan also show the greatest degree of cranial orientation of the infraspinatus facet, corresponding to their greater degree of humeral torsion (Larson, 1995) and to their higher dependence on the infraspinatus muscle to act as synergist to the deltoid in arm‐rising behaviors. The infraspinatus of these two taxa also shows an increase in size in respect to the other insertions, being the main cause of the distinctive triangular disposition of the rotator cuff insertions of the greater tubercle in the knuckle‐walkers. The infraspinatus has an important role in aiding the supraspinatus to assist the deltoid in arm‐rising behaviors, as well as exerting antigravitational forces during the stance phase of knuckle‐walking (Larson, 1993, 1995; Larson & Stern, 1986, 1987). Hence, these two muscles bear the responsibility of maintaining stability in a highly mobile joint (Larson & Stern, 1987).
Pan seem to generally exhibit enlarged rotator cuff muscle masses respect to the other hominoids (Kikuchi, Takemoto, & Kuraoka, 2012; Mathewson, Kwan, Eng, Lieber, & Ward, 2014; Oishi, Ogihara, Endo, Ichihara, & Asari, 2009; Potau et al., 2009), which could have resulted in a need of increase of insertion site space, contributing to their distinctive triangular disposition in the greater tubercle; however, this hypothesis has not been tested as such to date. Debate sparks whenever rotator cuff muscle mass enters the fray, especially regarding the question of whether hominoids have enlarged supraspinatus or infraspinatus muscles, with much of the data being derived from reported increased sizes of their attachment sites (ratios) at the scapula respect to each other (Bello‐Hellegouarch et al., 2013; Green, 2013; Roberts, 1974; Taylor, 1997; Young, 2008;). However, a recent study by Larson (2015a) suggests that muscle masses do not necessarily correspond to increased scapular fossae area, with variation between dorsal rotator cuff muscles mass not influencing the functional roles of such muscles, all of it showing a degree of dissociation between soft tissue properties and hard tissue morphology. In short, the functional roles in locomotion played by the individual rotator cuff muscles do not substantially differ among hominoids even if their masses do. The differences, then, would be brought about by the variation of each species in their specific locomotor repertoire (Larson, 2015a).
At the lesser tubercle, hominoids (including Pan and Gorilla) and Ateles exhibit the same narrow and spindle‐shaped morphology for the subscapularis insertion, in contrast to the rounded insertion exhibited by largely pronograde taxa (e.g., Colobus, Cebus, Papio and Lagothrix; Figure 4). As discussed above, the subscapularis muscle does not act as a unit in hominoids, but as three separated portions (lower, middle and upper; Larson & Stern, 1986; Larson, 1988, 1995). The shape of the insertion of the subscapularis in hominoids and Ateles reflects this differentiation, with the muscular fibers that originate from the most proximal part of the tendon (and therefore from the proximal part of the lesser tubercle) being involved in abduction and medial rotation, whereas those fibers originating from the distal parts being involved in adduction and medial rotation and not contributing to arm‐rising (Larson & Stern, 1986). The most caudal/distal fibers contribute to pulling the humeral head downward, toward the axilla (as a synergist to the infraspinatus). Such differentiation shows the versatility of this muscle in hominoids and Ateles, where it is extremely important in the stabilization of the glenohumeral joint during the support phase of climbing (Larson & Stern, 1986), as well as during the quadrupedal and knuckle‐walking stance phases (Tuttle & Basmajian, 1978b).
4. THE GLENOID CAVITY: MORPHOLOGY AND FUNCTION IN DEPTH
A recent study of the shape and function of the glenoid cavity determined that no morphological features are correlated to sex, activity level or side (Macias & Churchill, 2015). Among great hominoids, certain features of the glenoid cavity of Pan (laterally projecting articular glenoid rim and a central orientation of the deepest aspect of the fossa) could be related to vertical climbing (Macias & Churchill, 2015). These features might contribute to the stabilization of the glenohumeral joint at hind limb push‐off phase in climbing, where Pan protract their shoulders.
However, caution is needed when drawing conclusions from scapular glenoid shape until more studies are conducted, as the shape of the glenoid cavity seems to not be driven by locomotor constraints as much as the proximal humerus, according to a recent study by Arias‐Martorell et al. (2015b). For instance, in all the analyses orangutans exhibited morphological similarities of the glenoid cavity with Lagothrix, when these groups do not share the same locomotor repertoire. The shape of the glenoid cavity of orangutans was narrower and more curved than those of the other hominoids and exhibited a lip‐like reminiscent elongation of the cranial aspect, lightly resembling those of quadrupedal monkeys. In fact, the 3D GM analysis of the shape of the glenoid cavity, including all the hominoids and a suite of Old and New World monkeys, shows a complete overlap between Pongo and Lagothrix (Supporting Information Figure S1). However, the distinctive morphology of the glenoid cavity of Pongo could be related to a greater passive stabilization of the joint in abducted postures of the arm, permitting ball‐and‐socket joint contact in the medial and superior aspect of the proximal humerus (Kapandji, 2007). Such equivocal overlap between Lagothrix and Pongo, with the consequent relatively monkey‐like morphological affinities of the latter suggests that caution should be exercised when locomotor inferences are attempted based on the glenoid cavity alone, especially in cases where extinct taxa are involved (see below).
5. EVOLUTION OF HOMINOID GLENOHUMERAL MORPHOLOGY: THE MIOCENE APES
The amount of proximal humeri remains and scapular fragments with intact glenoid cavities from Miocene apes is scarce. This review considers the shoulder girdle and locomotor behavior of Miocene apes in general and, when possible, specifically their glenohumeral joint (either component).
The better‐known taxa from this period (both in general terms and in shoulder girdle remains numbers) are the Early Miocene (ca. 23–16 million years ago, Mya) apes, in particular Proconsul—including the recently erected Ekembo genus (E. nyanzae and E. heseloni), formerly considered proconsulids (McNulty, Begun, Kelley, Manthi, & Mbua, 2015)—and similar forms, such as Nyanzapithecus and Afropithecus. Early Miocene apes were powerful‐grasping, above‐branch quadrupeds/cautious climbers that retained a pronograde body plan and were morphologically generalized, showing no living ape‐like adaptations for below‐branch suspensory behaviors (Begun, Teaford, & Walker, 1994; Corruccini, Ciochon, & McHenry, 1975; Dunsworth, 2006; Fleagle, 1983; Morbeck, 1975; Rose, 1983, 1993; Walker & Pickford, 1983; Ward, 1997, 2015). When compared to extant taxa, Early Miocene apes show a combination of traits of arboreal quadrupedal Old World Monkeys and large New World Monkeys (spider, howler, and woolly monkey), and apes (Arias‐Martorell et al., 2015b; Rein, Harrison, & Zollikofer, 2011; Rose, 1983). They differ from earlier forms, such as the propliopithecoid Aegyptopithecus, in having the shoulder adapted to a wider range of loading, with a somewhat laterally projected acromion, an oblique spine and a cranially directed glenoid fossa, implying that (nonsuspensory) overhead positions of the forelimb could be easily achieved (Rose, 1983; Ward, 2015). The proximal half of their humerus is characterized by a shallow bicipital groove and a flat deltoid plane with well‐developed deltopectoral and deltotriceps crests, as observed in extant arboreal Old World monkeys (Napier & Davis, 1959; Rose, 1983). It is in the shape of the distal humerus that early Miocene apes most resemble hominoids, as well as in some aspects of the hands (Rose, 1983, 1988, 1992; Begun et al., 1994), which makes the combined features of the forelimb of these early apes suitable for an extended range of movement compared to earlier taxa (Rose, 1983).
For this period, there exists at least one evidence of a possible orthograde taxon, the early Miocene ape Morotopithecus bishopi (ca. 20 Mya, Uganda), which, according to the literature, exhibits orthograde features combining them with below‐branch locomotion (MacLatchy, 2004; MacLatchy et al., 2000; Nakatsukasa, 2008; Sanders & Bodenbender, 1994). While it is true that M. bishopi bears a great resemblance to modern hominoids in several aspects of its postcranium (MacLatchy, 2004; Nakatsukasa, 2008; Ward, 2015) and could possibly be an orthograde, the assumption that it might have been a suspensory ape mainly derives from the finding of a fragment of scapula preserving the glenoid cavity and scapular neck (MacLatchy et al., 2000). The glenoid fossa is oval and overall shallow, it lacks a notch in the craniodorsal surface of the glenoid margin (perimeter) and a lip, and the presence of glenoid labrum and proximal origin of the scapular spine is inferred (MacLatchy, 2004), thus resembling extant apes and the spider monkey. However, as argued above, functional and locomotor inferences derived from glenoid cavity morphology alone must be made with great care (or not made at all), especially if such claims cannot be supported by other elements of the forelimb that, as seen, might be better suited for it. Therefore, the claim that M. bishopi was a suspensory ape cannot be sustained on the basis of the morphology of its glenoid cavity alone.
There is, unfortunately, a gap in the African Miocene ape postcranial record from the Middle Miocene (ca. 16–11.6 Mya) until the advent of extant great apes (gorillas and chimpanzees) ca. 8–6 Mya. There is, however, at least one ape that shows adaptations toward enhanced forelimb‐dominated behaviors (nonsuspensory but heightened respect to earlier forms; Ishida, Kunimatsu, Takano, Nakano, & Nakatsukasa, 2004; Ishida et al., 1999; Nakatsukasa & Kunimatsu, 2009; Nakatsukasa, Yamanaka, Kunimatsu, Shimizu, & Ishida, 1998; Senut et al., 2004) and for which we have partial shoulder girdle remains. Nacholapithecus (ca. 15 Mya, Kenya) is well‐known from a multitude of remains and, especially, from the adult partial skeleton KNM‐BG 35250 (Ishida et al., 2004). This individual shows an unusual combination of features unlike any other (extant or extinct) ape (Ishida et al., 2004; Nakatsukasa & Kunimatsu, 2009; Ward, 2015) in being an overall pronograde but with adaptations resembling extant apes, probably related to the de‐emphasis of lumbar flexion–extension (dorsal stability; Nakatsukasa & Kunimatsu, 2009). The forelimb of Nacholapithecus shows mosaic characters (mixture of primitive and derived characters) but, unfortunately, there are no complete proximal humeri known for this taxon, and only scapular and clavicular features are known for the shoulder girdle (Ishida et al., 2004; Senut et al., 2004). The glenoid fossa is pear‐shaped and large and the acromion is projected beyond the glenoid, as seen in arboreal primates, and the clavicle is long and slender. These features, combined with a narrow trunk, suggest that the scapulae were laterally positioned, with the long clavicles either positioned in a cranial angle (as seen in orangutans) or with a proportionally large upper thorax with large muscles (Senut et al., 2004). The long clavicle with ligament insertions also suggests that protraction of the humerus in overhead postures would have been emphasized over abduction (Senut, 2003; Senut et al., 2004), with clambering and nonstereotypical arboreal behaviors (bridging, reaching, hoisting, transferring) being put forward as locomotor modes for this ape.
During the Middle Miocene the first apes appear in Eurasia, their dispersal bringing about a diversification in the range of locomotor repertoires not seen to date. There are evidences of, for example, the persistence of generalized locomotor behaviors, as seen in the Middle‐Late Miocene ape Sivapithecus (ca. 12–7 Mya; Madar, Rose, Kelley, MacLatchy, & Pilbeam, 2002; Pilbeam, Rose, Barry, & Shah, 1990); the appearance of the first undisputed orthograde (Pierolapithecus catalaunicus, ca. 15 Mya, Spain; Moyà‐Solà, Köhler, Alba, Casanovas‐Vilar, & Galindo, 2004, 2005); and the advent of the first unequivocal evidence of orthogrady paired with suspensory behaviors in the Late Miocene ape Hispanopithecus laietanus (ca. 9.5 Mya, Spain; Almécija, Alba, Moyà‐Solà, & Köhler, 2007; Alba, Almécija, Casanovas‐Vilar, Méndez, & Moyà‐Solà, 2012; Moyà‐Solà & Köhler, 1996; Pina, Alba, Almécija, Fortuny, & Moyà‐Solà, 2012). It is unfortunate that there are no glenohumeral remains preserved for any of these apes (but if there were, their glenohumeral remains should be expected to support the locomotor repertoires inferred for them from other postcranial remains).
The study of the glenohumeral remains from nonhominoid extinct Eurasian primates is of help in answering central questions regarding the morphological pathways to the acquisition of suspensory behaviors and orthogrady (Arias‐Martorell et al., 2015b). Pliopithecoids, a group of extinct primates that inhabited Eurasia from ca. 18 to 7 Mya, were initially considered to be related to hylobatids due to superficial resemblances such as relatively small body mass or slender forelimb long bones (e.g., Hürzeler, 1954; Zapfe, 1958). However, pliopithecoids retain primitive features indicating a much earlier divergence (Begun, 2002; Harrison, 2005, 2010). Epipliopithecus vindobonensis (Early Middle Miocene, Slovakia) is one of the best‐known pliopithecoids, with abundant postcranial remains from various individuals (Zapfe, 1958). The locomotor repertoire of E. vindobonensis has been diversely interpreted, from an arboreal generalist and terrestrial quadruped to an agile above‐branch walker and runner displaying significant climbing, as well as hind limb and forelimb suspension (Fleagle, 1983; Harrison, 2013; Rein et al., 2011; Rose, 1983, 1989, 1994; Zapfe, 1958). Recent studies (Rein et al., 2011) reemphasized the importance of quadrupedalism in this taxon by quantitatively reexamining various characters of its forelimb (length of the olecranon process relative to the size of the ulna). However, the relative high degree of humeral torsion of E. vindobonensis would predict a low frequency of quadrupedalism—a combination most similar to that displayed by New World suspensory monkeys—whereas phalangeal curvature would support a significant amount of climbing behaviors as well (Rein et al., 2011). Shape analyses undertaken on the proximal humerus of E. vindobonensis indicate that it has its closest morphological affinities with the woolly monkey (Arias‐Martorell et al., 2015b). E. vindobonensis exhibits, like the woolly monkey, a fairly globular articular surface, rounder on the superior aspect of the articular surface compared to the other arboreal quadrupeds (capuchins and colobus monkeys), but clearly differing from the protruding and extremely globular articular morphology of extant apes and the spider monkey (Figure 5). The tubercles of E. vindobonensis also closely resemble generalized arboreal quadrupeds (Arias‐Martorell et al., 2015b), particularly a round subscapularis insertion, which stresses the role of this muscle as a powerful internal rotator and stabilizer of the joint during the quadrupedal gait (Larson, 1988, 2007a), as well as relatively large tubercles with respect to the articular surface, as seen in more quadrupedal taxa.
Figure 5.
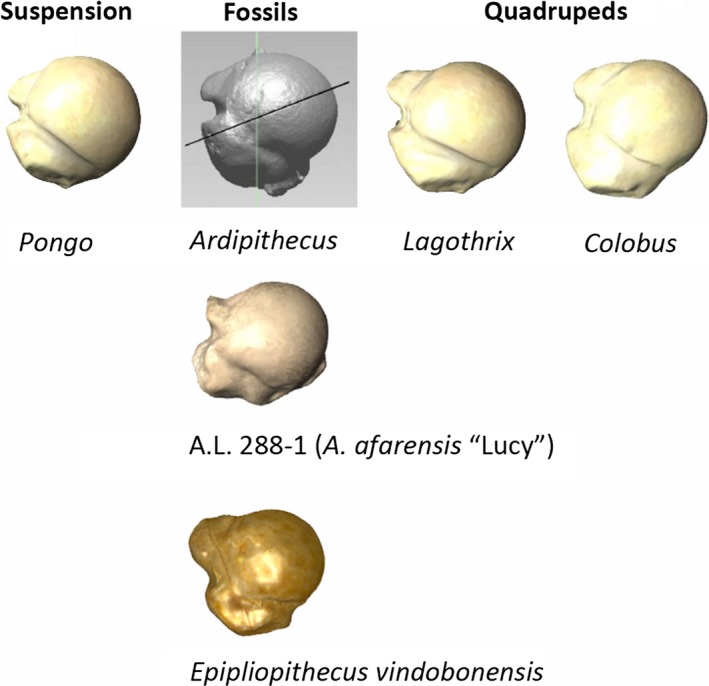
Comparison between fossil and extant primates proximal humeral morphologies, including the Middle Miocene pliopithecus Epipliopithecus vindobonensis, and the hominins A.L. 288‐1r (A. afarensis) and ARA‐VP‐7/2‐A (Ardipithecus ramidus). Note the striking similarity between Ardipithecus and Pongo (Image of Ardipithecus modified after Lovejoy et al., 2009a)
Although its main positional behavior would have consisted of generalized arboreal quadrupedalism, E. vindobonensis shows some forelimb suspensory adaptations, quite like the generalized (but still suspension‐capable) woolly monkey. From a wide‐ranging evolutionary perspective, the presence of these two features highlights the decoupling between the acquisition of suspensory adaptations (at least, at the glenohumeral joint) and that of an overall orthograde body plan, as the latter is lacking in both taxa. Both E. vindobonensis and the woolly monkey display proximal humeral morphologies enabling greater circumduction ranges in overhead limb positions than those of generalized arboreal quadrupeds, and as such, a fair amount of forelimb suspensory behaviors, without a concomitant shift toward an overall orthograde morphotype in torso and lumbar spine morphology (Fleagle, 1983; Harrison, 2013; Rein et al., 2011; Rose, 1983; Rose 1988; Rose, 1994; Rose et al., 1992). This suggests that the evolution of some suspensory adaptations, superimposed to an otherwise pronograde body plan suitable for generalized arboreal quadrupedalism (as in E. vindobonensis), might have been more common among extinct catarrhines than is generally assumed.
The presence of suspension has been traditionally linked to a series of derived morphological traits known as orthogrady, including a broad and shallow thorax, spinal invagination, long clavicles, dorsally placed scapulae with laterally oriented glenoid fossae, highly mobile shoulder joints, ulnar deviation of the hand, lack of ulnocarpal joint, a short lumbar column with dorsally placed transverse processes, visceral fixation, and loss of an external tail (e.g., Andrews & Groves, 1976; Gebo, 1996, 2010; Keith, 1903, 1923; Ward, 2015; Williams, 2012). However, with the expanding record of Miocene and Plio‐Pleistocene hominoid and pliopithecoid fossils, questions have been raised as to (a) the overall homology of those traits, exclusive of hominoids, thus constituting their morphological ancestral condition (Crompton, Vereecke, & Thorpe, 2008; Gebo et al., 1997; MacLatchy, 2004; MacLatchy et al., 2000; Williams, 2012), and (b) the assumption of presence of suspensory behaviors when orthograde features are recognized (mostly in the fossil record) and vice versa (Almécija, Alba, & Moyà‐Solà, 2009; Moyà‐Solà et al., 2004, 2005).
Regarding homology, the early hominoid Morotopithecus reviewed above poses two interesting scenarios for the evolution of orthogrady, depending on its phylogenetic position. If Morotopithecus is more closely related to crown hominoids (all the apes that would share a common ancestor), to the exclusion of the other Early to Middle Miocene African apes (Afropithecus, Proconsul, Nacholapithecus), the “orthograde” body plan would have been acquired as far as 20 million years ago, and would certainly be a crown hominoid synapomorphy (a derived characteristic shared by all subsequent members of the group; Gebo et al., 1997; MacLatchy et al., 2000; MacLatchy, 2004). However, if this is the case, the primitive condition of a pronograde body plan exhibited by the later ape Sivapithecus, which is regarded as a direct ancestor of orangutans based on cranial evidence, would have to be explained as an independent acquisition, having “re‐evolved” orthogrady later (as orangutans are orthogrades). The alternative explanation would be that Morotopithecus is not more closely related to crown hominoids than to any other particular taxon (i.e., it is a sister taxon to crown hominoids, not its common ancestor), and it would become an example of independent acquisition of orthogrady in general, and an evidence of homoplasy (in relation to orthogrady) within the ape lineage (Harrison, 2002, 2005). To this regard, morphological differences between the lesser and great apes have been interpreted as suggesting that suspension evolved in parallel in gibbons and siamangs and great apes (Larson, 1998). This seems to be further supported by the more primitive morphology (nonsuspensory) displayed by early members of the crown group (e.g., Pierolapithecus) or Sivapithecus (Alba, 2012; Madar et al., 2002; Moyà‐Solà et al., 2004; Pilbeam et al., 1990). This second scenario is even further supported by the glenohumeral evidence of the spider monkey and the apes, which show convergent suspensory adaptations (Arias‐Martorell et al., 2015a; Larson, 1998).
Regarding the correlation between suspensory behavior and orthogrady, the fact that all extant apes practice to some extent both vertical climbing and below‐branch suspension (Hunt, 1991a, 2004) has led to conflicting hypotheses on the adaptive role of suspension in the origin of orthograde features, with some authors stressing it (Gebo, 1996) and others favoring vertical and/or cautious climbing instead (Cartmill & Milton, 1977; Sarmiento, 1995). As discussed above, suspension has been further inferred for some extinct catarrhines retaining a pronograde body plan, most notably E. vindobonensis. On the opposite side of the spectrum is the case of Pierolapithecus, which displays a torso morphology that reflects an orthograde body plan, but lacks some key suspensory adaptations in the forelimbs, further suggesting a decoupling of the two features (Almécija et al., 2009; Moyà‐Solà et al., 2004, 2005).
To date, then, the earliest evidence for suspensory adaptations coupled with an orthograde body plan in the hominoid fossil record corresponds to Hispanopithecus laietanus (Alba, 2012; Alba, Almécija, & Moyà‐Solà, 2010; Alba et al., 2012; Almécija et al., 2007; Moyà‐Solà & Köhler, 1996; Susanna, Alba, Almécija, & Moyà‐Solà, 2014). As seen, Morotopithecus could challenge the latter statement, seeing that this ape seemingly exhibits orthograde features in the lumbar vertebrae coupled with suspensory adaptations (MacLatchy et al., 2000; Nakatsukasa, 2008; Sanders & Bodenbender, 1994). However, its postcranium would more likely suggest an instance of independent evolution of orthogrady (Alba, 2012; Harrison, 2010; Ward, 2015), based on the fact that (a) the evidence for suspension is drawn from the glenoid cavity alone and the issues that entails (it is worth keeping in mind that the morphology hailed as suspension‐diagnostic for primates is also found in cursorial mammals such as the horse; Roberts, 1974), and that (b) this ape exhibits an afropithecus‐like facial morphology (lacking hominid facial synapomorphies; Harrison, 2010).
Thus, based on current undisputed evidence available, orthogrady and suspension would have independently arisen several times throughout ape evolution. Among large‐bodied apes, the acquisition of an orthograde body plan seems to have taken place first (probably originally related to vertical climbing), with a later acquisition of suspension (which would have even appeared independently in some great ape lineages; Alba, 2012; Almécija et al., 2007, 2009; Cartmill, 1985; Crompton et al., 2008; Fleagle, 1976; Nakatsukasa, Kunimatsu, Nakano, Takano, & Ishida, 2003; Moyà‐Solà et al., 2004, 2005; Sarmiento, 1998). In contrast, small‐bodied primates (including extinct catarrhines such as E. vindobonensis) seem to have followed the reverse path, with suspensory adaptations being acquired on an otherwise pronograde body plan, which poses an interesting scenario for the evolution of gibbons and siamangs. The small size of these apes has been hypothesized to have been brought about through a process of dwarfism (e.g., Pilbeam & Young, 2004), but there are virtually no remains for lesser apes ancestors other than Yuanmoupithecus (ca. 7–8 Mya, China; Pan, 2006; Harrison, Ji, & Zheng, 2008), which is still largely unknown. This, then, does not exclude the possibility that they evolved from small‐bodied, pronograde stem hominoids, similar perhaps to E. vindobonensis, with their orthograde and suspensory adaptations having evolved in parallel with those of great apes. A new fossil (Pliobates cataloniae, 11.6 Mya, Spain; Alba et al., 2015) from the rich site of Abocador de Can Mata (Sabadell, Spain) has even brought its discoverers to put forward the hypothesis that the last common ancestor of crown hominoids might have been more gibbon‐like (or small‐bodied, generally quadrupedal but displaying use of forelimb suspension to some degree) than previously assumed.
The Miocene, thus, remains a fascinating period where ape locomotion diversified beyond any subsequent or past scope, and where the basis of the morphological changes that would finally lead to the rise of hominins and, ultimately, humans, were set.
6. EVOLUTION OF HOMINOID GLENOHUMERAL MORPHOLOGY: HOMININS
The fossil gap in Africa unfortunately covers part of the later Miocene period (ca. 10–8 Mya), when gorillas diverged from the hominin lineage to the chimpanzee–human split (ca. 6–5 Mya). Evidence available to characterize the last common ancestor (LCA) between humans and chimpanzees is that of the Late Miocene/Pleistocene putative early members of the hominin lineage: Ardipithecus, Shaelanthropus, and Orrorin. The debate has been mainly centered in the identification of bipedalism in each of these taxa (e.g., Almécija et al., 2013; Lovejoy, Suwa, Spurlock, Asfaw, & White, 2009c; White et al., 2009; Zollikofer et al., 2005), from which the bipedalism of latter hominins could have evolved, but in at least one case, there are known glenohumeral remains (although, unfortunately, not available for study to the wider scientific community; Lovejoy et al., 2009c; White et al., 2009).
The postcranium of the putative hominin Ardipithecus—as described by Tim White's team in a series of studies (White et al., 2009)—from the early Pliocene of Ethiopia (ca. 5.5–4.4 Mya), exhibits a modern‐looking pelvic morphology suggestive of habitual facultative bipedalism. The foot exhibits an amalgam of primitive features with specialized traits for habitual bipedality (Lovejoy et al., 2009c; White et al., 2009), the elbow shows full range of flexion–extension but lacks suspensory characters, and the hand exhibits adaptations consistent with above‐branch palmigrade behaviors (Lovejoy, Simpson, White, Asfaw, & Suwa, 2009a; Lovejoy, Suwa, Simpson, Matternes, & White, 2009b; White et al., 2009). Overall, the locomotor behavior of Ardipithecus is described as bipedalism with a large arboreal component, mainly above‐branch palmigrade quadrupedalism, clambering and bridging, resembling other Miocene taxa (especially Nacholapithecus), without the presence of suspension (Lovejoy et al., 2009a,b; White, Lovejoy, Asfaw, Carlson, & Suwa, 2015; White et al., 2009). There is at least one well‐preserved proximal humerus (ARA‐VP‐7/2‐A; Lovejoy et al., 2009a; White, Suwa, & Asfaw, 1994) which shows, according to the scholars that studied the remains, typical hominid characters, including “an elliptical head and shallow bicipital groove” (Lovejoy et al., 2009a; : 70e6), plus a minimum amount of torsion. Seeing how the proximal humeri of extant and extinct hominoids differ (however subtly) among taxa and how that reflects on locomotor behavior to a great extent, a detailed analysis of the proximal humerus of Ardipithecus would most likely render a wealth of information. For what can be qualitatively observed from the images provided in the publications of the remains (Figure 5), the morphology of the proximal humerus of A. ramidus appears well‐rounded and protruding in its articular surface, with tubercles appearing (to the naked eye) to be something in between those of the orangutan and the woolly monkey—as seen above in this review, both suspensory taxa, albeit being in opposite extremes of the spectrum of suspension‐using taxa. At the very least, the images provided for this proximal humerus would suggest a great range of circumduction at the shoulder level and possibly well‐developed arm‐rising capabilities. This would, of course, have to be tested conducting the adequate analysis of the remains to either confirm it or reject it, but such analysis is not possible at the moment.
Australopiths are one of the best represented and studied early hominin clades ranging from roughly 4 to 2 Mya, from Australopithecus anamensis to A. sediba, and covering territories from East Africa through South Africa. Among the australopiths, A. afarensis (ca. 4–3 Mya, Eastern Africa) is particularly well represented, followed by A. africanus (ca. 3–2 Mya, South Africa) and the more recent A. sediba (ca. 2 Mya, South Africa; Berger et al., 2010; Ward, 2013). Overall, the postcranial evidence clearly points to the hypothesis that committed bipedalism was their main locomotor behavior when on the ground, with fully orthograde bodies, but retaining the presence of arboreal traits in the forelimbs—including high intermembral and brachial indices, long and curved manual phalanges and a cranially oriented glenoid fossa (high glenoid‐bar index). The adaptive significance of such arboreal traits is highly debated, however, with some authors arguing in favor of australopiths being partly arboreal (exhibiting vertical climbing behaviors or even suspension; Jungers, 1982, 1991; Jungers & Stern, 1983; Larson, 1988, 2007a, 2012, 2013; Rein, Harrison, Carlson, & Harvati, 2017; Rose, 1984, 1991; Senut, 1980; Stern, 2000; Stern & Susman, 1981, 1983; Susman & Stern, 1991; Susman, Stern, & Jungers, 1984), exhibiting a compromise behavior of bipedal progression and some arboreality stemming from the retained arboreal characters (Cartmill & Schmitt, 1996; MacLatchy, 1996; Stern, 1999; Susman et al., 1984), or interpreting the arboreality‐related morphology of the forelimbs as primitive retentions without adaptive significance (Tardieu & Preuschoft, 1996; Ward, 2002, 2013).
Arias‐Martorell et al. (2015c), in an analysis of the proximal humeral morphology of one of the best‐preserved A. afarensis individuals, A.L. 288‐1 (“Lucy”), showed that the left humerus (A.L. 288‐1r) of this australopith female shows mixed characteristics between the derived condition of humans and a more generalized arboreal pattern. The analysis included another two australopith representatives, Sts 7 (A. africanus) and Omo 119‐73‐2718 (Australopithecus sp.), which also showed mixed arboreal traits, combining some orangutan‐like features with more generalized characteristics resembling the woolly monkey (especially Omo 119‐73‐2718). The patterns found in the proximal humerus would have indeed enabled these specimens to use a relatively significant amount of below‐branch positional behaviors (e.g., Larson, 2007a, 2013; Rose, 1991; Senut, 1980; Stern, 2000; Susman et al., 1984). None of the three australopiths shared the morphological condition of the African great hominoids (Gorilla and Pan), thus building on the contention that the LCA between hominins and panins could have exhibited a more generalized arboreal locomotor repertoire, instead of knuckle‐walking. Both A.L. 288‐1 and Sts 7 preserve the glenoid cavity of the scapula, hence morphological analyses of both were attempted, rendering, unfortunately but unsurprisingly, equivocal results (Arias‐Martorell et al., 2015c). In general, the glenoid cavities of both specimens showed more affinities to the great hominoids. The generally cranial orientation of the glenoid facets of these hominins (measured repeatedly in studies for Sts 7 and estimated for A.L. 288‐1; Campbell, 1966; Larson, 2007a; Oxnard, 1968; Robinson, 1972; Stern & Susman, 1983; Vrba, 1979), pattern also found in the juvenile A. afarensis scapula DIK‐1‐1 (Alemseged et al., 2006; Green & Alemseged, 2012) as well as in A. sediba (specimen MH2; Churchill et al., 2013), coupled with several other shoulder girdle characteristics (laterally placed supraglenoid tubercle, an ape‐like angle between the scapular spine and the axillary border, and a clavicle that lacks the characteristic human curvature), indicate that these hominins maintained a high shoulder position in a funnel‐shaped thorax. Such characteristic further suggests the capacity of sustaining abducted positions of the arm without the need of rotating the scapula upwards after the first 90 degrees of arm abduction, like the suspensory hominoids, putting these early hominins at an advantage position for niche exploitation. Thus, their locomotor behaviors would have consisted of full adaptation to bipedal terrestriality while on the ground, and to suspension/climbing while on the trees (Sylvester, 2006), with possible variations of the amount of suspension displayed (based on both its glenoid and scapular shape as well as other forelimb elements—e.g., ulnar shape—it has been argued that A. sediba was more suspensory than A. afarensis, for example; Churchill et al., 2013; Rein et al., 2017). The recently published partial skeleton of A. afarensis KSD‐VP‐1/1 (3.6 Mya, Ethiopia; Haile‐Selassie & Su, 2016) preserves most of the right scapula and is described by the authors as having a cranially oriented fossa as well (Melillo, 2016; Ryan & Sukhdeo, 2016). Melillo's (2016) analysis on shoulder girdle morphology (including qualitative observations, traditional metrics and geometric morphometrics) suggests that, when compared to all the other hominoids, A. afarensis shows more affinities to orangutans than any other group, and that its morphology is departed from that of the gorilla–chimpanzee cluster, making it highly unlikely for the LCA to have exhibited an African ape‐like shoulder girdle, agreeing with what has become the majority view (but see Young, Capellini, Roach, & Alemseged, 2015 for a different view on scapular shape). However, it is subsequently proposed that given her reconstructed morphology of the chimpanzee–human LCA (from scapular and clavicular traits only of extant hominoids) and the intermediate morphological condition of certain aspects between modern humans and orangutans of the KSD‐VP‐1/1 shoulder girdle, the forelimb of A. afarensis would seem to have been functionally selected for manipulative functions over locomotor ones. While this may possibly be partly true, it is worth keeping in mind that in the study conducted by Arias‐Martorell et al. (2015c), australopiths did not only exhibit morphological affinities with humans and the arboreal hominoids, but displayed similarities with more generalized monkeys as well (particularly with the woolly monkey). This stresses the importance of contextualizing the debate about the morphological affinities of early hominins with the inclusion of more generalized primate taxa that better characterize the evolutionary background of the hominoid lineage. As most studies stand now, only extant ape comparison samples are used, but due to the mosaic nature of the postcranial configurations of hominins, such studies might render relatively limited morphofunctional inferences because of the modern‐hominoid contained comparative sample.
The advent of the Homo clade (ca. 2–3 Mya) did not seem to reciprocate a morphological shift toward the modern human‐like condition right away (Larson, 2007a). The earliest species (H. habilis and, possibly, H. rudolfensis, ca. 2–1.5 Mya) retained australopith‐like overall postcranial morphologies, even though tool‐making abilities and manipulative capabilities are already present in these species—although the shoulder girdle remains are scant for H. habilis and nonexistent for H. rudolfensis (Larson, 2009). Early H. erectus depicts a distinct morphology not quite australopith‐like, not quite human (Larson, 2007a, 2009; Larson et al., 2007). Although no proximal humeri are known for H. erectus, it would seem that their shoulder girdle (of at least KNM‐WT 15000, relatively well‐preserved despite being juvenile) combined comparatively short clavicles, low humeral torsion and a protracted (lateralized) scapula position with an overall modern‐looking scapula (no upward‐facing glenoid cavity) that would have been sitting on a barrel‐shaped thorax (unlike the funnel‐shaped thorax of hominoids). Moreover, Arias‐Martorell et al. (2015c) report that the glenoid cavity of KNM‐WT 15000 is not quite human‐like either; its morphology was unlike any of the studied taxa (which included all hominoids plus the New World monkeys), adding to the contention that early H. erectus had a distinct morphology of its own—either transitional, as per Larson (2007a, 2009) or mosaic, retaining primitive and modern‐looking traits to comprise a morphology not seen in any extant species (Arias‐Martorell et al., 2015c). The major shift toward the modern human morphotype seems to have occurred in H. heildebergensis (ca. 0.6–0.2 Mya, Eurasia and Africa) and H. neanderthalensis (ca. 0.4 Mya, Europe), as well as the possible ancestor of both, H. antecessor (1.2–0.8 Mya, Spain; Larson, 2007a, 2009).
The analysis of the H. neanderthalensis proximal humerus of Tabun 1 (Arias‐Martorell et al., 2015c) virtually showed the same morphotype than that of modern humans, exhibiting a lowered neutral position of the arm, as discussed above (Kapandji, 2007; Larson, 1995, 2007b). The Tabun 1 specimen shows reduced insertion sites for the rotator cuff muscles, maybe indicating an early decrease of the above‐discussed reliance on the active stabilizers of the glenohumeral joint and diminished importance of the arm abductors (Bello‐Hellegouarch et al., 2013; Larson, 1995, 2007a; Potau et al., 2009; Roberts, 1974).
Neanderthal glenoids seem to be most similar to those of orangutans (superoinferiorly elongated, again signifying a possible lack of functional signal), which could result from developmental differences between Neanderthals and humans (Di Vincenzo, Churchill, & Manzi, 2012; Macias & Churchill, 2015). Di Vincenzo et al. (2012) study found that the differences on glenoid morphology between species of the Homo genus are related to a differential degree of development between the centers of ossification of the glenoid (Scheuer & Black, 2004) due to an enlarged growth period in modern humans (Di Vincenzo et al., 2012), and therefore not related to locomotion or to any functional constraint at that.
To a number of researchers, morphology at the glenohumeral level (and the whole of the shoulder girdle) at this stage in human evolution seems to be geared toward throwing effectiveness (Roach, Venkadesan, Rainbow, & Lieberman, 2013), with a few characters being essential—namely, humeral torsion and laterally oriented glenoid cavities. However, humeral torsion and some of the claims that have been derived from it (Roach & Richmond, 2015; Roach et al., 2013) are highly controversial, mostly because there is no consensus on what truly constitutes humeral torsion and what exactly the term stands for (this issue is beyond the scope of this review; for some of the debate see Cowgill, 2007; Larson, 1996; Larson, 2007b; Larson, 2015b; Rhodes, 2006; Roach & Richmond, 2015; Roach et al., 2013 and referenced therein). It is interesting that humeral torsion seems to be driven by the lateral rotation of the lesser tubercle to allocate for the bigger articular surface of the humerus, instead of being a result of an effective torsion of the humeral shaft (Fleagle & Simons, 1982). According to Rose (1989), however, more extensive articular surfaces are a separate trait from tubercle migration, although both may be combined, and thus, expansion can be brought about by the migration of one or both tubercles, combination of which (both tubercles migrating in the same direction or being brought closer together) would play a role in the final amount of torsion displayed. It is remarkable that Larson (1998) found that gibbons display the lowest humeral torsion of all living hominoids, while showing well‐rounded, big and protruding humeral articular surfaces, which she suggested is a compromise between the need of repositioning the scapula onto the back of the ribcage and extreme positioning of the elbow when engaging in brachiation (especially during ricochetal brachiation), and thus low torsion might be associated with the display of suspensory locomotion.
Overall, hominin glenohumeral joint remains play a key part on answering important questions about the morphological pathways that led to the acquisition of bipedalism, truly a defining characteristic of becoming human. But not only that, it also helps to understand that not all that seems uniquely human might be, and that hominins were part of the morphological stream that includes the hominoids that preceded them, and with whom they share more than previously thought to be possible.
7. FUTURE DIRECTIONS
Areas of future research within glenohumeral morphology could be circumscribed to anatomical and evolutionary. On the anatomical side, there is still a lot we do not know about soft tissue function and the role of passive stabilization at the joint. The glenoid cavity of the scapula remains an understudied aspect of it, and a very interesting one at that from a morphological standpoint. It is a region where soft tissue could be playing a fundamental role on function, that is, the presence of the cartilaginous rim (labrum) surrounding the cavity completely changes its shape and depth (personal observation), and thus possibly, its functional properties. From an evolutionary perspective, there is still a lot to be said about the adaptive role of glenohumeral morphology, and to what extent it shaped the hominoids evolutionary history. Questions such as what was the locomotor behavior of the LCA between humans and chimpanzees, or what was the extent of the role suspension played in the early days of the hominins only the discovery of new fossils (especially of the critical period of 8–6 mya) and the proper analysis of old ones will tell.
8. CONCLUSIONS
The glenohumeral joint remains a highly interesting and informative aspect of the postcranial skeleton to attempt morphofunctional explorations of extant and extinct primate taxa. Detailed and in‐depth analyses of the features of this joint provide insights into primate locomotor abilities, as well as offering a framework for contextualizing the evolutionary history of the groups under study. The proximal humerus, in particular, is of great importance, as its external morphology seems to be driven by the functional demands of locomotion. On the contrary, the glenoid cavity of the scapula does not seem to offer great insights into function, and any such conclusions should be considered with utmost caution.
It is important that the glenohumeral joint can help shed light on the evolutionary history of primates and hominoids in particular, being of special interest when dealing with topics such as homoplasy, orthogrady, the evolutionary role of suspension, and the morphology and locomotor behaviors of LCA nodes. For instance, there seems to be a decoupling between orthogrady and suspension, as the Eurasian small‐bodied catarrhines and the South American woolly monkey show clear adaptations to suspensory locomotion at the shoulder girdle and specifically at the glenohumeral joint without the acquisition of an orthograde body plan. The reverse condition seems to occur in some of the large‐bodied crown hominoids (Pierolapithecus), where the lack of suspensory adaptations goes together with an orthograde body plan. This also evidences the possibility that orthogrady and suspension may have arisen independently several times during hominoid and primate evolution.
Early (putative) representatives of the human branch (Ardipithecus) might have shown a degree of below‐branch positional behaviors while still bipedal on the ground, as seen in later hominins (Australopithecus), although such contention will need to await an in‐depth analysis of the whole fossil record.
At last, it is important to stress that any attempt to elucidate early hominin morphology from postcranial remains and derive behavioral hypothesis from such attempts would greatly benefit from including a wider range of comparative taxa, from the semisuspensory New World Monkeys to extinct hominoids (fossil record permitting), as the morphological affinities of early hominins are not as clear‐cut extant ape‐like as might have been assumed in the past.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
JAM conceived and designed the study, and wrote the manuscript.
Supporting information
ACKNOWLEDGMENTS
I am thankful to T. L. Kivell and J.J. García‐Granero for their invaluable comments and discussion on earlier versions of this manuscript and to J.M. Potau for his encouragement. This research is supported by the European Commission (MSCA 2015, Grant No. 703608). Authors are required to archive their data in a publicly accessible repository such as Dryad, FigShare, GenBank.
Arias‐Martorell J. The morphology and evolutionary history of the glenohumeral joint of hominoids: A review. Ecol Evol. 2019;9:703–722. 10.1002/ece3.4392
References
- Alba, D. M. (2012). Fossil apes of the Vallès‐Penedès Basin. Evolutionary Anthropology, 21, 254–296. 10.1002/evan.21312 [DOI] [PubMed] [Google Scholar]
- Alba, D. M. , Almécija, S. , Casanovas‐Vilar, I. , Méndez, J. M. , & Moyà‐Solà, S. (2012). A partial skeleton of the fossil great ape Hispanopithecus laietanus from Can Feu and mosaic evolution of crown‐hominoid positional behaviors. PLoS One, 7, e39617 10.1371/journal.pone.0039617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba, D. M. , Almecija, S. , DeMiguel, D. , Fortuny, J. , de los Rios, M. P. , Pina, M. , … Moya‐Sola, S. (2015). Miocene small‐bodied ape from Eurasia sheds light on hominoid evolution. Science, 350, aab2625‐1–aab2625‐11. [DOI] [PubMed] [Google Scholar]
- Alba, D. M. , Almécija, S. , & Moyà‐Solà, S. (2010). Locomotor inferences in Pierolapithecus and Hispanopithecus: Reply to Deane and Begun (2008). Journal of Human Evolution, 59, 143–149. 10.1016/j.jhevol.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Alemseged, Z. , Spoor, F. , Kimbel, W. H. , Bobe, R. , Geraads, D. , Reed, D. , & Wynn, J. G. (2006). A juvenile early hominin skeleton from Dikika, Ethiopia. Nature, 443, 296–301. 10.1038/nature05047 [DOI] [PubMed] [Google Scholar]
- Almécija, S. , Alba, D. M. , & Moyà‐Solà, S. (2009). Pierolapithecus and the functional morphology of Miocene ape hand phalanges: Paleobiological and evolutionary implications. Journal of Human Evolution, 57, 284–297. 10.1016/j.jhevol.2009.02.008 [DOI] [PubMed] [Google Scholar]
- Almécija, S. , Alba, D. M. , Moyà‐Solà, S. , & Köhler, M. (2007). Orang‐like manual adaptations in the fossil hominoid Hispanopithecus laietanus: First steps towards great ape suspensory behaviours. Proceedings of the Royal Society B, 274, 2375–2384. 10.1098/rspb.2007.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almécija, S. , Tallman, M. , Alba, D. M. , Pina, M. , Moyà‐Solà, S. , & Jungers, W. L. (2013). The femur of Orrorin tugenensis exhibits morphometric affinities with both Miocene apes and later hominins. Nature Communications, 4, 2888 10.1038/ncomms3888 [DOI] [PubMed] [Google Scholar]
- Andrews, P. , & Groves, C. P. (1976). Gibbons and brachiation In Rumbaugh D. M. (Ed.), Gibbon and siamang, Vol. 4: Suspensory behaviour, locomotion and other behaviors of captive gibbons, pp. 167–218. Karger, Basel, Switzerland. [Google Scholar]
- Arias‐Martorell, J. , Alba, D. M. , Potau, J. M. , Bello‐Hellegouarch, G. , & Pérez‐Pérez, A. (2015b). Morphological affinities of Epipliopithecus vindobonensis and Pliopithecus antiquus: Suspensory inferences based on a 3D geometric morphometric approach. Journal of Human Evolution, 80, 83–95. 10.1016/j.jhevol.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Arias‐Martorell, J. , Potau, J. M. , Bello‐Hellegouarch, G. , Pastor, J. F. , & Pérez‐Pérez, A. (2012). 3D geometric morphometric analysis of the proximal epiphysis of the hominoid humerus. Journal of Anatomy, 221, 394–405. 10.1111/j.1469-7580.2012.01560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias‐Martorell, J. , Potau, J. M. , Bello‐Hellegouarch, G. , & Pérez‐Pérez, A. (2015c). Like father, like son: Assessment of A.L. 288‐1 (A. afarensis), Sts 7 (A. africanus) and Omo 119‐73‐2718 (Australopithecus sp.) through a three‐dimensional shape analysis of the shoulder joint. PLoS One, 10, e0117408 10.1371/journal.pone.0117408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias‐Martorell, J. , Tallman, M. , Potau, J. M. , Bello‐Hellegouarch, G. , & Pérez‐Pérez, A. (2015a). Shape analysis of the proximal humerus in orthograde and semi‐orthograde primates: Correlates of suspensory behaviors. American Journal of Primatology, 77, 1–19. 10.1002/ajp.22306 [DOI] [PubMed] [Google Scholar]
- Ashton, E. H. , & Oxnard, C. E. (1963). The musculature of the primate shoulder. The Transactions of the Zoological Society of London, 29, 553–650. [Google Scholar]
- Basmajian, J. V. , & De Luca, C. J. (1985). Muscles alive: Their functions revealed by electromyography. Baltimore, MD: Williams & Wilkins. [Google Scholar]
- Begun, D. R. (2002). The Pliopithecoidea In Hartwig W. C. (Ed.), The Primate fossil record (pp. 231–253). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Begun, D. R. , Teaford, M. F. , & Walker, A. (1994). Comparative and functional anatomy of Proconsul phalanges from Kaswanga primate site, Rusinga Islandm, Kenya. Journal of Human Evolution, 26, 89–165. 10.1006/jhev.1994.1008 [DOI] [Google Scholar]
- Bello‐Hellegouarch, G. , Potau, J. M. , Arias‐Martorell, J. , Pastor, J. F. , Diogo, R. , & Pérez‐Pérez, A. (2012). The rotator cuff muscles in hominoidea: Evolution and adaptations to different types of locomotion In Hughes E. F. & Mills M. E. (Eds.), Primates: Classification, evolution and behavior (pp. 111–134). Hauppauge, NY: Nova Science Publishers. [Google Scholar]
- Bello‐Hellegouarch, G. , Potau, J. M. , Arias‐Martorell, J. , Pastor, J. F. , & Pérez‐Pérez, A. (2013). A comparison of qualitative and quantitative approaches to characterizing the dorsal side of the scapula in Hominoidea and its relationship to locomotion. International Journal of Primatology, 34, 315–336. 10.1007/s10764-013-9660-5 [DOI] [Google Scholar]
- Berger, R. L. , de Ruiter, D. J. , Churchill, S. E. , Schmid, P. , Carlson, K. J. , Dirks, P. H. G. M. , & Kibii, J. M. (2010). Australopithecus sediba: A new species of Homo‐like australopith from South Africa. Science, 328, 195–204. 10.1126/science.1184944 [DOI] [PubMed] [Google Scholar]
- Campbell, B. G. (1966). Human evolution. London, UK: Heinemann. [Google Scholar]
- Cant, J. G. H. , Youlatos, D. , & Rose, M. D. (2001). Locomotor behavior of Lagothrix lagothricha and Ateles belzebuth in Yasuní National Park, Ecuador: General patterns and nonsuspensory modes. Journal of Human Evolution, 41, 141–166. 10.1006/jhev.2001.0485 [DOI] [PubMed] [Google Scholar]
- Cant, J. G. H. , Youlatos, D. , & Rose, M. D. (2003). Suspensory locomotion of Lagothrix lagothricha and Ateles belzebuth in Yasuní National Park, Ecuador. Journal of Human Evolution, 44, 685–699. 10.1016/S0047-2484(03)00060-5 [DOI] [PubMed] [Google Scholar]
- Cartmill, M. (1985). Climbing In Hildebrand M., Bramble D., Liem K., & Wake D. (Eds.), Functional vertebrate morphology (pp. 73–88). Cambridge, UK: Belknap Press. [Google Scholar]
- Cartmill, M. , & Milton, K. (1977). The lorisiform wrist joint and the evolution of “brachiating” adaptations in the Hominoidea. American Journal of Physical Anthropology, 47, 249–272. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Cartmill, M. , & Schmitt, D. (1996). Pelvic rotation in human walking and running: Implications for early hominid bipedalism. American Journal of Physical Anthropology, 48, 25–44. [Google Scholar]
- Chan, L. K. (2007). Glenohumeral mobility in primates. Folia Primatologica, 78, 1–18. 10.1159/000095682 [DOI] [PubMed] [Google Scholar]
- Chan, L. K. (2008). The range of passive arm circumduction in primates: Do hominoids really have more mobile shoulders? American Journal of Physical Anthropology, 136, 265–277. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Churchill, S. E. , Holliday, T. W. , Carlson, K. J. , Jashashvili, T. , Macias, M. E. , Mathews, S. , … Berger, L. R. (2013). The upper limb of Australopithecus sediba . Science, 340, 1233477‐1–1233477‐6. [DOI] [PubMed] [Google Scholar]
- Corruccini, R. S. , & Ciochon, R. L. (1976). Morphometric affinities of the human shoulder. American Journal of Physical Anthropology, 45, 19–38. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Corruccini, R. S. , Ciochon, R. L. , & McHenry, H. M. (1975). Osteometric shape relationships in the wrist joint of some anthropoids. Folia Primatologica, 24, 250–274. 10.1159/000155696 [DOI] [PubMed] [Google Scholar]
- Cowgill, L. W. (2007). Humeral torsion revisited: A functional and ontogenetic model for population variation. American Journal of Physical Anthropology, 134, 472–480. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Crompton, R. H. , Vereecke, E. E. , & Thorpe, S. K. S. (2008). Locomotion and posture from the common hominoid ancestor to fully modern hominins, with special reference to the last common panin/hominin ancestor. Journal of Anatomy, 212, 501–543. 10.1111/j.1469-7580.2008.00870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vincenzo, F. , Churchill, S. E. , & Manzi, G. (2012). The Vindija neanderthal scapular glenoid fossa: Comparative shape analysis suggests evo‐devo changes among Neanderthals. Journal of Human Evolution, 62, 274–285. 10.1016/j.jhevol.2011.11.010 [DOI] [PubMed] [Google Scholar]
- Dunn, R. H. , Tocheri, M. W. , Orr, C. M. , & Jungers, W. L. (2014). Ecological divergence and talar morphology in gorillas. American Journal of Physical Anthropology, 153, 526–541. 10.1002/ajpa.22451 [DOI] [PubMed] [Google Scholar]
- Dunsworth, H. M. (2006). Proconsul heseloni feet from Rusinga Island, Kenya. PhD Dissertation, University of Pennsylvania, Pennsylvania.
- Fleagle, J. G. (1976). Locomotion and posture of the Malayan siamang and implications for hominid evolution. Folia Primatologica, 26, 245–269. 10.1159/000155756 [DOI] [PubMed] [Google Scholar]
- Fleagle, J. G. (1983). Locomotor adaptations of Oligocene and Miocene hominoids and their phyletic implications In Ciochon R. L. & Corruccini R. S. (Eds.), New interpretations of ape and human ancestry (pp. 301–324). New York, NY: Plenum Press; 10.1007/978-1-4684-8854-8 [DOI] [Google Scholar]
- Fleagle, J. G. , & Simons, E. L. (1982). The humerus of Aegyptopithecus zeuxis: A primitive anthropoid. American Journal of Physical Anthropology, 59, 175–193. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Gebo, D. L. (1996). Climbing, brachiation, and terrestrial quadrupedalism: Historical precursors of hominid bipedalism. American Journal of Physical Anthropology, 101, 55–92. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Gebo, D. L. (2010). Locomotor function across primates (including humans) In Larson C. S. (Ed.), A companion to biological anthropology (pp. 530–544). New York, NY: Wiley‐Blackwell; 10.1002/9781444320039 [DOI] [Google Scholar]
- Gebo, D. L. , MacLatchy, L. , Kityo, R. , Deino, A. , Kingston, J. , & Pilbeam, D. (1997). A hominoid genus from the early Miocene of Uganda. Science, 276, 401–404. 10.1126/science.276.5311.401 [DOI] [PubMed] [Google Scholar]
- Green, D. J. (2013). Ontogeny of the hominoid scapula: The influence of locomotion on morphology. American Journal of Physical Anthropology, 152, 239–260. [DOI] [PubMed] [Google Scholar]
- Green, D. J. , & Alemseged, Z. (2012). Australopithecus afarensis scapular ontogeny, function, and the role of climbing in human evolution. Science, 338, 514–517. 10.1126/science.1227123 [DOI] [PubMed] [Google Scholar]
- Haile‐Selassie, Y. , & Su, D. F. (2016). The postcranial anatomy of Australopithecus afarensis. Netherlands, Dordrecht: Springer; 10.1007/978-94-017-7429-1 [DOI] [Google Scholar]
- Harrison, T. (1989). New postcranial remains of Victoriapithecus from the Middle Miocene of Kenya. Journal of Human Evolution, 18, 3–54. 10.1016/0047-2484(89)90022-5 [DOI] [Google Scholar]
- Harrison, T. (2002). Late Oligocene to Middle Miocene catarrhines from Afro‐Arabia In Hartwig W. C. (Ed.), The primate fossil record (pp. 311–338). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Harrison, T. (2005). The zoogeographic and phylogenetic relationships of early catarrhine primates in Asia. Anthropological Science, 113, 43–51. 10.1537/ase.04S006 [DOI] [Google Scholar]
- Harrison, T. (2010). Dendropithecoidea, Proconsuloidea and Hominoidea In Werdelin L., & Sanders W. J. (Eds.), Cenozoic mammals of Africa (pp. 429–469). Berkeley, CA: University of California Press; 10.1525/california/9780520257214.001.0001 [DOI] [Google Scholar]
- Harrison, T. (2013). Catarrhine origins In Begun D. R. (Ed.), A companion to paleoanthropology (pp. 376–396). Oxford, UK: Blackwell Publishing; 10.1002/9781118332344.ch20 [DOI] [Google Scholar]
- Harrison, T. , Ji, X. , & Zheng, L. (2008). Renewed investigations at the late Miocene hominoid locality of Leilao, Yunnan, China. American Journal of Physical Anthropology, 135, 113. [Google Scholar]
- Hunt, K. D. (1991a). Positional behavior in the Hominoidea. International Journal of Primatology, 12, 95–118. 10.1007/BF02547576 [DOI] [Google Scholar]
- Hunt, K. D. (1991b). Mechanical implications of chimpanzee positional behavior. American Journal of Physical Anthropology, 86, 521–536. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Hunt, K. D. (2004). The special demands of great ape locomotion and posture In Russo A. E. & Begun D. R. (Eds.), The evolution of thought: Evolutionary origins of great ape intelligence (pp. 172–189). Cambridge, UK: Cambridge University Press; 10.1017/CBO9780511542299 [DOI] [Google Scholar]
- Hürzeler, J. (1954). Contribution a l'odontologie et a la phylogénèse du genre Pliopithecus gervais . Annales de Paleontologie, 40, 5–63. [Google Scholar]
- Inman, V. T. , Saunders, J. B. , & Abbot, L. C. (1944). Observations on the function of the shoulder joint. Journal of Bone and Joint Surgery. American Volume, 26, 1–30. [Google Scholar]
- Ishida, H. , Kunimatsu, Y. , Nakatsukasa, M. , & Nakano, Y. (1999). New hominoid genus from the Middle Miocene of Nachola, Kenya. Anthropological Science, 107, 189–191. [Google Scholar]
- Ishida, H. , Kunimatsu, Y. , Takano, T. , Nakano, Y. , & Nakatsukasa, M. (2004). Nacholapithecus skeleton from the Middle Miocene of Kenya. Journal of Human Evolution, 46, 69–103. 10.1016/j.jhevol.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Jungers, W. L. (1982). Lucy's limbs: Skeletal allometry and locomotion in Australopithecus afarensis . Nature, 297, 676–678. 10.1038/297676a0 [DOI] [Google Scholar]
- Jungers, W. L. (1991). Scaling postcranial joint size in hominoid primates. Journal of Human Evolution, 6, 391–399. 10.1007/BF02435532 [DOI] [Google Scholar]
- Jungers, W. L. , & Stern, J. T. (1981). Preliminary electromyographical analysis of brachiation in gibbon and spider monkey. International Journal of Primatology, 2, 19–33. 10.1007/BF02692297 [DOI] [Google Scholar]
- Jungers, W. L. , & Stern, J. T. (1983). Body proportions, skeletal allometry and locomotion in the Hadar hominins. A reply to Wolpoff. Journal of Human Evolution, 12, 673–684. 10.1016/S0047-2484(83)80007-4 [DOI] [Google Scholar]
- Kagaya, M. (2007). Glenohumeral joint surface characters and its relation to forelimb suspensory behavior in three ateline primates, Ateles, Lagothrix, and Alouatta . Anthropological Science, 115, 17–23. 10.1537/ase.041209 [DOI] [Google Scholar]
- Kapandji, I. A. (2007). The physiology of the joints, volume I: Upper limb. Edinburgh, UK: Churchill Livingstone. [Google Scholar]
- Keith, A. (1903). The extent to which the posterior segments of the body have been transmuted and suppressed in the evolution of man and allied primates. Journal of Anatomy, 37, 18–40. [PMC free article] [PubMed] [Google Scholar]
- Keith, A. (1923). Hunterian lectures on Man's posture: Its evolution and disorders. Lecture I. Theories concerning the evolution of Man's posture. BMJ, 1, 451–454. 10.1136/bmj.1.3246.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, Y. , Takemoto, H. , & Kuraoka, A. (2012). Relationship between humeral geometry and shoulder muscle power among suspensory, knuckle‐walking, and digitigrade/palmigrade quadrupedal primates. Journal of Anatomy, 220, 29–41. 10.1111/j.1469-7580.2011.01451.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, T. (1985). Bipedal and quadrupedal walking of primates: Comparative dynamics In Kondo S. (Ed.), Primate morphophysiology, locomotor analyses and human bipedalism (pp. 81–104). Tokyo, Japan: University of Tokyo Press. [Google Scholar]
- Knigge, R. P. , Tocheri, M. W. , Orr, C. M. , & McNulty, K. P. (2015). Three‐dimensional geometric morphometric analysis in extant gorilla taxa from highland and lowland habitats. Anatomical Record, 298, 277–290. 10.1002/ar.23069 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. (1988). Subscapularis function in gibbons and chimpanzees: Implications for the interpretation of humeral head torsion in hominoids. American Journal of Physical Anthropology, 76, 449–462. 10.1002/(ISSN)1096-8644 [DOI] [Google Scholar]
- Larson, S. G. (1993). Functional morphology of the shoulder in primates In Gebo D. L. (Ed.), Postcranial adaptation in nonhuman primates (pp. 45–69). DeKalb, IL: Northern Illinois University Press. [Google Scholar]
- Larson, S. G. (1995). New characters for the functional interpretation of primate scapulae and proximal humeri. American Journal of Physical Anthropology, 98, 13–35. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. (1996). Estimating humeral torsion on incomplete fossil anthropoid humeri. Journal of Human Evolution, 31, 239–257. 10.1006/jhev.1996.0059 [DOI] [Google Scholar]
- Larson, S. G. (2007a). Notes and Comments: The definition of humeral torsion: A comment on Rhodes (2007). American Journal of Physical Anthropology, 133, 819–821. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. (2007b). Evolutionary transformation of the hominin shoulder. Evolutionary Anthropology, 16, 172–187. 10.1002/evan.20149 [DOI] [Google Scholar]
- Larson, S. G. (2009). Evolution of the hominin shoulder: Early Homo In Grine F. E., Fleagle J. G., & Leakey R. E. (Eds.), The first humans: Origin and early evolution of the genus Homo (pp. 65–76). New York, NY: Springer; 10.1007/978-1-4020-9980-9 [DOI] [Google Scholar]
- Larson, S. G. (2012). Did australopiths climb trees? Science, 338, 478–479. 10.1126/science.1230128 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. (2013). Shoulder morphology in early hominin evolution In Reed K., Fleagle J. G., & Leakey R. (Eds.), The paleobiology of Australopithecus (pp. 247–261). Netherlands, Dordrecht: Springer; 10.1007/978-94-007-5919-0 [DOI] [Google Scholar]
- Larson, S. G. (2015a). Rotator cuff muscle size and the interpretation of scapular shape in primates. Journal of Human Evolution, 80, 96–106. 10.1016/j.jhevol.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. (2015b). Humeral torsion and throwing efficiency in early human evolution. Journal of Human Evolution, 85, 198–205. 10.1016/j.jhevol.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. , Jungers, W. L. , Morwood, M. J. , Sutikna, T. , Wahyu Saptomo, J. E. , Awe Due, R. , & Djubiantono, T. (2007). Homo floresiensis and the evolution of the hominin shoulder. Journal of Human Evolution, 53, 718–731. 10.1016/j.jhevol.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. , & Stern, J. T. (1986). EMG of scapulohumeral muscles in the chimpanzee during reaching and arboreal locomotion. The American Journal of Anatomy, 176, 171–190. 10.1002/(ISSN)1553-0795 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. , & Stern, J. T. (1987). EMG of chimpanzee shoulder muscles during knuckle‐walking: Problems of terrestrial locomotion in a suspensory adapted primate. Journal of Zoology, 212, 629–655. 10.1111/j.1469-7998.1987.tb05961.x [DOI] [Google Scholar]
- Larson, S. G. , & Stern, J. T. (1989). Role of supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): Implications for interpretation of humeral morphology. American Journal of Physical Anthropology, 79, 369–377. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. , & Stern, J. T. (1992). Further evidence for the role of supraspinatus in quadrupedal monkeys. American Journal of Physical Anthropology, 87, 359–363. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Larson, S. G. (1998). Parallel evolution in the hominoid trunk and forelimb. Evolutionary Anthropology, 6, 87–99. [Google Scholar]
- Larson, S. G. , & Stern, J. T. (2013). Rotator cuff muscle function and its relation to scapular morphology in apes. Journal of Human Evolution, 65, 391–403. 10.1016/j.jhevol.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Lovejoy, C. O. , Simpson, S. W. , White, T. D. , Asfaw, B. , & Suwa, G. (2009a). Careful climbing in the Miocene: The forelimbs of Ardipithecus ramidus and humans are primitive. Science, 326, 70e1–70e8. [PubMed] [Google Scholar]
- Lovejoy, C. O. , Suwa, G. , Simpson, S. W. , Matternes, J. H. , & White, T. D. (2009b). The great divides: Ardipithecus ramidus reveals the postcrania of our last common ancestor. Science, 326, 101–106. [PubMed] [Google Scholar]
- Lovejoy, C. O. , Suwa, G. , Spurlock, L. , Asfaw, B. , & White, T. D. (2009c). The pelvis and femur of Ardipithecus ramidus: The emergence of upright walking. Science, 326, 71e1–71e6. [PubMed] [Google Scholar]
- Macias, M. E. , & Churchill, S. E. (2015). Functional morphology of the Neanderthal scapular glenoid fossa. Anatomical Record, 298, 168–179. 10.1002/ar.23072 [DOI] [PubMed] [Google Scholar]
- MacLatchy, L. (1996). Another look at the australopith hip. Journal of Human Evolution, 31, 455–476. 10.1006/jhev.1996.0071 [DOI] [Google Scholar]
- MacLatchy, L. (2004). The oldest ape. Evolutionary Anthropology, 13, 90–103. 10.1002/evan.10133 [DOI] [Google Scholar]
- MacLatchy, L. , Gebo, D. , Kityo, R. , & Pilbeam, D. (2000). Postcranial functional morphology of Morotopithecus bishopi, with implications for the evolution of modern ape locomotion. Journal of Human Evolution, 39, 159–183. 10.1006/jhev.2000.0407 [DOI] [PubMed] [Google Scholar]
- Madar, S. I. , Rose, M. D. , Kelley, J. , MacLatchy, L. , & Pilbeam, D. (2002). New Sivapithecus postcranial specimens from the Siwaliks of Pakistan. Journal of Human Evolution, 42, 705–752. 10.1006/jhev.2002.0554 [DOI] [PubMed] [Google Scholar]
- Mathewson, M. A. , Kwan, A. , Eng, C. M. , Lieber, R. L. , & Ward, S. R. (2014). Comparison of rotator cuff muscle architecture between humans and other selected vertebrate species. Journal of Experimental Biology, 217, 261–273. 10.1242/jeb.083923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty, K. P. , Begun, D. R. , Kelley, J. , Manthi, F. K. , & Mbua, E. N. (2015). A systematic revision of Proconsul with the description of a new genus of early Miocene hominoid. Journal of Human Evolution, 84, 42–61. 10.1016/j.jhevol.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Melillo, S. E. (2016). The shoulder girdle of KSD‐VP‐1/1 In Haile‐Selassie Y. & Su D. F. (Eds.), The postcranial anatomy of Australopithecus afarensis (pp. 113–141). Netherlands, Dordrecht: Springer; 10.1007/978-94-017-7429-1 [DOI] [Google Scholar]
- Michilsens, F. , Vereecke, E. E. , D'Août, K. , & Aerts, P. (2009). Functional anatomy of the gibbon forelimb: Adaptations to a brachiating lifestyle. Journal of Anatomy, 215, 335–354. 10.1111/j.1469-7580.2009.01109.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michilsens, F. , Vereecke, E. E. , D'Août, K. , & Aerts, P. (2010). Muscle movement arms and function of the siamang forelimb during brachiation. Journal of Anatomy, 217, 521–535. 10.1111/j.1469-7580.2010.01272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbeck, M. (1975). Dryopithecus africanus forelimb. Journal of Human Evolution, 4, 39–46. 10.1016/0047-2484(75)90088-3 [DOI] [Google Scholar]
- Moyà‐Solà, S. , & Köhler, M. (1996). A Dryopithecus skeleton and the origins of great‐ape locomotion. Nature, 397, 156–159. 10.1038/379156a0 [DOI] [PubMed] [Google Scholar]
- Moyà‐Solà, S. , Köhler, M. , Alba, D. , Casanovas‐Vilar, I. , & Galindo, J. (2004). Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain. Science, 306, 1339–1344. 10.1126/science.1103094 [DOI] [PubMed] [Google Scholar]
- Moyà‐Solà, S. , Köhler, M. , Alba, D. , Casanovas‐Vilar, I. , & Galindo, J. (2005). Response to comment on “Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain”. Science, 308, 203 10.1126/science.1108433 [DOI] [PubMed] [Google Scholar]
- Nakatsukasa, M. (1994). Morphology of the humerus and femur in African mangabeys and guenons: Functional adaptation and implications for the evolution of positional behavior. African Study Monographs, S21, 1–61. [Google Scholar]
- Nakatsukasa, M. (2008). Comparative study of Moroto vertebral specimens. Journal of Human Evolution, 55, 581–588. 10.1016/j.jhevol.2008.04.009 [DOI] [PubMed] [Google Scholar]
- Nakatsukasa, M. , & Kunimatsu, Y. (2009). Nacholapithecus and its importance for understanding hominoid evolution. Evolutionary Anthropology, 18, 103–119. 10.1002/evan.20208 [DOI] [Google Scholar]
- Nakatsukasa, M. , Kunimatsu, Y. , Nakano, Y. , Takano, T. , & Ishida, H. (2003). Comparative and functional anatomy of phalanges in Nacholapithecus keirioi, a Middle Miocene hominoid from northern Kenya. Primates, 44, 371–412. 10.1007/s10329-003-0051-y [DOI] [PubMed] [Google Scholar]
- Nakatsukasa, M. , Yamanaka, A. , Kunimatsu, Y. , Shimizu, D. , & Ishida, H. (1998). A newly discovered Kenyapithecus skeleton and its implications for the evolution of positional behavior in Miocene East African hominoids. Journal of Human Evolution, 34, 657–664. 10.1006/jhev.1998.0228 [DOI] [PubMed] [Google Scholar]
- Napier, J. R. , & Davis, P. R. (1959). The fore‐limb skeleton and associated remains of Proconsul africanus. Fossil Mammals of Africa, No. 16. London, UK: British Museum. [Google Scholar]
- Oishi, M. , Ogihara, N. , Endo, H. , Ichihara, N. , & Asari, M. (2009). Dimensions of forelimb muscles in orangutans and chimpanzees. Journal of Anatomy, 215, 373–382. 10.1111/j.1469-7580.2009.01125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxnard, C. E. (1968). The architecture of the shoulder in some mammals. Journal of Morphology, 126, 249–290. 10.1002/(ISSN)1097-4687 [DOI] [PubMed] [Google Scholar]
- Pan, Y. (2006). Mammalian fauna associated with Lufengpithecus hudiensis. Primates Linnaeus, 1785 In Qi G. & Dong W. (Eds), Lufengpithecus hudiensis Site, pp. 131–148. Beijing, China: Science Press. [Google Scholar]
- Patton, K. T. , & Thibodeau, G. A. (2010). Anatomy and physiology. New York, NY: Elsevier. [Google Scholar]
- Pilbeam, D. , Rose, M. D. , Barry, J. C. , & Shah, S. M. (1990). New Sivapithecus humeri from Pakistan and the relationship of Sivapithecus and Pongo . Nature, 348, 237–239. 10.1038/348237a0 [DOI] [PubMed] [Google Scholar]
- Pilbeam, D. , & Young, N. (2004). Hominoid evolution: synthesizing disparate data. Comptes Rendus Palevol, 3, 305–321. [Google Scholar]
- Pina, M. , Alba, D. M. , Almécija, S. , Fortuny, J. , & Moyà‐Solà, S. (2012). Brief communication: Paleobiological inferences on the locomotor repertoire of extinct hominoids based on femoral neck cortical thickness: The fossil great ape Hispanopithecus laietanus as a test‐case study. American Journal of Physical Anthropology, 149, 142–148. 10.1002/ajpa.22109 [DOI] [PubMed] [Google Scholar]
- Potau, J. M. , Bardina, X. , & Ciurana, N. (2007). Subacromial space in African Great Apes and subacromial impingement syndrome in humans. International Journal of Primatology, 28, 865–880. 10.1007/s10764-007-9167-z [DOI] [Google Scholar]
- Potau, J. M. , Bardina, X. , Ciurana, N. , Camprubí, D. , Pastor, J. F. , de Paz, F. , & Barbosa, M. (2009). Quantitative analysis of the deltoid and rotator cuff muscles in humans and great apes. International Journal of Primatology, 30, 697–708. 10.1007/s10764-009-9368-8 [DOI] [Google Scholar]
- Preuschoft, H. (1973). The functional anatomy of the upper extremity In Bourne J. (Ed.), The chimpanzee, Vol. 6 (pp. 34–120). Basel, Switzerland: Karger. [Google Scholar]
- Preuschoft, H. (2004). Mechanisms for the acquisition of habitual bipedality: Are there biomechanical reasons for the acquisition of upright bipedal posture? Journal of Anatomy, 204, 363–384. 10.1111/j.0021-8782.2004.00303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoft, H. , Hohn, B. , Scherf, H. , Schmidt, M. , Krause, C. , & Witzel, U. (2010). Functional analysis of the primate shoulder. International Journal of Primatology, 31, 301–320. 10.1007/s10764-010-9399-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty, K. L. , & Ruff, C. B. (1994). Articular structure and function in Hylobates, Colobus and Papio . American Journal of Physical Anthropology, 94, 395–408. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Rein, T. R. , Harrison, T. , Carlson, K. J. , & Harvati, K. (2017). Adaptation to suspensory locomotion in Australopithecus sediba . Journal of Human Evolution, 104, 1–12. 10.1016/j.jhevol.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Rein, T. R. , Harrison, T. , & Zollikofer, C. P. E. (2011). Skeletal correlates of quadrupedalism and climbing in the anthropoid forelimb: Implications for inferring locomotion in Miocene catarrhines. Journal of Human Evolution, 61, 564–574. 10.1016/j.jhevol.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Rhodes, J. A. (2006). Adaptations to humeral torsion in medieval Britain. American Journal of Physical Anthropology, 130, 160–166. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Richmond, B. G. , & Strait, D. S. (2000). Evidence that humans evolved from a knuckle‐walking ancestor. Nature, 404, 382–385. 10.1038/35006045 [DOI] [PubMed] [Google Scholar]
- Roach, N. T. , & Richmond, B. G. (2015). Clavicle length, throwing performance and the reconstruction of the Homo erectus shoulder. Journal of Human Evolution, 80, 107–113. 10.1016/j.jhevol.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Roach, N. T. , Venkadesan, M. , Rainbow, M. J. , & Lieberman, D. E. (2013). Elastic energy storage in the shoulder and the evolution of high‐speed throwing in Homo . Nature, 489, 483–486. 10.1038/nature12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, D. (1974). Structure and function of the primate scapula In Jenkins F. (Ed.), Primate locomotion (pp. 171–200). New York, NY: Academics Press. [Google Scholar]
- Robinson, J. T. (1972). Early hominid posture and locomotion. Chicago, IL: University Press. [Google Scholar]
- Rose, M. D. (1983). Miocene hominoid postcranial morphology. Monkey‐like, ape‐like, neither or both? In Ciochon R. L. & Corruccini R. S. (Eds.), New interpretations of ape and human ancestry, pp. 405–417. New York, NY: Plenum Press; 10.1007/978-1-4684-8854-8 [DOI] [Google Scholar]
- Rose, M. D. (1984). Hominoid postcranial specimens from the Middle Miocene Chinji Formation, Pakistan. Journal of Human Evolution, 13, 503–516. 10.1016/S0047-2484(84)80004-4 [DOI] [Google Scholar]
- Rose, M. D. (1988). Another look at the anthropoid elbow. Journal of Human Evolution, 17, 193–224. [Google Scholar]
- Rose, M. D. (1989). New postcranial specimens of Catarrhines from Middle Miocene Chinji formation, Pakistan: Descriptions and a discussion of proximal humeral functional morphology in anthropoids. Journal of Human Evolution, 18, 131–162. 10.1016/0047-2484(89)90067-5 [DOI] [Google Scholar]
- Rose, M. D. (1991). The process of bipedalization in hominids In Coppens Y., & Senut B. (Eds.), Origine(s) de la bipéde chez les hominindés (pp. 37–48). Paris, France: CNRS. [Google Scholar]
- Rose, M. D. (1992). Kinematics of the trapezium‐1st metacarpal joint in extant anthropoids and Miocene hominoids. Journal of Human Evolution, 22, 255–266. [Google Scholar]
- Rose, M. D. (1993). Locomotor anatomy of Miocene hominoids In Gebo D. L. (Ed.), Postcranial adaptation in nonhuman primates (pp. 252–272). DeKalb, IL: Northern Illinois University Press. [Google Scholar]
- Rose, M. D. (1994). Quadrupedalism in some Miocene catarrhines. Journal of Human Evolution, 26, 387–411. 10.1006/jhev.1994.1025 [DOI] [Google Scholar]
- Rouviére, H. , & Delmas, A. (2002). Anatomie humaine descriptive, topographique et fonctionelle. Tome 3: Membres. Paris, France: Masson. [Google Scholar]
- Ruff, C. B. , & Runestad, J. A. (1992). Primate limb bone structural adaptations. Annual Review of Anthropology, 21, 407–433. 10.1146/annurev.an.21.100192.002203 [DOI] [Google Scholar]
- Ryan, T. M. , & Sukhdeo, S. (2016). KDS‐VP‐1/1: Analysis of the postcranial skeleton using high‐resolution computed tomography In Haile‐Selassie Y. & Su D. F. (Eds.), The postcranial anatomy of Australopithecus afarensis (pp. 39–62). Netherlands, Dordrecht: Springer; 10.1007/978-94-017-7429-1 [DOI] [Google Scholar]
- Sanders, W. J. , & Bodenbender, B. E. (1994). Morphometric analysis of lumbar vertebra UMP 67‐28: Implications for spinal function and phylogeny of the Miocene Moroto hominoid. Journal of Human Evolution, 26, 203–237. 10.1006/jhev.1994.1012 [DOI] [Google Scholar]
- Sarmiento, E. E. (1995). Cautious climbing and folivory: A model of hominoid differentiation. Journal of Human Evolution, 10, 289–321. 10.1007/BF02438967 [DOI] [Google Scholar]
- Sarmiento, E. E. (1998). Generalized quadrupeds, committed bipeds, and the shift to open habitats: An evolutionary model of hominid divergence. American Museum Novitates, 3250, 1–78. [Google Scholar]
- Scheuer, L. , & Black, S. (2004). The juvenile skeleton. London, UK: Academic Press/Elsevier. [Google Scholar]
- Senut, B. (1980). New data on the humerus and its joints in Plio‐Pleistocene hominids. Collegium Antropologicum, 4, 87–93. [Google Scholar]
- Senut, B. (2003). Palaeontological approach to the evolution of hominid bipedalism: The evidence revisited. Courier Forschungs‐Institut Senckenberg, 243, 125–244. [Google Scholar]
- Senut, B. , Nakatsukasa, M. , Kunimatsu, Y. , Nakano, Y. , Takano, T. , Tsujikawa, H. , … Ishida, H. (2004). Preliminary analysis of Nacholapithecus scapula and clavicle from Nachola, Kenya. Primates, 45, 97–104. 10.1007/s10329-003-0073-5 [DOI] [PubMed] [Google Scholar]
- Stern, J. T. (1999). The cost of bent‐knee, bent‐hip, bipedal gait: A reply to Crompton et al . Journal of Human Evolution, 36, 567–570. 10.1006/jhev.1999.0290 [DOI] [PubMed] [Google Scholar]
- Stern, J. T. (2000). Climbing to the top: A personal memoir of Australopithecus afarensis . Evolutionary Anthropology, 9, 113–133. 10.1002/(ISSN)1520-6505 [DOI] [Google Scholar]
- Stern, J. T. , & Susman, R. L. (1981). Electromyography of the gluteal muscles in Hylobates, Pongo and Pan: Implications for the evolution of hominid bipedality. American Journal of Physical Anthropology, 55, 153–166. 10.1002/(ISSN)1096-8644 [DOI] [Google Scholar]
- Stern, J. T. , & Susman, R. L. (1983). The locomotor anatomy of Australopithecus afarensis . American Journal of Physical Anthropology, 60, 279–317. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Susanna, I. , Alba, D. M. , Almécija, S. , & Moyà‐Solà, S. (2014). The vertebral remains of the Late Miocene great ape Hispanopithecus laietanus from Can Llobateres 2 (Vallès‐Penedès Basin, NE Iberian Peninsula). Journal of Human Evolution, 73, 15–34. 10.1016/j.jhevol.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Susman, R. L. , & Stern, J. T. (1991). Locomotor behavior of early hominids: Epistemology and fossil evidence In Coppens Y. & Senut B. (Eds.), Origine(s) de la bipéde chez les hominindés (pp. 121–132). Paris, France: CNRS. [Google Scholar]
- Susman, R. L. , Stern, J. T. , & Jungers, W. L. (1984). Arboreality and bipedality in the Hadar hominids. Folia Primatologica, 43, 113–156. 10.1159/000156176 [DOI] [PubMed] [Google Scholar]
- Sylvester, A. D. (2006). Locomotor decoupling and the origin of bipedalism. Journal of Theoretical Biology, 242, 581–590. 10.1016/j.jtbi.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Tardieu, C. , & Preuschoft, H. (1996). Ontogeny of the knee joint in humans, great apes and fossil hominids: Pelvi‐femoral relationships during postnatal growth in humans. Folia Primatologica, 66, 68–81. 10.1159/000157186 [DOI] [PubMed] [Google Scholar]
- Taylor, A. B. (1997). Scapula form and biomechanics in gorillas. Journal of Human Evolution, 33, 529–553. 10.1006/jhev.1997.0147 [DOI] [PubMed] [Google Scholar]
- Terry, G. C. , & Chopp, T. M. (2000). Functional anatomy of the shoulder. Journal of Athletic Training, 35, 248–255. [PMC free article] [PubMed] [Google Scholar]
- Testut, L. , & Latarjet, A. (1975). Tratado de anatomía humana. Tomo I: Osteología, artología y miología. Barcelona, Spain: Salvat. [Google Scholar]
- Thorpe, S. K. S. , Holder, R. L. , & Crompton, R. H. (2007). Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science, 316, 1328–1331. 10.1126/science.1140799 [DOI] [PubMed] [Google Scholar]
- Tocheri, M. W. , Solhan, C. R. , Orr, C. M. , Femiani, J. , Frohlich, B. , Groves, C. P. , … Jungers, W. L. (2011). Ecological divergence and medial cuneiform morphology in gorillas. Journal of Human Evolution, 60, 171–184. 10.1016/j.jhevol.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Tuttle, R. H. , & Basmajian, J. V. (1978a). Electromyography of the pongid shoulder muscles. Part II: Deltoid, rhomboid and “rotator cuff”. American Journal of Physical Anthropology, 49, 47–56. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Tuttle, R. H. , & Basmajian, J. V. (1978b). Electromyography of Pongid shoulder muscles III. Quadrupedal positional behavior. American Journal of Physical Anthropology, 49, 57–70. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Veeger, H. E. J. , & van der Helm, F. C. T. (2007). Shoulder function: The perfect compromise between mobility and stability. Journal of Biomechanics, 40, 2119–2129. 10.1016/j.jbiomech.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Vrba, E. S. (1979). A new study of the scapula of Australopithecus africanus from Sterkfontein. American Journal of Physical Anthropology, 51, 117–130. 10.1002/(ISSN)1096-8644 [DOI] [Google Scholar]
- Walker, A. , & Pickford, M. (1983). New postcranial fossils of Proconsul africanus and Proconsul nyanzae In Ciochon R. L. & Corruccini R. S. (Eds.), New Interpretations of Ape and Human Ancestry (pp. 325–351). New York, NY: Plenum Press; 10.1007/978-1-4684-8854-8 [DOI] [Google Scholar]
- Ward, C. V. (1997). Functional anatomy and phyletic implication of the hominoid trunk and hindlimb In Begun D. R., Ward C. V., & Rose M. D. (Eds.), Function, Phylogeny, and Fossils (pp. 101–130). Berlin, Germany: Springer; 10.1007/978-1-4899-0075-3 [DOI] [Google Scholar]
- Ward, C. V. (2002). Interpreting the posture and locomotion of Australopithecus afarensis: Were do we stand? Yearbook of Physical Anthropology, 45, 185–215. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Ward, C. V. (2013). Postural and locomotor adaptations of Australopithecus species In Reed K. E., Fleagle J. G., & Leakey R. E. (Eds.), The paleobiology of Australopithecus (pp. 235–245). New York, NY: Springer; 10.1007/978-94-007-5919-0 [DOI] [Google Scholar]
- Ward, C. V. (2015). Postcranial and locomotor adaptations of hominoids In Henke W. & Tattersall I. (Eds.), Handbook of Paleoanthropology Part 2 (pp. 1011–1030). Berlin, Germany: Springer. [Google Scholar]
- White, T. D. , Asfaw, B. , Beyene, Y. , Haile‐Selassie, Y. , Lovejoy, C. O. , Suwa, G. , & WoldeGabriel, G. (2009). Ardipithecus ramidus and the paleobiology of early hominins. Science, 326, 64–86. 10.1126/science.1175802 [DOI] [PubMed] [Google Scholar]
- White, T. D. , Lovejoy, O. C. , Asfaw, B. , Carlson, J. P. , & Suwa, G. (2015). Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both. Proceedings of the National Academy of Sciences of the United States of America, 112, 4877–4884. 10.1073/pnas.1403659111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, T. D. , Suwa, G. , & Asfaw, B. (1994). Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature, 371, 306–312. 10.1038/371306a0 [DOI] [PubMed] [Google Scholar]
- Williams, S. A. (2012). Evolution of the hominoid vertebral column. PhD Dissertation, University of Illinois at Urbana‐Campaign, Illinois.
- Young, N. M. (2008). A comparison of the ontogeny of shape variation in the anthropoid scapula: Functional and phylogenetic signal. American Journal of Physical Anthropology, 136, 247–254. 10.1002/(ISSN)1096-8644 [DOI] [PubMed] [Google Scholar]
- Young, N. M. , Capellini, T. D. , Roach, N. T. , & Alemseged, Z. (2015). Fossil hominin shoulder support an African ape‐like last common ancestor of humans and chimpanzees. Proceedings of the National Academy of Sciences of the United States of America, 112, 11829–11834. 10.1073/pnas.1511220112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapfe, H. (1958). The skeleton of Pliopithecus (Epipliopithecus) vindobonensis Zapfe and Hürzeler. American Journal of Physical Anthropology, 16, 441–457. 10.1002/(ISSN)1096-8644 [DOI] [Google Scholar]
- Zollikofer, C. P. E. , Ponce de León, M. S. , Lieberman, D. E. , Guy, F. , Pilbeam, D. , Likius, A. , … Brunet, M. (2005). Virtual cranial reconstruction of Sahelanthropus tchadensis . Nature, 434, 755–759. 10.1038/nature03397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


