Abstract
Background:
Cerebrospinal fluid (CSF) biomarkers can distinguish Alzheimer’s disease (AD) patients from normal controls; however, their interpretation and potential for use in patients with mild cognitive impairment (MCI) remains unclear.
Objective:
To examine whether biomarker levels allow for risk stratification among MCI patients who are at increased risk to develop AD, thus allowing for improved targeting of early interventions for those whose risk are higher.
Methods:
We analyzed data from the Alzheimer’s Disease Neuroimaging Initiative on MCI patients (n = 195) to estimate their risk of developing AD for up to 6 years on the basis of baseline CSF biomarkers. We used time-dependent receiver operating characteristic analysis to identify the best combination of biomarkers to discriminate those who converted to AD from those who remained stable. We used these data to construct a multi-biomarker score and estimated the risk of progression to AD for each quintile of the multi-biomarker score.
Results:
We found that Aβ1–42 and P-tau181p were the best combination among CSF biomarkers to predict the overall risk of developing AD among MCI patients (area under the curve = 0.77). The hazard ratio of developing AD among MCI patients with high-risk (3rd–5th quintiles) biomarker levels was about 4 times greater than MCI patients with low-risk (1st quintile) levels (95% confidence interval, 1.93–7.26).
Conclusion:
Our study identifies MCI patients at increased risk of developing AD by applying a multi-biomarker score using CSF biomarker results. Our findings may be of value to MCI patients and their clinicians for planning purposes and early intervention as well as for future clinical trials.
Keywords: Alzheimer’s disease, cerebrospinal fluid, discriminatory ability, mild cognitive impairment, risk stratification
INTRODUCTION
Much of the focus of Alzheimer’s disease (AD) research has turned to the pre-dementia stages of the disease. Patients in the prodromal stage of AD, referred to as mild cognitive impairment (MCI) [1], are at increased risk of developing AD. Evidence has emerged suggesting that such individuals [2] are most likely to benefit from disease-modifying therapies once they become available [3, 4].
Blood pressure and cholesterol levels provide physicians and patients with a quantification of the risk of experiencing heart disease, which can be used to inform treatment decisions. Similarly, risk stratification of MCI patients using biomarker levels could be useful in identifying higher-risk patients early in the disease course with the goal of providing early intervention. While currently available pharmacological treatments for MCI patients provide modest benefits in terms of preventing the onset of AD [4–7], knowledge of a patient’s risk could also trigger care planning strategies for patients and their caregivers.
Several biomarkers have been proposed to facilitate an accurate diagnosis of AD during the MCI stage, such as hippocampal atrophy on magnetic resonance imaging (MRI), amyloid imaging using positron emission tomography (PET), and changes in cerebrospinal fluid (CSF) [8, 9]. CSF concentration of Aβ1–42 (a biomarker of amyloid-β deposition in the brain) and biomarkers of neurodegeneration, including the CSF concentrations of total tau (T-tau) and phosphorylated tau (P-tau181p) proteins, are reflected in the currently proposed diagnostic criteria [7] for AD and MCI [10].
A National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroup proposed criteria for the specific definition of MCIdueto AD by combining clinical symptoms with CSF biomarker evidence [7, 11], denoting the presence of a positive Aβ biomarker and a positive biomarker of neuronal injury (T-tau or P-tau181p) as a high likelihood that the MCI syndrome is due to AD. Also, the research criteria proposed at an International Working Group (IWG) of dementia experts considered abnormalities in CSF biomarkers as one of four supportive diagnostic features of AD [12–14]. Both of these groups acknowledge the importance of CSF biomarkers in informing the likelihood of the progression of AD among MCI patients [15].
Decreased levels of Aβ1–42, and elevated levels of T-tau or P-tau181P in CSF have been established as useful indicators for early ADdiagnosis [16–19]. Although there have been several possible cut-off values proposed [5, 20–22], there is a lack of agreement on cut-off thresholds due to the variability in CSF measurements between laboratories [23] and across techniques [24].
Combining CSF biomarkers into a single score has been shown to better discriminate between patients with an AD diagnosis compared with healthy controls than an individual biomarker [16, 23, 25, 28]. Examples include the Innotest Amyloid-Tau Index (IATI) defined by the ratio Aβ1–42/(240+1.18×tau) [25, 26], the AD-CSF-Index [4, 16, 27], and the ratios T-tau/Aβ1–42 or P-tau181p/Aβ1–42. These proposed diagnostic algorithms, however, were constructed initially to discriminate AD patients from cognitively normal controls but not to distinguish between MCI patients who have developed AD over time and those who remained stable. We have extended this logic to assess how well a combined prognostic biomarker measured at baseline could distinguish between MCI patients who develop AD over time and those who do not.
MATERIALS AND METHODS
Subjects
All data were obtained from the AD Neuroimaging Initiative (ADNI) database October 26, 2013 (https://ida.loni.usc.edu). The ADNI is a non-treatment, observational study aimed at setting standards for brain imaging and chemical biomarkers for diagnosis and treatment trials. The study was launched in 2003 and is supported by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies, and non-profit organizations. The study (ADNI 1) enrolled 192 patients with mild AD, 398 with MCI, and 229 with no cognitive impairment [29]. Six month or one year clinical, imaging, and biomarker assessments were conducted over a study period.
The primary goal of the ADNI is to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. We included MCI subjects with complete data on CSF biomarkers at study entry in our analysis (n = 195). We extracted all assessments at baseline and the disease status at each follow-up.
CSF measurement
The methods for CSF acquisition and biomarker measurement used in the ADNI study have been reported previously [22]. The CSF concentration of Aβ1–42, T-tau, and P-tau181p were measured in the baseline CSF samples using Innogenetics reagents (research use only AlzBio3 immuno-assay kits, Ghent, Belgium) and the multiplex xMAP Luminex platform (Lumnix Corporation, Austin, TX) at the Penn ADNI Biomarker Core Laboratory [30]. This is not directly comparable with another commonly used analytical platform in European countries, the Innotest (enzyme-linked immunosorbent assay [ELISA]) [18]. More details on data collection of the CSF samples can be found at on the ADNI website (http://www.adni-info.org).
Statistical analysis
To examine the presence of selection bias between MCI patients with CSF information and those without, we compared baseline demographic and clinical data between groups, using a non-parametric Kruskal-Wallis test for continuous variables and Pearson’s χ2 test for dichotomous variables.
Receiver operator characteristic (ROC) curves are standard summaries of diagnostic accuracy for continuously valued test results [31, 32] and dichotomous disease status. In our study, however, disease status is defined as the development of AD, which can change during follow-up. Accordingly, we used time-dependent ROC analysis to characterize the predictive accuracy of CSF biomarkers with continuous values and the time-dependent outcome of interest [31, 33]. Hence, we sought to characterize the prognostic accuracy of combinations of CSF biomarkers among MCI patients with potential for progression to AD and further estimate the AD risk in terms of CSF biomarker values measured at study entry. To our knowledge, this was the first study to examine the predictive accuracy of CSF biomarkers on MCI patients using time-dependent ROC analysis.
We first fit Cox proportional hazards (PH) models using time to AD as the dependent variable and Aβ1–42, T-tau, and P-tau181p as the primary independent variables to assess the discriminatory ability of these biomarkers on the progression to AD. We summarized the discrimination potential of the combinations of CSF biomarkers, measured at baseline (t = 0), to distinguish between MCI patients who developed AD by a particular time t and those who remained stable by calculating ROC curves for cumulative AD cases at each follow-up time t [31].
Using time-dependent ROC methods we derived combinations of sensitivity and specificity by comparing the predicted probabilities of developing AD (estimated from the fitted Cox PH model of CSF biomarkers mentioned above) and the actual outcomes at each follow-up time (t = 1–6 years). More importantly, censored observations were included in the calculation of sensitivity and specificity. For each time t, we calculated the area under the ROC curve (AUC (t)), which can be interpreted as the probability that a randomly selected MCI patient who developed AD at time t has a larger predicted risk than a randomly selected MCI patient who remained stable. We used AUC(t) to examine the best combination of CSF biomarkers for longitudinal predictive ability for the progression of AD for MCI patients. After the most optimal combination was chosen, we used the coefficients from the fitted Cox PH model to construct a multi-biomarker score (S) for MCI patients using the following equation: S = Ʃ(βi × biomarker Ai), where βi denotes the estimated beta coefficients for biomarkers Ai.
We then divided MCI patients into quintiles based on their multi-biomarker scores, and computed the cumulative risk of progression to AD for each group using Kaplan-Meier methods. We compared the observed risk functions estimated from the Kaplan Meier methods graphically to those estimated from Cox PH regression methods using the same five groups of patients to assess model fit.
We then illustrated the longitudinal risk of developing AD for each quintile or risk group using Kaplan-Meier methods to establish a prediction model for MCI patients and calculate the probabilities of progression to AD by each group at each time point. We also calculated covariate-adjusted hazard ratios by incorporating potential confounding variables into the Cox PH model. The analyses were done with and without adjustment for potential confounding of age, gender, marital status, education level, apoliopoprotein (APOE) ε4 alleles carrier status, baseline clinical dementia rating sum of boxes (CDR-SB), baseline Mini-Mental State Exam (MMSE) score, baseline Alzheimer’s Disease Assessment Scale (ADAS 13) score, baseline hippocampus volume, baseline ventricles volume, and the anti-dementia medication history. We further used a multivariate backward selection Cox regression model to estimate the impact of the potential confounders (p-values for removal from the model was defined as 0.05).
We used log-rank test to compare the risk of progression to AD among quintiles. The proportional hazards assumption was assessed using the log (-log) plots of the survival function using Schoenfeld residuals [34]. The Wilcoxon (Breslow‒Gehan) test was performed when hazard functions were thought to vary in ways other than proportionally. Risk groups were collapsed if no significantly different risk was presented between quintiles.
We compared the prognostic power of the multi-biomarker score to that of each individual CSF biomarker alone and to other diagnostic indices that are commonly used such as the ratio tau/Aβ1–42 (T-tau or P-tau181p), the index described by Hulstaert et al.[25] computed as Aβ1–42/(240+1.18 × tau) (T-tau or P-tau181p), and the AD-CSF-index developed by Molinuevo et al. [16] by applying these indices on the MCI sample in our study and then computing the AUC at each time point separately by time-dependent ROC analyses described earlier. The latter two indices were constructed using AD patients and cognitively normal controls.
All the analyses were done by using Stata version 12 (StataCorp, College Station, TX) and R software (version 3.0.3; R Foundation for Statistical Computing, Vienna, Austria) with the survivalROC and riskset ROC libraries.
RESULTS
Descriptive statistics
In total, 195 of 398 MCI patients with complete CSF information at baseline were included (Table 1). Among those with complete CSF data, the mean age was 74 years (range: 67–81 years old), the majority of the sample was men (67%), about 70% received no anti-dementia medication at study entry, 2.5% received either cholinesterase inhibitors or menantine, and the rest had no information available. The median follow-up period was 30 months (range, 9–58 months). With the mean conversion time of 24.5 months, 102 out of 195 MCI patients have converted to AD. The cumulative risk of developing AD by 6 years was 66%, which is similar to previous studies where 80% of MCI patients developed AD within 8 years [35]. No significant differences were found between MCI patients with and without complete CSF biomarker information at baseline (Table 1). (Supplementary Table 1 presents demographic characteristics of MCI patients with CSF information between those who have converted to AD and those who remained stable within 6 years).
Table 1.
Demographic characteristics of the Alzheimer’s disease Neuroimaging Initiative (ADNI 1) MCI subjects with and without complete CSF biomarker information at baseline*
| Covariate | With CSF data (n = 195) |
Without CSF data (n = 203) |
p-value |
|---|---|---|---|
| Demographic factors | |||
| Age, mean + SD, y | 74 ± 7 | 75 ± 7 | 0.16 |
| Male, % | 66.7 | 62.6 | 0.39 |
| Education, mean + SD, y | 16 ± 3 | 15 ± 3 | 0.43 |
| Marital status, % | 0.12 | ||
| Married | 84.1 | 76.4 | |
| Widowed | 9.2 | 14.8 | |
| Divorced | 6.2 | 6.4 | |
| Never married | 0.5 | 2.5 | |
| With Family history of dementia, % | 4.6 | 2.0 | 0.14 |
| APOE ε4 carrier, % | 53.8 | 52.7 | 0.82 |
| Baseline cognitive test, mean ± SD | |||
| MMSE score | 26.91 ± 1.79 | 27.14 ± 1.76 | 0.33 |
| CDR sum of boxes | 1.56 ± 0.89 | 1.64 ± 0.89 | 0.90 |
| ADAS 13 | 18.85 ± 6.23 | 18.45 ± 6.32 | 0.41 |
| Anti-dementia medications history, % | 0.65 | ||
| None | 70.3 | 54.7 | |
| ChEI only | 1.0 | 1.5 | |
| Memantine only | 1.5 | 2.0 | |
| NA | 27.2 | 41.9 | |
| Baseline MRI volumetric measures | |||
| Hippocampus volume (mm3) | 6,355 ± 1,085 | 6,448 ± 1,077 | 0.86 |
| Ventricles volume (ml) | 43,751 ± 24,574 | 44,266 ± 24,876 | 0.60 |
No significance difference was found in terms of covariates listed above between MCI patients with and without complete CSF biomarker information. MCI, mild cognitive impairment; APOE, apolipoprotein E; MMSE, Mini-Mental State Examination; CDR-SB, Clinical Dementia Rating–sum of boxes subscale; ADAS-cog, AD Assessment Scale–cognitive subscale; MRI, magnetic resonance imaging; ChEI, cholinesterase inhibitor; SD: standard deviation; NA, not available.
Time-dependent ROC analysis
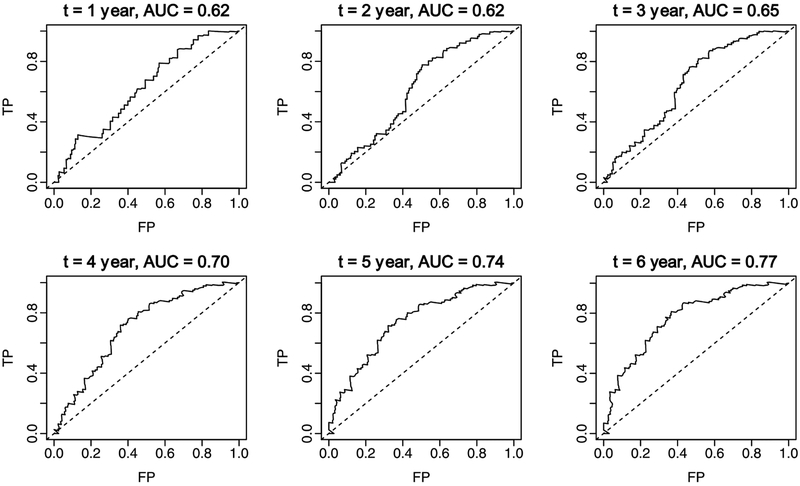
We assessed the discriminatory ability of CSF biomarkers by generating ROC curves at annual time points. The results of fitting a Cox PH model with the three biomarkers showed that Aβ1–42 and P-tau181p were significantly associated with the risk of developing AD (Supplementary Table 2). Furthermore, the time-dependent ROC analyses showed no difference between the AUC(t) values using the combination of all three CSF biomarkers compared with the combination of Aβ1–42 and P-tau181p only (the AUCs were 0.65 and 0.77 for year 3 and year 6, respectively, regardless of whether T-tau was included or not). The combination of Aβ1–42 and P-tau181p discriminated reasonably well among those MCI patients who developed AD during follow-up and those who remained stable (Fig. 1). The AUC(t) values ranged from 0.61 at 2 years to 0.77 at 6 years. Accordingly, we chose Aβ42 and P-tau181p to construct the multi-biomarker score in patients with MCI.
Fig. 1.
Time-dependent ROC curves by follow-up period and the combinations of CSF biomarkers (Aβ1–42 + P-tau181p) estimated from a Cox proportional hazards model. TP: true positive = sensitivity; FP: false positive = 1-specificity.
Predictive discrimination of CSF multi-biomarker score
We calculated a multi-biomarker score for each MCI patient using the coefficients derived from the Cox PH model. The score was calculated as (− 0.006) × Aβ1–42 +0.012 × P-tau181p. The mean multi-biomarker score was −0.56 ± 0.49, and the distribution of the scores appeared to be bimodal (Supplementary Figure 1). We divided MCI patients into quintiles according to their multi-biomarker score and then estimated the hazard ratio of the progression to AD for each quintile relative to the first quintile group (Table 2), controlling for the baseline risk factors listed in Table 1 (the result of fitting initial three CSF biomarkers and other baseline risk factors is presented in Supplementary Table 3). Among the covariates considered, only ADAS 13 score and hippocampus volume showed significant impact on the progression to AD.
Table 2.
Hazard ratio of each covariate using the Cox proportional hazards model*
| Covariate | HR | SE | p-value |
|---|---|---|---|
| Multi-bio marker score in thea | |||
| 2nd quintile | 1.82 | 0.86 | 0.206 |
| 3rd quintile | 2.24 | 1.03 | 0.078 |
| 4th quintile | 1.79 | 0.80 | 0.194 |
| 5th quintile | 1.63 | 0.81 | 0.327 |
| Age | 0.97 | 0.02 | 0.125 |
| Male | 1.02 | 0.34 | 0.949 |
| Education | 0.99 | 0.04 | 0.850 |
| Married (reference) | |||
| Widowed | 0.81 | 0.39 | 0.664 |
| Divorced | 1.63 | 0.94 | 0.394 |
| Never married | 3.82 | 4.21 | 0.224 |
| Having family history of dementia | 0.76 | 0.39 | 0.591 |
| APOE ε4 carrier | 1.21 | 0.33 | 0.491 |
| Baseline MMSE score | 0.95 | 0.08 | 0.501 |
| Baseline CDR sum of boxes | 1.29 | 0.19 | 0.085 |
| Baseline ADAS 13 | 1.09 | 0.03 | 0.001 |
| Baseline hippocampus volume (mm3) | 0.999566 | 0.00 | 0.005 |
| Baseline ventricles volume (ml) | 1.000003 | 0.00 | 0.628 |
n = 148.
Quintiles were defined by the equation: (–0.006)× Aβ1–42 + 0.012×P-tau181p. HR, hazard ratio; APOE, apolipoprotein E; MMSE, Mini-Mental State Examination; CDR-SB, Clinical Dementia Rating–sum of boxes subscale; ADAS 13, AD Assessment Scale; SE: standard error.
The univariate Cox PH model showed a significant difference in the probability of progression from MCI to AD between quintiles of the multi-biomarker score (Table 3). We found that MCI patients with a biomarker score in the third quintile had the highest risk of developing AD when adjusting for demographic or MRI imaging variables but not cognitive tests. In unadjusted analyses, those with a biomarker score in the fifth quin-tile appeared to have the highest risk.
Table 3.
Proportional hazards model results of patients with MCI by quintiles of multi-biomarker scores*
| Adjusted | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quintile of multi-biomarker scores | Unadjusted | demographica | cognitive testb | MRI imagingc | backward selectiond | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| 1st quintile (reference group) | ||||||||||
| 2nd quintile | 1.93 | 0.86–4.35 | 1.94 | 0.85–4.44 | 1.63 | 0.70–3.78 | 2.10 | 0.85–5.16 | 2.03 | 0.84–4.92 |
| 3rd quintile | 3.82 | 1.83–7.99 | 3.73 | 1.74–8.02 | 1.24 | 1.41–6.79 | 2.73 | 1.17–6.33 | 2.43 | 1.03–5.71 |
| 4th quintile | 3.40 | 1.64–7.05 | 3.21 | 1.49–6.92 | 2.41 | 1.11–5.25 | 2.37 | 1.03–5.44 | 1.94 | 0.85–4.43 |
| 5th quintile | 4.10 | 1.97–8.54 | 3.73 | 1.69–8.24 | 3.03 | 1.39–6.62 | 2.44 | 1.05–5.64 | 1.93 | 0.83–4.47 |
The included covariates in each adjusted categories are listed in Table 1.
Covariates included age, sex, education level, marital status, and APOE ε4 carrier status.
Covariates included baseline MMSE, baseline CDR sum of boxes, and baseline ADAS 13.
Covariates included baseline hippocampus volume and baseline ventricles volume.
Covariates included baselines CDR-SB, baseline ADAS13 and baseline hippocampus volumes. P-value for removal from the model was defined as 0.05. MCI, mild cognitive impairment; HR, hazard ratio; CI, confidence interval; MRI, magnetic resonance imaging.
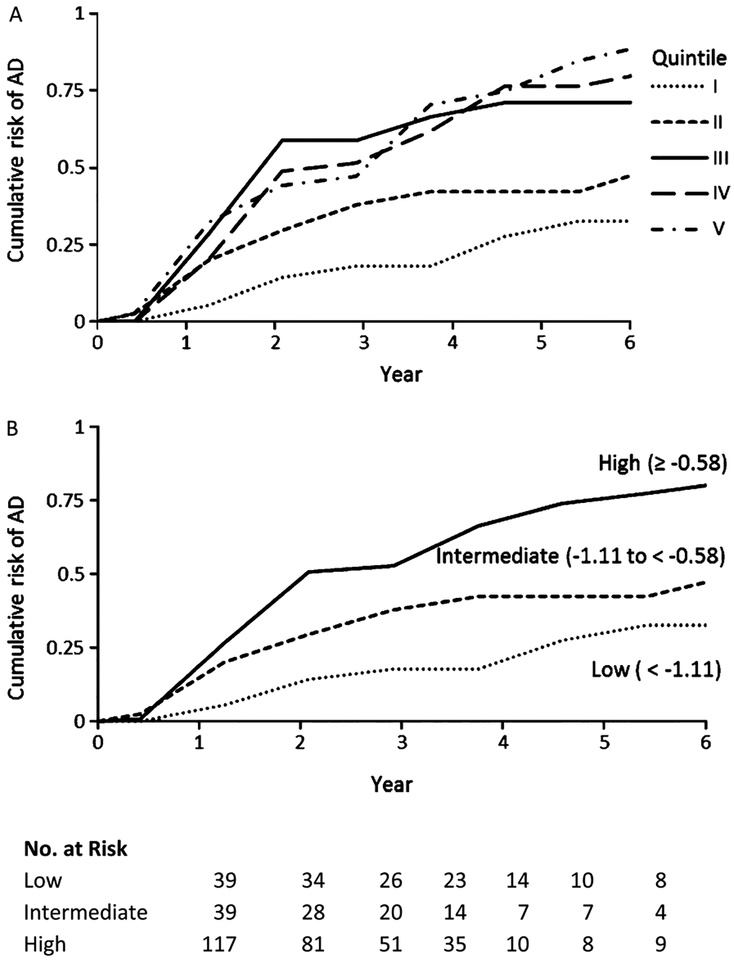
We compared the observed risk of progression to AD by quintiles of the multi-biomarker score using Kaplan-Meier survival methods (i.e., the cumulative risk of developing AD is 1 minus the Kaplan-Meier estimate for the proportion of MCI patients remaining stable at time t). The cumulative risk of developing AD by 6 years associated with multi-biomarker scores in the 1st through 5th quintile were 33%, 50%, 71%, 81%, and 90%, respectively (the log-rank test, p-value <0.0002; the Wilcoxon test, p-value <0.0004). We compared the observed Kaplan-Meier survival curves graphically with those predicted by the Cox PH model when using the same quintile groups of biomarker scores in order to assess model fit (not shown). The model exhibited good fit with the 6-year risk of developing AD- risk increasing monotonically as the multi-biomarker score increased, and the proportional hazard assumption was not violated (p-value = 0.24).
We found a clear gap between the group of the third, fourth, and fifth quintiles and the first and second quin-tiles (Fig. 2A), and we found no significantly different risk among the top three quintiles by either the log-rank tests or the Wilcoxon (Breslow–Gehan) test. Thus, we further collapsed the top three quintiles and labeled this group as high risk, we labeled the second quin-tile as intermediate risk and the first quintile as low risk. We then estimated the longitudinal variation of cumulative risk on the progression to AD (Fig. 2B), which showed the clear classification of AD risk by multi-biomarker scores categorized as three risk levels (high, intermediate, and low) with a follow-up of up to 6 years. The univariate Cox PH model using these three risk groups showed a significant difference in the probability of progression from MCI to AD (Table 4). The unadjusted hazard ratio of developing AD among MCI patients with high-risk biomarkers levels was about 4 times greater than MCI patients with low-risk levels (95% confidence interval [CI], 1.93–7.26), whereas the hazard ratios were 3.5, 2.8, and 2.5 respectively when controlling for demographic, cognitive test, and MRI imaging covariates.
Fig. 2.
Cumulative probability of AD, according to quintile of multi-biomarker scores (Panel A) and risk levels (Panel B). Multi-biomarker scores were classified as low (1st quintile), intermediate (2nd quintile), or high (3rd, 4th, and 5th quintiles). The parenthesis presented the range the multi-biomarker scores by risk levels.
Table 4.
Relationship between baseline covariates and the risk of developing AD in patients with MCI
| Adjusted | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk levele | Unadjusted | demographica | COgnitive testb | MRI imagingc | backward selectiond | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Low (reference group) | |||||||||||
| Intermediate | 1.93 | 0.86–4.35 | 1.93 | 0.85–4.42 | 1.63 | 0.70–3.79 | 2.08 | 0.85–5.11 | 2.02 | 0.83–4.91 | |
| High | 3.75 | 1.93–7.26 | 3.53 | 1.75–7.12 | 2.8 | 1.38–5.71 | 2.5 | 1.18–5.31 | 2.06 | 0.97–4.38 | |
The included covariates in each adjusted categories are listed in Table 1.
Covariates included age, sex, education level, marital status, and APOE ε4 carrier status.
Covariates included baseline MMSE, baseline CDR sum of boxes, and baseline ADAS 13.
Covariates included baseline hippocampus volume and baseline ventricles volume.
Covariates included baselines CDR-SB, baseline ADAS13 and baseline hippocampus volumes. P-value for removal from the model was defined as 0.05.
The lowest quintile is labeled low risk, the second quintile is labeled intermediate risk, and the top three quintiles are labeled high risk. MCI, mild cognitive impairment; HR, hazard ratio; CI, confidence interval; MRI, magnetic resonance imaging.
The multivariate backward selection model results indicated that CDR-SB, ADAS13, and hippocampus volume were significantly associated with the progression to AD in patients with MCI, considering all baseline covariates simultaneously. Other risk factors, such as APOE ε4 carrier status and MMSE score did not contribute to the explanatory power of the model. However, the hazard ratio for high-risk biomarker levels was only about 2 times greater than low-risk levels and was only borderline significant (95% CI, 0.97–4.38) when we adjusted for three significant covariates selected from the multivariate backward selection analysis (Table 4).
Comparison of discrimination power
Table 5 presents the AUC values at year 1 through year 6 of the time-dependent ROC analyses applying several combinations of biomarkers as well as several published indices [16, 25] on the MCI sample in our study. We found no difference in AUC values at year 3, whereas AUC values ranged from 0.69 to 0.77 at year 6 (although these results were similar). We found that the multi-biomarker score estimated in our study on MCI patients and the AD-CSF-index (P-tau) developed by Molinuevo et al. [16] that compared AD patients to healthy controls were associated with the best AUC value at year 6 among all tested diagnostic indices that predicted the longitudinal progression to AD. Specifically, their discriminative power between MCI patients who converted to AD and those who remained stable were 0.77 at year 6. With regards to the remainder of the indices, combined biomarkers presented better discriminative ability (higher AUC) than individual CSF indices.
Table 5.
Prognostic power of the AD indices based on CSF biomarkers by time-dependent ROC analysis
| Index | AUC | |||||
|---|---|---|---|---|---|---|
| Year l | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | |
| Aβl-42 | 0.58 | 0.60 | 0.63 | 0.66 | 0.68 | 0.74 |
| P-tau181p | 0.63 | 0.61 | 0.65 | 0.68 | 0.72 | 0.72 |
| T-tau | 0.54 | 0.61 | 0.65 | 0.68 | 0.68 | 0.69 |
| P-tau181p/Aβl-42 | 0.63 | 0.62 | 0.65 | 0.70 | 0.74 | 0.76 |
| T-tau/Aβl-42 | 0.57 | 0.62 | 0.66 | 0.70 | 0.70 | 0.73 |
| Hulstaert (P-tau), Aβl-42/(240+1.18 × P-tau181p),a [25] | 0.60 | 0.60 | 0.64 | 0.68 | 0.71 | 0.75 |
| Hulstaert (T-tau), Aβl-42/(240+1.18 × T-tau),a [25] | 0.58 | 0.61 | 0.66 | 0.69 | 0.70 | 0.74 |
| AD-CSF-index (P-tau181p),a,b [16] | 0.63 | 0.62 | 0.66 | 0.70 | 0.74 | 0.77 |
| AD-CSF-index (T-tau),a,b [16] | 0.57 | 0.62 | 0.66 | 0.70 | 0.71 | 0.74 |
| Current studyc | 0.62 | 0.62 | 0.65 | 0.70 | 0.74 | 0.77 |
Indices were derived from AD patients versus healthy controls.
. tau was referred to either P-tau181p or T-tau in this case.
(−0.006)×Aβ1–42 + 0.012×P-tau181p. AD, Alzheimer’s disease; CSF, cerebrospinal fluid; ROC, receiver operator characteristic; AUC, area under curve.
DISCUSSION
Our study sought to enhance the estimation of probability of progression from MCI to AD by creating a biomarker-based prognostic index. We found that a combined multi-CSF biomarker score, as categorized using quintiles or risk levels, provides a good estimate of the risk of developing AD up to 6 years. The hazard ratio of developing AD among MCI patients with high-risk biomarker levels was about 4 times greater than MCI patients with low-risk levels (95% CI, 1.93–7.26). Furthermore, the result of applying our index on AD patients and healthy controls from ADNI 1 (n = 216) showed the similar cut-off values of quintiles of multi-biomarker scores as those from MCI patients (Supplementary Table 4).
In our case, the combination of Aβ1–42 and P-tau181p showed predictive results similar to the combination of all three CSF biomarkers together, which may due to P-tau181p and T-tau status as neurodegeneration markers. We estimated the AUCs of published diagnostic indices developed from AD patients and healthy controls by applying those indices to our MCI sample and compared them with the AUC estimated with our index. The multi-biomarker score of combing Aβ1–42 and P-tau181p together showed better and comparable discriminative abilities than those relying on single CSF biomarker and published indices [4, 16, 25, 36], respectively (Table 5). However, diagnostic indices developed in the previous studies [4, 16, 25, 36, 37] may not be applicable to the current study since our index was designed based on the MCI population, whereas the former indices were based on comparisons between AD patients and healthy controls. The interpretation of any comparative results should be made with caution due to the heterogeneity of the study populations used in each study.
We demonstrated that the multi-biomarker score using the ADNI dataset with the Luminex-xMAP analytical platform resulted in AUCs and discriminative ability similar to those diagnostic indices developed using CSF biomarkers analyzed from different platforms or assays (ELISA or mesoscale) applied on AD patients versus healthy controls [4, 16, 25]. Moreover, our study used a time-dependent ROC method, which is able to capture censored observations in the calculation of sensitivity and specificity better than a logistic regression model used in other studies. This approach allowed us to accurately evaluate the discriminative capacity of CSF biomarkers measured at baseline over time.
It is well known that decreased Aβ1–42 and elevated tau levels predict progression from MCI to AD [18, 38–41], but there is a lack of the agreement regarding potential cut-off thresholds [24]. In other words, individuals with MCI who exhibit low levels of Aβ1–42 and high levels of T-tau or P-tau181P have higher risk of developing AD compared to those with higher levels of Aβ42 or lower levels of T-tau or P-tau181P, but the relationship between quintiles of our index derived from CSF biomarker concentration level and the progression of AD on MCI patients might not be ordinal. While the unadjusted data for quintiles showed that MCI patients with the composite biomarker score at the top quintile had a highest risk, we found that MCI patients with the score at the middle quintile tended to have a higher risk of developing AD after adjusting for MRI imaging (hippocampus volume and ventricles volume), which was shown to be a good predictor of MCI to AD conversion [42, 43]. This might be attributable to the heterogeneity of the study population as well as the discrepant continuum between the pathophysiological process of AD and its clinical symptomatology, as studies have shown that altered Aβ metabolism precedes tau-related pathology, neuronal degeneration, and clinical symptoms [44, 45]. It is also unclear if the APOE genotype influences the CSF biomarkers-based risk classification of AD in some studies [4, 46]; however we found no significant difference of APOE ε4 carrier status by quintile/risk level groups and no significant interaction between APOE ε4 carrier status and quin-tiles of multi-biomarker scores using Cox PH model. Validation in a larger sample would be informative in this regard.
The consensus from the Alzheimer’s Biomarkers Standardization Initiative (ABSI) is to consider CSF biomarker analysis as a routine clinical test in patients with early-onset dementia, either at the prodromal stage or with atypical AD [4]. With a low frequency of complications for lumbar puncture [47], especially in the elderly population [48, 49], routine analysis of CSF as part of the clinical workup for patients with possible AD has been advocated [6, 50, 51]. In addition to pharmacological treatments, other interventions, such as cognitive rehabilitation or participation in social activities, are also recommended for MCI patients [52]. Several cognitive interventions, such as cognitive stimulation, cognitive training, and cognitive rehabilitation have shown some effect on improving learning abilities and cognition among MCI patients [53]. Thus, properly selecting candidates for earlier treatment is necessary. Our results showed that MCI patients in the 3rd, 4th, and 5th quintiles of multi-biomarker score (the high-risk group) were most likely to convert to AD, which should qualify them as the primary target population if initiating a treatment program MCI patients was applicable. The multi-biomarker score developed in our study using an MCI population constitutes a reasonable measure with regard to the risk stratification of MCI patients for targeted interventions, such as potentially effective treatments or life management strategies. Furthermore, cost-effectiveness analysis for CSF biomarkers and subsequent interventions could be performed to show the utility of our risk stratification approach to payers as suggested by the ABSI [4] by targeting different intervention strategies based on the risk level determined by the multi-biomarker score here (with accurate diagnosis of MCI as the premise).
There are limitations to our study. First, neither the baseline biomarker level when cognitively normal persons develop MCI nor the disease history of MCI patients was known. This might resulted in the non-ordinal risk pattern by quintiles of multi-biomarker score. It is also possible that the MCI subjects (late MCI) [54, 55] recruited in the first phase of the ADNI were nearing progression to AD, and their biomarker levels were close to the threshold of AD. Further validation studies should be applied on a population with relatively early stage of MCI, such as early MCI defined in the second phase of ADNI (i.e., objective memory loss documented with scores approximately 0.5–1.5 SD below the mean of healthy controls on delayed paragraph recall performance from the Wechsler Memory Scale Logical Memory II) [56], to fully describe the continuum of CSF biomarker levels and the disease progression of MCI for better discriminatory performance if possible. Second, changes in the concentrations of CSF tau and Aβ1–42 are early events in the pathogenesis of AD and levels of Aβ1–42 are already fully decreased at least 5 to 10 years before conversion to AD, whereas T-tau and P-tau181p seem to act as later markers [45, 57]. This means that patients with MCI may not be an optimal target population to apply the CSF analysis since CSF biomarkers (especially Aβ1–42) convert to pathologic values several years before the first appearance of clinical signs [6]. Finally, the current results demonstrate that the discriminatory accuracy of the composite biomarker model is not yet clinically satisfactory with an insufficient sample size and the heterogeneity of study samples.
In summary, our study examined the feasibility of distinguishing MCI patients with higher risk of developing AD from those at lower-risk through the creation of a multi-biomarker score. We did not attempt to define a universal cut-off value on CSF biomarker concentration levels, which would be difficult due to assay platforms generating different absolute values [24] and intra-center or inter-center variability of CSF concentration level [50]. However, we did find similar cut-off results of our index, derived from MCI patients, applying on AD patients and healthy control. Our findings demonstrate that MCI patients could be effectively categorized into different risk groups of developing AD through the use of multiple CSF biomarkers, thus potentially identifying persons with MCI who are best suited for pharmacological or non-pharmacological treatment.
Supplementary Material
ACKNOWLEDGMENTS
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bio-engineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; Bio-Clinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0066r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150066.
REFERENCES
- [1].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56, 303–308. Erratum in: Arch Neurol 56, 760. [DOI] [PubMed] [Google Scholar]
- [2].Morris JC, Price JL (2001) Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci 17, 101–118. [DOI] [PubMed] [Google Scholar]
- [3].Tarawneh R, Holtzman DM (2009) Critical issues for successful immunotherapy in Alzheimer’s disease: Development of biomarkers and methods for early detection and intervention. CNS Neurol Disord Drug Targets 8, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, Perret-Liaudet A, Teunissen CE, Parnetti L (2014) The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 10, 808–817. [DOI] [PubMed] [Google Scholar]
- [5].De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H (2010) Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 67, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hampel H, Lista S, Teipel SJ, Garaci F, Nistico R, Blennow K, Zetterberg H, Bertram L, Duyckaerts C, Bakardjian H (2014) Perspective on future role of biological markers in clinical therapy trials of Alzheimer’s disease: A long-range point of view beyond 2020. Biochem Pharmacol 88, 426–449. [DOI] [PubMed] [Google Scholar]
- [7].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, Herholz K, Bokde AL, Jessen F, Hoessler YC (2010) Biomarkers for Alzheimer’s disease: Academic, industry and regulatory perspectives. Nat Rev Drug Discov 9, 560–574. [DOI] [PubMed] [Google Scholar]
- [9].Blennow K, Zetterberg H (2013) The application of cerebrospinal fluid biomarkers in early diagnosis of Alzheimer disease. Med Clin North Am 97, 369–376. [DOI] [PubMed] [Google Scholar]
- [10].Lewczuk P (2014) Currently available biomarkers and strategies for the validation of novel candidates for neurochemical dementia diagnostics in Alzheimer’s disease and mild cognitive impairment. Adv Geriatr 2014, Article ID 891780. [Google Scholar]
- [11].Petersen R, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L (2014) Mild cognitive impairment: A concept in evolution. J Intern Med 275, 214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D (2010) Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol 9, 1118–1127. [DOI] [PubMed] [Google Scholar]
- [13].Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G (2007) Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol 6, 734–746. [DOI] [PubMed] [Google Scholar]
- [14].Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R (2014) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13, 614–629. [DOI] [PubMed] [Google Scholar]
- [15].Morris J, Blennow K, Froelich L, Nordberg A, Soininen H, Waldemar G, Wahlund LO, Dubois B (2014) Harmonized diagnostic criteria for Alzheimer’s disease: Recommendations. J Intern Med 275, 204–213. [DOI] [PubMed] [Google Scholar]
- [16].Molinuevo JL, Gispert JD, Dubois B, Heneka MT, Lleo A, Engelborghs S, Pujol J, de Souza LC, Alcolea D, Jessen F (2013) The AD-CSF-Index discriminates Alzheimer’s disease patients from healthy controls: A validation study. J Alzheimers Dis 36, 67–77. [DOI] [PubMed] [Google Scholar]
- [17].Zetterberg H, Blennow K (2013) Cerebrospinal fluid biomarkers for Alzheimer’s disease: More to come? J Alzheimers Dis 33, S361–S369. [DOI] [PubMed] [Google Scholar]
- [18].Le Bastard N, Coart E, Vanderstichele H, Vanmechelen E, Martin J-J, Engelborghs S (2013) Comparison of two analytical platforms for the clinical qualification of Alzheimer’s disease biomarkers in pathologically-confirmed dementia. J Alzheimers Dis 33, 117–131. [DOI] [PubMed] [Google Scholar]
- [19].Fagan AM, Perrin RJ (2012) Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomarkers 6, 455–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka S-K, van der Flier WM, Blankenstein MA, Ewers M (2009) CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393. [DOI] [PubMed] [Google Scholar]
- [21].Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol 5, 228–234. [DOI] [PubMed] [Google Scholar]
- [22].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schoonenboom N, Reesink F, Verwey N, Kester M, Teunissen C, Van De Ven P, Pijnenburg Y, Blankenstein M, Rozemuller A, Scheltens P (2012) Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 78, 47–54. [DOI] [PubMed] [Google Scholar]
- [24].Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, Coart E, Morris JC, Holtzman DM (2011) Comparison of analytical platforms for cerebrospinal fluid measures of beta-amyloid 1–42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol 68, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H (1999) Improved discrimination of AD patients using beta-amyloid(1–42) and tau levels in CSF. Neurology 52, 1555–1562. [DOI] [PubMed] [Google Scholar]
- [26].Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund L-O, Freund-Levi Y, Tsolaki M, Minthon L, Wallin ÅK, Hampel H (2009) Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol 8, 619–627. [DOI] [PubMed] [Google Scholar]
- [27].Molinuevo JL, Gispert JD, Pujol J, Rojas S, Lladó A, Balasa M, Antonell A, Sanchez-Valle R, Rami L (2012) [A new approach to the Alzheimer’s disease diagnosis with biomarkers: Description of the AD-CSF-Index]. Rev Neurol 54, 513–522. [PubMed] [Google Scholar]
- [28].Tapiola T, Alafuzoff I, Herukka S-K, Parkkinen L, Hartikainen P, Soininen H, Pirttil¨a T (2009) Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66, 382–389. [DOI] [PubMed] [Google Scholar]
- [29].Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR Jr, Jagust WJ, Shaw LM, Toga AW (2010) Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 74, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR Jr, Feldman HH, Bokde AL, Alexander GE, Scheltens P (2012) Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging 33, 1203–1214. e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heagerty PJ, Lumley T, Pepe MS (2000) Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 56, 337–344. [DOI] [PubMed] [Google Scholar]
- [32].Pencina MJ, D’Agostino RB (2004) Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med 23, 2109–2123. [DOI] [PubMed] [Google Scholar]
- [33].Heagerty PJ, Zheng Y (2005) Survival model predictive accuracy and ROC curves. Biometrics 61, 92–105. [DOI] [PubMed] [Google Scholar]
- [34].Grambsch PM, Therneau TM (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81, 515–526. [Google Scholar]
- [35].Petersen RC, Morris JC (2005) Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 62, 1160–1163. [DOI] [PubMed] [Google Scholar]
- [36].Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ (2007) CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: A follow-up study. Neurology 69, 631–639. [DOI] [PubMed] [Google Scholar]
- [37].Lehmann S, Schraen S, Quadrio I, Paquet C, Bombois S, Delaby C, Dorey A, Dumurgier J, Hirtz C, Krolak-Salmon P, Laplanche JL, Moreaud O, Peoc’h K, Rouaud O, Sablonniere B, Thouvenot E, Touchon J, Vercruysse O, Hugon J, Gabelle A, Pasquier F, Perret-Liaudet A (2014) Impact of harmonization of collection tubes on Alzheimer’s disease diagnosis. Alzheimers Dement 10, S390–S394. e392. [DOI] [PubMed] [Google Scholar]
- [38].Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256, 183–194. [DOI] [PubMed] [Google Scholar]
- [39].Selkoe DJ (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 399, A23–A31. [DOI] [PubMed] [Google Scholar]
- [40].Hebert LE, Scherr PA, Beckett LA, Albert MS, Pilgrim DM, Chown MJ, Funkenstein HH, Evans DA (1995) Age-specific incidence of Alzheimer’s disease in a community population. JAMA 273, 1354–1359. [PubMed] [Google Scholar]
- [41].Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C (2003) Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Arch Neurol 60, 1385. [DOI] [PubMed] [Google Scholar]
- [42].Vemuri P, Wiste H, Weigand S, Shaw L, Trojanowski J, Weiner M, Knopman D, Petersen R, Jack C (2009) MRI and CSF biomarkers in normal, MCI, and AD subjects predicting future clinical change. Neurology 73, 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Davatzikos C, Bhatt P, Shaw LM, Batmanghelich KN, Trojanowski JQ (2011) Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol Aging 32, 2322.e2319–2322.e2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vemuri P, Wiste H, Weigand S, Shaw L, Trojanowski J, Weiner M, Knopman D, Petersen R, Jack C (2009) MRI and CSF biomarkers in normal, MCI, and AD subjects Diagnostic discrimination and cognitive correlations. Neurology 73, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O (2012) Cerebrospinal fluid levels of beta-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 69, 98–106. [DOI] [PubMed] [Google Scholar]
- [46].Lewczuk P, Zimmermann R, Wiltfang J, Kornhuber J (2009) Neurochemical dementia diagnostics: A simple algorithm for interpretation of the CSF biomarkers. J Neural Transmn 116, 1163–1167. [DOI] [PubMed] [Google Scholar]
- [47].Peskind ER, Riekse R, Quinn JF, Kaye J, Clark CM, Farlow MR, Decarli C, Chabal C, Vavrek D, Raskind MA, Galasko D (2005) Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord 19, 220–225. [DOI] [PubMed] [Google Scholar]
- [48].Zetterberg H, Tullhog K, Hansson O, Minthon L, Londos E, Blennow K (2010) Low incidence of post-lumbar puncture headache in 1,089 consecutive memory clinic patients. Eur Neurol 63, 326–330. [DOI] [PubMed] [Google Scholar]
- [49].Blennow K, Wallin A, Hager O (1993) Low frequency of post-lumbar puncture headache in demented patients. Acta Neurol Scand 88, 221–223. [DOI] [PubMed] [Google Scholar]
- [50].Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, Parnetti L, Perret-Liaudet A, Shaw LM, Teunissen C, Wouters D, Blennow K (2012) Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 8, 65–73. [DOI] [PubMed] [Google Scholar]
- [51].Tabaraud F, Leman JP, Milor AM, Roussie JM, Barriere G, Tartary M, Boutros-Toni F, Rigaud M (2012) Alzheimer CSF biomarkers in routine clinical setting. Acta Neurol Scand 125, 416–423. [DOI] [PubMed] [Google Scholar]
- [52].Petersen RC (2011) Mild cognitive impairment. N Engl J Med 364, 2227–2234. [DOI] [PubMed] [Google Scholar]
- [53].Simon SS, Yokomizo JE, Bottino C (2012) Cognitive intervention in amnestic mild cognitive impairment: A systematic review. Neurosci Biobehav Rev 36, 1163–1178. [DOI] [PubMed] [Google Scholar]
- [54].Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA (2010) Clinical core of the Alzheimer’s Disease Neuroimaging Initiative: Progress and plans. Alzheimers Dement 6, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vemuri P, Wiste H, Weigand S, Knopman D, Trojanowski J, Shaw L, Bernstein M, Aisen P, Weiner M, Petersen R (2010) Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology 75, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].ADNI GO protocol, http://www.adni-info.org/Scientists/Pdfs/ADNIGOprotocol.pdf, Accessed February 23.
- [57].Toledo JB, Xie SX, Trojanowski JQ, Shaw LM (2013) Longitudinal change in CSF Tau and Abeta biomarkers for up to 48 months in ADNI. Acta Neuropathol 126, 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.