Abstract
Increased plant biomass is observed in terrestrial systems due to rising levels of atmospheric CO2, but responses of marine macroalgae to CO2 enrichment are unclear. The 200% increase in CO2 by 2100 is predicted to enhance the productivity of fleshy macroalgae that acquire inorganic carbon solely as CO2 (non‐carbon dioxide‐concentrating mechanism [CCM] species—i.e., species without a carbon dioxide‐concentrating mechanism), whereas those that additionally uptake bicarbonate (CCM species) are predicted to respond neutrally or positively depending on their affinity for bicarbonate. Previous studies, however, show that fleshy macroalgae exhibit a broad variety of responses to CO2 enrichment and the underlying mechanisms are largely unknown. This physiological study compared the responses of a CCM species (Lomentaria australis) with a non‐CCM species (Craspedocarpus ramentaceus) to CO2 enrichment with regards to growth, net photosynthesis, and biochemistry. Contrary to expectations, there was no enrichment effect for the non‐CCM species, whereas the CCM species had a twofold greater growth rate, likely driven by a downregulation of the energetically costly CCM(s). This saved energy was invested into new growth rather than storage lipids and fatty acids. In addition, we conducted a comprehensive literature synthesis to examine the extent to which the growth and photosynthetic responses of fleshy macroalgae to elevated CO2 are related to their carbon acquisition strategies. Findings highlight that the responses of macroalgae to CO2 enrichment cannot be inferred solely from their carbon uptake strategy, and targeted physiological experiments on a wider range of species are needed to better predict responses of macroalgae to future oceanic change.
Keywords: carbon uptake strategy, carbon dioxide‐concentrating mechanism, CCM, CO2 enrichment, macroalgae, non‐CCM, ocean acidification, physiology
1. INTRODUCTION
Since the beginning of the industrial era, atmospheric CO2 concentrations have increased by 40% (IPCC, 2013). In terrestrial systems, there is evidence of a “CO2 enrichment” effect that is resulting in a higher plant biomass (Zhu et al., 2016) as the availability of CO2 for carbon fixation in photosynthetic organisms via the enzyme Rubisco increases. In coastal marine systems, macroalgae fulfill the same functional role as terrestrial plants; they are foundation species that provide food, habitat, and shelter to higher trophic levels (e.g., shellfish and fish), as well as a range of ecosystem services including nutrient cycling. In addition, they have a potential role in the blue carbon economy and climate mitigation (Bennett et al., 2015; Chung, Beardall, Mehta, Sahoo, & Stojkovic, 2011; Tuya, Wernberg, & Thomsen, 2008). By the end of the century, CO2 concentrations in seawater will increase by 200% under the RCP (Representative Concentration Pathway) 6.0 scenario (IPCC, 2013) and, like terrestrial plants, macroalgal productivity is predicted to increase (Koch, Bowes, Ross, & Zhang, 2013; Sunday et al., 2016).
The absorption of CO2 by seawater and the subsequent chemical reactions cause a suite of changes to the seawater carbonate system, termed ocean acidification: By 2100, the bicarbonate ion will additionally increase by 14% with a concurrent decline in pH of 0.3 units (RCP6.0 scenario; Hurd, Hepburn, Currie, Raven, & Hunter, 2009; IPCC, 2013; The Royal Society, 2005). In addition to taking up CO2 via diffusion, ~65% of macroalgae can utilize the bicarbonate ion directly from seawater (Kübler & Dudgeon, 2015). Bicarbonate use is an energy‐consuming process, and the derivation of CO2 from bicarbonate (for subsequent carbon fixation) is termed a carbon dioxide‐concentrating mechanism (CCM). Species that solely rely on the diffusive uptake of CO2, termed non‐CCM species (mostly Phylum Rhodophyta), were thought to be rare (Kübler & Dudgeon, 2015; Raven, Ball, Beardall, Giordano, & Maberly, 2005). However, Cornwall, Revill, and Hurd (2015) discovered that up to 90% of some populations were non‐CCM species in subtidal reef habitats in Tasmania, southern Australia, and on average 35% of macroalgae globally are now considered non‐CCM (Kübler & Dudgeon, 2015).
Extensive field surveys have been conducted to study CCM and non‐CCM species in temperate (Cornwall et al., 2015; Hepburn et al., 2011) and tropical systems (Diaz‐Pulido, Cornwall, Gartrell, Hurd, & Tran, 2016), and along a gradient of CO2/pH in a volcanic vent in Italy (Cornwall et al., 2017). These surveys indicate that the responses of macroalgae to CO2 enrichment will depend on their mechanism(s) of inorganic carbon uptake, that is, their carbon uptake strategies (Hepburn et al., 2011). For CCM species, the predominance of the bicarbonate ion in seawater (90% under current pH) may mean that their growth is not limited by dissolved inorganic carbon (DIC) supply and there will be no future CO2 enrichment effect. However, field surveys have illustrated that within CCM species we can further distinguish between (a) those that are able to use the 200% additional CO2 predicted for the future and (b) those that cannot (Cornwall et al., 2017; Hepburn et al., 2011; Kübler & Dudgeon, 2015): (a) Species able to use additional CO2 either exhibit a low affinity for DIC (carbon‐limited CCM species) or have CCMs that can be downregulated (i.e., decreased CCM activity); (b) CCM species that are considered unresponsive to future CO2 concentrations have either a high affinity for CO2 or CCMs that cannot be downregulated under elevated CO2 conditions (Cornwall et al., 2017). In contrast, growth and photosynthesis of non‐CCM species are thought to be limited under current CO2 concentrations and they are predicted to benefit from the future 200% increase in dissolved CO2 (Cornwall et al., 2015; Hepburn et al., 2011; Kübler & Dudgeon, 2015).
Carbon uptake strategies in macroalgae can be determined by analyzing their carbon stable isotope composition (δ13C) (Maberly, Raven, & Johnston, 1992): CO2 is more depleted in 13C than HCO3 − and thus has a lower δ13C value. Macroalgae that possess a CCM typically have δ13C values ranging between −30‰ and −10‰, whereas a lack of a CCM is indicated by δ13C <−30‰. A change in the δ13C value of the macroalgal tissue indicates a change in carbon uptake strategy and can also indicate biochemical changes in the macroalgae such as the use of sugars and lipid synthesis (Raven et al., 2002). Macroalgae generally exhibit significant changes in both lipids and fatty acids with changing environmental conditions. Such biochemical changes can be observed in the biomass very shortly after exposure (≤7 days) as an acclimation mechanism in membrane and/or storage lipids (Al‐Hasan, Hantash, & Radwan, 1991; Gosch, Lawton, Paul, Nys, & Magnusson, 2015). Elevated CO2 concentrations have been shown to either increase or decrease the lipid production of several species of algae, especially when combined with nutrient limitation (Bermúdez et al., 2015; Sun, Chen, & Du, 2016). CO2 enrichment could therefore not only result in increased growth rates, but also lead to changes in lipid storage. Whether a species will invest in growth or lipid storage may depend on their carbon uptake strategy. Species that are currently saturated for DIC (i.e., CCM species) may use the elevated DIC to invest in lipid storage, since their growth rates are not limited by carbon. Species whose growth rates are currently limited by DIC (i.e., non‐CCM species) may increase their growth rates first before investing in storage compounds.
Given that both CO2 and bicarbonate concentrations are increasing in seawater, and macroalgae have a variety of inorganic carbon uptake strategies (Diaz‐Pulido et al., 2016), determining the physiological and growth responses of macroalgae to future CO2 enrichment is complex. Despite the ecological, economic, and cultural importance of macroalgae, we know very little about their mechanistic physiological responses to future CO2 enrichment, particularly compared to microalgae and terrestrial plants (Hibberd & Covshoff, 2010; Jungnick et al., 2014; Sandrini, Matthijs, Verspagen, Muyzer, & Huisman, 2014).
While field surveys have proved useful in identifying the general carbon uptake strategies of macroalgae, detailed knowledge of photosynthetic and growth responses to CO2 enrichment is unknown for most species and mainly focused on CCM species (Britton, Cornwall, Revill, Hurd, & Johnson, 2016; Fernández, Roleda, & Hurd, 2015; Israel & Hophy, 2002) and calcareous species (Hall‐Spencer et al., 2008; Jokiel et al., 2008). The effect of CO2 enrichment on non‐CCM species has been tested only twice, for Lomentaria articulata (Kübler, Johnston, & Raven, 1999) and Amansia rhodantha (Ho & Carpenter, 2017), with results being inconsistent between studies: Lomentaria articulata exhibited an increase in growth, while no response was detected for Amansia rhodantha. Likewise, for CCM species, both increased growth (Kim et al., 2016), no response (Fernández et al., 2015; Ho & Carpenter, 2017; Israel & Hophy, 2002; Rautenberger et al., 2015), and decreased growth have been reported (García‐Sánchez, Fernández, & Niell, 1994; Kim et al., 2016). Therefore, the question remains as to whether or not carbon uptake strategy can be used as a predictor of macroalgal responses to CO2 enrichment.
In this study, we compare the effects of CO2 enrichment on the growth, physiological, and biochemical responses of two temperate red macroalgae: a CCM species, Lomentaria australis (Kützing) Levring, and a non‐CCM species, Craspedocarpus ramentaceus (C. Agardh) Min‐Thein & Womersley. Both species are ecological dominants in the subtidal waters of eastern Tasmania, Australia. As this is only the third non‐CCM species to be included in a CO2 enrichment experiment, as well as the first study to compare biochemical responses of a non‐CCM species versus a CCM species, we aim to advance our understanding of the mechanisms that underlie macroalgal responses to CO2 enrichment. In addition, we conduct a comprehensive literature survey, including studies conducted prior to ocean acidification becoming a research field, to better understand the role that carbon uptake strategy plays in effecting the response of fleshy macroalgae to CO2 enrichment.
We hypothesized that: (a) CO2 enrichment will have no effect on the growth and photosynthetic rate of L. australis (CCM species), if the species used in this study has a high affinity for DIC. However, if the species has a low affinity for DIC, we predict a downregulation of energy‐costly uptake of HCO3 −, with an increased passive CO2 uptake resulting in higher rates of growth and photosynthesis. (b) The net photosynthesis and growth of C. ramentaceus (non‐CCM species) will increase with CO2 enrichment, based on the likelihood that the species is currently carbon‐limited. (c) An elevated DIC availability will be used for the accumulation of storage compounds such as lipids in L. australis (CCM species), resulting in composition changes in lipid classes and fatty acids, whereas C. ramentaceus (non‐CCM species) will primarily invest in growth rather than storage of lipids.
2. MATERIALS AND METHODS
2.1. Macroalgal collection and culture maintenance
Twelve individual specimens of Lomentaria australis (a CCM species) and Craspedocarpus ramentaceus (a non‐CCM species) were collected from Tinderbox, Tasmania (S43°03'30.722 E147°19'52.583), on 21 October 2016 at 6 m depth using Scuba and were transported to the laboratory (30 min) in plastic bags containing seawater. Carbon uptake strategy of both species was confirmed by conducting pH‐drift experiments following Cornwall et al. (2015) (Supporting information Table S1). In the laboratory, the macroalgae were carefully rinsed with filtered seawater and visible epiphytes were gently removed. Prior to the experiment, individuals were acclimated to laboratory conditions in 20‐L aquaria for 48 hr, with constant aeration and a 12:12 light:dark photoperiod and a photon flux density (PFD) of 18–20 µmol photons m−2 s−1. During the pretreatment (24 hr) and experiment (7 days), the PFD was raised to 25–30 µmol photons m−2 s−1 which mimicked the PFD that was measured at the collection site at 6 m using integrating PAR sensors (Odyssey, Dataflow Systems Pty Ltd, Christchurch, New Zealand). In addition, light saturation curves were conducted to ensure that the light levels used in the experiment were sufficient for saturating photosynthesis (Supporting information Figure S1, Table S2). Seawater used in the experiment was collected south of Bruny Island, Tasmania, and was filtered to 1 μm and UV‐sterilized (Emperor Aquatics Smart HO UV Sterilizer, 025050‐2, 50 W lamp). The ammonium and nitrate concentrations were 0.46 and 0.20 µM, respectively, measured using a QuickChem 8500 Series 2 Automated Ion Analyzer (Lachat Instrument, Loveland, USA).
2.2. Experimental conditions
For each species, ~1 g of alga was randomly assigned to an individual container (650 ml) of an automated, pH‐controlled culture system. For both species, there were two experimental CO2 treatments: “current” (pHT = 8.00, pCO2 = 365.17) and “future” (pHT = 7.70, pCO2 = 1,015.24) at 12.5°C (detailed in the Supporting information Table S3). This corresponds to the local ambient pH/CO2 and the expected future seawater conditions, with n = 6 replicates for each species and CO2 treatment. The future pH was based on the RCP6.0, which is a “business as usual” modeling projection (IPCC, 2013). A magnetic stirrer in each container (set at 650 rpm) mixed the seawater to minimize the diffusion boundary layer around the blades of the macroalgae.
Target CO2 levels were achieved in each of the 24 culture containers using a system similar to that described in Bockmon, Frieder, Navarro, White‐Kershek, and Dickson (2013) with the modifications presented in Reidenbach et al. (2017). Briefly, every 4 hr, seawater in each 650‐ml culture container was replaced by flushing the container with ~1 L of seawater using Jebao DP‐4 Auto Dosing aquarium pumps. CO2 was added to this incoming seawater as it entered each culture container using a mixture of air and CO2, which was briefly in contact with seawater entering each culture container using a membrane contactor (Micromodule, model 0.5 × 1, Membrana, USA) placed near each culture container inlet. The contactors contain a microporous hollow fiber membrane, which allows efficient mixing of liquids and gases. Since the seawater and air/CO2 were in contact for only a few seconds, full equilibration of the air/CO2 mixture was not possible and air/CO2 ratios of approximately 400:1–1,000:1 were needed to achieved target CO2 levels in the seawater. These ratios were achieved using mass flow controllers (FMA5418A for air, FMA5402A for CO2; Omega Engineering, USA), whose flow rates were controlled by an analog output module housed in a USB chassis (NI9264 and cDAQ‐9174, National Instruments, USA). To maintain target CO2 levels, a feedback system monitored pHT and adjusted the flow rates of the mass flow controllers automatically.
Seawater pHT was measured using a modified version of the spectrophotometric pHT system developed by McGraw et al. (2010). Briefly, a syringe pump and rotary valves were used to sample seawater (V6 pump, 24090 and 24493 valves, 23425 valve driver, Norgren, UK) while minimizing gas exchange. Spectra were acquired using an LED light source and a UV‐Vis spectrometer (BluLoop and USB2000+, Ocean Optics, USA) with a 1‐cm flow‐through quartz cuvette. Reference spectra were obtained from a 25 ml seawater sample; sample spectra were obtained by mixing 24.80 ml of seawater and 200 µl of 2 mM metacresol purple dye (857890, Sigma‐Aldrich, Australia) within the syringe pump. The temperature of each sample was recorded with a PT100 temperature sensor and a high‐precision data logger (PT‐104, PICO Technology, UK). All instrument control, spectra manipulations, and pHT calculations were done using LabVIEW 2014 (National Instruments, USA).
pHT was calculated from the temperature, salinity, and the absorbance spectra, which was calculated from the individual reference and sample spectra. To improve measurement precision, the absorbance at 434, 578, and 750 nm was determined using the quadratic fits of the absorbance spectra between 429–439 nm and 573–583 nm and a background signal averaged between 750 and 760 nm (McGraw et al., 2010). Each recorded measurement of pHT was the average of four replicate measurements, which took ~2 min to obtain. The pH system was standardized using certified reference materials provided by Andrew Dickson, Scripps Institute for Oceanography, San Diego, USA (Dickson, Sabine, & Christian, 2007). A 0.03 pH unit offset was added to each measurement based on the difference between the measured pH and that calculated from the known DIC and AT of the certified reference material. The appropriateness of this correction was verified through pHT measurements of the Tris buffer.
2.3. Biotic responses
Growth rate was determined from changes in both thallus length and wet weight. Linear extension (distance from the apices to the main axis) was calculated from photographs of each individual placed on a grid on day 1 and day 7 using the software ImageJ (Schneider, Rasband, & Eliceiri, 2012). Relative growth rate (RGR) on a wet weight basis was calculated for each individual according to Yong, Yong, and Anton (2013): RGR = ln(Wt/W 0) × t −1, where W 0 is the wet weight at day 1 and Wt is the final wet weight after 7 days. To remove surface water, blades were carefully blotted dry with tissue paper before weighing.
Net photosynthetic rates were measured at the end of the experiment (day 7) at the beginning of the light cycle in each culture tank under experimental conditions using an Orion RDO Probe 087010MD. Initial O2 measurements were conducted immediately after the culture tanks received a fresh seawater supply, and final O2 measurements were conducted two hours later. The probe was calibrated using a standard of 100% air saturation achieved by bubbling with air for 10 min. Oxygen production was calculated as final O2 measurements – initial O2 measurements and was standardized to tissue wet weight (g) per hour.
The maximum quantum yield (Fv/Fm) was used as an indicator of the performance of photosystem II (PSII) at the beginning and at the end of the experiment. Fv/Fm was measured using a pulse amplitude modulation (PAM) chlorophyll fluorescence meter (Diving PAM, Walz, Germany) using individuals that had been dark‐adapted for 10 min. The gain was set to 2, and F 0 ranged between 200 and 1,000 for each measurement.
The content of photosynthetic pigments (chlorophyll a and phycobiliproteins) was analyzed using ~0.2 g (wet weight) of each individual on day 7. Chlorophyll a was extracted at room temperature according to Seely, Duncan, and Vidaver (1972) and Schmidt, Maraschin, and Bouzon (2010) using acetone and dimethylsulfoxide (DMSO). Pigment content was quantified spectrophotometrically with a S‐22 UV/Vis Spectrophotometer (Boeco, Germany), and concentrations (mg/g of wet weight) were calculated using the equations in Seely et al. (1972). To determine phycobiliprotein content (mg/g of wet weight), samples were flash frozen by immersion in liquid nitrogen and ground into a fine powder with a mortar and pestle. The extraction was carried out at 4°C using a 0.1 M phosphate buffer (pH = 7.2) according to the methods of Schmidt et al. (2010) and Sampath‐Wiley and Neefus (2007). All extractions were conducted in the dark.
Tissue samples from the apices were taken during the pretreatment and at the end of the experiment on day 7 from each individual sample to determine δ13C, C:N ratios, and lipid class and fatty acid content and composition. The samples were rinsed in Type I ultrapure water, frozen at −80°C, freeze‐dried (Labconco FreeZone 4.5), and ground with a mortar and pestle. δ13C, C and N content, and C:N were determined following the methods in Cornwall et al. (2015) using a NA1500 elemental analyzer coupled to a Thermo Scientific Delta V Plus via a ConFlo IV. Combustion and reduction were achieved at 1,020°C and 650°C, respectively. Values were normalized to the VPDB scale (Vienna Pee Dee Belemnite) via a 3‐point calibration using certified reference material. Both precision and accuracy were ±0.1 ‰ (1 SD). For the analyses, change in δ13C was calculated as the difference in δ13C between day 7 and day 1.
Total lipids were extracted following a modified version of Bligh and Dyer (1959) of the dried and weighed (ca. 40 mg) macroalgal tissue. Lipids were extracted overnight using a one‐phase methanol (MeOH): dichloromethane (DCM): Milli‐Q (2:1:08 v/v/v) solvent mixture. Phase separation was achieved by addition of 10 ml of DCM and 10 ml of Milli‐Q water the next day. The lower lipid‐containing layer was drained into a round bottom flask. After addition of few drops of MeOH, solvent was removed using a rotary evaporator (ca. 40°C). The lipid extracts were transferred to preweighed vials, and solvents were evaporated under a constant stream of nitrogen gas. The lipid‐containing vials were weighed for determination of total lipids. Samples were redissolved in DCM and stored at −20°C until further analysis.
To determine lipid class composition, an aliquot of the total lipid extract was spotted on SIII chromarods (5 µm particle size). Samples were co‐eluted with a standard mix to determine the lipid class composition including hydrocarbons (HC), steryl and wax esters (WE), triacylglycerols (TAG), sterols (ST), free fatty acids (FFA), and polar lipids which includes glycolipids and phospholipids (PL). The mobile phase consisted of hexane: diethyl ether (DEE): glacial acetic acid (GAA) (70:10:0.1 v/v/v). Chromarods were developed for 25 min and then dried at 100°C for 10 min. The dried rods were analyzed using an Iatroscan Mark V TH10 thin layer chromatography (TLC) with a flame ionization detector (FID). Peak identification was achieved by comparison with retention times of co‐eluted standards. For quantification, the SIC480II IatroscanTM integrating software (System Instruments, Mitsubishi Chemical Instruments) was used. The integrated areas were transformed to mass per µl spotted using pre‐determined linear regression calculations.
To analyze the fatty acids, an aliquot of the total lipid extract was saponified by addition of 2 ml of 5% KOH in 80% MeOH and placed on a heating block (80°C for 3 hr). After cooling, 1 ml of H2O was added and the mixture was extracted with hexane:DCM (4:1 v/v) to yield a nonsaponifiable neutral lipid fraction in the upper organic layer and free fatty acids in the lower aqueous layer. The aqueous layer was acidified by addition of 0.3 ml concentrated HCl and extracted three times with hexane:DCM (4:1, v/v). Solvents were removed under a stream of inert nitrogen gas, and methylation was performed by addition of MeOH:DCM:conc. HCl (10:1:1, v/v/v) and heating for 1 hr at 80°C. After cooling, 1 ml H2O was added and the resulting fatty acid methyl esters (FAME) were extracted three times into hexane:DCM (4:1, v/v). Solvents were removed under a stream of nitrogen gas. The samples were analyzed using gas chromatography (GC) using an Agilent Technologies 7890 (Palo Alto, California, USA) GC coupled with a flame ionization detector (FID) analyzer and equipped with a nonpolar EquityTM‐1 fused silica capillary column (15 m × 0.1 mm internal diameter, 0.1 µm film thickness). Agilent ChemStation software was used for quantification of FAME peaks. The individual fatty acids were identified using a GC–mass spectrometer (MS) using a Finnigan ThermoQuest GCQ GC‐MS System fitted with an on‐column injector and using ThermoQuest Xcalibur software.
To determine how macroalgae modify the carbonate chemistry of their local environment, seawater samples (13 ml) were collected from each replicate culture tank immediately after a change in seawater and again four hours later. These samples were collected both at day 1 and day 7. Simultaneously, pHT was measured in the culture tanks with a Thermo Scientific Orion VERSA STAR 90 meter and pH electrode Orion 8107BNUMD Ross Ultra pH/ATC Triode, calibrated with a Tris buffer. Seawater samples were immediately poisoned with HgCl2 and stored under constant darkness. DIC concentrations of the water samples were measured using a DIC analyzer (Apollo SciTech DIC Analyzer Model AS‐C3) with an inbuilt CO2 analyzer (LI‐COR LI‐7000 CO2/H2O Analyzer). The CO2 analyzer was calibrated with a certified reference material provided by Andrew Dickson, Scripps Institute for Oceanography, San Diego, USA (Dickson et al., 2007). AT was calculated using the constants of Mehrbach, Culberson, Hawley, and Pytkowicz (1973), refitted by Dickson and Millero (1987).
2.4. Statistical analyses
All statistical analyses were performed using the software R 3.1.2 (R Core Team, 2016). A multivariate analysis of variance (MANOVA) was used to assess whether there were differences in biological responses (linear extension, wet weight RGR, Chl a and phycobiliprotein content, net photosynthesis, lipid content, fatty acid content, change in δ13C, change in C:N ratios, and change in carbon and nitrogen tissue content) between species and CO2 treatments. A second MANOVA was performed on the change in carbonate parameters ([H+], [HCO3 −], [CO3 2−], [CO2], and total DIC). The data passed the tests for MANOVA assumptions (normality and homogeneity of variance). When the interaction or main factor pH was significant (at α = 0.05), Tukey's honestly significant difference (THSD) post hoc tests were used to determine which treatments differed from each other (using the aov() and TukeyHSD() function in R). The Hedge's g is used as a measure of effect size (a g of 1 indicates that two groups differ by 1 SD). Hedge's g is calculated as follows: (M1‐M2)/SDpooled, where M1‐M2 is the difference in means of two groups and SDpooled is the pooled standard deviation. Differences in Fv/Fm values were tested with a generalized linear model using the lme4 package, with treatment as fixed factor and start/end of the experiment as random factor (Bates, Mächler, Bolker, & Walker, 2015). A correspondence analysis (CA) was performed to detect differences in fatty acid composition between species and treatments using the Vegan package v.2.3‐5 (Oksanen et al., 2016). In the CA plot, objects (specimens) that are close to one another are likely to have a similar fatty acid composition. Specimens found near the point representing a fatty acid are likely to contain a higher relative level of that fatty acid. For this analysis, only fatty acids that were on average >0.5% of the TFA profile were used. Graphs were customized using the packages ggplot2 (Wickham, 2011), plyr (Wickham, 2011), and reshape2 (Wickham, 2007).
2.5. Literature synthesis
To further determine whether or not patterns in the responses of fleshy macroalgae to CO2 enrichment depend on their carbon uptake strategy, we conducted a comprehensive literature survey. We searched the databases Web of Science and Google Scholar using the keywords “CO2 enrichment”, “CO2 fertilization”, “ocean acidification”, “macroalgae”, and “seaweed” in various combinations. Fifty‐six studies published between 1989 and 2018 were analyzed, giving a total of 61 species. Studies were only examined if they met specific criteria. (a) The study had to report a growth response to CO2 enrichment (in addition, photosynthetic response and δ13C response were noted if those were reported); (b) the study needed to be a manipulative experiment comparing one or several macroalgal species in different pCO2 treatments (thus, we excluded field studies that were conducted in natural pCO2 gradients); (c) the study had to report whether the species used in the experiment were able to utilize HCO3 − or not (i.e., if the species possessed a CCM); (d) only studies on marine fleshy macroalgae were considered; (e) the macroalgae used in the experiment had to be identified to species level (i.e., no “turf”‐forming mats or community responses were included); (f) studies testing the effect of CO2 enrichment on microscopic early developmental stages and spore germination were excluded; and (g) the study had to be published in a peer‐reviewed journal. Many studies also tested additional experimental conditions (e.g., irradiance or nutrient concentrations), in combination with elevated pCO2. Those studies were included in our literature synthesis, but care was taken to only take into account the single‐term effects of elevated pCO2.
3. RESULTS
3.1. Growth
For L. australis (the CCM species), linear extension under the future CO2 treatment (pH = 7.70) was twofold greater (a Hedge's g effect size of 1.5) than the current conditions treatment (pH = 8.04) (Table 1, Figure 1), whereas for C. ramentaceus (the non‐CCM species), there was no significant effect of CO2 enrichment on growth (Table 1, Figure 1). Growth rates of L. australis under the future treatment were ca. eightfold and sevenfold greater (a Hedge's g effect size of 2.8) than C. ramentaceus under both current and future treatments, respectively (p < 0.0001 for both comparisons, Tukey's honestly significant difference tests). Growth rate on a wet weight basis showed a similar CO2 treatment effect (p = 0.095, Table 1; Supporting information Figure S2).
Table 1.
Multivariate analysis of variance (MANOVA) table displaying p‐values and F‐values for all measured responses (linear extension, wet weight relative growth rate, net photosynthesis, chlorophyll a content, phycobiliprotein content, change in δ13C, change in C:N ratio, change in carbon tissue content, change in nitrogen tissue content, total lipid content, total fatty acid content, change in [H+], change in [HCO3 −], change in [CO2], change in [CO3 2−], and change in total DIC)
| Response | Interaction | Factor | ||||
|---|---|---|---|---|---|---|
| Species | CO2 treatment | |||||
| F‐value | p‐value | F‐value | p‐value | F‐value | p‐value | |
| Linear extension | 6.474 | 0.021 | – | – | – | – |
| Wet weight RGR | 3.125 | 0.095 | 15.743 | 0.001 | 2.133 | 0.162 |
| Chlorophyll a content (mg per g wet weight) | 0.509 | 0.485 | 20.563 | <0.001 | 0.169 | 0.686 |
| Phycobiliprotein content (mg per g wet weight) | 1.375 | 0.257 | 4.271 | 0.054 | 0.020 | 0.889 |
| Net photosynthesis (µmol O2 hr− 1 g− 1) | 1.813 | 0.196 | 1.385 | 0.256 | 0.075 | 0.788 |
| Change in δ13C | 1.229 | 0.283 | 8.528 | 0.010 | 7.975 | 0.012 |
| Change in C:N ratio | 0.274 | 0.608 | 1.350 | 0.261 | 0.083 | 0.777 |
| Change in carbon tissue content | 0.012 | 0.914 | 1.051 | 0.320 | 0.110 | 0.744 |
| Change in nitrogen tissue content | 0.031 | 0.861 | 5.813 | 0.028 | 0.140 | 0.713 |
| Total lipid content | 0.134 | 0.719 | 2.458 | 0.135 | 0.121 | 0.733 |
| Total fatty acid content | 0.220 | 0.645 | 35.466 | <0.001 | 3.363 | 0.084 |
| Change in [H+] | 0.659 | 0.427 | 0.549 | 0.468 | 133.993 | <0.001 |
| Change in [HCO3 −] (µmol/kg) | 0.418 | 0.526 | 0.580 | 0.456 | 0.013 | 0.910 |
| Change in [CO2] (µmol/kg) | 0.501 | 0.488 | 0.395 | 0.537 | 134.789 | <0.001 |
| Change in [CO3] (µmol/kg) | 0.025 | 0.877 | 1.671 | 0.212 | 0.171 | 0.684 |
| Change in total DIC (µmol/kg) | 0.754 | 0.396 | 0.119 | 0.734 | 2.572 | 0.125 |
p‐values and F‐values of separate factors are not shown when the interaction between factors is significant (α = 0.05).
p‐values in bold have a significance of p < 0.05.
Figure 1.
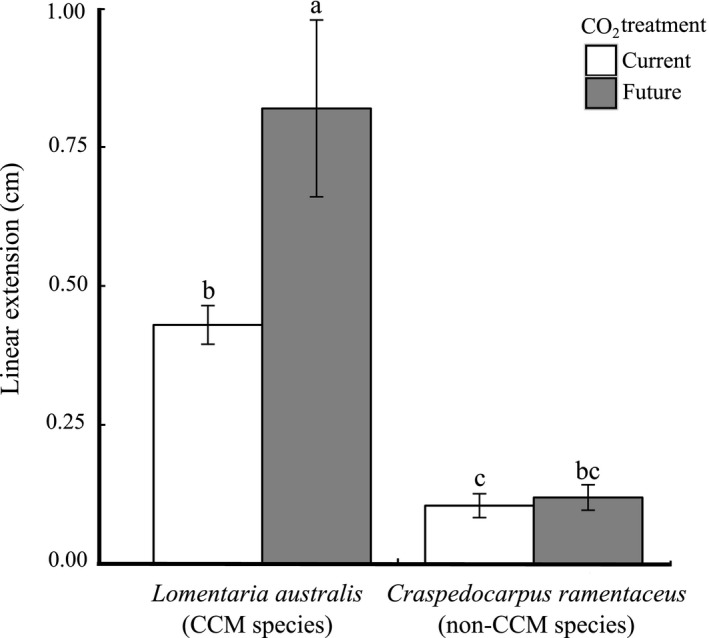
Linear extension (cm) of an algal species with carbon‐concentrating mechanism (Lomentaria australis; CCM species) and a species without (Craspedocarpus ramentaceus; non‐CCM species), with current (8.0) and future (7.7) CO2 treatment. Data are displayed as mean ± standard error, n = 6. Bars sharing a letter are not significantly different (Tukey's honestly significant difference tests, α = 0.05)
3.2. Net photosynthesis, pigments, and FV/FM
Net photosynthetic rates were similar for L. australis and C. ramentaceus, ranging from 0.3 to 0.4 µmol hr−1 g−1, and were unaffected by the CO2 treatment (Table 1, Figure 2). C. ramentaceus (the non‐CCM species) had a higher pigment content of both chlorophyll a and phycobiliproteins compared to L. australis (the CCM species), but pigment content was not affected by CO2 treatment (Supporting information Figure S3, Figure S4). The Fv/Fm value ranged between 0.51–0.54 and 0.49–0.58 for L. australis and C. ramentaceus, respectively, throughout the experiment and was not significantly different between treatments (p > 0.05 for all comparisons, linear mixed model Wald chi‐square test).
Figure 2.
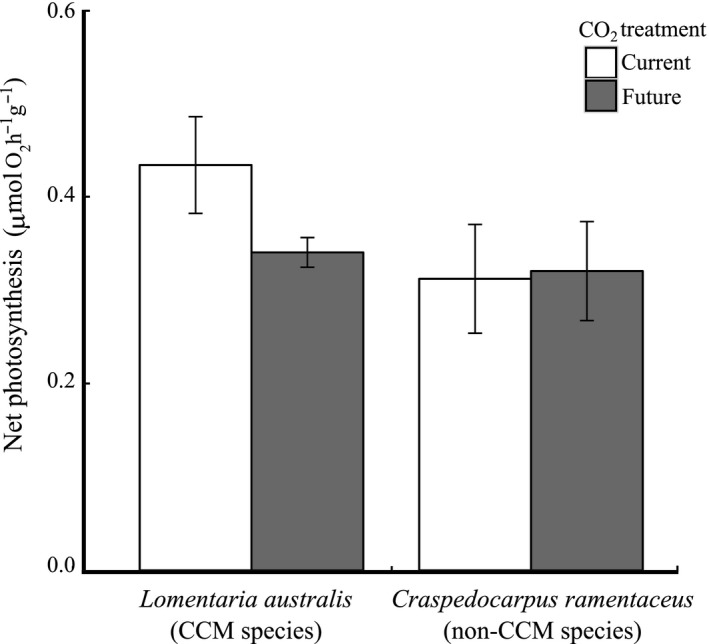
Net photosynthesis rates of a species with carbon‐concentrating mechanism (Lomentaria australis; CCM species) and a species without (Craspedocarpus ramentaceus; non‐CCM species), with current (8.0) and future (7.7) CO2 treatment. Data are displayed as mean ± standard error, n = 6. There were no significant differences between species and treatments
3.3. Stable isotopes and C:N ratios
L. australis (the CCM species) under the future treatment had a ca. twofold greater decrease (a Hedge's effect size of 1.2) in δ13C, compared to the individuals of the same species in current conditions (Figure 3, p < 0.05, Tukey's honestly significant difference test). CO2 treatment did not affect the degree of δ13C change of C. ramentaceus (the non‐CCM species) (p > 0.05, Tukey's honestly significant difference tests). C:N ratios increased from ~6.5 w/w at the start to ~8.5 w/w at the end of the experiment for each of the four treatment–species combinations, due to an increase in carbon content of the tissue (Supporting information Table S4). The magnitude of change in C:N ratio and C content did not differ between species and CO2 treatment (Table 1). The nitrogen content of the tissue remained relatively constant for both L. australis and C. ramentaceus, with less than 0.39% w/w difference from the start to end of the experiment (Supporting information Table S4).
Figure 3.
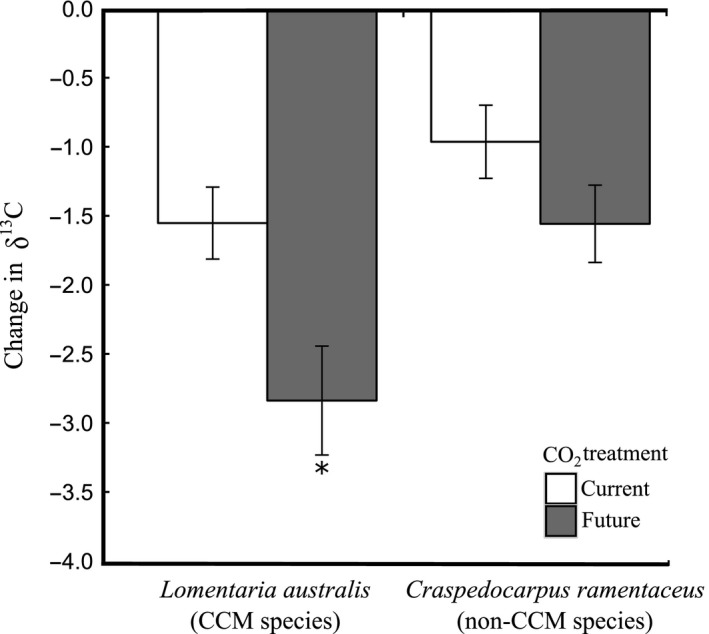
Change in δ13C ratios between day 1 (start of the experiment) and day 7 (end of the experiment) of a species with carbon‐concentrating mechanism (Lomentaria australis; CCM species) and a species without (Craspedocarpus ramentaceus; non‐CCM species), in current (8.0) and future (7.7) CO2 treatment. Data are displayed as mean ± standard error, n = 5–6 (with n = 5 for the ambient CCM treatment, and n = 6 for all other treatments). * denotes significantly different treatments (Tukey's honestly significant difference tests, α = 0.05)
3.4. Biochemical responses
L. australis (the CCM species) had a higher total fatty acid content than C. ramentaceus (the non‐CCM species), but this was not affected by experimental treatment (Table 1; Supporting information Figure S5). Both species had similar total lipid content (Table 1; Supporting information Figure S6). Polar lipids dominated the lipid class composition of both L. australis and C. ramentaceus (Supporting information Figure S7). There were no differences in lipid class percentage between species and CO2 treatment (Supporting information Figure S7). A correspondence analysis (CA) showed that fatty acid composition differed significantly between species, but that the composition in each species did not change with CO2 treatment (Figure 4). The first CA axis explained 98.2% of the variation, and the second axis explained 0.8% of the variation. The difference in fatty acid composition was mainly driven by 16:1n‐7, 18:1n‐9, 18:0, 20:4n‐6, 20:3n‐6, and 20:5n‐3, where the former four fatty acids were more associated with C. ramentaceus and the latter two with L. australis.
Figure 4.
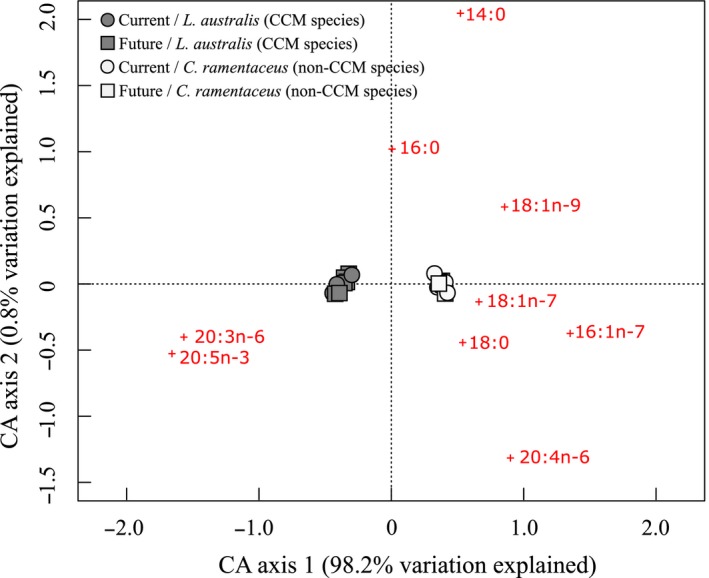
Correspondence analysis based on fatty acid composition of a species with carbon dioxide‐concentrating mechanism (Lomentaria australis; CCM species; gray) and a species without (Craspedocarpus ramentaceus; non‐CCM species; white), under current (circles) and future (squares) conditions. Crosses denote fatty acids. The nine most important fatty acids (the fatty acids that explain most of the variability) have been labeled
3.5. Modification of seawater carbonate chemistry by algae
Over a period of four hours, the algae modified the pH, total DIC, CO2, HCO3 −, and CO3 2− of the water in their culture tank, before they received a fresh supply of seawater (Supporting information Table S5). The [H+] decreased (i.e., pH increased), more so in the future treatments than in current treatments, but without differences between species (Table 1). The total DIC, HCO3 –, and CO2 decreased, and the CO3 2− increased over the 4 hr. There were no significant differences between species and CO2 treatments for the increase in CO32− and the decrease in HCO3 – and total DIC. The decrease in pH and CO2 was greater in future conditions, but did not differ between species (Table 1).
3.6. Literature synthesis
Approximately 40% of the CCM species that were tested (21 out of 55 species) showed no growth response to CO2 enrichment, five species had decreased growth rates and 16 species increased growth with elevated CO2 (Table 2). For 13 CCM species, mixed responses were reported (i.e., several species were tested in multiple studies, and the responses reported were not concordant). Net photosynthetic rates either increased or did not respond to CO2 enrichment for the majority of the CCM species tested (11 and 10 species, respectively); in four species, photosynthesis decreased; nine species showed mixed responses; and for 21 species, photosynthetic responses were not reported (Table 2). Only three non‐CCM species (including our work) have been studied in relation to CO2 enrichment, with contrasting results in terms of both growth and net photosynthesis (Table 2). Overall, δ13C values were measured in only a minority of the studies published (10 out of 56 studies) (Table 2). None of the species increased δ13C values in response to elevated CO2, but no clear pattern in relation to growth and photosynthesis can be inferred.
Table 2.
Response to CO2 enrichment reported in the literature for fleshy macroalgal species, regarding growth, photosynthesis, and δ13C values (“I” = increase, “D” = decrease, “NR” = no response, “–” = unreported)
| Phylum and species | Enriched pCO2 level (μatm) | Response to CO2 enrichment | Putative carbon uptake strategy | Reference | ||
|---|---|---|---|---|---|---|
| Growth | Photosynthesis | δ13C | ||||
| Rhodophyta (Red algae) | ||||||
| Amansia rhodantha | ~1,000 | NR | – | – | Non‐CCM | [1] |
| Chondrus crispus | ~800 | NR & I | NR, D & I | – | CCM | [2] |
| Craspedocarpus ramentaceus | ~1,000 | NR | NR | NR | Non‐CCM | current study |
| Gelidium crinale | ~750 | NR | – | – | CCM | [3] |
| Gracilaria chilensis | 650 & 1,250 | I | I | – | CCM | [4] |
| G. conferta | ~750 | NR | – | – | CCM | [3] |
| G. secundata | – | I | – | – | CCM | [5] |
| G. tenuistipitata | – | D | D | – | CCM | [6] |
| G. tikvahiae | (pH = 6.0) | NR | I | – | CCM | [7] |
| Gracilariopsis lemaneiformis* | ~700, ~1,000 & ~1,400 | NR & I | NR & I | – | CCM | [8–12] |
| Grateloupia cornea | 900 & 1,900 | D | D | – | CCM | [13] |
| Hypnea cornuta | ~750 | NR | – | – | CCM | [3] |
| H. musciformis | ~750 | D | – | – | CCM | [3] |
| H. spinella | 700 & 1,600 | I | I | – | CCM | [14] |
| Laurencia intricata | ~1,000 | NR | I | D | CCM | [15] |
| Lomentaria articulata | 700–1,800 (range) | I | – | D | Non‐CCM | [16] |
| L. australis | ~1,000 | I | NR | D | CCM | current study |
| Melanothamnus harveyi | ~800 & ~1,500 | NR & I | NR & I | – | CCM | [17] |
| Palmaria palmata | ~1,000 | NR & D | D | – | CCM | [18,19] |
| Phycodrys rubens | ~1,000 | NR | – | – | Possibly non‐CCM | [20] |
| Plocamium cartilagineum | 900 | D & I | – | – | CCM | [21] |
| Porphyra linearis | ~750 | D | NR | – | CCM | [22] |
| Ptilota gunneri | ~1,000 | NR | – | – | Possibly non‐CCM | [20] |
| Pterocladiella capillacea | ~750 | NR | – | – | CCM | [3] |
| Pyropia haitanensis | 1,000 | I | D & I | – | CCM | [23,24] |
| P. leucosticta | – | D | I | – | CCM | [25] |
| P. yezoensis | 1,000 & 1600 | I | I | – | CCM | [26] |
| Chlorophyta (Green algae) | ||||||
| Chaetomorpha linum | (pH = 6.73) | I | – | – | CCM | [27] |
| Cladophora coelothrix | (pH = 6.73) | I | – | – | CCM | [27] |
| C. patentiramea | (pH = 6.73) | NR | – | – | CCM | [27] |
| C. vagabunda | (pH = 6.0) | I | I | – | Possibly non‐CCM | [7] |
| Codium fragile | 900 & 1,900 | NR | NR | – | CCM | [13] |
| Monostroma grevillei var. arctica | ~1,000 | NR | – | – | CCM | [20] |
| Ulva australis* | ~900 & ~1,000 & ~1,900 | NR & I | NR & I | – | CCM | [13,28–30] |
| U. lactuca | ~700 | NR | NR | – | CCM | [10,31] |
| U. linza | ~750 & ~1,000 | NR | D | – | CCM | [3,32] |
| U. prolifera | ~1,000 | I | NR | – | CCM | [33–35] |
| U. pulchra | – | I | – | – | CCM | [36] |
| U. reticulata | – | NR | – | – | CCM | [36] |
| U. rigida* | ~1,200 | NR & I | NR & D | NR | CCM | [36–39] |
| Ochrophyta (Brown algae) | ||||||
| Alaria esculenta* | ~1,000 & ~1,300 | NR, D & I | NR | D | CCM | [20,40,41] |
| Chnoospora implexa | ~1,000 | NR | I | D | CCM | [15] |
| Desmarestia aculeata | ~1,000 & ~1,300 | D & I | NR & I | D & NR | CCM | [20,40,42] |
| Dictyopteris undulata | 900 | I | – | – | CCM | [21] |
| Dictyota bartayresiana | ~1,000 | NR | – | – | CCM | [1] |
| Fucus vesiculosus | ~1,200 | NR & D | – | – | CCM | [43–45] |
| F. vesiculosus f mytili | 1,000 | I | – | – | CCM | [46] |
| Laminaria solidungula | ~1,200 | NR | NR | NR | CCM | [47] |
| Lobophora variegata | ~1,000 | NR | – | – | CCM | [1] |
| Macrocystis pyrifera | ~1,200 | NR | NR | NR | CCM | [48] |
| Nereocystis luetkeana | ~3,000 | I | I | – | CCM | [49,50] |
| Padina pavonica | ~750 | NR | – | – | CCM | [3] |
| Saccharina japonica | ~1800 | NR | I | – | CCM | [51] |
| S. latissima* | ~1,000 & ~1,200 & ~3,000 | NR, D & I | NR & I | NR & D | CCM | [18,41,47,49,52] |
| Saccorhiza dermatodea | ~1,000 | I | – | – | CCM | [20] |
| Sargassum fusiforme* | ~700 & ~1,000 | D & I | NR & I | – | CCM | [53–55] |
| S. horneri | 900 & 1,900 | NR & I | NR | – | CCM | [13,21] |
| S. muticum | 1,000 | I | I | – | CCM | [56] |
| S. thunbergii | 900 & 1,900 | I | I | – | CCM | [13] |
| S. vulgare | ~750 | NR | – | – | CCM | [3] |
| Turbinaria ornata | ~1,000 | NR | NR | NR | CCM | [15] |
The elevated pCO2 level (which was compared to ambient control levels in each study), the carbon uptake strategy (non‐CCM or CCM), and references are also noted. * indicates species of which detailed physiological and biochemical regulatory mechanisms are known.
References: [1] Ho and Carpenter (2017); [2] Sarker, Bartsch, Olischläger, Gutow, and Wiencke (2013); [3] Israel and Hophy (2002); [4] Gao et al. (1993); [5] Lignell and Pedersén (1989); [6] García‐Sánchez et al. (1994); [7] Rivers and Peckol (1995); [8] Chen, Zou, Zhu, and Yang (2017); [9] Xu, Zou, and Gao (2010); [10] Liu, Zou, and Yang (2018); [11] Zou and Gao (2009); [12] Kang, Kambey, Shen, Yang, and Chung (2017); [13] Kim et al. (2016); [14] Suárez‐Álvarez, Gómez‐Pinchetti, and García‐Reina (2012); [15] Bender‐Champ, Diaz‐Pulido, and Dove (2017); [16] Kübler et al. (1999); [17] Olischläger and Wiencke (2013); [18] Nunes et al. (2016); [19] Sebök, Herppich, and Hanelt (2017); [20] Gordillo, Carmona, Viñegla, Wiencke, and Jiménez (2016); [21] Kram et al. (2016); [22] Israel, Katz, Dubinsky, Merrill, and Friedlander (1999); [23] Liu and Zou (2015a); [24] Xu, Chen, et al. (2017); [25] Mercado, Javier, Gordillo, Xavier Niell, and Figueroa (1999); [26] Gao et al. (1991); [27] de Paula Silva, Paul, Nys, and Mata (2013); [28] Reidenbach et al. (2017); [29] Kang and Chung (2017); [30] Kang and Kim (2016); [31] Liu and Zou (2015b); [32] Gao et al. (2018); [33] Xu and Gao (2012); [34] Li, Xu, and He (2016); [35] Li, Zhong, Zheng, Zhuo, and Xu (2018); [36] Björk, Haglund, Ramazanov, and Pedersén (1993); [37] Gordillo, Niell, and Figueroa (2001); [38] Rautenberger et al. (2015); [39] Gordillo, Figueroa, and Niell (2003); [40] Iñiguez et al. (2016a); [41] Gordillo et al. (2015); [42] Iñiguez, Heinrich, Harms, and Gordillo (2017); [43] Gutow et al. (2014); [44] Kawamitsu and Boyer (1999); [45] Ober and Thornber (2017); [46] Mensch et al. (2016); [47] Iñiguez et al, (2016b); [48] Fernández et al. (2015); [49] Swanson and Fox (2007); [50] Thom (1996); [51] Kang and Chung (2018); [52] Olischläger, Iñiguez, Koch, Wiencke, and Gordillo (2017); [53] Zou (2005); [54] Zou, Gao, and Luo (2011); [55] Jiang, Zou, Lou, and Gong (2018); [56] Xu, Gao, Gao, Xu, and Wu (2017).
4. DISCUSSION
Carbon uptake strategy has been a key concept in developing hypotheses related to how fleshy macroalgae will respond to CO2 enrichment (Gutow et al., 2014; Hepburn et al., 2011; Ho & Carpenter, 2017; Hurd, Lenton, Tilbrook, & Boyd, 2018; Israel & Hophy, 2002; Johnson, Comeau, Lantz, & Smith, 2017; Koch et al., 2013). The aim of this study was to compare the physiological and biochemical responses of L. australis (a CCM species) with C. ramentaceus (a non‐CCM species) to CO2 enrichment in order to broaden our knowledge on whether or not carbon uptake strategy is a useful predictor of the responses of fleshy macroalgae to CO2 enrichment. Our results, along with a careful scrutiny of the literature, reveal that fleshy macroalgal species exhibit a wide array of responses to CO2 enrichment in terms of both growth and photosynthesis (Table 2). As these responses also vary between species with the same carbon uptake strategy, we suggest that it is not currently possible to predict the responses of macroalgae to a higher CO2 ocean based solely on the presence/absence or type of CCM: A greater mechanistic understanding is needed before such predictions can be made.
4.1. The response of CCM species
For CCM species that responded positively to CO2 enrichment, the range of growth responses varied from a ~ 1.2‐fold increase in Ulva australis (Kim et al., 2016) to a ~ 4‐fold increase in Gracilaria chilensis (Gao, Aruga, Asada, & Kiyohara, 1993), fitting well with the twofold response of Lomentaria australis (the CCM species in our study). However, it is noteworthy that mixed growth responses to CO2 enrichment were reported for 13 of the CCM species and mixed photosynthetic responses for nine of the CCM species included in our literature survey (Table 2), as several species have been tested in multiple studies and the results did not always match. This inconsistency is most likely because other environmental factors also affect growth and physiological responses. For example, the kelp Saccharina latissima showed either no response or increased growth rates to elevated pCO2, depending on levels of UV radiation (Gordillo, Aguilera, Wiencke, & Jiménez, 2015). In another study, S. latissima had decreased growth rates in response to CO2 enrichment (Swanson & Fox, 2007), but the pCO2 used (~3,000 µatm) was much higher (3×) than that typically used in OA experiments and may be unrealistic, as the corresponding decrease in pH is larger than the worst‐case IPCC scenario (RCP8.5; IPCC, 2013). Overall, our synthesis indicates that many (~40%) CCM species are likely to be saturated for inorganic carbon, but that the benefits of CO2 enrichment for those that are not saturated for inorganic carbon could be substantial in terms of increased growth and productivity.
The reason for the decreased growth rate recorded for five CCM species with CO2 enrichment is unknown, but may be due to a sensitivity to increasing H+ concentrations. This sensitivity is known to exist in calcifying organisms (e.g., coccolithophores and zooplankton), as elevated H+ directly decreases calcification, and in fish, where increasing H+ causes acidosis and behavioral changes (Hurd et al., 2018). As H+ is key in regulating cellular homeostasis, changing H+ concentrations could affect macroalgal metabolic processes and CCM activity. The effect of increasing H+ concentrations has not been addressed for fleshy macroalgae, with the exception of Roleda, Morris, McGraw, and Hurd (2012), who found an interactive effect of pH (i.e., H+) and DIC on early microscopic life‐history stages of Macrocystis pyrifera. As H+ concentrations will increase by 200% by 2100 (RCP6.0 scenario; IPCC, 2013), this calls for future research on how increasing H+ concentrations, and the interaction with DIC enrichment, affect macroalgal physiology.
The increase in growth rate of Lomentaria australis with elevated CO2 most likely occurred as a result of the downregulation of an energy‐costly CCM. This idea is supported by the greater shift to more negative δ13C values under future CO2 conditions. The increase in tissue carbon content for all treatments and lack of change in lipid accumulation both indicate that the decrease in δ13C values is not related to sugar use or lipid accumulation, but was caused by a change in carbon uptake strategy and the relative use of HCO3 − and CO2. As with growth rates and net photosynthesis, mixed results were reported for δ13C in the current literature (Table 2). For example, an increased growth rate was combined with decreased δ13C in Lomentaria australis (current study), whereas growth rates and δ13C simultaneously decreased in Desmarestia aculeata (Iñiguez et al., 2016a). This shows that it is important to measure physiological processes and parameters (e.g., net photosynthetic rates, C:N ratios, and δ13C) in addition to measuring the response (i.e., growth), if we are to understand how the response to CO2 enrichment is regulated.
4.2. The response of non‐CCM species
The lack of response of Craspedocarpus ramentaceus to CO2 enrichment suggests that growth and photosynthesis were saturated at current CO2 levels, as there was no evidence of nitrogen or light limitation in this experiment. C:N ratios were typical of N‐sufficient Rhodophyta (Atkinson & Smith, 1983; Supporting information Table S4), the growth rates of the specimens in our experiment are within the healthy range of fleshy Rhodophyta (Kübler et al., 1999; Nishihara, Terada, & Noro, 2004), and the light levels were sufficient for saturating photosynthesis (Supporting information Figure S1, Table S2). Only three non‐CCM species (including our work) have been studied in relation to CO2 enrichment, with contrasting results (Table 2). This indicates that, as with CCM species, non‐CCM species could have different affinities for CO2. For non‐CCM species, the affinity for DIC will mainly depend on Rubisco activity and kinetics (as this is the enzyme involved in carbon fixation). The amount of Rubisco, the V max (maximal Rubisco activity) and Km (affinity of Rubisco for CO2) vary greatly between species (Israel & Hophy, 2002). Red algae, for example, have a higher affinity for CO2 relative to O2 compared to green and brown algae, and, in general, studies show that Rubisco enzymes in CO2‐only users have evolved a higher affinity for CO2 than species with a CCM (Badger et al., 1998). In addition, environmental conditions play an important role in Rubisco kinetics. High temperatures, for example, decrease the affinity for CO2 in algae (Beardall, Stojkovic, & Larsen, 2009) and the activation state of Rubisco changes with light and CO2 concentrations, as is also known from terrestrial plants (Salvucci & Crafts‐Brandner, 2004). The capacity of non‐CCM species to fix carbon is often thought to be adapted to low rates of light absorption, in order to avoid photodamage (Maberly et al., 1992), and it is possible that non‐CCM species are therefore unable to take advantage of elevated CO2, rather than being limited in carbon. Studying how Rubisco kinetics change with CO2 enrichment could give more insight into the response of non‐CCM species. Given that 35% of red macroalgae are non‐CCM (Kübler & Dudgeon, 2015), we emphasize the need to incorporate more non‐CCM species in future laboratory studies if we are to determine how macroalgae will respond to future global change.
4.3. Biochemical responses
Our study provides the first findings on the impacts of CO2 enrichment on the lipid content, lipid class, and fatty acid composition in a CCM species compared to a non‐CCM species. Both species invested in growth rather than storing lipids, which supported our hypothesis for C. ramentaceus (the non‐CCM species), but did not support the hypothesis that lipid content and fatty acid composition would change in L. australis (the CCM species). There are several possible explanations for the lack of biochemical response of L. australis: (a) Previous work, mainly on microalgae, has shown that it is the combination of excess carbon and limiting nitrogen (i.e., insufficient nitrogen for protein synthesis) that provides optimal conditions for an increase in carbon flow toward acetyl coenzyme A (acetyl Co‐A) and NADPH for FA synthesis (Sun et al., 2016). However, the nitrogen concentration used in this experiment was not likely to be limited, as the concentrations were that of ambient seawater and C:N ratios in the algal tissue were in the range consistent with nitrogen sufficiency (Atkinson & Smith, 1983). (b) Results from the lipid class composition showed that most of the lipid occurred as structural polar lipids. This indicates that both species accumulate storage lipids only in very low amounts. The ability to accumulate storage lipids might be exhibited only by certain macroalgal taxa, while others, including some red algae, generally exhibit low storage lipid concentrations and predominantly change the composition and concentration of structural polar lipids and therefore no changes were observed (Schmid, Guihéneuf, & Stengel, 2017).
4.4. DIC limitation as a predictor of macroalgal responses to future high CO2 oceans
The results from this study and prior studies (Table 2) highlight that—even though carbon use strategy will play a role—the responses of macroalgae to CO2 enrichment cannot be inferred solely from the carbon uptake strategy as has been implicated in field studies (Cornwall et al., 2017, 2015 ; Diaz‐Pulido et al., 2016; Hepburn et al., 2011). We suggest that the key to predicting the response(s) of macroalgae to a future high CO2 ocean is to understand which species have growth rates that are limited by DIC availability under current CO2 levels, and which species are saturated. In addition, the ability of CCM species to downregulate their CCM will be a decisive factor in their response to future CO2 enrichment conditions. This view is summarized in Figure 5, which synthesizes the predicted responses of fleshy macroalgae based on their putative limitation by DIC (with high‐affinity species being saturated and low‐affinity species being limited) and carbon uptake strategy, expanding on the figure of Cornwall et al. (2017). We additionally suggest that some species may be sensitive to higher [H+] also predicted for the future, and a mechanistic understanding of the interactions between DIC and [H+] is needed (Bach et al., 2013; Roleda et al., 2012). To be able to predict the responses of macroalgae to CO2 enrichment, future studies should focus on providing a better understanding of the physiological mechanisms that underlie DIC acquisition: Details of physiological and biochemical regulatory mechanisms are known for only a few of the studied species listed in Table 2 (indicated with *), and these are mostly species with high commercial value or fouling species that inhibit growth of species with commercial value. This emphasizes an urgent need for targeted physiological experiments, as well as molecular studies, on a wide range of macroalgal species from the tropics to the poles, if we are to gain a mechanistic understanding of macroalgal responses to a future higher CO2 world, and move beyond predictions based on field observational studies.
Figure 5.
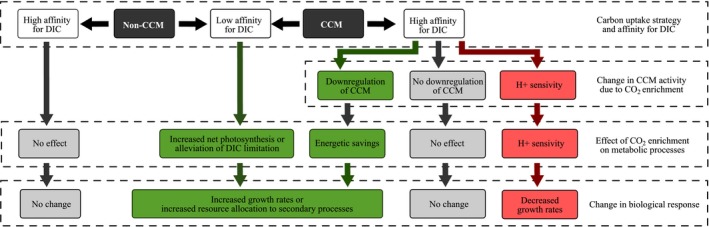
Predicted physiological and growth response of fleshy macroalgae to CO2 enrichment based on their carbon uptake strategy and affinity for DIC. Species with a low affinity for DIC are likely to be limited in DIC under current conditions, and species with a high affinity are likely to be saturated for DIC. There is literature evidence that some CCM species are sensitive to increased H+ concentrations, illustrated on the right hand side of the figure: Although not illustrated, H+ sensitivity for non‐CCM species may also be possible. This figure builds on that of Cornwall et al. (2017)
DATA ACCESSIBILITY STATEMENT
All data created during this research are available at the IMAS data management system and at FigShare (https://doi.org/10.6084/m9.figshare.7189382 and https://doi.org/10.6084/m9.figshare.7189292).
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
L.M.L. carried out laboratory experiment and analyzed the data with assistance by M.S, P.P.L., and D.B. L.M.L., M.S, P.P.L., and C.L.H. designed the study. L.M.L., M.S., and C.L.H. wrote the original draft, with subsequent contributions by all other authors. A.T.R., P.V., P.D.N., and C.M.M. collected laboratory data, contributed materials, and assisted with carbonate chemistry calculations.
Supporting information
ACKNOWLEDGMENTS
L.M.L. was funded by Holland Scholarship and the Groningen University Fund. M.S. was funded by the Deutsche Forschungsgemeinschaft (DFG, grant ID: SCHM 3335/1‐1). We thank Dr Toby Bolton for his expert support. The authors thank the editor and two anonymous reviewers for their helpful comments on the manuscript
van der Loos LM, Schmid M, Leal PP, et al. Responses of macroalgae to CO2 enrichment cannot be inferred solely from their inorganic carbon uptake strategy. Ecol Evol. 2019;9:125–140. 10.1002/ece3.4679
Holland Scholarship; Groningen University
Fund; M.S. was funded by the Deutsche Forschungsgemeinschaft (DFG, grant ID: SCHM 3335/1‐1)
REFERENCES
- Al‐Hasan, R. H. , Hantash, F. M. , & Radwan, S. S. (1991). Enriching marine macroalgae with eicosatetraenoic (arachidonic) and eicosapentaenoic acids by chilling. Applied Microbiology and Biotechnology, 35, 530–535. 10.1007/BF00169763 [DOI] [Google Scholar]
- Atkinson, M. J. , & Smith, S. V. (1983). C:N: P ratios of benthic marine plants. Limnology and Oceanography, 28, 568–574. 10.4319/lo.1983.28.3.0568. [DOI] [Google Scholar]
- Bach, L. T. , Mackinder, L. C. M. , Schulz, K. G. , Wheeler, G. , Schroeder, D. C. , Brownlee, C. , & Riebesell, U. (2013). Dissecting the impact of CO2 and pH on the mechanisms of photosynthesis and calcification in the coccolithophore Emiliania huxleyi . New Phytologist, 199, 121–134. 10.1111/nph.12225. [DOI] [PubMed] [Google Scholar]
- Badger, M. R. , Andrews, T. J. , Whitney, S. M. , Ludwig, M. , Yellowlees, D. C. , Leggat, W. , & Price, G. D. (1998). The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast‐based CO2‐concentrating mechanisms in algae. Canadian Journal of Botany, 76, 10.1139/b98-074. [DOI] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 48 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Beardall, J. , Stojkovic, S. , & Larsen, S. (2009). Plant ecology & diversity living in a high CO2 world: Impacts of global climate change on marine phytoplankton. Plant Ecology & Diversity 2(2), 191–205. 10.1080/17550870903271363 [DOI] [Google Scholar]
- Bender‐Champ, D. , Diaz‐Pulido, G. , & Dove, S. (2017). Effects of elevated nutrients and CO2 emission scenarios on three coral reef macroalgae. Harmful Algae, 65, 40–51. 10.1016/j.hal.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Bennett, S. , Wernberg, T. , Connell, S. D. , Hobday, A. J. , Johnson, C. R. , & Poloczanska, E. S. (2015). The “Great Southern Reef”: Social, ecological and economic value of Australia’s neglected kelp forests. Marine and Freshwater Research, 67, 47–56. 10.1071/MF15232 [DOI] [Google Scholar]
- Bermúdez, R. , Feng, Y. , Roleda, M. Y. , Tatters, A. O. , Hutchins, D. A. , Larsen, T. , … Winder, M. (2015). Long‐term conditioning to elevated pCO2 and warming influences the fatty and amino acid composition of the diatom Cylindrotheca fusiformis . PLoS ONE, 10, 1–15. 10.1371/journal.pone.0123945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk, M. , Haglund, K. , Ramazanov, Z. , & Pedersén, M. (1993). Inducible mechanisms for HCO3‐ utilization and repression of photorespiration in protoplast and thalli of three species of Ulva (Chlorophyta). Journal of Phycology, 29, 166–173. [Google Scholar]
- Bligh, E. , & Dyer, W. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry, 37, 911–917. 10.1139/y59-099 [DOI] [PubMed] [Google Scholar]
- Bockmon, E. E. , Frieder, C. A. , Navarro, M. O. , White‐Kershek, L. A. , & Dickson, A. G. (2013). Technical Note: Controlled experimental aquarium system for multi‐stressor investigation of carbonate chemistry, oxygen saturation, and temperature. Biogeosciences, 10, 5967–5975. 10.5194/bg-10-5967-2013. [DOI] [Google Scholar]
- Britton, D. , Cornwall, C. E. , Revill, A. T. , Hurd, C. L. , & Johnson, C. R. (2016). Ocean acidification reverses the positive effects of seawater pH fluctuations on growth and photosynthesis of the habitat‐forming kelp. Ecklonia Radiata. Scientific Reports, 6, 26036 10.1038/srep26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B. , Zou, D. , Zhu, M. , & Yang, Y. (2017). Effects of CO2 levels and light intensities on growth and amino acid contents in red seaweed Gracilaria lemaneiformis . Aquaculture Research, 48, 2683–2690. 10.1111/are.13100. [DOI] [Google Scholar]
- Chung, I. K. , Beardall, J. , Mehta, S. , Sahoo, D. , & Stojkovic, S. (2011). Using marine macroalgae for carbon sequestration: A critical appraisal. Journal of Applied Phycology, 23, 877–886. 10.1007/s10811-010-9604-9. [DOI] [Google Scholar]
- Cornwall, C. E. , Revill, A. T. , Hall‐Spencer, J. M. , Milazzo, M. , Raven, J. A. , & Hurd, C. L. (2017). Inorganic carbon physiology underpins macroalgal responses to elevated CO2 . Scientific Reports, 7, 10.1038/srep46297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall, C. E. , Revill, A. T. , & Hurd, C. L. (2015). High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynthesis Research, 124, 181–190. 10.1007/s11120-015-0114-0. [DOI] [PubMed] [Google Scholar]
- de Paula Silva, P. H. , Paul, N. A. , de Nys, R. , & Mata, L. (2013). Enhanced production of green tide algal biomass through additional carbon supply. PLoS ONE, 8, e81164 10.1371/journal.pone.0081164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz‐Pulido, G. , Cornwall, C. , Gartrell, P. , Hurd, C. , & Tran, D. V. (2016). Strategies of dissolved inorganic carbon use in macroalgae across a gradient of terrestrial influence: Implications for the Great Barrier Reef in the context of ocean acidification. Coral Reefs, 35, 1327–1341. 10.1007/s00338-016-1481-5. [DOI] [Google Scholar]
- Dickson, A. G. , & Millero, F. J. (1987). A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Research Part A, Oceanographic Research Papers, 34, 1733–1743. 10.1016/0198-0149(87)90021-5. [DOI] [Google Scholar]
- Dickson, A. G. , Sabine, C. L. , & Christian, J. R. 2007. Guide to best practices for ocean CO2 measurements. PICES Special Publication 3.
- Fernández, P. A. , Roleda, M. Y. , & Hurd, C. L. (2015). Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera . Photosynthesis Research, 124, 293–304. 10.1007/s11120-015-0138-5. [DOI] [PubMed] [Google Scholar]
- Gao, K. , Aruga, Y. , Asada, K. , Ishihara, T. , Akano, T. , & Kiyohara, M. (1991). Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2 concentrations. Journal of Applied Phycology, 3, 355–362. 10.1007/BF00026098. [DOI] [Google Scholar]
- Gao, K. , Aruga, Y. , Asada, K. , & Kiyohara, M. (1993). Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis . Journal of Applied Phycology, 5, 563–571. 10.1007/BF02184635 [DOI] [Google Scholar]
- Gao, G. , Beardall, J. , Bao, M. , Wang, C. , Ren, W. , & Xu, J. (2018). Ocean acidification and nutrient limitation synergistically reduce growth and photosynthetic performances of a green tide alga Ulva linza . Biogeosciences, 15, 3409–3420. 10.5194/bg-15-3409-2018. [DOI] [Google Scholar]
- García‐Sánchez, M. J. , Fernández, J. A. , & Niell, X. (1994). Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata . Planta, 194, 55–61. 10.1007/BF00201034. [DOI] [Google Scholar]
- Gordillo, F. J. L. , Aguilera, J. , Wiencke, C. , & Jiménez, C. (2015). Ocean acidification modulates the response of two Arctic kelps to ultraviolet radiation. Journal of Plant Physiology, 173, 41–50. 10.1016/j.jplph.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Gordillo, F. J. L. , Carmona, R. , Viñegla, B. , Wiencke, C. , & Jiménez, C. (2016). Effects of simultaneous increase in temperature and ocean acidification on biochemical composition and photosynthetic performance of common macroalgae from Kongsfjorden (Svalbard). Polar Biology, 39, 1993–2007. 10.1007/s00300-016-1897-y. [DOI] [Google Scholar]
- Gordillo, F. J. L. , Figueroa, F. L. , & Niell, F. X. (2003). Photon‐ and carbon‐use efficiency in Ulva rigida at different CO2 and N levels. Planta, 218, 315–322. 10.1007/s00425-003-1087-3. [DOI] [PubMed] [Google Scholar]
- Gordillo, F. J. L. , Niell, F. X. , & Figueroa, F. L. (2001). Non‐photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Biomedical and Life Sciences, 213, 64–70. 10.1007/s004250000468. [DOI] [PubMed] [Google Scholar]
- Gosch, B. J. , Lawton, R. J. , Paul, N. A. , Nys, R. D. , & Magnusson, M. (2015). Environmental effects on growth and fatty acids in three isolates of Derbesia tenuissima (Bryopsidales, Chlorophyta). Algal Research, 9, 82–93. 10.1016/j.algal.2015.02.022 [DOI] [Google Scholar]
- Gutow, L. , Rahman, M. M. , Bartl, K. , Saborowski, R. , Bartsch, I. , & Wiencke, C. (2014). Ocean acidification affects growth but not nutritional quality of the seaweed Fucus vesiculosus (Phaeophyceae. Fucales). PANGAEA. 10.1594/PANGAEA.835334 [DOI]
- Hall‐Spencer, J. M. , Rodolfo‐Metalpa, R. , Martin, S. , Ransome, E. , Fine, M. , Turner, S. M. , … Buia, M.‐C. (2008). Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature, 454, 96–99. 10.1038/nature07051. [DOI] [PubMed] [Google Scholar]
- Hepburn, C. D. , Pritchard, D. W. , Cornwall, C. E. , Mcleod, R. J. , Beardall, J. , Raven, J. A. , & Hurd, C. L. (2011). Diversity of carbon use strategies in a kelp forest community: Implications for a high CO2 ocean. Global Change Biology, 17, 2488–2497. 10.1111/j.1365-2486.2011.02411.x. [DOI] [Google Scholar]
- Hibberd, J. M. , & Covshoff, S. (2010). The regulation of gene expression required for C4 photosynthesis. Annual Review of Plant Biology, 61, 181–207. 10.1146/annurev-arplant-042809-112238. [DOI] [PubMed] [Google Scholar]
- Ho, M. , & Carpenter, R. C. (2017). Differential growth responses to water flow and reduced pH in tropical marine macroalgae. Journal of Experimental Marine Biology and Ecology, 491, 58–65. 10.1016/j.jembe.2017.03.009. [DOI] [Google Scholar]
- Hurd, C. L. , Hepburn, C. D. , Currie, K. I. , Raven, J. A. , & Hunter, K. A. (2009). Testing the effects of ocean acidification on algal metabolism: Considerations for experimental designs. Journal of Phycology, 45, 1236–1251. 10.1111/j.1529-8817.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- Hurd, C. L. , Lenton, A. , Tilbrook, B. , & Boyd, P. W. (2018). Current understanding and challenges for oceans in a higher‐CO2 world. Nature Climate Change, 8, 686–694. 10.1038/s41558-018-0211-0. [DOI] [Google Scholar]
- Iñiguez, C. , Carmona, R. , Lorenzo, M. R. , Niell, F. X. , Wiencke, C. , & Gordillo, F. J. L. (2016a). Increased CO2 modifies the carbon balance and the photosynthetic yield of two common Arctic brown seaweeds: Desmarestia aculeata and Alaria esculenta . Polar Biology, 39, 1979–1991. 10.1007/s00300-015-1724-x. [DOI] [Google Scholar]
- Iñiguez, C. , Carmona, R. , Lorenzo, M. R. , Niell, F. X. , Wiencke, C. , & Gordillo, F. J. L. (2016b). Increased temperature, rather than elevated CO2, modulates the carbon assimilation of the Arctic kelps Saccharina latissima and Laminaria solidungula . Marine Biology, 163, 10.1007/s00227-016-3024-6. [DOI] [Google Scholar]
- Iñiguez, C. , Heinrich, S. , Harms, L. , & Gordillo, F. J. L. (2017). Increased temperature and CO2 alleviate photoinhibition in Desmarestia anceps: From transcriptomics to carbon utilization. Journal of Experimental Botany, 68, 3971–3984. 10.1093/jxb/erx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC . (2013). Climate Change 2013: the physical science basis In: Contribution of Working Group I to the Fifth Assessment Report (AR5) of the Intergovernmental Panel on Climate Change (pp. 1029–1136). [Google Scholar]
- Israel, A. , & Hophy, M. (2002). Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Global Change Biology, 8, 831–840. 10.1046/j.1365-2486.2002.00518.x. [DOI] [Google Scholar]
- Israel, A. , Katz, S. , Dubinsky, Z. , Merrill, J. E. , & Friedlander, M. (1999). Photosynthetic inorganic carbon utilization and growth of Porphyra linearis (Rhodophyta). Journal of Applied Phycology, 11, 447–453. 10.1023/A:1008122306268 [DOI] [Google Scholar]
- Jiang, H. , Zou, D. , Lou, W. , & Gong, J. (2018). Effects of CO2 supply on growth and photosynthetic ability of young sporophytes of the economic seaweed Sargassum fusiforme (Sargassaceae, Phaeophyta). Journal of Applied Phycology., 10.1007/s10811-018-1569-0. [DOI] [Google Scholar]
- Johnson, M. D. , Comeau, S. , Lantz, C. A. , & Smith, J. E. (2017). Complex and interactive effects of ocean acidification and temperature on epilithic and endolithic coral‐reef turf algal assemblages. Coral Reefs, 36, 1059–1070. 10.1007/s00338-017-1597-2. [DOI] [Google Scholar]
- Jokiel, P. L. , Rodgers, K. S. , Kuffner, I. B. , Andersson, A. J. , Cox, E. F. , & Mackenzie, F. T. (2008). Ocean acidification and calcifying reef organisms: A mesocosm investigation. Coral Reefs, 27, 473–483. 10.1007/s00338-008-0380-9. [DOI] [Google Scholar]
- Jungnick, N. , Ma, Y. , Mukherjee, B. , Cronan, J. C. , Speed, D. J. , Laborde, S. M. , … Moroney, J. V. (2014). The carbon concentrating mechanism in Chlamydomonas reinhardtii: Finding the missing pieces. Photosynthesis Research, 121, 159–173. 10.1007/s11120-014-0004-x. [DOI] [PubMed] [Google Scholar]
- Kang, J. W. , & Chung, I. K. (2017). The effects of eutrophication and acidification on the ecophysiology of Ulva pertusa Kjellman. Journal of Applied Phycology, 29, 2675–2683. 10.1007/s10811-017-1087-5. [DOI] [Google Scholar]
- Kang, J. W. , & Chung, I. K. (2018). The interactive effects of elevated CO2 and ammonium enrichment on the physiological performances of Saccharina japonica (Laminariales. Phaeophyta). Ocean Science Journal, 53(3), 487–497. 10.1007/s12601-018-0014-2 [DOI] [Google Scholar]
- Kang, J. W. , Kambey, C. , Shen, Z. , Yang, Y. , & Chung, I. K. (2017). The short‐term effects of elevated CO2 and ammonium concentrations on physiological responses in Gracilariopsis lemaneiformis (Rhodophyta). Fisheries and Aquatic Sciences, 20, 10.1186/s41240-017-0063-y. [DOI] [Google Scholar]
- Kang, E. J. , & Kim, K. Y. (2016). Effects of future climate conditions on photosynthesis and biochemical component of Ulva pertusa (Chlorophyta). Algae, 31, 49–59. 10.4490/algae.2016.31.3.9 [DOI] [Google Scholar]
- Kawamitsu, Y. , & Boyer, J. S. (1999). Photosynthesis and carbon storage between tides in a brown alga, Fucus vesiculosus . Marine Biology, 133, 361–369. 10.1007/s002270050475 [DOI] [Google Scholar]
- Kim, J. H. , Kang, E. J. , Edwards, M. S. , Lee, K. , Jeong, H. J. , & Kim, K. Y. (2016). Species‐specific responses of temperate macroalgae with different photosynthetic strategies to ocean acidification: A mesocosm study. Algae, 31, 243–256. 10.4490/algae.2016.31.8.20. [DOI] [Google Scholar]
- Koch, M. , Bowes, G. , Ross, C. , & Zhang, X. H. (2013). Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology, 19, 103–132. 10.1111/j.1365-2486.2012.02791.x. [DOI] [PubMed] [Google Scholar]
- Kram, S. L. , Price, N. N. , Donham, E. M. , Johnson, M. D. , Kelly, E. L. A. , Hamilton, S. L. , & Smith, J. E. (2016). Variable responses of temperate calcified and fleshy macroalgae to elevated pCO2 and warming. ICES Journal of Marine Science: Journal Du Conseil, 73, 693–703. 10.1093/icesjms/fsv168. [DOI] [Google Scholar]
- Kübler, J. E. , & Dudgeon, S. R. (2015). Predicting effects of ocean acidification and warming on algae lacking carbon concentrating mechanisms. PLoS ONE, 10, 1–19. 10.1371/journal.pone.0132806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler, J. E. , Johnston, A. M. , & Raven, J. A. (1999). The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata . Plant, Cell and Environment, 22, 1303–1310. 10.1046/j.1365-3040.1999.00492.x [DOI] [Google Scholar]
- Li, X. , Xu, J. , & He, P. (2016). Comparative research on inorganic carbon acquisition by the macroalgae Ulva prolifera (Chlorophyta) and Pyropia yezoensis (Rhodophyta). Journal of Applied Phycology, 28, 491–497. 10.1007/s10811-015-0603-8. [DOI] [Google Scholar]
- Li, Y. , Zhong, J. , Zheng, M. , Zhuo, P. , & Xu, N. (2018). Photoperiod mediates the effects of elevated CO2 on the growth and physiological performance in the green tide alga Ulva prolifera . Marine Environmental Research, 10.1016/j.marenvres.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Lignell, Å. , & Pedersén, M. (1989). Effects of pH and inorganic carbon concentration on growth of Gracilaria secundata . British Phycological Journal, 24, 83–89. 10.1080/00071618900650071 [DOI] [Google Scholar]
- Liu, C. , & Zou, D. (2015a). Do increased temperature and CO2 levels affect the growth, photosynthesis, and respiration of the marine macroalga Pyropia haitanensis (Rhodophyta)? An experimental study. Hydrobiologia, 745, 285–296. 10.1007/s10750-014-2113-0 [DOI] [Google Scholar]
- Liu, C. , & Zou, D. (2015b). Responses of elevated CO2 on photosynthesis and nitrogen metabolism in Ulva lactuca (Chlorophyta) at different temperature levels. Marine Biology Research, 11, 1043–1052. 10.1080/17451000.2015.1062520 [DOI] [Google Scholar]
- Liu, C. , Zou, D. , & Yang, Y. (2018). Comparative physiological behaviors of Ulva lactuca and Gracilariopsis lemaneiformis in responses to elevated atmospheric CO2 and temperature. Environmental Science and Pollution Research, 25, 27493–27502. 10.1007/s11356-018-2792-6 [DOI] [PubMed] [Google Scholar]
- Maberly, S. C. , Raven, J. A. , & Johnston, A. M. (1992). Discrimination between 12C and 13C by marine plants. Oecologia, 91, 481–492. 10.1007/BF00650320 [DOI] [PubMed] [Google Scholar]
- McGraw, C. M. , Cornwall, C. E. , Reid, M. R. , Currie, K. I. , Hepburn, C. D. , Boyd, P. W. , … Hunter, K. A. (2010). An automated pH‐controlled culture system for laboratory‐based ocean acidification experiments. Limnology and Oceanography: Methods, 8, 686–694. 10.4319/lom.2010.8.686. [DOI] [Google Scholar]
- Mehrbach, C. , Culberson, C. H. , Hawley, J. E. , & Pytkowicz, R. M. (1973). Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnology and Oceanography, 18, 897–907. [Google Scholar]
- Mensch, B. , Neulinger, S. C. , Graiff, A. , Pansch, A. , Künzel, S. , Fischer, M. A. , & Schmitz, R. A. (2016). Restructuring of epibacterial communities on Fucus vesiculosus forma mytili in response to elevated pCO and increased temperature levels. Frontiers in Microbiology, 7, 1–15. 10.3389/fmicb.2016.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado, J. M. , Javier, F. , Gordillo, L. , Xavier Niell, F. , & Figueroa, F. L. (1999). Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta . Journal of Applied Phycology, 11, 455–461. [Google Scholar]
- Nishihara, G. N. , Terada, R. , & Noro, T. (2004). Photosynthesis and growth rates of Laurencia brongniartii J. Agardh (Rhodophyta, Ceramiales) in preparation for cultivation. Journal of Applied Phycology, 16, 303–308. 10.1023/B. [DOI] [Google Scholar]
- Nunes, J. , McCoy, S. J. , Findlay, H. S. , Hopkins, F. E. , Kitidis, V. , Queiros, A. M. , … Widdicombe, S. (2016). Two intertidal, non‐calcifying macroalgae (Palmaria palmata and Saccharina latissima) show complex and variable responses to short‐term CO2 acidification. ICES Journal of Marine Science, 73, 887–896. 10.1093/icesjms/fsv081. [DOI] [Google Scholar]
- Ober, G. T. , & Thornber, C. S. (2017). Divergent responses in growth and nutritional quality of coastal macroalgae to the combination of increased pCO2 and nutrients. Marine Environmental Research, 131, 69–79. 10.1016/j.marenvres.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Olischläger, M. , Iñiguez, C. , Koch, K. , Wiencke, C. , & Gordillo, F. J. L. (2017). Increased pCO2 and temperature reveal ecotypic differences in growth and photosynthetic performance of temperate and Arctic populations of Saccharina latissima . Planta, 245, 119–136. 10.1007/s00425-016-2594-3. [DOI] [PubMed] [Google Scholar]
- Olischläger, M. , & Wiencke, C. (2013). Ocean acidification alleviates low‐temperature effects on growth and photosynthesis of the red alga Neosiphonia harveyi (Rhodophyta). Journal of Experimental Botany, 64, 5587–5597. 10.1093/jxb/ert329. [DOI] [PubMed] [Google Scholar]
- Oksanen, J. , Guillaume Blanchet, F. , Friendly, M. , Kindt, R. , Legendre, P. , McGlinn, D. , … Wagner, H. (2016). Vegan: Community ecology package. R package version 2.3‐4. Retreieved from http://CRAN.R-project.org/package=vegan [Google Scholar]
- Rautenberger, R. , Fernández, P. A. , Strittmatter, M. , Heesch, S. , Cornwall, C. E. , Hurd, C. L. , & Roleda, M. Y. (2015). Saturating light and not increased carbon dioxide under ocean acidification drives photosynthesis and growth in Ulva rigida (Chlorophyta). Ecology and Evolution, 5, 874–888. 10.1002/ece3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, J. A. , Ball, L. A. , Beardall, J. , Giordano, M. , & Maberly, S. C. (2005). Algae lacking carbon‐concentrating mechanisms. Canadian Journal of Botany, 83, 879–890. 10.1139/b05-074. [DOI] [Google Scholar]
- Raven, J. A. , Johnston, A. M. , Kübler, J. E. , Korb, R. , McInroy, S. G. , Handley, L. L. , … Walker, D. I. (2002). Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Functional Plant Biol., 29, 335–378. 10.1071/PP01201. [DOI] [PubMed] [Google Scholar]
- Reidenbach, L. B. , Fernandez, P. A. , Leal, P. P. , Noisette, F. , McGraw, C. M. , Revill, A. T. , … Kübler, J. E. (2017). Growth, ammonium metabolism, and photosynthetic properties of Ulva australis (Chlorophyta) under decreasing pH and ammonium enrichment. PLoS ONE, 12, 1–20. 10.1371/journal.pone.0188389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers, J. S. , & Peckol, P. (1995). Interactive effects of nitrogen and dissolved inorganic carbon on photosynthesis, growth, and ammonium uptake of the macroalgae Cladophora vagabunda and Gracilaria tikvahiae . Marine Biology, 121, 747–753. 10.1007/BF00349311 [DOI] [Google Scholar]
- Roleda, M. Y. , Morris, J. N. , McGraw, C. M. , & Hurd, C. L. (2012). Ocean acidification and seaweed reproduction: Increased CO2 ameliorates the negative effect of lowered pH on meiospore germination in the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae). Global Change Biology, 18, 854–864. 10.1111/j.1365-2486.2011.02594.x. [DOI] [Google Scholar]
- Salvucci, M. E. , & Crafts‐Brandner, S. J. (2004). Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiologia Plantarum, 120, 179–186. 10.1111/j.0031-9317.2004.0173.x [DOI] [PubMed] [Google Scholar]
- Sampath‐Wiley, P. , & Neefus, C. D. (2007). An improved method for estimating R‐phycoerythrin and R‐phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta). Journal of Applied Phycology, 19, 123–129. 10.1007/s10811-006-9118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini, G. , Matthijs, H. C. P. , Verspagen, J. M. H. , Muyzer, G. , & Huisman, J. (2014). Genetic diversity of inorganic carbon uptake systems causes variation in CO2 response of the cyanobacterium Microcystis. The ISME Journal, 8, 589–600. 10.1038/ismej.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker, M. Y. , Bartsch, I. , Olischläger, M. , Gutow, L. , & Wiencke, C. (2013). Combined effects of CO2, temperature, irradiance and time on the physiological performance of Chondrus crispus (Rhodophyta). Botanica Marina, 56, 63–74. 10.1515/bot-2012-0143. [DOI] [Google Scholar]
- Schmid, M. , Guihéneuf, F. , & Stengel, D. B. (2017). Plasticity and remodelling of lipids support acclimation potential in two species of low‐intertidal macroalgae, Fucus serratus (Phaeophyceae) and Palmaria palmata (Rhodophyta). Algal Research, 26, 104–114. 10.1016/j.algal.2017.07.004. [DOI] [Google Scholar]
- Schmidt, É. C. , Maraschin, M. , & Bouzon, Z. L. (2010). Effects of UVB radiation on the carragenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales): Changes in ultrastructure, growth, and photosynthetic pigments. Hydrobiologia, 649, 171–182. 10.1007/s10750-010-0243-6. [DOI] [Google Scholar]
- Schneider, C. A. , Rasband, W. S. , & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebök, S. , Herppich, W. B. , & Hanelt, D. (2017). Development of an innovative ring‐shaped cultivation system for a land‐based cultivation of marine macroalgae. Aquacultural Engineering, 77, 33–41. 10.1016/j.aquaeng.2017.01.005. [DOI] [Google Scholar]
- Seely, G. R. , Duncan, M. J. , & Vidaver, W. E. (1972). Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Marine Biology, 12, 184–188. 10.1007/BF00350754. [DOI] [Google Scholar]
- Suárez‐Álvarez, S. , Gómez‐Pinchetti, J. L. , & García‐Reina, G. (2012). Effects of increased CO2 levels on growth, photosynthesis, ammonium uptake and cell composition in the macroalga Hypnea spinella (Gigartinales, Rhodophyta). Journal of Applied Phycology, 24, 815–823. 10.1007/s10811-011-9700-5. [DOI] [Google Scholar]
- Sun, Z. , Chen, Y. F. , & Du, J. (2016). Elevated CO2 improves lipid accumulation by increasing carbon metabolism in Chlorella sorokiniana . Plant Biotechnology Journal, 14, 557–566. 10.1111/pbi.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday, J. M. , Fabricius, K. E. , Kroeker, K. J. , Anderson, K. M. , Brown, N. E. , Barry, J. P. , … Harley, C. D. G. (2016). Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nature Climate Change, 1, 1–6. 10.1038/nclimate3161. [DOI] [Google Scholar]
- Swanson, A. K. , & Fox, C. H. (2007). Altered kelp (Laminariales) phlorotannins and growth under elevated carbon dioxide and ultraviolet‐B treatments can influence associated intertidal food webs. Global Change Biology, 13, 1696–1709. 10.1111/j.1365-2486.2007.01384.x [DOI] [Google Scholar]
- The Royal Society (2005). Ocean acidification due to increasing atmospheric carbon dioxide. The Royal Society Policy document 12/05.
- Thom, R. M. (1996). CO2‐Enrichment effects on eelgrass (Zostera marina L.) and bull kelp (Nereocystis luetkeana (mert.) P & R.). Water, Air, and Soil Pollution, 88, 383–391. 10.1007/BF00294113. [DOI] [Google Scholar]
- Tuya, F. , Wernberg, T. , & Thomsen, M. S. (2008). The spatial arrangement of reefs alters the ecological patterns of fauna between interspersed algal habitats. Estuarine, Coastal and Shelf Science, 78, 774–782. 10.1016/j.ecss.2008.02.017. [DOI] [Google Scholar]
- Wickham, H. (2007). Reshaping data with the reshape package. Journal of Statistical Software, 21, 1–20. [Google Scholar]
- Wickham, H. (2009). ggplot2: Elegant graphics for data analysis (pp. 1–213). New York, NY: Springer‐Verlag. [Google Scholar]
- Wickham, H. (2011). The split‐apply‐combine strategy for data analysis. Journal of Statistical Software, 40, 1–29. 10.1039/np9971400083 [DOI] [Google Scholar]
- Xu, K. , Chen, H. , Wang, W. , Xu, Y. , Ji, D. , Chen, C. , & Xie, C. (2017). Responses of photosynthesis and CO2 concentrating mechanisms of marine crop Pyropia haitanensis thalli to large pH variations at different time scales. Algal Research, 28, 200–210. 10.1016/j.algal.2017.10.023. [DOI] [Google Scholar]
- Xu, J. , & Gao, K. (2012). Future CO2‐induced ocean acidification mediates the physiological performance of a green tide alga. Plant Physiology, 160, 1762–1769. 10.1104/pp.112.206961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Gao, G. , Xu, J. , & Wu, H. (2017). Physiological response of a golden tide alga (Sargassum muticum) to the interaction of ocean acidification and phosphorus enrichment. Biogeosciences, 14, 671–681. 10.5194/bg-14-671-2017. [DOI] [Google Scholar]
- Xu, Z. , Zou, D. , & Gao, K. (2010). Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Botanica Marina, 53, 123–129. 10.1515/BOT.2010.012 [DOI] [Google Scholar]
- Yong, Y. S. , Yong, W. T. L. , & Anton, A. (2013). Analysis of formulae for determination of seaweed growth rate. Journal of Applied Phycology, 25, 1831–1834. 10.1007/s10811-013-0022-7 [DOI] [Google Scholar]
- Zhu, Z. , Piao, S. , Myneni, R. B. , Huang, M. , Zeng, Z. , Canadell, J. G. , … Zeng, N. (2016). Greening of the Earth and its drivers. Nature Climate Change, 6, 791–795. 10.1038/nclimate3004 [DOI] [Google Scholar]
- Zou, D. (2005). Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture, 250, 726–735. 10.1016/j.aquaculture.2005.05.014 [DOI] [Google Scholar]
- Zou, D. , & Gao, K. (2009). Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia, 48, 510–517. 10.2216/08-99.1 [DOI] [Google Scholar]
- Zou, D. , Gao, K. , & Luo, H. (2011). Short‐ and long‐term effects of elevated CO2 on photosynthesis and respiration in the marine macroalga Hizikia Fusiformis (Sargassaceae, Phaeophyta) grown at low and high N supplies. Journal of Phycology, 47, 87–97. 10.1111/j.1529-8817.2010.00929.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data created during this research are available at the IMAS data management system and at FigShare (https://doi.org/10.6084/m9.figshare.7189382 and https://doi.org/10.6084/m9.figshare.7189292).


