Abstract
Purpose
Adherence to medication can be assessed by various self-report questionnaires. One could hypothesize that survey respondents tend to answer questions in a manner that will be viewed favorably by others. We aimed to answer if anonymous and nonanonymous responses to a questionnaire on medication adherence differ.
Patients and methods
Adherence was assessed with the German Stendal Adherence with Medication Score (SAMS), which includes 18 questions with responses based on a 5-point Likert scale. Anonymous data from 40 subjects were collected during a symposium for patients with Parkinson’s disease (PD), and nonanonymous data were obtained from 40 outpatient-clinic PD patients at the Department of Neurology.
Results
The two groups (anonymous self-reported questionnaire and nonanonymous) did not differ in terms of demographical characteristics and the SAMS sum score. However, anonymously collected data showed significant higher scoring for the item 6 (“Do you forget your medications?”) than the data collected nonanonymously (P=0.017). All other items of the SAMS did not significantly differ between both groups.
Conclusion
Overall assessment of adherence does not depend on whether the patient remains anonymous or not. There seems to be no relevant social desirability bias in nonanonymous responses.
Keywords: self-report, adherence questionnaire, Parkinson’s disease, anonymous, nonadherence
Introduction
Treatment of neurological disorders commonly includes long-term pharmacological therapy. However, for various reasons, many people do not follow the instructions they are given for prescribed treatments (nonadherence).2–6 Nonadherence can be assessed with direct or indirect methods. Direct methods include visually observed therapy or metabolites in the blood and have limited practicality within routine clinical use. Many researchers justify indirect methods including self-report questionnaires.1,7–10 However, does it make a difference when adherence was assessed anonymously or nonanonymously and when the patients know the researcher/physician? To answer this question, we aimed to compare anonymous vs nonanonymous responses to a questionnaire on medication adherence. We chose a cohort of patients with Parkinson’s disease (PD), because it involves a common chronical disorder with patients ingesting several drugs per day.14
Methods
The study was approved by the local ethics committee of the Jena University Hospital. Written informed consent was obtained from all participating patients prior to enrolment in the study, and the study was conducted in accordance with the Declaration of Helsinki. Anonymous data from 40 subjects were collected during a symposium for PD patients in the Jena University organized by the Department of Neurology at the Jena University Hospital. This regular symposium is open for all patients with PD from Thuringia and neighboring regions. In preparation of the symposium, invitations were sent to regional Patient Support Groups as well as neurological outpatient clinics. Nonanonymous data from patients known to the researchers (TP, DS, CP) were obtained from 40 outpatient-clinic PD patients at the Department of Neurology between September and October 2017. Additional demographical data collected are listed in Table 1. We used the German Stendal Adherence with Medication Score (SAMS) questionnaire, an extension of the validated German Essen Compliance Score, to assess adherence (Table 2).11–13 The SAMS includes 18 questions with responses based on a 5-point Likert scale. In accordance with the cumulative scale 0–72, 0 indicates complete adherence and 72 indicates complete nonadherence (SAMS items are given in Table 1). SPSS (version 23.0; IBM Corporation, Armonk, NY, USA) was used for statistical analyses. There is no established threshold to determine nonadherence. It is generally considered that suboptimal adherence becomes clinically significant when <80% of prescribed medication is taken.15–17 In our study, the highest 25% of all SAMS scores were categorized as nonadherent.15 This leads to a study and sample-specific SAMS cutoff of 7 points for a clinical meaningful nonadherence. The patients were then categorized into 1) fully adherent (SAMS=0), 2) moderate nonadherent (SAMS=1–7), and 3) nonadherent (SAMS >7). Sample size calculation was performed for the SAMS sum score as the main outcome. A sample size of 34 participants per group was found to determine if both groups are equivalent (power=0.8, α=0.05, sampling ratio=1). After checking for outliers and normality, either the t-test or chi-squared test were used for comparison between both groups with Bonferroni correction for multiple comparisons. Kolmogorov–Smirnov test was used to determine similarity between both groups. Statistical significance was set at P<0.05. The datasets generated during the current study are available from the corresponding author on reasonable request for scientific purpose only.
Table 1.
Characteristics of both groups (nonanonymous self-report and anonymous self-report) and items of the Stendal Adherence to Medication Score
| Demographical characteristics | Self-report questionnaire | P-value | |
|---|---|---|---|
| Nonanonymous | Anonymous | ||
| Age | 70±9 45–82 |
72±9 44–83 |
0.370 |
| Number of drugs per day | 6±3 2–14 |
6±3 2–15 |
0.371 |
| Sum Stendal Adherence to Medication Score | 5.4±6.8 0–33 |
5.4±5.8 0–31 |
0.874 |
| Sex | 1.000 | ||
| Male | 24 | 24 | |
| Female | 16 | 16 | |
| Marital status | 0.583 | ||
| Married | 30 | 31 | |
| Missing value | 0 | 1 | |
| Single | 1 | 2 | |
| Divorced or widowed | 9 | 6 | |
| Graduation | 0.522 | ||
| High | 15 | 15 | |
| Low | 13 | 9 | |
| Middle | 12 | 16 | |
| Occupation | 0.185 | ||
| Employed | 4 | 3 | |
| Not employed | 3 | 0 | |
| Pensioned | 33 | 37 | |
| Organizes daily medication to be taken | 0.216 | ||
| Caregiver | 6 | 10 | |
| Health care service | 2 | 0 | |
| Patient | 32 | 30 | |
Note: Metric data are given as mean ± SD and range, categorical data are given as number, and t-test or chi-squared test was used for comparison between both groups.
Table 2.
Stendal Adherence to Medication Score (SAMS)
| For all | For most | For half | For some | For none | ||
| 0 | 1 | 2 | 3 | 4 | ||
| 1 | Do you know the reason for taking your medication? | |||||
| 2 | Do you know the dosages of your medication? | |||||
| 3 | Are you familiar with the timing for taking the medication? | |||||
| All | Most | Half | Some | None | ||
| 0 | 1 | 2 | 3 | 4 | ||
| 4 | Do you take your medication regularly? | |||||
| 5 | Do you know the names of medications you are taking? | |||||
| Never | Rare | Sometimes | Often | Mostly | ||
| 0 | 1 | 2 | 3 | 4 | ||
| 6 | Do you forget to take your medication? | |||||
| 7 | Are you untroubled about taking the medication? | |||||
| 8 | Do you stop taking your medication when you feel better? | |||||
| 9 | Do you stop taking your medication if you sometimes feel worse after taking the medication? | |||||
| 10 | Do you take any wrong or other/unprescribed medications (such as those of your partner)? | |||||
| If you think you have side effects due to of the medications (such as tremors, nausea, etc) | ||||||
| 11 | Do you reduce the dose without consulting a doctor? | |||||
| 12 | Do you not take the medication for a while, ie, take a break? | |||||
| 13 | If you feel you have to take too many, do you stop taking those medications you consider to be less important than the others without consulting your doctor? | |||||
| If you forget or omit your medication, do you forget it… | ||||||
| 14 | in the morning? | |||||
| 15 | at noon? | |||||
| 16 | in the evening? | |||||
| 17 | Do you deliberately not take medications you do not consider important, but take the rest? | |||||
| 18 | If you take medication as a syringe or a weekly tablet, have you ever forgotten it? |
Results
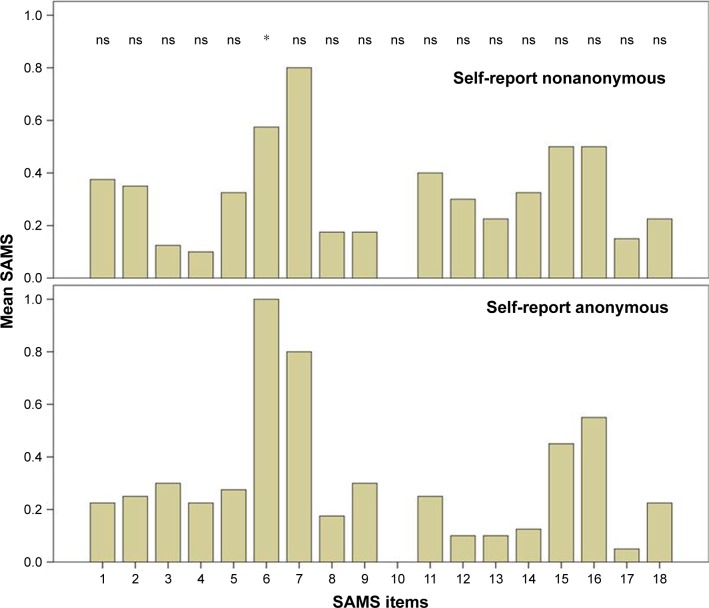
Overall 32 female and 48 male patients with PD with a mean age of 71 years (SD=9, range 44–83) were included in the analysis. The two groups (anonymous self-reported questionnaire and nonanonymous) did not differ in terms of age, gender, marital status, graduation, occupation, the number of drugs used per day, and the mean SAMS sum score (chi-squared test and t-test, respectively; P>0.05) (detailed in Table 1). The mean SAMS sum score of both groups did not differ in the Kolmogorov–Smirnov test (P=0.91). According to the SAMS, 13 (16%) patients were fully adherent (SAMS=0), 46 (56.8%) showed moderate nonadherence, and 21 (25.9%) were found to be clinically meaningful nonadherent. The numbers of fully adherent, moderate nonadherent, and nonadherent patients did not significantly differ between anonymously and nonanonymously collected data (chi-squared test, P>0.05). Anonymously collected data showed significantly higher scoring for the item 6 (“Do you forget your medications?”) than the data collected nonanonymously (P=0.017 with Bonferroni correction). All other items of the SAMS did not significantly differ between both groups (Figure 1).
Figure 1.
Comparison of each item of the Stendal Adherence to Medication Score (SAMS) between both groups.
Notes: Only item 6 (“Do you forget your medications?”) was significantly (*P=0.017) higher in the anonymously collected data. All other items of the SAMS did not significantly differ between both groups (ns).
Discussion
Pharmacotherapy in patients with PD is often suboptimal, and nonadherence is influenced by various factors, such as disease stage, motor complications, complexity of therapeutic schedule, or the presence of depression.18 The prevalence of the observed nonadherence agrees with other epidemiological studies.18,19 Our study indicates that overall assessment of adherence does not depend on whether the patient remains anonymous or not. Solely, for the item “Do you forget your medications?” did patients more frequently report forgetfulness if they were asked anonymously. This also implies that this general question probably does not add significant knowledge about the individual adherence of a patient. It seems more appropriate to convey to the patient that forgetting is a normal human trait and to rephrase the question to, “If you forget your medication, is it more likely to happen in the morning, at noon or in the evening?” Our data suggest that adherence questionnaires can also be used by physicians for regular care to determine reasons of nonadherence.
This study is not free of limitations. Although the results are likely to be transferable to other groups of patients, it should be noted that it was restricted to patients with PD, because they usually need long-term medical treatment with several drugs per day. Due to the study design, we cannot guarantee that other cofounders, such as disease severity or disease stage (which were not assessed in the anonymous cohort), were perfectly matched. However, to the group with the nonanonymous self-report, we only included patients from our outpatient hospital who were mobile and able to walk in order to make them comparable to the anonymous patients who had participated in the symposium.
Conclusion
We found that the overall assessment of adherence, as indicated by the SAMS sum score, does not depend on whether the patient remains anonymous or not. There seems to be no social desirability bias in nonanonymous responses. Therefore, the SAMS questionnaire can be used as valid tool for physicians to detect nonadherence in their patients.
Acknowledgments
We thank Nasim Kroegel for proofreading and Sarah Mendorf, Jenny Wilde, Maria-Theresa Gruber, and Juliane Nößler for acquisition of data.
Footnotes
Author contributions
Research idea and study design: TP. Data collection: TP, DS, CP. Data analysis/interpretation: TP, DS, GHF, AK. Preparation of the manuscript: TP. Revision and approval of the manuscript: CP, OW, JG. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pérez-Escamilla B, Franco-Trigo L, Moullin JC, Martínez-Martínez F, García-Corpas JP. Identification of validated questionnaires to measure adherence to pharmacological antihypertensive treatments. Patient Prefer Adherence. 2015;9:569–578. doi: 10.2147/PPA.S76139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yap AF, Thirumoorthy T, Kwan YH. Systematic review of the barriers affecting medication adherence in older adults. Geriatr Gerontol Int. 2016;16(10):1093–1101. doi: 10.1111/ggi.12616. [DOI] [PubMed] [Google Scholar]
- 4.Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med. 2017;99:269–276. doi: 10.1016/j.ypmed.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence. JAMA. 2014;312(12):1237–1247. doi: 10.1001/jama.2014.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slomski A. Pill reminders don’t improve adherence. JAMA. 2017;317(24):2476. doi: 10.1001/jama.2017.7588. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwlaat R, Wilczynski N, Navarro T. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother. 2004;38(9):1363–1368. doi: 10.1345/aph.1E071. [DOI] [PubMed] [Google Scholar]
- 11.Franke GH, Jagla M, Reimer J, Haferkamp L, Türk T, Witzke O. Erfassung von Medikamenten-compliance bei erfolgreich nierentransplanti-erten mit einer erweiterten version des morisky-scores – dem Essener Compliance Score (ECS) [Detection of adherence to medication with an extended version of the Morisky score (Essener Compliance Score, ECS) in kidney transplanted patients] PPmP – Psychotherapie Psychosomatik Medizinische Psychologie. 2009;59(02):79–93. [Google Scholar]
- 12.Jäger SFG, Reimer J, Gall C, Haferkamp L, Türk T, Witzke O. Der Zusammenhang zwischen Medikamenten-Compliance und gesundheitsbezogener Lebensqualität bei Nierentransplantierten [Relations-ship between adherence to medication and health related quality of life in kidney transplanted patients] AK Klinische Psychologie im BDP: Psychische Störungen in der somatischen Rehabilitation. 2009:79–93. [Google Scholar]
- 13.Türk TFG, Jagla M, Haferkamp L, Reimer J, Kribben A, Witzke O. Development of the Essen compliance score – measurement of adherence in kidney transplant patients. Am J Transpl. 2009;9(Suppl 2):A296. [Google Scholar]
- 14.Bainbridge JL, Ruscin JM. Challenges of treatment adherence in older patients with Parkinson’s disease. Drugs Aging. 2009;26(2):145–155. doi: 10.2165/0002512-200926020-00006. [DOI] [PubMed] [Google Scholar]
- 15.Dimatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 16.Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurologica Scandinavica. 2017;135(5):507–515. doi: 10.1111/ane.12703. [DOI] [PubMed] [Google Scholar]
- 17.Offord S, Lin J, Wong B, Mirski D, Baker RA. Impact of oral anti-psychotic medication adherence on healthcare resource utilization among schizophrenia patients with medicare coverage. Commun Mental Health J. 2013;49(6):625–629. doi: 10.1007/s10597-013-9638-y. [DOI] [PubMed] [Google Scholar]
- 18.Straka I, Minar M, Gazova A, Valkovic P, Kyselovic J. Clinical aspects of adherence to pharmacotherapy in Parkinson disease: a PRISMA-compliant systematic review. Medicine. 2018;97(23):e10962. doi: 10.1097/MD.0000000000010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand H, Hayes P, Morrissey EC, et al. Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J Hypertens. 2017;35(12):2346–2357. doi: 10.1097/HJH.0000000000001502. [DOI] [PubMed] [Google Scholar]