Abstract
Urban landscapes are associated with abiotic and biotic environmental changes that may result in potential stressors for wild vertebrates. Urban exploiters have physiological, morphological, and behavioral adaptations to live in cities. However, there is increasing evidence that urban exploiters themselves can suffer from urban conditions, especially during specific life‐history stages. We looked for a link between the degree of urbanization and the level of developmental stress in an urban exploiter (the house sparrow, Passer domesticus), which has recently been declining in multiple European cities (e.g., London, UK). Specifically, we conducted a large‐scale study and sampled juvenile sparrows in 11 urban and rural sites to evaluate their feather corticosterone (CORT) levels. We found that juvenile feather CORT levels were positively correlated with the degree of urbanization, supporting the idea that developing house sparrows may suffer from urban environmental conditions. However, we did not find any correlation between juvenile feather CORT levels and body size, mass, or body condition. This suggests either that the growth and condition of urban sparrows are not impacted by elevated developmental CORT levels, or that urban sparrows may compensate for developmental constraints once they have left the nest. Although feather CORT levels were not correlated with baseline CORT levels, we found that feather CORT levels were slightly and positively correlated with the CORT stress response in juveniles. This suggests that urban developmental conditions may potentially have long‐lasting effects on stress physiology and stress sensitivity in this urban exploiter.
Keywords: birds, CORT, development, morphology, stress, urbanization
1. INTRODUCTION
Urbanization induces profound and rapid landscape changes, which are associated with a reduction and alteration of natural habitats (Adams & Klobodu, 2017; Gil & Brumm, 2014; Marzluff, Bowman, & Donnelly, 2001; McKinney, 2002; Xu, Xie, Qi, Luo, & Wang, 2018). In cities, species have to cope with intense biotic and abiotic changes in their environment, such as urban features including buildings and roads (McKinney, 2002; Seress & Liker, 2015), different types or quantities of food (Haverland & Veech, 2017; Newsome et al., 2014; Seress & Liker, 2015; Vuorisalo et al., 2003), modifications to vegetation and the presence of exotic plants (Chace & Walsh, 2006; McKinney, 2006), and changes in the local climate due to anthropogenic activities (Seress & Liker, 2015). Urban species also have to face biochemical (Cai & Calisi, 2016; Gorissen, Snoeijs, Duyse, & Eens, 2005; Grimm et al., 2008; Seress & Liker, 2015), noise (Francis, Kleist, Ortega, & Cruz, 2012; Halfwerk et al., 2011; Meillère, Brischoux, & Angelier, 2015; Slabbekoorn et al., 2010), light (Kempenaers, Borgström, Loës, Schlicht, & Valcu, 2010; Navara & Nelson, 2007; Ouyang et al., 2017; Stone, Harris, & Jones, 2015), and/or microwave pollution (Balmori, 2009; Everaert & Bauwens, 2007). Considering these environmental modifications, the presence or absence of a given species in the urban landscape mainly depends on its ability to adjust to these novel environmental conditions (Candolin & Wong, 2012). Many species cannot cope with these changes (i.e., “urban avoiders”; Blair, 1996), and species richness decreases as the level of urbanization increases, leading to homogenized communities in cities (Gil & Brumm, 2014; McKinney, 2006). However, a few species can survive and reproduce in cities, and they may even benefit from urban conditions (i.e., “urban exploiters”; Blair, 1996; Kark, Iwaniuk, Schalimtzek, & Banker, 2006).
Avian species are known to exhibit important changes in behavior, physiology, and morphology in urbanized areas (Gil & Brumm, 2014; Kark et al., 2006; Seress & Liker, 2015). For example, noise and light pollution are known to affect the reproductive behavior of small passerines like European robins (Erithacus rubecula), great tits (Parus major), pied flycatchers (Ficedula hypoleuca) and house sparrows (Passer domesticus; Miller, 2006; Halfwerk & Slabbekoorn, 2009; Kempenaers et al., 2010; de Jong et al., 2015; Meillère, Brischoux, & Angelier, 2015) and, consequently, their reproductive performance (Francis, Ortega, & Cruz, 2009; Halfwerk et al., 2011; Kempenaers et al., 2010; Kight & Swaddle, 2011; Kleist, Guralnick, Cruz, Lowry, & Francis, 2017). Similarly, an urban diet can have detrimental effects on reproductive performance (Demeyrier, Charmantier, Lambrechts, & Grégoire, 2017; Peach, Mallord, Ockendon, Orsman, & Haines, 2015; Plummer, Bearhop, Leech, Chamberlain, & Blount, 2013) and lead to nutritional stress in urban birds such as corvids (Heiss, Clark, & McGowan, 2009; Jones & Reynolds, 2008). These nutritional constraints can also affect development. For example, in great tits and house sparrows, urban individuals are also usually smaller and lighter than rural ones (Biard et al., 2017; Meillère et al., 2017; Meillère, Brischoux, Parenteau, & Angelier, 2015).
A small body size in urban individuals may be a result of developmental conditions. Corticosterone (CORT) is an important hormone that is relevant when evaluating the constraints that may occur during development. CORT is a glucocorticoid hormone that is secreted by the Hypothalamus–Pituitary–Adrenal axis (HPA) in response to unpredictable events and mediates allostasis in birds (i.e., stability through changes; McEwen & Wingfield, 2003; Landys, Ramenofsky, & Wingfield, 2006). High levels of CORT in birds are often associated with lower performance. In particular, high levels of CORT are associated with poor developmental conditions in chicks (Love, McGowan, & Sheriff, 2012; Wada, Salvante, Wagner, Williams, & Breuner, 2009), and a higher risk of reproductive failure in breeders (Angelier, Wingfield, Weimerskirch, & Chastel, 2010; Bonier, Martin, Moore, & Wingfield, 2009; Bonier, Moore, Martin, & Robertson, 2009; Cyr & Romero, 2007; Wingfield & Romero, 2001). Consequently, the study of CORT levels may be used to assess the ability of individuals and species to cope with urban conditions (Bonier, 2012; Zhang et al., 2011). For example, studies have shown that CORT secretion increases with nutritional stress in multiple species, such as white‐crowned sparrows (Zonotrichia leucophrys), eastern bluebirds (Sialia sialis), and snow petrels (Pagodroma nivea; Lynn, Breuner, & Wingfield, 2003; Lynn, Prince, & Phillips, 2010; Angelier, Wingfield, Parenteau, Pellé & Chastel, 2015). CORT levels are also correlated with other urban constraints, such as pollution, predation pressure, and/or human disturbance (Bonier, 2012; Cyr & Romero, 2007; Foltz et al., 2015; Love et al., 2012; Meillère et al., 2016; Ouyang et al., 2015; Zhang et al., 2011).
The house sparrow is commensal with humans and is considered to be one of the most highly adapted species to urban conditions (Anderson, 2006). Although this species was previously a widespread avian urban exploiter, urban populations of house sparrows have been strongly declining in European cities in the past few decades, particularly in highly urbanized cities like London (UK) and Antwerp (Crick, Robinson, Appleton, Clark, & Rickard, 2002; De Coster, Laet, Vangestel, Adriaensen, & Lens, 2015; Laet & Summers‐Smith, 2007; Shaw, Chamberlain, & Evans, 2008; Summers‐Smith, 2003). Recently, it has been suggested that urban conditions could be especially detrimental to developing sparrows: Urban conditions correlate negatively with growth, body size, and feather quality (Meillère et al., 2017; Meillère, Brischoux, Parenteau, et al., 2015; Seress et al., 2012). Previously, circulating blood CORT levels and body condition have been measured in adults and juveniles of both urban and rural populations, but no difference has been found (Bókony, Seress, Nagy, Lendvai, & Liker, 2012; Meillère et al., 2017; Meillère, Brischoux, Parenteau, et al., 2015). In a recent study, Hudin et al. (2018) compared feather CORT levels between urban and rural sparrows, and they did not find any significant difference in feather CORT levels between these populations. However, they focused on a specific geographical area and considered urbanization as a categorical factor without precisely quantifying the degree of urbanization (see Liker, Papp, Bókony, & Lendvai, 2008). Such a quantitative approach would be beneficial to generalize these previously reported results (Hudin et al., 2018) and to test whether the effect of urbanization on CORT levels may vary according to the degree of urbanization.
In this study, we investigated the impact of urbanization on the stress physiology of growing house sparrows. It is difficult to measure CORT levels in house sparrow nestlings because plasma CORT levels vary throughout the developmental period (Wada et al., 2008, 2009), and nests are very difficult to access in cities. To solve these problems, we used feather samples. House sparrow nestlings retain the feathers that they grow during development for several weeks after fledging, so it is possible to sample feathers after juveniles leave the nest. Further, circulating CORT is incorporated into the feather matrix during feather growth (Bortolotti, Blas, & German, 2008; Jenni‐Eiermann et al., 2015) and, therefore, feathers that were grown in the nest provide an integrative measure of developmental CORT levels. However, it is still important to measure circulating plasma CORT levels, because they can help us understand the long‐term effects of a nestling's exposure to stress. Plasma CORT levels give information about juvenile condition by measuring the normal expression of CORT (baseline CORT level), and the functionality of the HPA axis later in life by measuring their response to stress (stress‐induced CORT level). Using this method, we investigated (a) the link between urbanization and feather CORT levels in juvenile sparrows, (b) the link between feather CORT levels and morphological attributes of juveniles (body size, mass, and condition), and (c) the link between feather CORT levels and plasma CORT levels. To do so, we led a large‐scale sampling effort (feather plucking and morphological measurements of juvenile sparrows) thanks to a network of volunteers and qualified ornithologists who sampled 11 sites located through an urbanization gradient (from rural areas to large cities). First, we predicted that if urbanization is associated with important developmental constraints, feather CORT levels would increase as the degree of urbanization increased. Second, if developmental stress affects both CORT regulation and growth, we predicted that body size and condition would correlate negatively with feather CORT levels in juvenile sparrows. Finally, if developmental stress affects the HPA axis with long‐lasting effects on CORT regulation, we expected that feather CORT levels may be positively correlated with plasma CORT levels in juveniles. Alternatively, feather CORT levels and juvenile plasma CORT levels may be independent if such long‐lasting effects are not apparent.
2. MATERIALS AND METHODS
2.1. Ethics statement
This study was carried out in accordance with all applicable institutional and/or national guidelines for the care and use of animals. All experimental procedures were approved by the “Comité d'Ethique en Expérimentation Animale Poitou‐Charentes”, France (authorization number: CE2012–7). Permits for the capture, sampling and banding of house sparrows were issued by the “Centre de Recherches sur la Biologie des Populations d'Oiseaux” (CRBPO) to all the ringers involved in the sampling.
2.2. Study sites and captures
In 2013 (June–August), a total of 111 juvenile house sparrows were captured with mist nets at 11 sites in France (Figure 1; geographic coordinates of the capture sites and sample sizes for each population are summarized in Table 1). The 11 sites differed in urbanization level, ranging from sparsely populated areas (e.g., isolated farms, small villages) to highly urbanized city centers. To quantify the degree of urbanization of each capture site, we used the method developed by Liker et al. (2008) for house sparrows (see also Meillère, Brischoux, Parenteau, et al., 2015; Meillère et al., 2017). Briefly, we used digital aerial photographs of 1 km2 areas around each capture site that we divided into 100 cells. For each capture site, we extracted five habitat characteristics as follows: mean building density score, number of cells with high building density, mean vegetation density score, number of cells with high vegetation density, and number of cells with roads. Then, we used the PC1 value from a principal component analysis (PCA) of these five variables to attribute an urbanization score to each site (Table 1). The PC1 accounted for 93.3% of the total variance and was strongly positively correlated with artificial surfaces (building density and roads; all r > 0.806) and negatively with vegetation cover (all r < −0.980).
Figure 1.
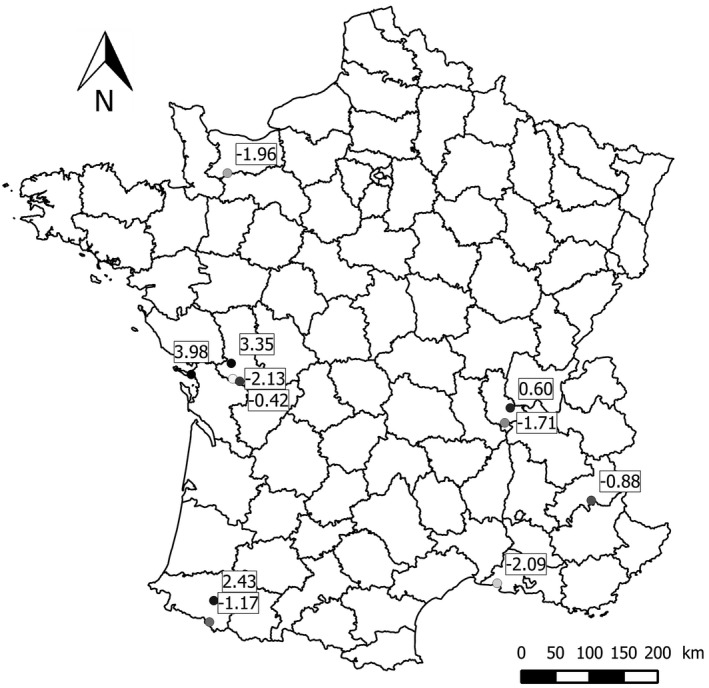
Geographical localization of the 11 capture sites sampled in this study
Table 1.
Habitat characteristics of the capture sites and corresponding sample sizes. Sites are ordered from the most to the least urbanized site (in bold: PC1 values from a principal component analysis conducted on the five habitat variables). Sites with * are those used for additional morphological and physiological measurements. The sites are shown in the map in Figure 1
| Capture site (geographic coordinates) | Habitat characteristics | Sample sizes | |||||
|---|---|---|---|---|---|---|---|
| Mean building density score | Number of cells with high building density | Mean vegetation density | Number of cells with high vegetation density | Number of cells with road | Urbanization score (PC1) | Juveniles | |
| La Rochelle* (46.148; −1.154) | 1.22 | 36 | 0.62 | 11 | 95 | 3.98 | 13 |
| Niort* (46.313; −0.479) | 1.18 | 24 | 0.82 | 11 | 97 | 3.35 | 10 |
| Oloron‐Ste‐Marie (43.196; −0.611) | 1.11 | 31 | 1.17 | 32 | 68 | 2.43 | 11 |
| Lyon (45.777; 4.854) | 0.52 | 7 | 1.52 | 57 | 89 | 0.60 | 7 |
| Villefollet* (46.127; −0.268) | 0.45 | 11 | 1.72 | 74 | 48 | −0.42 | 8 |
| Chorges (44.546; 6.266) | 0.30 | 8 | 1.71 | 81 | 42 | −0.88 | 10 |
| Etsaut (42.913; −0.572) | 0.34 | 3 | 1.92 | 92 | 54 | −1.17 | 3 |
| Givors (45.560; 4.772) | 0.17 | 0 | 2.00 | 100 | 50 | −1.71 | 15 |
| Rully (48.824; −0.715) | 0.15 | 2 | 1.97 | 97 | 27 | −1.96 | 8 |
| Saintes‐Maries‐de‐la‐mer (43.490; 4.401) | 0.12 | 0 | 2.00 | 100 | 30 | −2.09 | 13 |
| Villiers‐en‐Bois* (46.147; −0.426) | 0.11 | 1 | 1.98 | 98 | 23 | −2.13 | 13 |
2.3. Feather collection (11 sites), morphological measurements, and blood sampling (4 sites)
Feathers were collected from all study sites. The two innermost rectrices of each individual were collected and stored in dry paper envelopes until laboratory analyses. We weighed (high‐resolution balance: ±0.01 mg) and measured the length (digital caliper: ±0.01 mm; see Meillère et al., 2017) of all feathers. In addition to feather collections, morphological measurements and blood samples were collected at 4 of the 11 sites (two urban and two rural; see Table 1). For all juveniles captured at these four sites, body mass (electronic balance: ±0.1 g), wing length (steel rule: ±1 mm), tarsus length, and bill length (caliper: ±0.1 mm) were measured. Additionally, fat and muscle scores were recorded as detailed in Kaiser (1993), Brown (1996) and Leloutre, Gouzerh, and Angelier (2014). For consistency and to avoid potential methodological bias, morphological measurements were all collected by the same person. In addition to morphological measurements, juveniles from these four sites were bled within 3 min of capture (mean ± SE: 2 min 39 s ± 2 s; range: 1 min 13 s – 3 min 45 s) to quantify baseline CORT concentration (Romero & Reed, 2005). Following this first blood sample, birds were kept in cloth bags and sampled a second time 30 min after capture to obtain stress‐induced CORT levels (Wingfield, Davey, Peter, & Tobe, 1994). CORT levels are at a maximum after 30‐min of restraint in this species (Romero, Cyr, & Romero, 2006), and therefore, this allowed us to assess the CORT stress response (Wingfield et al., 1998). We defined the “increase in plasma CORT” as the difference between stress‐induced CORT and baseline CORT levels. All blood samples were collected from the alar vein using a 25‐gauge needle and heparinized microcapillary tubes (up to 150 µl for CORT assay). Blood samples were centrifuged (4,500 rpm, 7 min), and plasma and red blood cells were separated (plasma for CORT assay and red blood cells for molecular sexing). Then, they were kept at −20°C until laboratory analyses at the “Centre d'Etudes Biologiques de Chizé” (hereafter CEBC).
To assess juvenile body condition, we used the “scaled mass index” (SMI) as recommended by Peig & Green (2009, 2010). The SMI adjusts the mass of all individuals to that which would be expected if they all had the same body size (Peig & Green, 2009). We used tarsus length to calculate the SMI because it had the greatest correlation with body mass (tarsus length: r = 0.588, p < 0.001; wing length: r = 0.313, p = 0.041). The SMI was computed for each individual as follows:
where M i and L i are body mass and tarsus length of the individual i, respectively, L 0 is the arithmetic mean value of tarsus length for the whole study population (L 0 = 18.18 mm, n = 43), and b is the slope estimate of a standardized major axis (SMA) regression of log‐transformed body mass on log‐transformed tarsus length (b = 1.86).
2.4. Molecular sexing and CORT analyses
The sex of juveniles was determined by molecular sexing as detailed in Fridolfsson and Ellegren (1999). DNA was extracted from blood samples, and PCR reactions were performed in order to amplify genomic DNA. DNA sequences with only the CHDIZ fragment correspond to males, whereas DNA sequences with the CHDIZ and/or the CHDIW fragment correspond to females. Plasma concentrations of CORT were measured in duplicate by radio‐immunoassay, as previously described (Lormée, Jouventin, Trouve, & Chastel, 2003). Feather CORT assays were conducted at the CEBC by following previously validated methods (Bortolotti et al., 2008) with minor improvements (see Meillère et al., 2016). Specifically, 10 ml of methanol (HPLC grade) were added to each feather sample to extract CORT from the whole feather. Then, feathers were incubated at 50°C overnight in a shaking water bath, the methanol was separated from feather material, and the feather remnants were washed before being added to the original methanol extract. Then, feather extracts were analyzed by radio‐immunoassay at the CEBC as previously described (Lormée et al., 2003). Feather CORT levels were expressed either as ng of CORT per mg of feather (ng/mg) or as ng of CORT per mm of feather (ng/mm). The minimum detectable CORT level was 0.83 ng/ml. All samples were run in three assays, and the intra‐ and inter‐assay coefficients of variation were 7.07% and 9.99%, respectively.
2.5. Statistical analyses
All statistical analyses were performed in R 3.4.3 (R Core Team, 2016). First, to test the correlation of urbanization with feather CORT levels in juveniles, we fitted linear mixed models (LMMs, normal error distribution, identity link function) with “capture site” (a total of 11 sites) as a random factor to control for the non‐independence of individuals captured at the same site. We used “urbanization” (PC1 score) and “capture date” as fixed effects in our model. We did not include the “sex” in this analysis because it was unknown for all individuals that were not bled (60 birds from 7 sites). Second, to test whether developmental stress affects both CORT regulation and growth, we fitted LMMs (normal error distribution, identity link function) with “SMI,” “tarsus length,” “body mass,” “baseline CORT,” “stress‐induced CORT levels,” or “increase in plasma CORT” as response variables. We used “capture site” as a random factor, and “feather CORT level” and “sex” as fixed effects in these models. We verified that all models met the assumptions of equal variances and normal residuals. Baseline CORT levels were log10‐transformed to ensure the normality of model residuals, but we present non‐transformed values to facilitate interpretation. Feather CORT levels in ng/mm were highly correlated with feather CORT levels in ng/mg (r = 0.837; p < 0.001), and all the results were qualitatively similar when using one measure or the other. Therefore, we only present the feather CORT data expressed in ng/mg in the rest of the manuscript (but see Supporting Information Table S1 and Figure S1 for all results and graph with feather CORT expressed in ng/mm).
3. RESULTS
3.1. Urbanization and feather CORT levels (11 sites)
Feather CORT levels were significantly and positively correlated with urbanization score (LMM: t = 3.79, p = 0.004; Figure 2). Feather CORT levels were in mean 19.45% higher in the most urban site relative to the most rural site. Feather CORT levels were not explained by the capture date (t = −0.03, p = 0.974).
Figure 2.
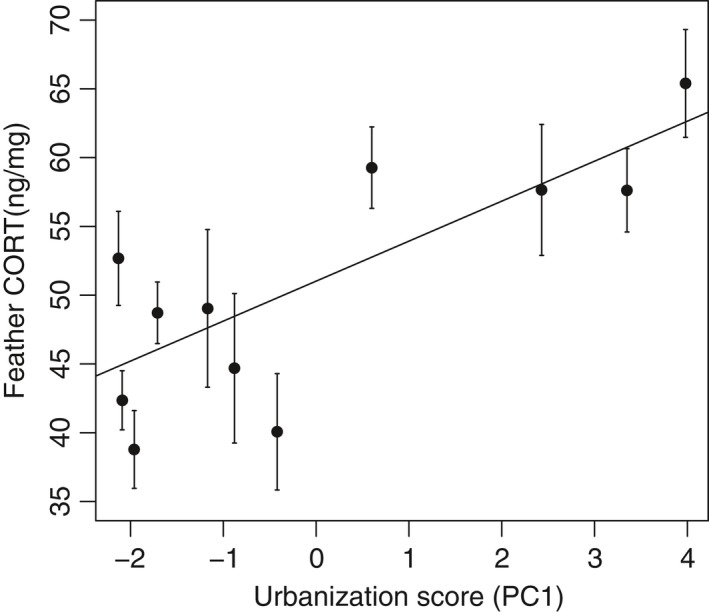
Relationship between the degree of urbanization and feather CORT level expressed in ng/mg in juvenile sparrows. The line represents a significant relationship between feather CORT level and urbanization score
3.2. Relationship between feather CORT levels and body size and condition (4 sites)
Neither SMI, tarsus length, nor body mass were explained by feather CORT levels, sex, or the “CORTf x Sex” interaction (Table 2a–c).
Table 2.
Results of the linear mixed models (LMMs) when investigating the influence of feather CORT levels on body size, body condition, and plasma CORT levels, with feather CORT expressed in ng/mg. All models include capture site as a random factor. Estimates are for males in comparison to females
| Model | Parameter | Estimate ±SE | t | p | 95% CI |
|---|---|---|---|---|---|
| (a) SMI | Intercept | 25.49 ± 1.40 | 18.18 | <0.001 | 22.64; 28.34 |
| CORTf | −0.01 ± 0.02 | −0.57 | 0.571 | −0.06; 0.03 | |
| Sex | −1.96 ± 1.98 | −0.99 | 0.330 | −5.98; 2.07 | |
| CORTf x Sex | 0.03 ± 0.03 | 0.98 | 0.332 | −0.04; 0.10 | |
| (b) Tarsus length | Intercept | 18.21 ± 0.55 | 33.16 | <0.001 | 17.15; 19.27 |
| CORTf | −0.004 ± 0.009 | −0.48 | 0.637 | −0.02; 0.01 | |
| Sex | 1.13 ± 0.78 | 1.44 | 0.159 | −0.38; 2.64 | |
| CORTf x Sex | −0.01 ± 0.01 | −1.006 | 0.322 | −0.04; 0.01 | |
| (c) Body mass | Intercept | 25.56 ± 1.46 | 17.54 | <0.001 | 22.60; 28.52 |
| CORTf | −0.02 ± 0.02 | −1.02 | 0.317 | −0.07; 0.02 | |
| Sex | 0.88 ± 2.08 | 0.42 | 0.675 | −3.35; 5.11 | |
| CORTf x Sex | 0.0003 ± 0.04 | 0.009 | 0.993 | −0.07; 0.07 | |
| (d) Baseline CORT level | Intercept | 1.30 ± 1.25 | 1.05 | 0.305 | 1.10; 3.71 |
| CORTf | −0.02 ± 0.02 | −0.81 | 0.428 | −0.06; 0.02 | |
| Sex | −1.57 ± 1.91 | −0.82 | 0.419 | −5.26; 2.12 | |
| CORTf x Sex | 0.01 ± 0.03 | 0.30 | 0.764 | −0.05 0.07 | |
| (e) Stress‐induced CORT level | Intercept | 12.59 ± 5.61 | 2.24 | 0.031 | 1.18; 24.01 |
| CORTf | 0.17 ± 0.09 | 1.87 | 0.070 | −0.01; 0.35 | |
| Sex | 5.77 ± 7.65 | 0.75 | 0.456 | −9.79; 21.33 | |
| CORTf x Sex | −0.20 ± 0.13 | −1.52 | 0.138 | −0.47; 0.07 | |
| (f) Increase in plasma CORT | Intercept | 9.72 ± 5.33 | 1.82 | 0.080 | −1.24; 20.68 |
| CORTf | 0.18 ± 0.08 | 2.18 | 0.039 | 0.01; 0.36 | |
| Sex | 7.25 ± 7.28 | 1.00 | 0.329 | −7.71; 22.21 | |
| CORTf x Sex | −0.21 ± 0.13 | −1.63 | 0.115 | −0.48; 0.05 |
3.3. Relationship between feather CORT level and plasma CORT levels (4 sites)
Neither baseline CORT levels nor stress‐induced CORT levels were explained by feather CORT levels (Figure 3a,b), sex, or the “CORTf x Sex” interaction (Table 2d,e), although stress‐induced CORT levels were positively but weakly associated with feather CORT levels (Table 2e). However, the increase in plasma CORT in juveniles was significantly and positively related to feather CORT levels (Table 2f; Figure 3c). Moreover, this increase was not significantly affected by sex or the “CORTf x Sex” interaction, suggesting the effect of feather CORT levels on the increase in plasma CORT did not significantly differ between males and females (Table 2f).
Figure 3.
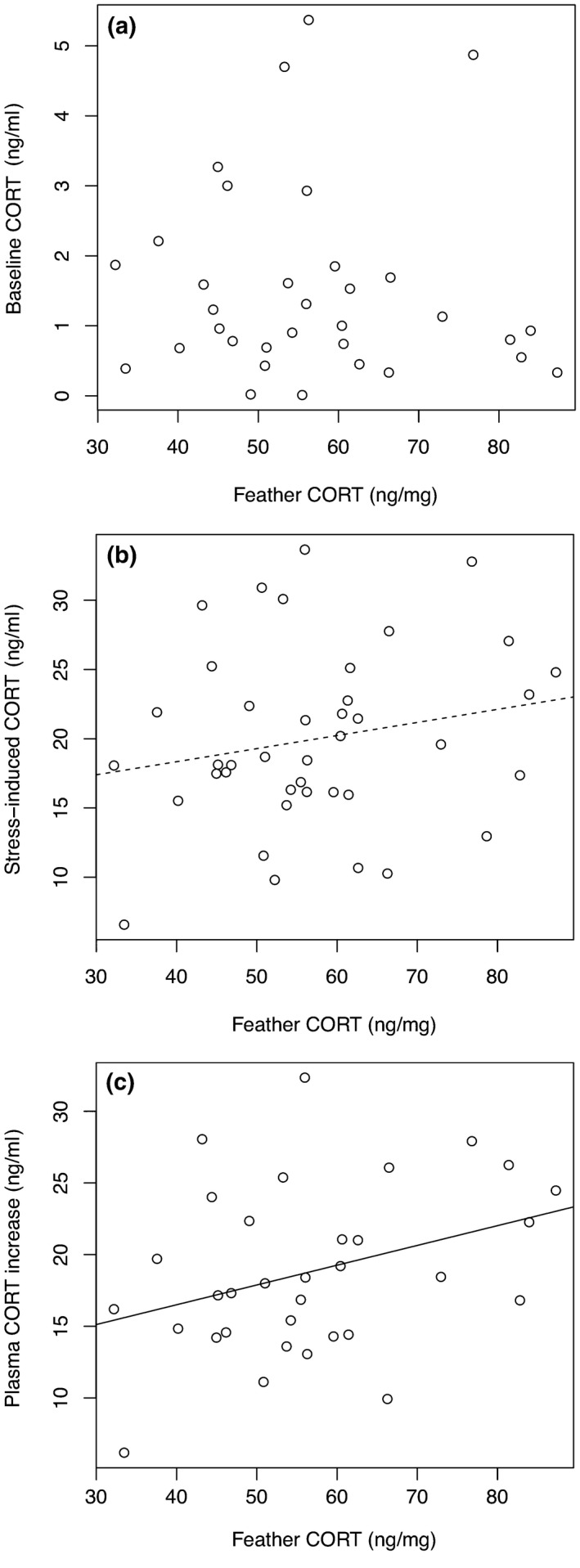
Relationship between feather CORT levels expressed in ng/mg and (a) baseline CORT level, (b) stress‐induced CORT level, and (c) increase in plasma CORT in juvenile sparrows. The dotted line represents a marginal and non‐significant relationship (p < 0.10); the solid line represents a significant relationship (p < 0.05)
4. DISCUSSION
The house sparrow may represent a sentinel species of the urban environment and, thus, is a good model to study the functional constraints of living in cities for urban exploiters. To better understand the potential causes of the recent decline of urban house sparrow populations, we conducted a large‐scale study to evaluate the impact of urbanization on the stress physiology of juvenile house sparrows. By using an integrative measure of CORT from feathers sampled across a rural–urban gradient, we found that urban juveniles had higher feather CORT levels than rural ones. These feathers grew during the developmental phase (i.e., in the nest); thus, our study highlights the functional constraints imposed by a city lifestyle and suggests that these constraints are most likely to occur during the developmental period prior to fledging. Feather CORT levels were not correlated to any juvenile attribute (body size, mass, or condition) except for the CORT stress response (the increase in CORT levels in response to a standardized stress protocol), suggesting that urban environmental conditions may affect the ontogeny of the CORT stress response in house sparrow nestlings.
4.1. Impact of urbanization on feather CORT level
We showed that an increasing level of urbanization was associated with higher feather CORT levels in juvenile house sparrows. This result partly contrasts with those from Hudin et al. (2018). In their study, they found no difference in feather CORT levels between urban and rural juvenile sparrows. Contrary to our study, they captured birds in early fall, when the post‐juvenile molt has already occurred in house sparrows. This could explain the discrepancy because most urban‐related constraints seem to occur during the developmental period when sparrows are growing their feathers in the nest (Meillère et al., 2017; Seress et al., 2012). Elevated feather CORT levels usually result from elevated circulating plasma CORT levels at the time of feather growth (Bortolotti et al., 2008), as experimentally demonstrated in pigeons (Columbia livia domestica) and European starlings (Sturnus vulgaris; Lattin, Reed, DesRochers, & Romero, 2011; Jenni‐Eiermann et al., 2015). Consequently, elevated feather CORT levels have been associated with challenging environmental conditions in various species (e.g., geese and eiders in Legagneux et al., 2013; tree and barn swallows (Tachycineta bicolor, Hirundo rustica) in Fairhurst et al., 2015, 2011; rhinoceros auklet (Cerorhinca monocerata) in Will et al., 2015; mallard duckling (Anas platyrhynchos) in Johns, Marchant, Fairhurst, Speakman, & Clark, 2017) and are associated with reduced survival in house sparrows, specifically (e.g., Koren et al., 2012). Our results support the hypothesis that urban sparrows are constrained during their development and suggest that urban juvenile sparrows may have a lower survival probability than rural ones (Meillère et al., 2017; Meillère, Brischoux, Parenteau, et al., 2015; Seress et al., 2012). This interpretation is supported by multiple ecological studies, which have reported a negative impact of urbanization on house sparrow populations (e.g., population decline: De Coster et al., 2015; reduced breeding success: Seress et al., 2012; reduced body size and feather quality: Meillère et al., 2017). However, exposure to moderate CORT levels during development can also be beneficial under some circumstances (reviewed in Madliger & Love, 2014). For example, Grava et al. (2013) found that elevated juvenile feather CORT levels were associated with potential benefits later in life (i.e., post‐fledging habitat quality, syrinx mass). Future studies should examine the fitness consequences of slight inter‐individual variation in feather CORT levels in juvenile sparrows.
Interestingly, visual inspection of our data suggests that urbanization may only be a constraint when it reaches a specific threshold (see. Figure 2). However, we lack statistical power to run non‐linear models (e.g., broken‐stick models), and sampling additional populations would be required to properly test this hypothesis.
There are numerous potential sources of stress for birds developing in an urban environment, which could impact nestlings either directly or indirectly through the modification of environmental constraints (Bonier, 2012; Chace & Walsh, 2006; Meillère, Brischoux, & Angelier, 2015; Seress & Liker, 2015). Elevated feather CORT levels could result from a change in diet and food quality in urban environments. Recently, Hudin et al. (2018) experimentally showed that an urban diet is associated with elevated feather CORT levels in juvenile house sparrows, supporting the idea that the urban diet may not be suitable for developing house sparrow nestlings (Meillère et al., 2017). Indeed, nestling house sparrows need a protein‐rich diet to grow properly (Anderson, 2006; White, 2008), but the availability of invertebrates is lower in cities relative to rural areas (Chace & Walsh, 2006; Hudin et al., 2017; Moudrá, Zasadil, Moudrý, & Šálek, 2018; Paker, Yom‐Tov, Alon‐Mozes, & Barnea, 2014; Summers‐Smith, 2003), and urban house sparrow parents feed their chicks a lower quality diet than their rural counterparts (Seress & Liker, 2015). In addition, urban food may contain heavy metals, which may contaminate nestlings (Dauwe, Janssens, Bervoets, Blust, & Eens, 2004; Raupp, Shrewsbury, & Herms, 2010; Zvereva & Kozlov, 2010) and lead to potential effects on growth, behavior or the immune system (e.g., pied flycatchers in Eeva, Hasselquist, Tummeleht, Nikinmaa, & Ilmonen, 2005; great tits in Gorissen et al., 2005; feral pigeons (Columbia livia) in Chatelain, Gasparini, & Frantz, 2016; reviewed in Montiglio & Royauté, 2014). A low‐quality and/or contaminated diet could be associated with nutritional stress and, therefore, with elevated feather CORT levels in urban chicks (e.g., food restriction in rhinoceros auklets: Will et al., 2014, 2015; heavy metal contamination in blackbirds (Turdus merula): Meillère et al., 2016), especially in house sparrows (Hudin et al., 2018). Feather CORT levels can also be affected by weather conditions, as previously shown in several species (Legagneux et al., 2013), including the house sparrow (Treen, Hobson, Marchant, & Bortolotti, 2015). Unfortunately, climate data were not available for most sites, so we could not test this hypothesis. However, our results are unlikely to be biased by inter‐site variation in climate because the rural and urban sites were not spatially segregated (see Figure 1).
Higher feather CORT levels in urban nestlings could also be a result of environmental perturbations, such as noise and light pollution. Noise pollution could indirectly affect nestlings by disrupting aspects of parental care such as incubation commitment or brood provisioning (Injaian, Taff, & Patricelli, 2018; Meillère, Brischoux, & Angelier, 2015; Schroeder, Nakagawa, Cleasby, & Burke, 2012). Noise pollution could also directly disturb the nestlings, and as a result, increase nestling CORT secretion as previously shown in a few bird species (Kleist et al., 2017; Davies, Beck, & Sewall, 2018; but see Angelier, Meillère, Grace, Trouvé, & Brischoux, 2016). Previous studies have demonstrated that urban noise is associated with changes in nestlings growth, metabolism, and stress response, and with a reduced probability of survival in several bird species (e.g., tree swallows in Leonard & Horn, 2008; Injaian et al., 2018; white‐crowned sparrows (Zonotrichia leucophrys orienta) in Crino, Johnson, Blickley, Patricelli, & Breuner, 2013; house sparrows in Meillère, Brischoux, Ribout, & Angelier, 2015; Brischoux, Meillère, Dupoué, Lourdais, & Angelier, 2017; western bluebirds (Sialia mexicana) in Kleist et al., 2017). Light pollution may also result in an increase in feather CORT because it artificially increases the amount of time nestlings are active. For instance, an experimental study on great tits showed that urban light was associated with increased brood provisioning (Titulaer, Spoelstra, Lange, & Visser, 2012) and, therefore, with increased begging activity in urban nestlings (Ouyang et al., 2015; Titulaer et al., 2012). Increased activity (especially begging activity) has been associated with increased CORT levels in wild birds (Kitaysky, Kitaiskaia, Piatt, & Wingfield, 2003; Loiseau, Sorci, Dano, & Chastel, 2008; reviewed in Landys et al., 2006). Since high‐quality food may be limited in cities (Seress & Liker, 2015), urban nestlings may be expending more energy by begging than they are receiving, leading to nutritional stress and increased feather CORT levels (Will et al., 2014).
4.2. Feather CORT levels and body size
In previous studies, we found that body size decreases as the degree of urbanization increases in house sparrows (Meillère et al., 2017; Meillère, Brischoux, Parenteau, et al., 2015). Although we found a positive correlation between feather CORT levels and the degree of urbanization, we surprisingly did not find any significant relationship between feather CORT levels and body size in juvenile house sparrows. As feather and structural growth occur simultaneously in the nest, our results suggest that CORT exposure during the post‐hatching period is not directly correlated with nestling growth. Previous studies have shown that elevated plasma and feather CORT levels can be correlated with reduced body size and mass in pigeon and sparrow nestlings (Grace, Froud, Meillère, & Angelier, 2017; Jenni‐Eiermann et al., 2015). Interestingly, this correlation between CORT levels, body size, and body mass seems to attenuate and even disappear as the nestlings develop, suggesting that nestlings can compensate for a slow initial growth despite stressful or challenging conditions (Grace et al., 2017; Jenni‐Eiermann et al., 2015). This may explain why feather CORT levels and juvenile body size were not correlated in our study, although this may also result from a lack of statistical power (tarsus length was only available from 4 sites). Furthermore, CORT is progressively and irreversibly incorporated into feathers during growth and, therefore, feather CORT levels represent a sum of CORT exposure during feather growth (Bortolotti et al., 2008; Jenni‐Eiermann et al., 2015; Romero & Fairhurst, 2016; Will et al., 2014). Growth and juvenile body size may be affected by acute and temporary stressful events during growth, which are not identifiable in the integrated feather CORT measurement.
4.3. Feather CORT level and juvenile body condition and CORT levels
Juvenile body mass and condition were not influenced by feather CORT levels. Unlike body size, body mass and condition are labile, and thus are likely to be more dependent on individual characteristics and the local environmental conditions that are present closer to the time of capture (e.g., food, weather, predation; Krebs & Singleton, 1993; Gosler, 1996; Schulte‐Hostedde, Millar, & Hickling, 2001; Peig & Green, 2009). In contrast, feather CORT is determined weeks prior to the time of capture. This may blur a potential relationship between feather CORT levels and condition in juvenile sparrows. An alternative explanation may be that there is a progressive selective disappearance of individuals with low body condition in the population. Post‐fledging mortality is high in small passerines including house sparrows (Leloutre et al., 2014). In addition, higher feather CORT concentrations are associated with lower survival probability in adult house sparrows (Koren et al., 2012). This selection may eliminate individuals with a lower body mass and condition (see Lendvai, Loiseau, Sorci, & Chastel, 2009) and may, therefore, mask a potential relationship between developmental conditions (e.g., feather CORT levels) and juvenile attributes (body size, body mass and condition, and baseline CORT levels).
We measured baseline CORT and stress‐induced CORT in order to test whether the HPA axis and juvenile sensitivity to stress may be affected by developmental conditions and CORT exposure during development (i.e., feather CORT). We did not find evidence for any correlation between feather CORT levels and baseline CORT levels. However, we found a non‐significant positive trend between stress‐induced CORT levels and feather CORT levels. We also found a significant positive relationship between feather CORT levels and the CORT stress response (i.e., the increase in CORT levels in response to a standardized stressor) in juvenile sparrows. Such result has previously been found with a positive relationship between stress‐induced CORT levels and feather CORT levels in partridges (Alectoris rufa; Bortolotti et al., 2008). These results suggest that the CORT stress response at the juvenile stage may be associated with developmental CORT exposure. Several laboratory studies have previously reported an effect of developmental CORT exposure on the CORT stress response of individuals later in life (e.g., Spencer, Buchanan, Goldsmith, & Catchpole, 2003; Spencer, Evans, & Monaghan, 2009; Haussmann, Longenecker, Marchetto, Juliano, & Bowden, 2011; Marasco, Robinson, Herzyk, & Spencer, 2012; Crino, Driscoll, & Breuner, 2014). For instance, Spencer et al. (2009) showed that CORT‐fed zebra finch nestlings had a steeper increase in plasma CORT levels in response to an acute stress as juveniles. Stressful conditions during development (e.g., nutritional restrictions) without any manipulation of plasma CORT levels can also increase plasma CORT levels of juveniles in response to stress, as shown in red‐legged kittiwakes and western scrub‐jays (Kitaysky, Kitaiskaia, Wingfield, & Piatt, 2001; Pravosudov & Kitaysky, 2006). Thus, developmental stress or developmental CORT exposure may have long‐lasting effects on the CORT stress response of house sparrows. This suggests that urban developmental conditions may modify the functioning of the HPA axis in urban birds. It is possible that an intense CORT stress response may provide some benefits to urban individuals during the juvenile or the adult stage (e.g., improved survival, Angelier et al., 2010). To test this hypothesis, future studies would need to experimentally investigate the impact of developmental CORT exposure on the ontogeny of the HPA axis and the ability of individuals to subsequently cope with their environment.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
EB, FB, PYH, and FA designed the study, conducted the statistical analyses, and drafted the manuscript; CP and CT assayed CORT levels in feather and blood. All authors gave final approval for publication.
DATA ACCESSIBILITY
Raw data are available on Dryad, https://doi.org/10.5061/dryad.r617g73.
Supporting information
ACKNOWLEDGMENTS
We are very grateful to all the volunteer bird ringers for their help in the field and for providing samples: A. Meillère, J. Bauwin, O. Benoit‐Gonin, N. Boileau, R. Garcin, G. Olioso, P. Ollivier, and B. Vollot. Bird processing and feather sampling were performed by trained bird ringers, and authorized by the French Bird Ringing Center (CRBPO, Muséum National d'Histoire Naturelle) under the SPOL PASDOM research project and personal CRBPO research program (N°: 335, F. Angelier) including blood sampling authorizations (Comité d'Ethique Poitou Charentes, N°: CE2013‐3). We also thank A. Meillère and P. Fiquet for helping with ringer recruitment for this project. We thank S. Hope and G. Names for correcting the English. Financial support was provided by the Fyssen foundation (grant to F. Angelier), by the Agence Nationale de la Recherche (ANR project Urbastress to F. Angelier, ANR‐16‐CE02‐0004‐01), by the CPER ECONAT, and by the Centre National de la Recherche Scientifique.
Beaugeard E, Brischoux F, Henry P‐Y, Parenteau C, Trouvé C, Angelier F. Does urbanization cause stress in wild birds during development? Insights from feather corticosterone levels in juvenile house sparrows (Passer domesticus). Ecol Evol. 2019;9:640–652. 10.1002/ece3.4788
REFERENCES
- Adams, S. , & Klobodu, E. K. M. (2017). Urbanization, democracy, bureaucratic quality, and environmental degradation. Journal of Policy Modeling, 39, 1035–1051. 10.1016/j.jpolmod.2017.04.006 [DOI] [Google Scholar]
- Anderson, T. R. (2006). Biology of the ubiquitous house sparrow: From genes to populations (p. 560). Oxford, NY: Oxford University Press. [Google Scholar]
- Angelier, F. , Meillère, A. , Grace, J. K. , Trouvé, C. , & Brischoux, F. (2016). No evidence for an effect of traffic noise on the development of the corticosterone stress response in an urban exploiter. General and Comparative Endocrinology, 232, 43–50. 10.1016/j.ygcen.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Angelier, F. , Wingfield, J. C. , Parenteau, C. , Pellé, M. , & Chastel, O. (2015). Does short‐term fasting lead to stressed‐out parents? A study of incubation commitment and the hormonal stress responses and recoveries in snow petrels. Hormones and Behavior, 67, 28–37. 10.1016/j.yhbeh.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Angelier, F. , Wingfield, J. C. , Weimerskirch, H. , & Chastel, O. (2010). Hormonal correlates of individual quality in a long‐lived bird: A test of the “corticosterone‐fitness hypothesis”. Biology Letters, 6, 846–849. 10.1098/rsbl.2010.0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmori, A. (2009). Electromagnetic pollution from phone masts. Effects on wildlife. Pathophysiology, 16, 191–199. 10.1016/j.pathophys.2009.01.007 [DOI] [PubMed] [Google Scholar]
- Biard, C. , Brischoux, F. , Meillère, A. , Michaud, B. , Nivière, M. , Ruault, S. , … Angelier, F. (2017). Growing in cities: An urban penalty for wild birds? A study of phenotypic differences between urban and rural great tit chicks (Parus major). Frontiers in Ecology and Evolution, 5, 79–92. 10.3389/fevo.2017.00079 [DOI] [Google Scholar]
- Blair, R. B. (1996). Land use and avian species diversity along an urban gradient. Ecological Applications, 6, 506–519. 10.2307/2269387 [DOI] [Google Scholar]
- Bókony, V. , Seress, G. , Nagy, S. , Lendvai, A. Z. , & Liker, A. (2012). Multiple indices of body condition reveal no negative effect of urbanization in adult house sparrows. Landscape and Urban Planning, 104, 75–84. 10.1016/j.landurbplan.2011.10.006 [DOI] [Google Scholar]
- Bonier, F. (2012). Hormones in the city: Endocrine ecology of urban birds. Hormones and Behavior, 61, 763–772. 10.1016/j.yhbeh.2012.03.016 [DOI] [PubMed] [Google Scholar]
- Bonier, F. , Martin, P. R. , Moore, I. T. , & Wingfield, J. C. (2009). Do baseline glucocorticoids predict fitness? Trends in Ecology & Evolution, 24, 634–642. 10.1016/j.tree.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Bonier, F. , Moore, I. T. , Martin, P. R. , & Robertson, R. J. (2009). The relationship between fitness and baseline glucocorticoids in a passerine bird. General and Comparative Endocrinology, 163, 208–213. 10.1016/j.ygcen.2008.12.013 [DOI] [PubMed] [Google Scholar]
- Bortolotti, M. T. A. , Blas, J. , & German, T. (2008). Corticosterone in feathers is a long‐term, integrated measure of avian stress physiology. Functional Ecology, 22, 494–500. 10.1111/j.1365-2435.2008.01387.x [DOI] [Google Scholar]
- Brischoux, F. , Meillère, A. , Dupoué, A. , Lourdais, O. , & Angelier, F. (2017). Traffic noise decreases nestlings’ metabolic rates in an urban exploiter. Journal of Avian Biology, 48, 905–909. 10.1111/jav.01139 [DOI] [Google Scholar]
- Brown, M. E. (1996). Assessing body condition in birds In Nolan V. & Ketterson E. D. (Eds), Current ornithology (pp. 67–135). Boston, MA: Springer. [Google Scholar]
- Cai, F. , & Calisi, R. M. (2016). Seasons and neighborhoods of high lead toxicity in New York City: The feral pigeon as a bioindicator. Chemosphere, 161, 274–279. 10.1016/j.chemosphere.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Candolin, U. , & Wong, B. B. M. (Eds.) (2012). Behavioural responses to a changing world: Mechanisms and consequences (p. 280). Oxford, NY: Oxford University Press. [Google Scholar]
- Chace, J. F. , & Walsh, J. J. (2006). Urban effects on native avifauna: A review. Landscape and Urban Planning, 74, 46–69. 10.1016/j.landurbplan.2004.08.007 [DOI] [Google Scholar]
- Chatelain, M. , Gasparini, J. , & Frantz, A. (2016). Do trace metals select for darker birds in urban areas? An experimental exposure to lead and zinc. Global Change Biology, 22, 2380–2391. 10.1111/gcb.13170 [DOI] [PubMed] [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing. Vienna, Austria: R Found. Stat. Comput; Retrieved from http://www.R-project.org/ [Google Scholar]
- Crick, H. , Robinson, R. , Appleton, G. , Clark, N. , & Rickard, A. D. (2002). Investigation into the causes of the decline of starlings and house sparrows in Great Britain. British Trust for Ornithology Research Report 290, Defra, London, UK.
- Crino, O. L. , Driscoll, S. C. , & Breuner, C. W. (2014). Corticosterone exposure during development has sustained but not lifelong effects on body size and total and free corticosterone responses in the zebra finch. General and Comparative Endocrinology, 196, 123–129. 10.1016/j.ygcen.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Crino, O. L. , Johnson, E. E. , Blickley, J. L. , Patricelli, G. L. , & Breuner, C. W. (2013). Effects of experimentally elevated traffic noise on nestling white‐crowned sparrow stress physiology, immune function and life history. Journal of Experimental Biology, 216, 2055–2062. 10.1242/jeb.081109 [DOI] [PubMed] [Google Scholar]
- Cyr, N. E. , & Romero, L. (2007). Chronic stress in free‐living European starlings reduces corticosterone concentrations and reproductive success. General and Comparative Endocrinology, 151, 82–89. 10.1016/j.ygcen.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Dauwe, T. , Janssens, E. , Bervoets, L. , Blust, R. , & Eens, M. (2004). Relationships between metal concentrations in great tit nestlings and their environment and food. Environmental Pollution, 131, 373–380. 10.1016/j.envpol.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Davies, S. , Beck, M. L. , & Sewall, K. B. (2018). Territorial aggression in urban and rural song sparrows is correlated with corticosterone, but not testosterone. Hormones and Behavior, 98, 8–15. 10.1016/j.yhbeh.2017.11.010 [DOI] [PubMed] [Google Scholar]
- De Coster, G. , De Laet, J. , Vangestel, C. , Adriaensen, F. , & Lens, L. (2015). Citizen science in action: Evidence for long‐term, region‐wide house sparrow declines in Flanders, Belgium. Landscape and Urban Planning, 134, 139–146. 10.1016/j.landurbplan.2014.10.020 [DOI] [Google Scholar]
- De Jong, M. , Ouyang, J. Q. , Da Silva, A. , van Grunsven, R. H. A. , Kempenaers, B. , Visser, M. E. , & Spoelstra, K. (2015). Effects of nocturnal illumination on life‐history decisions and fitness in two wild songbird species. Philosophical Transactions of the Royal Society B: Biological Sciences, 370, 20140128 10.1098/rstb.2014.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laet, J. D. , & Summers‐Smith, J. D. (2007). The status of the urban house sparrow Passer domesticus in north‐western Europe: A review. Journal of Ornithology, 148, 275–278. 10.1007/s10336-007-0154-0 [DOI] [Google Scholar]
- Demeyrier, V. , Charmantier, A. , Lambrechts, M. M. , & Grégoire, A. (2017). Disentangling drivers of reproductive performance in urban great tits: A food supplementation experiment. Journal of Experimental Biology, 220, 4195–4203. 10.1242/jeb.161067 [DOI] [PubMed] [Google Scholar]
- Eeva, T. , Hasselquist, D. , Tummeleht, L. , Nikinmaa, M. , & Ilmonen, P. (2005). Pollution related effects on immune function and stress in a free‐living population of pied flycatcher Ficedula hypoleuca . Journal of Avian Biology, 36, 405–412. 10.1111/j/0908-8857.2005.03449.x [DOI] [Google Scholar]
- Everaert, J. , & Bauwens, D. (2007). A possible effect of electromagnetic radiation from mobile phone base stations on the number of breeding house sparrows (Passer domesticus). Electromagnetic Biology and Medicine, 26, 63–72. 10.1080/15368370701205693 [DOI] [PubMed] [Google Scholar]
- Fairhurst, G. D. , Berzins, L. L. , Bradley, D. W. , Laughlin, A. J. , Romano, A. , Romano, M. , … Clark, R. G. (2015). Assessing costs of carrying geolocators using feather corticosterone in two species of aerial insectivore. Royal Society Open Science, 2, 150004 10.1098/rsos.150004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst, G. D. , Frey, M. D. , Reichert, J. F. , Szelest, I. , Kelly, D. M. , & Bortolotti, G. R. (2011). Does environmental enrichment reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective. PLoS One, 6, e17663 10.1371/journal.pone.0017663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz, S. L. , Davis, J. E. , Battle, K. E. , Greene, V. W. , Laing, B. T. , Rock, R. P. , … Moore, I. T. (2015). Across time and space: Effects of urbanization on corticosterone and body condition vary over multiple years in song sparrows (Melospiza melodia). Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 323, 109–120. 10.1002/jez.1906 [DOI] [PubMed] [Google Scholar]
- Francis, C. D. , Kleist, N. J. , Ortega, C. P. , & Cruz, A. (2012). Noise pollution alters ecological services: Enhanced pollination and disrupted seed dispersal. Proceedings of the Royal Society B: Biological Sciences, 279, 2727–2735. 10.1098/rspb.2012.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, C. D. , Ortega, C. P. , & Cruz, A. (2009). Noise pollution changes avian communities and species interactions. Current Biology, 19, 1415–1419. 10.1016/j.cub.2009.06.052 [DOI] [PubMed] [Google Scholar]
- Fridolfsson, A.‐K. , & Ellegren, H. (1999). A simple and universal method for molecular sexing of non‐ratite birds. Journal of Avian Biology, 30, 116–121. 10.2307/3677252 [DOI] [Google Scholar]
- Gil, D. , & Brumm, H. (Eds.) (2014). Avian urban ecology: Behavioural and physiological adaptations (p. 240). Oxford, NY: Oxford University Press. [Google Scholar]
- Gorissen, L. , Snoeijs, T. , Duyse, E. V. , & Eens, M. (2005). Heavy metal pollution affects dawn singing behaviour in a small passerine bird. Oecologia, 145, 504–509. 10.1007/s00442-005-0091-7 [DOI] [PubMed] [Google Scholar]
- Gosler, A. G. (1996). Environmental and social determinants of winter fat storage in the great tit Parus major . Journal of Animal Ecology, 65, 1 10.2307/5695 [DOI] [Google Scholar]
- Grace, J. K. , Froud, L. , Meillère, A. , & Angelier, F. (2017). House sparrows mitigate growth effects of post‐natal glucocorticoid exposure at the expense of longevity. General and Comparative Endocrinology, 253, 1–12. 10.1016/j.ygcen.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Grava, T. , Fairhurst, G. D. , Avey, M. T. , Grava, A. , Bradley, J. , Avis, J. L. , … Otter, K. A. (2013). Habitat quality affects early physiology and subsequent neuromotor development of juvenile black‐capped chickadees. PLoS One, 8, e71852 10.1371/journal.pone.0071852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, N. B. , Foster, D. , Groffman, P. , Grove, J. M. , Hopkinson, C. S. , Nadelhoffer, K. J. , … Peters, D. P. (2008). The changing landscape: Ecosystem responses to urbanization and pollution across climatic and societal gradients. Frontiers in Ecology and the Environment, 6, 264–272. 10.1890/070147 [DOI] [Google Scholar]
- Halfwerk, W. , Bot, S. , Buikx, J. , van der Velde, M. , Komdeur, J. , ten Cate, C. , & Slabbekoorn, H. (2011). Low‐frequency songs lose their potency in noisy urban conditions. Proceedings of the National Academy of Sciences of the United States of America, 108, 14549–14554. 10.1073/pnas.1109091108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfwerk, W. , & Slabbekoorn, H. (2009). A behavioural mechanism explaining noise‐dependent frequency use in urban birdsong. Animal Behavior, 78, 1301–1307. 10.1016/j.anbehav.2009.09.015 [DOI] [Google Scholar]
- Haussmann, M. F. , Longenecker, A. S. , Marchetto, N. M. , Juliano, S. A. , & Bowden, R. M. (2011). Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proceedings of the Royal Society B: Biological Sciences, 279, 1447–1456. 10.1098/rspb.2011.1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverland, M. B. , & Veech, J. A. (2017). Examining the occurrence of mammal species in natural areas within a rapidly urbanizing region of Texas, USA. Landscape and Urban Planning, 157, 221–230. 10.1016/j.landurbplan.2016.06.001 [DOI] [Google Scholar]
- Heiss, R. S. , Clark, A. B. , & McGowan, K. J. (2009). Growth and nutritional state of American crow nestlings vary between urban and rural habitats. Ecological Applications, 19, 829–839. 10.1890/08-0140.1 [DOI] [PubMed] [Google Scholar]
- Hudin, N. S. , Neve, L. D. , Strubbe, D. , Fairhurst, G. D. , Vangestel, C. , Peach, W. J. , & Lens, L. (2017). Supplementary feeding increases nestling feather corticosterone early in the breeding season in house sparrows. Ecology and Evolution, 7, 6163–6171. 10.1002/ece3.3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudin, N. , Teyssier, A. , Aerts, J. , Fairhurst, G. D. , Strubbe, D. , White, J. , … Lens, L. (2018). Do wild‐caught urban house sparrows show desensitized stress responses to a novel stressor? Biology Open, 7, bio031849 10.1242/bio.031849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Injaian, A. S. , Taff, C. C. , & Patricelli, G. L. (2018). Experimental anthropogenic noise impacts avian parental behaviour, nestling growth and nestling oxidative stress. Animal Behavior, 136, 31–39. 10.1016/j.anbehav.2017.12.003 [DOI] [Google Scholar]
- Jenni‐Eiermann, S. , Helfenstein, F. , Vallat, A. , Glauser, G. , Jenni, L. , & Fisher, D. (2015). Corticosterone: Effects on feather quality and deposition into feathers. Methods in Ecology and Evolution, 6, 237–246. 10.1111/2041-210X.12314 [DOI] [Google Scholar]
- Johns, D. W. , Marchant, T. A. , Fairhurst, G. D. , Speakman, J. R. , & Clark, R. G. (2017). Biomarker of burden: Feather corticosterone reflects energetic expenditure and allostatic overload in captive waterfowl. Functional Ecology, 32, 345–357. 10.1111/1365-2435.12988 [DOI] [Google Scholar]
- Jones, D. N. , & Reynolds, S. J. (2008). Feeding birds in our towns and cities: A global research opportunity. Journal of Avian Biology, 39, 265–271. 10.1111/j.0908-8857.2008.04271.x [DOI] [Google Scholar]
- Kaiser, A. (1993). A new multi‐category classification of subcutaneous fat deposits of songbirds. Journal of Field Ornithology, 64, 246–255. [Google Scholar]
- Kark, S. , Iwaniuk, A. , Schalimtzek, A. , & Banker, E. (2006). Living in the city: Can anyone become an ‘urban exploiter’? Journal of Biogeography, 34, 638–651. 10.1111/j.1365-2699.2006.01638.x [DOI] [Google Scholar]
- Kempenaers, B. , Borgström, P. , Loës, P. , Schlicht, E. , & Valcu, M. (2010). Artificial night lighting affects dawn song, extra‐pair siring success, and lay date in songbirds. Current Biology, 20, 1735–1739. 10.1016/j.cub.2010.08.028 [DOI] [PubMed] [Google Scholar]
- Kight, C. R. , & Swaddle, J. P. (2011). How and why environmental noise impacts animals: An integrative, mechanistic review. Ecology Letters, 14, 1052–1061. 10.1111/j.1461-0248.2011.01664.x [DOI] [PubMed] [Google Scholar]
- Kitaysky, A. S. , Kitaiskaia, E. V. , Piatt, J. F. , & Wingfield, J. C. (2003). Benefits and costs of increased levels of corticosterone in seabird chicks. Hormones and Behavior, 43, 140–149. 10.1016/S0018-506X(02)00030-2 [DOI] [PubMed] [Google Scholar]
- Kitaysky, A. S. , Kitaiskaia, E. V. , Wingfield, J. C. , & Piatt, J. F. (2001). Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red‐legged kittiwake chicks. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 171, 701–709. 10.1007/s003600100230 [DOI] [PubMed] [Google Scholar]
- Kleist, N. J. , Guralnick, R. P. , Cruz, A. , Lowry, C. A. , & Francis, C. D. (2017). Chronic anthropogenic noise disrupts glucocorticoid signaling and has multiple effects on fitness in an avian community. Proceedings of the National Academy of Sciences of the United States of America, 115, E648–E657. 10.1073/pnas.1709200115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren, L. , Nakagawa, S. , Burke, T. , Soma, K. K. , Wynne‐Edwards, K. E. , & Geffen, E. (2012). Non‐breeding feather concentrations of testosterone, corticosterone and cortisol are associated with subsequent survival in wild house sparrows. Proceedings of the Royal Society B: Biological Sciences, 279, 1560–1566. 10.1098/rspb.2011.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs, C. J. , & Singleton, G. R. (1993). Indices of condition for small mammals. Australian Journal of Zoology, 41, 317–323. [Google Scholar]
- Landys, M. M. , Ramenofsky, M. , & Wingfield, J. C. (2006). Actions of glucocorticoids at a seasonal baseline as compared to stress‐related levels in the regulation of periodic life processes. General and Comparative Endocrinology, 148, 132–149. 10.1016/j.ygcen.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Lattin, C. R. , Reed, J. M. , DesRochers, D. W. , & Romero, L. M. (2011). Elevated corticosterone in feathers correlates with corticosterone‐induced decreased feather quality: A validation study. Journal of Avian Biology, 42, 247–252. 10.1111/j.1600-048X.2010.05310.x [DOI] [Google Scholar]
- Legagneux, P. , Harms, N. J. , Gauthier, G. , Chastel, O. , Gilchrist, H. G. , Bortolotti, G. , … Soos, C. (2013). Does feather corticosterone reflect individual quality or external stress in Arctic‐nesting migratory birds? PLoS One, 8, e82644 10.1371/journal.pone.0082644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloutre, C. , Gouzerh, A. , & Angelier, F. (2014). Hard to fly the nest: A study of body condition and plumage quality in house sparrow fledglings. Current Zoology, 60, 449–459. 10.1093/czoolo/60.4.449 [DOI] [Google Scholar]
- Lendvai, A. Z. , Loiseau, C. , Sorci, G. , & Chastel, O. (2009). Early developmental conditions affect stress response in juvenile but not in adult house sparrows (Passer domesticus). General and Comparative Endocrinology, 160, 30–35. 10.1016/j.ygcen.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Leonard, M. L. , & Horn, A. G. (2008). Does ambient noise affect growth and begging call structure in nestling birds? Behavioral Ecology, 19, 502–507. 10.1093/beheco/arm161 [DOI] [Google Scholar]
- Liker, A. , Papp, Z. , Bókony, V. , & Lendvai, A. Z. (2008). Lean birds in the city: Body size and condition of house sparrows along the urbanization gradient. Journal of Animal Ecology, 77, 789–795. 10.1111/j.1365-2656.2008.01402.x [DOI] [PubMed] [Google Scholar]
- Loiseau, C. , Sorci, G. , Dano, S. , & Chastel, O. (2008). Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. General and Comparative Endocrinology, 155, 101–108. 10.1016/j.ygcen.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Lormée, H. , Jouventin, P. , Trouve, C. , & Chastel, O. (2003). Sex‐specific patterns in baseline corticosterone and body condition changes in breeding red‐footed boobies Sula sula . Ibis, 145, 212–219. 10.1046/j.1474-919X.2003.00106.x [DOI] [Google Scholar]
- Love, O. P. , McGowan, P. , & Sheriff, M. J. (2012). Maternal adversity and ecological stressors in natural populations: The role of stress axis programming in individuals, with implications for populations and communities. Functional Ecology, 27, 81–92. 10.1111/j.1365-2435.2012.02040.x [DOI] [Google Scholar]
- Lynn, S. E. , Breuner, C. W. , & Wingfield, J. C. (2003). Short‐term fasting affects locomotor activity, corticosterone, and corticosterone binding globulin in a migratory songbird. Hormones and Behavior, 43, 150–157. 10.1016/S0018-506X(02)00023-5 [DOI] [PubMed] [Google Scholar]
- Lynn, S. E. , Prince, L. E. , & Phillips, M. M. (2010). A single exposure to an acute stressor has lasting consequences for the hypothalamo–pituitary–adrenal response to stress in free‐living birds. General and Comparative Endocrinology, 165, 337–344. 10.1016/j.ygcen.2009.07.018 [DOI] [PubMed] [Google Scholar]
- Madliger, C. L. , & Love, O. P. (2014). The need for a predictive, context‐dependent approach to the application of stress hormones in conservation. Conservation Biology, 28, 283–287. 10.1111/cobi.12185 [DOI] [PubMed] [Google Scholar]
- Marasco, V. , Robinson, J. , Herzyk, P. , & Spencer, K. A. (2012). Pre‐ and post‐natal stress in context: Effects on the stress physiology in a precocial bird. Journal of Experimental Biology, 215, 3955–3964. 10.1242/jeb.071423 [DOI] [PubMed] [Google Scholar]
- Marzluff, J. , Bowman, R. , & Donnelly, R. (2001). Avian ecology and conservation in an urbanizing world. Boston, MA: Springer. [Google Scholar]
- McEwen, B. S. , & Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43, 2–15. 10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- McKinney, M. L. (2002). Urbanization, biodiversity, and conservation. BioScience, 52, 883–890. 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2 [DOI] [Google Scholar]
- McKinney, M. L. (2006). Urbanization as a major cause of biotic homogenization. Biological Conservation, 127, 247–260. 10.1016/j.biocon.2005.09.005 [DOI] [Google Scholar]
- Meillère, A. , Brischoux, F. , & Angelier, F. (2015). Impact of chronic noise exposure on antipredator behavior: An experiment in breeding house sparrows. Behavioral Ecology, 26, 569–577. 10.1093/beheco/aru232 [DOI] [Google Scholar]
- Meillère, A. , Brischoux, F. , Bustamante, P. , Michaud, B. , Parenteau, C. , Marciau, C. , & Angelier, F. (2016). Corticosterone levels in relation to trace element contamination along an urbanization gradient in the common blackbird (Turdus merula). Science of the Total Environment, 566–567, 93–101. 10.1016/j.scitotenv.2016.05.014 [DOI] [PubMed] [Google Scholar]
- Meillère, A. , Brischoux, F. , Henry, P.‐Y. , Michaud, B. , Garcin, R. , & Angelier, F. (2017). Growing in a city: Consequences on body size and plumage quality in an urban dweller, the house sparrow (Passer domesticus). Landscape and Urban Planning, 160, 127–138. 10.1016/j.landurbplan.2016.12.014 [DOI] [Google Scholar]
- Meillère, A. , Brischoux, F. , Parenteau, C. , & Angelier, F. (2015). Influence of urbanization on body size, condition, and physiology in an urban exploiter: A multi‐component approach. PLoS One, 10, e0135685 10.1371/journal.pone.0135685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meillère, A. , Brischoux, F. , Ribout, C. , & Angelier, F. (2015). Traffic noise exposure affects telomere length in nestling house sparrows. Biology Letters, 11, 20150559 10.1098/rsbl.2015.0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. W. (2006). Apparent effects of light pollution on singing behavior of American robins. The Condor, 108, 130–139. 10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2 [DOI] [Google Scholar]
- Montiglio, P. O. , & Royauté, R. (2014). Contaminants as a neglected source of behavioural variation. Animal Behavior, 88, 29–35. 10.1016/j.anbehav.2013.11.018 [DOI] [Google Scholar]
- Moudrá, L. , Zasadil, P. , Moudrý, V. , & Šálek, M. (2018). What makes new housing development unsuitable for house sparrows (Passer domesticus)? Landscape and Urban Planning, 169, 124–130. 10.1016/j.landurbplan.2017.08.017 [DOI] [Google Scholar]
- Navara, K. J. , & Nelson, R. J. (2007). The dark side of light at night: Physiological, epidemiological, and ecological consequences. Journal of Pineal Research, 43, 215–224. 10.1111/j.1600-079X.2007.00473.x [DOI] [PubMed] [Google Scholar]
- Newsome, T. M. , Dellinger, J. A. , Pavey, C. R. , Ripple, W. J. , Shores, C. R. , Wirsing, A. J. , & Dickman, C. R. (2014). The ecological effects of providing resource subsidies to predators. Global Ecology and Biogeography, 24, 1–11. 10.1111/geb.12236 [DOI] [Google Scholar]
- Ouyang, J. Q. , de Jong, M. , Hau, M. , Visser, M. E. , van Grunsven, R. H. A. , & Spoelstra, K. (2015). Stressful colours: Corticosterone concentrations in a free‐living songbird vary with the spectral composition of experimental illumination. Biology Letters, 11, 20150517 10.1098/rsbl.2015.0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang, J. Q. , de Jong, M. , van Grunsven, R. H. A. , Matson, K. D. , Haussmann, M. F. , Meerlo, P. , … Spoelstra, K. (2017). Restless roosts: Light pollution affects behavior, sleep, and physiology in a free‐living songbird. Global Change Biology, 23, 4987–4994. 10.1111/gcb.13756 [DOI] [PubMed] [Google Scholar]
- Paker, Y. , Yom‐Tov, Y. , Alon‐Mozes, T. , & Barnea, A. (2014). The effect of plant richness and urban garden structure on bird species richness, diversity and community structure. Landscape and Urban Planning, 122, 186–195. 10.1016/j.landurbplan.2013.10.005 [DOI] [Google Scholar]
- Peach, W. J. , Mallord, J. W. , Ockendon, N. , Orsman, C. J. , & Haines, W. G. (2015). Invertebrate prey availability limits reproductive success but not breeding population size in suburban house sparrows Passer domesticus . Ibis, 157, 601–613. 10.1111/ibi.12264 [DOI] [Google Scholar]
- Peig, J. , & Green, A. (2009). New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos, 118, 1883–1891. 10.1111/j.1600-0706.2009.17643.x [DOI] [Google Scholar]
- Peig, J. , & Green, A. (2010). The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Functional Ecology, 24, 1323–1332. 10.1111/j.1365-2435.2010.01751.x [DOI] [Google Scholar]
- Plummer, K. E. , Bearhop, S. , Leech, D. I. , Chamberlain, D. E. , & Blount, J. D. (2013). Fat provisioning in winter impairs egg production during the following spring: A landscape‐scale study of blue tits. Journal of Animal Ecology, 82, 673–682. 10.1111/1365-2656.12025 [DOI] [PubMed] [Google Scholar]
- Pravosudov, V. V. , & Kitaysky, A. S. (2006). Effects of nutritional restrictions during post‐hatching development on adrenocortical function in western scrub‐jays (Aphelocoma californica). General and Comparative Endocrinology, 145, 25–31. 10.1016/j.ygcen.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Raupp, M. J. , Shrewsbury, P. M. , & Herms, D. A. (2010). Ecology of herbivorous arthropods in urban landscapes. Annual Review of Entomology, 55, 19–38. 10.1146/annurev-ento-112408-085351 [DOI] [PubMed] [Google Scholar]
- Romero, L. M. , Cyr, N. E. , & Romero, R. C. (2006). Corticosterone responses change seasonally in free‐living house sparrows (Passer domesticus). General and Comparative Endocrinology, 149, 58–65. 10.1016/j.ygcen.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Romero, L. M. , & Fairhurst, G. D. (2016). Measuring corticosterone in feathers: Strengths, limitations, and suggestions for the future. Comparative Biochemistry and Physiology Part A Molecular Integrative Physiology, 202, 112–122. 10.1016/j.cbpa.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Romero, L. M. , & Reed, J. M. (2005). Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comparative Biochemistry and Physiology Part A Molecular Integrative Physiology, 140, 73–79. 10.1016/j.cbpb.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Schroeder, J. , Nakagawa, S. , Cleasby, I. R. , & Burke, T. (2012). Passerine birds breeding under chronic noise experience reduced fitness. PLoS One, 7, e39200 10.1371/journal.pone.0039200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte‐Hostedde, A. , Millar, J. S. , & Hickling, G. J. (2001). Evaluating body condition in small mammals. Canadian Journal of Zoology, 79, 1021–1029. 10.1139/z01-073 [DOI] [Google Scholar]
- Seress, G. , Bókony, V. , Pipoly, I. , Szép, T. , Nagy, K. , & Liker, A. (2012). Urbanization, nestling growth and reproductive success in a moderately declining house sparrow population. Journal of Avian Biology, 43, 403–414. 10.1111/j.1600-048X.2012.05527.x [DOI] [Google Scholar]
- Seress, G. , & Liker, A. (2015). Habitat urbanization and its effects on birds. Acta Zoologica Academiae Scientiarum Hungaricae, 61(4), 373–408. 10.17109/AZH.61.4.373.2015 [DOI] [Google Scholar]
- Shaw, L. M. , Chamberlain, D. , & Evans, M. (2008). The house sparrow Passer domesticus in urban areas: Reviewing a possible link between post‐decline distribution and human socioeconomic status. Journal of Ornithology, 149, 293–299. 10.1007/s10336-008-0285-y [DOI] [Google Scholar]
- Slabbekoorn, H. , Bouton, N. , van Opzeeland, I. , Coers, A. , ten Cate, C. , & Popper, A. N. (2010). A noisy spring: The impact of globally rising underwater sound levels on fish. Trends in Ecology & Evolution, 25, 419–427. 10.1016/j.tree.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Spencer, K. A. , Buchanan, K. L. , Goldsmith, A. R. , & Catchpole, C. K. (2003). Song as an honest signal of developmental stress in the zebra finch (Taeniopygia guttata). Hormones and Behavior, 44, 132–139. 10.1016/S0018-506X(03)00124-7 [DOI] [PubMed] [Google Scholar]
- Spencer, K. A. , Evans, N. P. , & Monaghan, P. (2009). Postnatal stress in birds: A novel model of glucocorticoid programming of the hypothalamic‐pituitary‐adrenal axis. Endocrinology, 150, 1931–1934. 10.1210/en.2008-1471 [DOI] [PubMed] [Google Scholar]
- Stone, E. L. , Harris, S. , & Jones, G. (2015). Impacts of artificial lighting on bats: A review of challenges and solutions. Mammalian Biology, 80, 213–219. 10.1016/j.mambio.2015.02.004 [DOI] [Google Scholar]
- Summers‐Smith, J. D. (2003). The decline of the house sparrow: A review. British Birds, 96, 439–446. [Google Scholar]
- Titulaer, M. , Spoelstra, K. , Lange, C. Y. M. J. G. , & Visser, M. E. (2012). Activity patterns during food provisioning are affected by artificial light in free living great tits (Parus major). PLoS One, 7, e37377 10.1371/journal.pone.0037377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treen, G. D. , Hobson, K. A. , Marchant, T. A. , & Bortolotti, G. R. (2015). Large‐scale spatial variation in feather corticosterone in invasive house sparrows (Passer domesticus) in Mexico is related to climate. Ecology and Evolution, 5, 3808–3817. 10.1002/ece3.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorisalo, T. , Andersson, H. , Hugg, T. , Lahtinen, R. , Laaksonen, H. , & Lehikoinen, E. (2003). Urban development from an avian perspective: Causes of hooded crow (Corvus corone cornix) urbanisation in two Finnish cities. Landscape and Urban Planning, 62, 69–87. 10.1016/S0169-2046(02)00124-X [DOI] [Google Scholar]
- Wada, H. , Salvante, K. G. , Stables, C. , Wagner, E. , Williams, T. D. , & Breuner, C. W. (2008). Adrenocortical responses in zebra finches (Taeniopygia guttata): Individual variation, repeatability, and relationship to phenotypic quality. Hormones and Behavior, 53, 472–480. 10.1016/j.yhbeh.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Wada, H. , Salvante, K. G. , Wagner, E. , Williams, T. D. , & Breuner, C. W. (2009). Ontogeny and individual variation in the adrenocortical response of zebra finch (Taeniopygia guttata) nestlings. Physiological and Biochemical Zoology, 82, 325–331. 10.1086/599320 [DOI] [PubMed] [Google Scholar]
- White, T. C. R. (2008). The role of food, weather and climate in limiting the abundance of animals. Biological Reviews of the Cambridge Philosophical Society, 83, 227–248. 10.1111/j.1469-185X.2008.00041.x [DOI] [PubMed] [Google Scholar]
- Will, A. P. , Suzuki, Y. , Elliott, K. H. , Hatch, S. A. , Watanuki, Y. , & Kitaysky, A. S. (2014). Feather corticosterone reveals developmental stress in seabirds. Journal of Experimental Biology, 217, 2371–2376. 10.1242/jeb.098533 [DOI] [PubMed] [Google Scholar]
- Will, A. , Watanuki, Y. , Kikuchi, D. M. , Sato, N. , Ito, M. , Callahan, M. , … Kitaysky, A. (2015). Feather corticosterone reveals stress associated with dietary changes in a breeding seabird. Ecology and Evolution, 5, 4221–4232. 10.1002/ece3.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield, J. , Davey, K. , Peter, R. , & Tobe, S. (1994). Modulation of the adrenocortical response to stress in birds. Perspectives in Comparative Endocrinology, 520–528, 10.1007/s00442-013-2598-7 [DOI] [Google Scholar]
- Wingfield, J. C. , Maney, D. L. , Breuner, C. W. , Jacobs, J. D. , Lynn, S. , Ramenofsky, M. , & Richardson, R. D. (1998). Ecological bases of hormone‐behavior interactions: The “emergency life history stage”. American Zoologist, 38, 191–206. 10.1093/icb/38.1.191 [DOI] [Google Scholar]
- Wingfield, J. C. , & Romero, L. M. (2001). Adrenocortical responses to stress and their modulation in free‐living vertebrates In McEwen B. S. (Ed.), Handbook of Physiology, section 7: The endocrine system. Vol. 4: Coping with the environment: Neural and endocrine mechanisms (pp. 211e236). Oxford, NY: Oxford University Press. [Google Scholar]
- Xu, X. , Xie, Y. , Qi, K. , Luo, Z. , & Wang, X. (2018). Detecting the response of bird communities and biodiversity to habitat loss and fragmentation due to urbanization. Science of the Total Environment, 624, 1561–1576. 10.1016/j.scitotenv.2017.12.143 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Lei, F. , Liu, S. , Li, D. , Chen, C. , & Wang, P. (2011). Variation in baseline corticosterone levels of tree sparrow (Passer montanus) populations along an urban gradient in Beijing, China. Journal of Ornithology, 152, 801–806. 10.1007/s10336-011-0663-8 [DOI] [Google Scholar]
- Zvereva, E. L. , & Kozlov, M. V. (2010). Responses of terrestrial arthropods to air pollution: A meta‐analysis. Environmental Science and Pollution Research International, 17, 297–311. 10.1007/s11356-009-0138-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available on Dryad, https://doi.org/10.5061/dryad.r617g73.


