Abstract
Background
Traumatic spinal cord injury (SCI) causes neuronal death, demyelination, axonal degeneration, inflammation, glial scar formation, and cystic cavitation resulting in interruption of neural signaling and loss of nerve function. Multifactorial targeted therapy is a promising strategy for SCI.
Methods
The anti-inflammatory peptide KAFAKLAARLYRKALARQLGVAA (KAFAK) and brain-derived neurotrophic factor (BDNF)-modified hyaluronan-methylcellulose (HAMC) hydrogel was designed for minimally invasive, localized, and sustained intrathecal protein delivery. The physical and biological characteristics of HAMC-KAFAK/BDNF hydrogel were measured in vitro. SCI model was performed in rats and HAMC-KAFAK/BDNF hydrogel was injected into the injured site of spinal cord. The neuronal regeneration effect was evaluated by inflammatory cytokine levels, behavioral test and histological analysis at 8 weeks post operation.
Results
HAMC-KAFAK/BDNF hydrogel showed minimally swelling property and sustained release of the KAFAK and BDNF. HAMC-KAFAK/BDNF hydrogel significantly improved the proliferation of PC12 cells in vitro without cytotoxicity. Significant recovery in both neurological function and nerve tissue morphology in SCI rats were observed in HAMC-KAFAK/BDNF group. HAMC-KAFAK/BDNF group showed significant reduction in proinflammatory cytokines expression and cystic cavitation, decreased glial scar formation, and improved neuronal survival in the rat SCI model compared to HAMC group and SCI group.
Conclusion
The HAMC-KAFAK/BDNF hydrogel promotes functional recovery of rats with spinal cord injury by regulating inflammatory cytokine levels and improving axonal regeneration.
Keywords: hyaluronan-methylcellulose hydrogel, anti-inflammatory peptide, neuroprotection, spinal cord injury
Introduction
Traumatic spinal cord injury (SCI) results in a devastating loss of motor and sensory function below the lesion site, with substantial impact on patient’s quality of life and life expectancy.1 Due to the complicated pathophysiology of SCI and the extremely limited spontaneous regeneration capacity of the central nervous system, there is still a lack of effective drugs or treatments.2,3 The pathophysiology of SCI involves a primary and secondary damage process. The primary damage is caused by the initial traumatic event and directly disrupts neurons, axons, blood vessels, and glia. A cascade of secondary injury follows the injury-associated vascular damage, characterized by multifaceted inflammation, hypoxia, edema, and oxidative stress resulting in widespread neuron death, axonal degeneration, demyelination, glial scar formation, and cystic cavitation at the lesion site in a delayed and progressive fashion.4–6 This series of adverse events ultimately leads to irreversible damage to the spinal cord, thereby blocking nerve signal transduction and recovery of neural function.7 Therefore, development of novel therapeutic strategies to attenuate detrimental outcomes in the secondary damage phase following SCI is essential for maintaining the remaining sensory and motor functions.
Recently, the local microenvironment after SCI has been shown to be an important factor in affecting nerve regeneration.8 The inflammatory environment is a key inhibitory factor for regeneration, which promotes neuronal apoptosis, inhibits neural stem cell differentiation, and accelerates glial cell necrosis and degeneration of axons.9 In addition, deficiency in local nerve growth factors after SCI is another important reason for the failure of axonal regeneration in the central nervous system (CNS).10 Thus, blocking of the inflammatory response and ensuring the delivery of appropriate growth factors supports the creation of a regenerative microenvironment and recovery of neural function. Several approaches have been employed for delivering anti-inflammatory medications and nerve growth factors to lesion site in the spinal cord, such as gene therapy,11 genetically engineered cells,12 and micro infusion pump.13 However, these strategies present some disadvantages including viral vector spread outside the target area, uncontrolled transgene expression, immune rejection of transplanted cells, pump implantation, and subsequent refill.14 Recent studies have revealed that composite hydrogel implantation has been considered as a promising strategy to promote functional recovery of nerve tissue after SCI by sustained release of biologically active substances and microenvironment remodeling.15,16
KAFAKLAARLYRKALARQLGVAA (KAFAK), which is an anti-inflammatory, cell-penetrating peptide (CPP), suppresses the syntheses of proinflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor (TNF)-α through mitogen-activated protein kinase–activated protein kinase 2 (MK2).17 Bartlett et al18 reported that treatment with KAFAK-loaded poly(NIPAm-AMPS) nanoparticles suppressed inflammation both in vitro, in a macrophage model with human monocytes, and ex vivo, in a bovine osteoarthritis model. As a biocompatible CPP, KAFAK is an ideal biomaterial for controlling local inflammation after SCI. In addition to improving the external microenvironment of injured neurons, it is also necessary to activate the neuron’s own powerful regenerative potential.19 Brain-derived neurotrophic factor (BDNF) is known as one of the ideal neurotrophic factors increasing synaptic plasticity, promoting the survival of existing neurons and axonal regeneration after SCI.20,21 Moreover, increasing the level of BDNF in the nerve tissue by local delivery can decrease the inhibitory nature of proteoglycans in the scar and encourage the differentiation of new neurons.22
To combine sustained release of KAFAK anti-inflammatory peptide and BDNF localization at the lesion site, we introduce a biodegradable, injectable, and biocompatible hydrogel as a biofunctionalized delivery platform for nerve regeneration. The hydrogel is composed of hyaluronan (HA) and methylcellulose (MC) that form a gel at physiologic temperatures with shear-thinning and minimally swelling properties.23 Hyaluronan-methylcellulose (HAMC) hydrogels, the crosslink density or polymer chain entanglement of which can be altered to match the characteristics of the spinal cord, have been applied directly to the intrathecal space as a drug delivery carrier.24 HA, which is a naturally occurring polysaccharide, is commonly found in the nervous system and can facilitate cell phenotype preservation, angiogenesis, and stem cell differentiation. MC has inverse thermal gelling properties and can be modified to allow bio-orthogonal coupling chemistry.25 In the present study, we conjugate both KAFAK peptide and recombinant rat BDNF to the injectable HAMC hydrogel using a facile strategy. Specifically, the maleimide-KAFAK and maleimide-streptavidin were covalently bonded to the MC and biotinylated BDNF was conjugated to the MC by strong and selective interaction between streptavidin and biotin. Then, we administered this injectable composite HAMC hydrogel to allow sustained release of KAFAK and BNDF in adult rats after SCI. We hypothesized that the HAMC-KAFAK/BDNF hydrogel would attenuate local inflammation, encourage neuron regeneration and axon elongation, and promote functional recovery in rat model of SCI.
Materials and methods
Preparation of HAMC-KAFAK/BDNF
Maleimide-KAFAK and maleimide-streptavidin were covalently bonded to the MC by thiol–maleimide click chemistry and biotinylated BDNF was conjugated to MC-streptavidin as previously described.25 Briefly, MC is carboxylated using 1.5 M sodium hydroxide and an overdose of bromoacetic acid. After purification by dialysis, sulfhydryl groups (100 mg of reactive thiols) were integrated into the carboxylated MC polymer (250 mg) main chain upon reaction with 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (750 mg) and 3,3′-dithiobis (propionic dihydrazide, 750 mg), followed by reduction of the disulfide bond with dithiothreitol (1.0 g). Maleimide-labeled streptavidin (mol maleimide: mol streptavidin =9:1, 1.1 mg) and maleimide-KAFAK peptide (59 mg) were then added and mixed with previously synthesized sulfhydryl-MC by stirring in 0.1 M PBS (100 mL) overnight at 4°C. N-ethylhydroxy maleimide (13 mg) was then added to quench unreacted free sulfhydryl groups, followed by the removal of unbound maleimide-containing reagent by dialysis. Then MC-KAFAK and MC-streptavidin were sterile-filtered using a 0.22 µm syringe filter and then lyophilized to amorphous white solids, respectively.
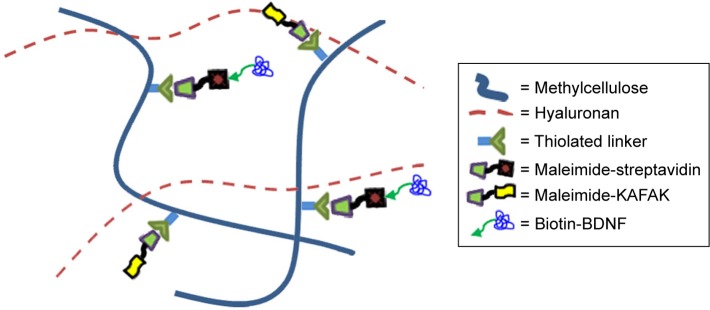
HAMC-KAFAK/BDNF hydrogels were prepared by physical blending of HA, MC-KAFAK, MC-streptavidin, and biotinylated-BDNF in artificial cerebrospinal fluid (aCSF) with a final composition of 1.5 wt% HA, 2.5 wt% MC-KAFAK, 0.5 wt% MC-streptavidin, and 0.2 wt% biotinylated BDNF. The HAMC hydrogels were prepared with 1.5 wt% HA and 3 wt% MC for comparison. The above ingredients were sterile-filtered before mixing and sequentially added to the aCSF using a high-speed centrifugal mixer (Eppendorf) for 25 seconds at 3,000 rpm/min and dissolved at 4°C for 12 hours. For in vivo studies, the HAMC-KAFAK/BDNF hydrogel compositions were sterilized by filtration and the preparation process was in sterile conditions. The time interval between preparation and use of the final composite hydrogels was less than 15 minutes, during which time the hydrogels were kept at 4°C (Figure 1).
Figure 1.
Schematic diagram of hydrogel composed of hyaluronan and MC for use as a drug delivery platform of KAFAK and BDNF.
Note: Taking advantages of chemical conjunction of thiol maleimide and biotin–streptavidin, MC is chemically modified with KAFAK and BDNF to enable the sustained release of them.
Abbreviations: BDNF, brain-derived neurotrophic factor; KAFAK, KAFAKLAARLYRKALARQLGVAA; MC, methylcellulose.
Swelling properties of HAMC-KAFAK/BDNF hydrogels
Swelling properties were measured in physiologic conditions. Hydrogels were added into 50 mL centrifuge tubes with PBS at 37°C. At different time points, the hydrogels were taken out, and the weight was measured after removal of excess water on the surface. The swelling ratio (SR) = (Wt − Wo)/Wo ×100%. Wt is the weight of the swollen hydrogel and Wo is the initial weight. The SR was calculated at 6, 12, 18, and 24 hours.
Cell culture and cell proliferation assay
PC12 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM (Gibco®; Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (Thermo Fisher Scientific), 0.1 mg/mL streptomycin, and 100 U/mL penicillin. Cultures were incubated at 37°C, 5% CO2, and 95% humidity. The cultivated medium was replaced every 2 days. Cells were passaged when confluence reached 80%–90%. HAMC-KAFAK/BDNF treatment was used to evaluate the effect of released BDNF on PC12 cells proliferation. The PC12 cells were seeded at the density of 1×104 cells/cm2 into 96-well plates and 100 µL medium per well. Cells were treated with HAMC-KAFAK/BDNF hydrogels, while the same volume of DMEM was added to the control group. The PC12 cells proliferation was evaluated with a CCK-8 cell proliferation kit (Dojindo Molecular Technologies, Inc., Japan). In brief, at 1, 3, and 7 days, 10 µL CCK-8 solution was applied to each well and incubated for 1 hour, and the absorbance at 450 nm was measured. The experiments were performed in triplicate.
KAFAK and BDNF release in vitro
To explore the profile of KAFAK release by the HAMC hydrogel, we added 25 µL of HAMC-KAFAK/BDNF hydrogels (the concentration of KAFAK: 0.6 µg/mL) to 2 mL sterile PBS. KAFAK release into solution was measured using a fluoraldehyde o-phthalaldehyde assay (Thermo Fisher Scientific), by fluorescence analysis. Fluorescence measurements of KAFAK release were taken at 6, 12, 24, 48, 72, and 96 hours. Three replicates were performed.
To explore the profile of BDNF release by HAMC hydrogel, we added 25 µL of HAMC-KAFAK/BDNF hydrogels (the concentration of BDNF: 1 µg/mL) to 2 mL sterile PBS. BDNF release into solution was measured using a rat BDNF ELISA kit (Beijing Solarbio Science & Technology Co., Ltd). Supernatant was collected at 6, 12, 24, 48, 72, and 96 hours. Three replicates were performed. Finally, the accumulated release ratio was calculated.
Animals and surgical procedures
All procedures were conducted according to protocols approved by Institutional Animal Care and Use Committee of Logistics College of PAP and all experiments were approved by the Institutional Animal Care and Use Committee of Logistics College of PAP. Adult female Sprague Dawley rats (200–230 g) obtained from the Laboratory Animal Center of the Military Medical Science Academy of China were anesthetized with a nitrous oxide/oxygen mixture (70%/30%) containing 1.4% isoflurane delivered by nose cone. All 72 rats were randomized to 4 groups: sham, SCI, HAMC, and HAMC-KAFAK/BDNF (n=18). A laminectomy was performed at the T10 vertebral level to expose the spinal cord and an aneurysm clip with a force of 25 × g was used for 1 minute to develop an SCI model. A HAMC-KAFAK/BDNF or HAMC hydrogel injection was administered after 5 minutes of SCI. A Hamilton syringe was inserted into the center of the traumatic area and 10 µL of the HAMC-KAFAK/BDNF or HAMC hydrogel was injected manually. Following implantation, the muscle and skin of the surgical wound were closed. Animals in the sham group received only the laminectomy without the SCI. The rats were then placed on warming pads until they completely recovered from anesthesia. Daily care of the animals included emptying of the bladder by manual compression and massage.
Functional assessments
All assessments were performed and analyzed by two observers blinded to each group. 1) Hindlimb locomotor function was determined before and after injury and transplantation, using the Basso Beattie Bresnahan (BBB) locomotor rating scale, as described previously.26 Rats were placed individually in the open field, and camera-recorded for 4 minutes. A score of 21 indicates that locomotor function was the same as normal uninjured rats, whereas a score of 0 indicates no hindlimb movement. 2) Motor function was determined biweekly, starting 4 weeks after SCI using the inclined plane test.27 Rats were placed on the inclined plane and the maximum angle at which they could maintain themselves for 5 seconds, without falling, was recorded. 3) Footprint analysis was used to evaluate stride length, base of support, and rotation angle, which measure regularity and relative paw placement.28 The average distance from both hindlimbs was measured to calculate the stride length. The base of support was defined as the width of the area between the left and right hindlimb. The hindlimb rotation angle was measured as the angle (degrees) of the hindlimb axis with respect to the runway axis. Rats were trained to walk across the runway until they finished the exercise voluntarily. The footprint test was performed at 8 weeks following SCI, as rats were capable of frequent, weight-supported stepping at this time.
Quantitative detection of TNF-α, IL-1β, IL-6, and IL-10 by ELISA
Six rats in each group were killed and segments of spinal cord (10 mm around lesion epicenter) were collected into Eppendorf tubes 7 days after surgery. Lysis buffer was added to dissolve the tissues after washing with PBS and then the tissues were sonicated for 10 seconds to isolate proteins. Subsequently, the tissues were centrifuged at a speed of 10,000 rpm for 10 minutes at 4°C to obtain the supernatant. The concentration of cytokines, such as TNF-α, IL-1β, IL-6, and IL-10, were measured by a microplate reader in accordance with the instructions of the ELISA kits and were compared to a standard curve (PeproTech, Inc., Rocky Hill, NJ, USA). The data are expressed as pg cytokine per mL tissue.
Tissue preparation and histochemistry
At 8 weeks after implantation, rats were killed by cardiac perfusion with PBS under anesthesia followed by 4% paraformaldehyde in 0.1 M PBS. The spinal cords were completely removed and postfixed overnight in 4% paraformaldehyde followed by 30% sucrose for 24 hours. Then, the spinal cords were frozen and longitudinally cut into 10 µm thick tissue sections using a cryostat microtome (CM 1900; Leica Microsystems, Wetzlar, Germany) and thaw-mounted onto slides (Thermo Fisher Scientific). The cryostat sections were stored at −20°C.
For immunofluorescence labeling, the tissue sections were rinsed with PBS, treated with blocking solution (0.25% Triton X-100, 5% bovine serum albumin in PBS) for 45 minutes and incubated in blocking solution with the following primary antibodies overnight at 4°C: rabbit anti-βIII-tubulin (1:150, Abcam, Cambridge, UK) to mark neurons and rabbit anti-glial fibrillary acidic protein (GFAP; 1:200, Abcam) to mark astrocytes. After rinsing with PBS, the sections were incubated for 2 hours at room temperature in blocking solution with Cy3 conjugated secondary antibodies (1:200, Proteintech Group, Inc., Illinois, USA). Sections were rinsed with PBS again and the nuclei were visualized using DAPI (1:100; Sigma-Aldrich Co., St Louis, MO, USA).
For cavity volume analysis, every twentieth of the tissue sections was selected and stained with H&E. The cavity volume of the spinal cord was calculated using the Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
Statistical analysis was performed using the software package SPSS 19.0 (IBM Corporation, Armonk, NY, USA). All data were presented as the mean ± standard error of the mean. Statistical significance of cell proliferation, BBB scores, and maximal angle of the inclined plane were determined using repeated-measures two-way ANOVA, and the significant of other measures were determined using one-way ANOVA. P-value <0.05 was considered to be statistically significant.
Results
Swelling properties of HAMC-KAFAK/BDNF hydrogels
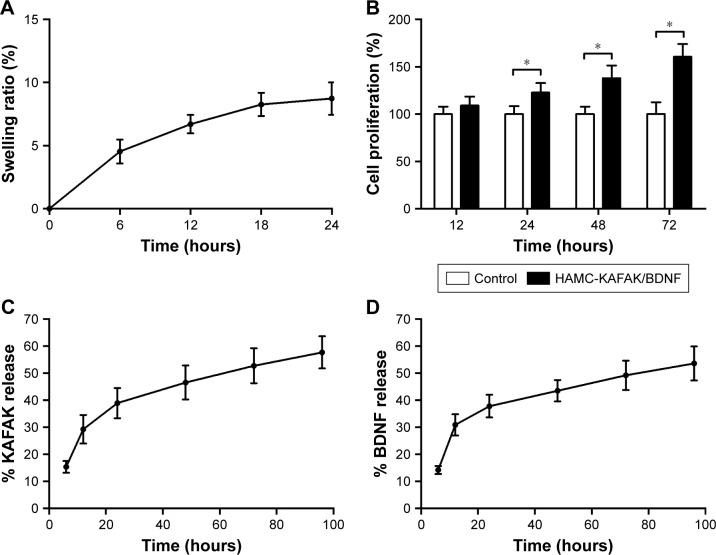
The swelling properties of HAMC-KAFAK/BDNF hydro-gels were evaluated by measuring the change of weight when incubated under physiologic conditions. As shown in Figure 2A, there was little change of the SR of HAMC-KAFAK/BDNF hydrogels in a specified period of time. The SR at 24 hours was only 8.7% ± 1.3%.
Figure 2.
(A) The swelling ratio of HAMC-KAFAK/BDNF hydrogels at the first 24 hours. (B) Effect of BDNF released from HAMC-KAFAK/BDNF hydrogels on the proliferation of PC12 cells at the first 72 hours. (C) KAFAK release pattern of HAMC-KAFAK/BDNF hydrogels in PBS at the first 96 hours. (D) KAFAK release pattern of HAMC-KAFAK/BDNF hydrogels in PBS at the first 96 hours. Data are presented as mean ± SEM, n=3. * in (B) indicates P<0.05, by two-way ANOVA.
Abbreviations: BDNF, brain-derived neurotrophic factor; HAMC, hyaluronan-methylcellulose; KAFAK, KAFAKLAARLYRKALARQLGVAA.
BDNF released from hydrogels showed bioactivity on PC12 cells
To test the bioactivity of BDNF released from HAMC-KAFAK/BDNF, PC12 cells were cultured with HAMC-KAFAK/BDNF. The cells’ proliferation results are shown as a relative ratio of the control group at different time points. The results of control group were considered to be 100%. As shown in Figure 2B, there were no significant differences in cell proliferation with the treatment of HAMC-KAFAK/BDNF hydrogel at the first 12 hours (P>0.05). However, the cell proliferation of HAMC-KAFAK/BDNF group was significantly higher than those in the control group since the 24-hour time point (HAMC-KAFAK/BDNF group: 122.8% ± 10.1%; P<0.05). After 72 hours, the cell proliferation of HAMC-KAFAK/BDNF group reached up 160.5% ± 13.5%. These results demonstrated that BDNF released from HAMC-KAFAK/BDNF hydrogel maintained their bioactivity and promoted the proliferation of PC12 cells.
KAFAK and BDNF release pattern in vitro
The profile of KAFAK release in vitro is shown in Figure 2C. The 96-hour kinetic measure of the peptide release from the HAMC-KAFAK/BDNF hydrogel showed an initially quick release of KAFAK during the first 24 hours in PBS (ratio 6 hours: 15.4% ± 2.2%; 12 hours: 29.3% ± 5.3%; 24 hours: 38.9% ± 5.6%). After 24 hours, the accumulated release of KAFAK was approximately 38.9%. However, the release speed gradually decreased from 24 to 96 hours (ratio 48 hours: 46.5% ± 6.3%; 72 hours: 52.7% ± 6.4%; 96 hours: 57.7% ± 5.9%).
The profile of BDNF release in vitro is shown in Figure 2D. Similar to the release pattern of KAFAK, the HAMC-KAFAK/BDNF hydrogel showed an initially quick release of BDNF during the first 24 hours in PBS (ratio 6 hours: 14.2% ± 1.5%; 12 hours: 30.9% ± 4.0%; 24 hours: 37.8% ± 4.2%), and the release speed gradually slowed down and tends to stabilize from 24 to 96 hours (ratio 48 hours: 43.5% ± 4.0%; 72 hours: 49.2% ± 5.4%; 96 hours: 53.6% ± 6.3%).
HAMC-KAFAK/BDNF promotes behavioral outcomes after SCI
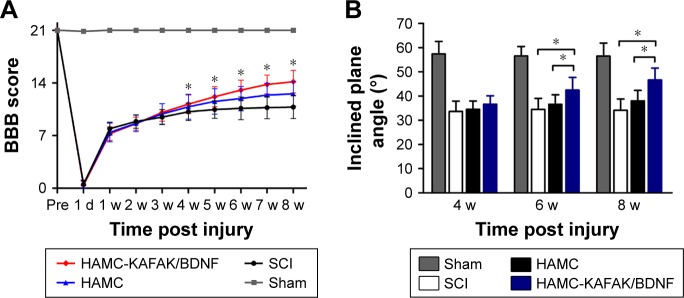
To examine the effect of HAMC-KAFAK/BDNF transplantation on the functional recovery of SCI rats, the BBB rating scale was used to measure locomotor functional recovery during the 8 weeks after surgery (Figure 3A). All animals performed normal during the locomotor behavior test before injury (21.00±0.00), with a significant decrease after clip compression of the spinal cord, where their hindlimbs were immediately paralyzed. BBB scores increased from 0.50±0.52 at 1 day to 10.79±1.53 at 8 weeks after surgery in the SCI group. The HAMC-KAFAK/BDNF group had a significant improvement in BBB score, from 0.42±0.51 at 1 day to 14.17±1.51 at 8 weeks post operation. Surprisingly, the HAMC-KAFAK/BDNF group showed sharply increased BBB scores starting at the 4-week time point compared to the SCI group (P<0.05). The scores in the sham group were maintained at 21 for 8 weeks post operation. Two-way ANOVA showed that different treatments and times had significant influence on BBB scores (all P<0.05), and there was also a significant effect of the combined factors of treatment and time on BBB score (P<0.05).
Figure 3.
Evaluation of locomotor recovery.
Notes: (A) Changes in the BBB locomotor scores of rats in HAMC-KAFAK/BDNF, HAMC, SCI, and Sham groups at different time points. (B) Inclined plane test performed 4, 6, and 8 weeks after SCI. Data are presented as mean ± SEM, n=12 per group. * indicates P<0.05, respectively, by two-way ANOVA.
Abbreviations: BBB, Basso Beattie Bresnahan; BDNF, brain-derived neurotrophic factor; d, days; HAMC, hyaluronan-methylcellulose; KAFAK, KAFAKLAARLYRKA-LARQLGVAA; SCI, spinal cord injury; w, weeks.
In addition to weekly locomotor evaluation, the inclined plane apparatus was used to assess motor function biweekly (Figure 3B). There was no significant difference in the maximal angles of inclined plane in the SCI group between 4 and 8 weeks post surgery (4 weeks: 33.62°±4.24° vs 8 weeks: 34.11°±4.66°). However, maximal angles increased from 36.60°±3.50° at 4 weeks to 46.64°±4.90° at 8 weeks in the HAMC-KAFAK/BDNF group (P<0.05). Remarkably, maximal angles in the HAMC-KAFAK/BDNF group were significantly higher at the 6- and 8-week time points compared to the SCI group (P<0.05). Two-way ANOVA showed that the HAMC-KAFAK/BDNF treatment had a significant effect on the maximal angles of the inclined plane (P<0.05). The interaction between treatment and time was also significant (P<0.05).
To assess comprehensive parameters, we tested hindlimb locomotor recovery by footprint analysis at 8 weeks after surgery (Figure 4D). The base of support in the HAMC-KAFAK/BDNF group (43.1±3.97 mm) was significantly decreased compared to the SCI group (50.20±5.41 mm; P<0.05; Figure 4B). There was also a significant decrease in angle of hindlimb rotation in the HAMC-KAFAK/BDNF group compared to the SCI group (HAMC-KAFAK/BDNF: 14.19°±2.01° vs SCI: 20.10°±4.02°; P<0.05; Figure 4C). Meanwhile, the stride length in the HAMC-KAFAK/BDNF group (108.37±9.75 mm) was significantly increased compared to the SCI group (90.06±9.09 mm; P<0.05; Figure 4A). Altogether, these results demonstrated that behavioral outcomes were negatively affected by SCI but could be great improved by HAMC-KAFAK/BDNF hydrogel implantation.
Figure 4.
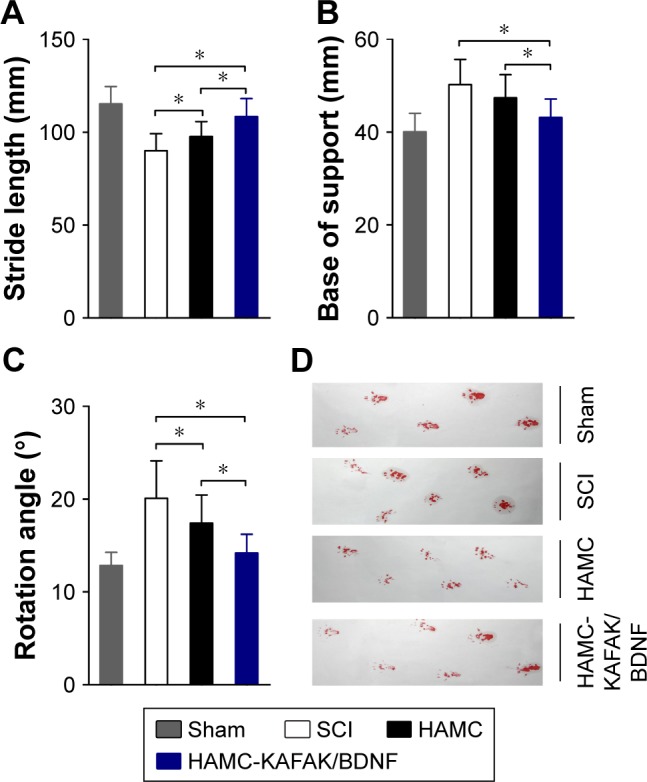
The quality of locomotor recovery was quantified by three parameters of footprint analysis performed at 8 weeks.
Notes: (A) Stride length. (B) Base of support. (C) Rotation angle. (D) Representative footprints. Data are presented as mean ± SEM, n=12 per group. * indicates P<0.05, respectively, by one-way ANOVA.
Abbreviations: BDNF, brain-derived neurotrophic factor; HAMC, hyaluronan-methylcellulose; KAFAK, KAFAKLAARLYRKALARQLGVAA; SCI, spinal cord injury.
HAMC-KAFAK/BDNF promotes functional recovery by regulating inflammatory cytokine levels after SCI
We conducted an ELISA to determine whether HAMC-KAFAK/BDNF inhibited inflammation levels at 7 days after surgery. As shown in Figure 5A–C, proinflammatory cytokines including TNF-α, IL-1β, and IL-6 were significantly suppressed by injection of HAMC-KAFAK/BDNF compared to the untreated SCI group (all P<0.05). TNF-α levels in the SCI group (44.27±5.73 pg/mL) were increased compared to those in the sham group (19.95±2.03 pg/mL; Figure 5A). However, HAMC significantly inhibited the expression of TNF-α (37.71±3.62 pg/mL) compared to the SCI group (44.27±5.73 pg/mL; P<0.05). The HAMC-KAFKA/BDNF group showed a more significant decrease in TNF-α levels (30.70±3.84 pg/mL) compared to the HAMC group (37.71±3.62 pg/mL; P<0.05). This inhibition of expression by HAMC-KAFAK/BDNF was also noted for IL-1β and IL-6 (Figure 5B–C). On the other hand, IL-10, an anti-inflammatory cytokine, was significantly increased in the HAMC-KAFAK/BDNF group (97.05±6.64 pg/mL; all P<0.05) compared to the SCI or HAMC group (Figure 5D). Altogether, these results dem onstrated that injection of HAMC-KAFAK/BDNF inhibited the expression of proinflammatory cyto-kines while upregulating the levels of anti-inflammatory cytokines.
Figure 5.
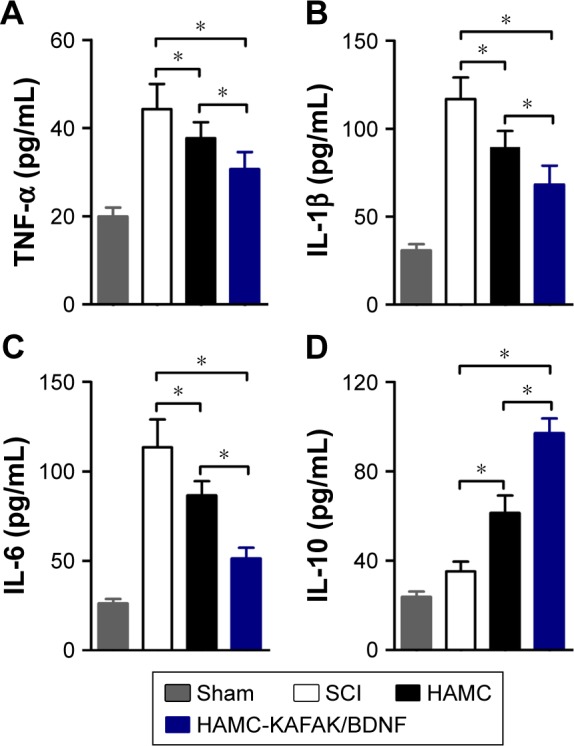
Effects of HAMC-KAFAK/BDNF hydrogel on cytokines expression in the injured spinal cord.
Notes: (A) TNF-α. (B) IL-1β. (C) IL-6. (D) IL-10. Data are presented as mean ± SEM, n=6 per group. * indicates P<0.05, respectively, by one-way ANOVA.
Abbreviations: BDNF, brain-derived neurotrophic factor; HAMC, hyaluronan-methylcellulose; KAFAK, KAFAKLAARLYRKALARQLGVAA; SCI, spinal cord injury; TNF, tumor necrosis factor.
HAMC-KAFAK/BDNF attenuates neurologic damage and promotes neuroregeneration after SCI
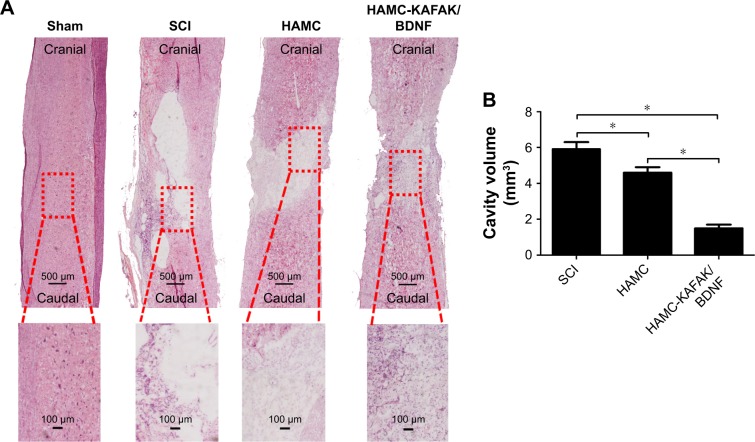
To further investigate the role of HAMC-KAFAK/BDNF in SCI-induced neurologic damage, the regenerated spinal cord sections were stained with H&E and analyzed by light microscope at 8 weeks after surgery (Figure 6A). An administration of HAMC-KAFAK/BDNF led to a substantial reduction in cavity volume (1.52±0.19 mm3) compared to the HAMC and SCI groups (4.58±0.33 mm3 and 5.87±0.42 mm3, respectively; all P<0.05; Figure 6B), suggesting that there are regenerative advantages of combining KAFAK and BDNF in terms of inhibiting inflammation, scar formation, and promoting CNS recovery.
Figure 6.
H&E staining and quantitative analysis of cavity volume.
Notes: (A) H&E staining of longitudinal cutting results at 8 weeks after SCI. (B) Quantitative analysis of H&E staining results. Data are presented as mean ± SEM, n=12 per group. * indicates P<0.05, respectively, by one-way ANOVA.
Abbreviations: BDNF, brain-derived neurotrophic factor; HAMC, hyaluronan-methylcellulose; KAFAK, KAFAKLAARLYRKALARQLGVAA; SCI, spinal cord injury.
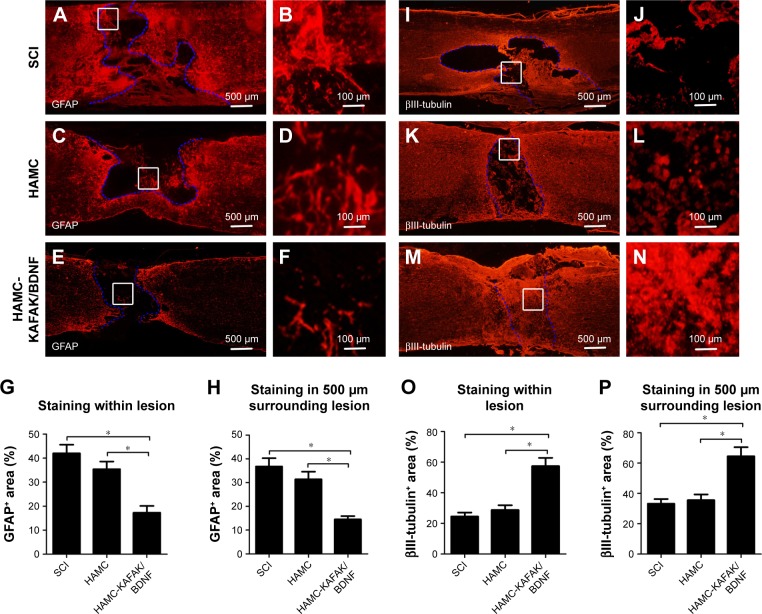
It has been previously shown that reactive astrocytes, in which GFAP is the hallmark protein, are typically observed forming a glial scar around the lesion site after SCI.29 Therefore, immunofluorescence staining was used to assess the activation and distribution of astrocytes using GFAP as a marker. Figure 7A–F shows that the typical GFAP-positive glia scar was present within, and surrounding, the lesion site 8 weeks after SCI. The glial scars in the SCI group were more obvious (42.03% ± 3.56% staining within the lesion and 36.82% ± 3.47% staining 500 µm surrounding the lesion) than those in the HAMC-KAFAK/BDNF group (17.28% ± 2.76% staining within the lesion and 14.52% ± 1.43% staining 500 µm surrounding the lesion; P<0.05; Figure 7G–H).
Figure 7.
Immunofluorescence staining of GFAP and βIII-tubulin performed 8 weeks after SCI.
Notes: (A–F) Representative images of GFAP expression in rats of SCI, HAMC, and HAMC-KAFAK/BDNF group. (G) Quantitative analysis of the percent of GFAP+ area within the lesion. (H) Quantitative analysis of the percent of GFAP+ area 500 µm surrounding the lesion. (I–N) Representative images of βIII-tubulin expression in rats of SCI, HAMC, and HAMC-KAFAK/BDNF group. (O) Quantitative analysis of the percent of GFAP+ area within the lesion. (P) Quantitative analysis of the percent of GFAP+ area 500 µm surrounding the lesion. Data are presented as mean ± SEM, n=12 per group. * indicates P<0.05, respectively, by one-way ANOVA.
Abbreviations: BDNF, brain-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; HAMC, hyaluronan-methylcellulose; KAFAK, KAFAKLAARLYRKALARQLGVAA; SCI, spinal cord injury.
To analyze axonal regeneration at the lesion site, βIII-tubulin was used to label the axons (Figure 7I–N). In the SCI group, axonal density was diminished, consistent with a previous study.30 However, in the HAMC-KAFAK/BDNF group, the extension of axons and their number from the border to the center of the injured site was obviously greater than those in the SCI group. The presence of high-density axons was increased in the HAMC-KAFAK/BDNF group (57.42% ± 5.38% within the lesion and 64.55% ± 5.91% staining 500 µm surrounding the lesion), in contrast with those in the SCI group (24.53% ± 2.56% within the lesion and 33.23% ± 3.08% staining 500 µm surrounding lesion; P<0.05; Figure 7O–P) where axons were almost not observed at 8 weeks post surgery, due to glial scar and cavity formation. Altogether, these results indicated that HAMC-KAFAK/BDNF attenuated neurologic damage and promoted neuroregeneration after SCI.
Discussion
In the adult mammalian CNS, traumatic injuries often lead to functional deficits, largely owing to insufficient regenerative and repair capacity.30 Over the past decades, many therapeutic approaches have been tested and performed to promote spinal cord regeneration in animal models or humans.11–13 Biomaterial-based hydrogels have shown potential promise in restoring connectivity and function after SCI as drug delivery systems, by regulating microenvironment and by providing favorable substrates and paths for axonal regeneration.31 Among these hydrogels, HAMC has been shown to be a flexible, localized drug delivery platform for several different therapeutic proteins in a sustained manner releasing at the injury site and supporting axonal extension across the lesion. In this study, a HAMC hydrogel was modified with the anti-inflammatory peptide KAFAK and BDNF and injected into a lesion region to suppress inflammation, promote the survival of existing neurons, and enhance axonal regeneration. We examined locomotor function and axonal regeneration at 8 weeks after SCI and demonstrated that injection of HAMC-KAFAK/BDNF hydrogel improved recovery of neural behavior and histologic outcomes. An administration of HAMC-KAFAK/BDNF hydrogel attenuated astrocyte reactive hyperplasia and inflammation in the lesion site. In addition, we found that this composite HAMC-KAFAK/BDNF hydrogel markedly reduced the cystic cavity in the lesion area and promoted axonal regeneration and functional recovery.
Targeting inflammation is believed to be important for treating SCI.32 Methylprednisolone has previously been used to suppress the immune system and decrease inflammation, and has been applied in the management of many conditions including acute SCI.33,34 However, methylprednisolone-associated adverse effects occur commonly, such as infection, gastrointestinal hemorrhage, sepsis, and pneumonia. Therefore, anti-inflammatory peptides and proteins have recently been exploited as alternative therapies for treatment of inflammatory conditions.35 In light of these findings, we utilized KAFAK as an anti-inflammatory agent following traumatic injury to the spinal cord. The anti-inflammatory peptide KAFAK has the capacity to decrease the synthesis of proinflammatory cytokines by suppressing MK2, which regulates the synthesis of several proinflammatory cytokines including TNF-α, IL-1, and IL-6.17 According to previous studies, there was an upregulation of proinflammatory cytokines IL-1β, IL-6, and TNF-α following SCI.36 The link between proinflammatory cytokines and neurotoxicity coupled to inhibition of axonal regeneration has been well reported.37 As expected, we observed a significant reduction in TNF-α, IL-1β, and IL-6 expression in rats injected with HAMC-KAFAK/BDNF and HAMC hydrogel compared to the SCI rats. Previous studies suggested that HA attenuates microglia/macrophage activation and thus reduces inflammation in injured spinal cord.38,39 Moreover, a more obvious reduction of inflammation was observed in the HAMC-KAFAK/BDNF group than in the HAMC group, which confirmed that our KAFAK sustained-release platform effectively attenuates inflammation for a longer period of time. The physical blend of HA and MC can quickly form a gel at physiologic temperatures with shear-thinning and minimal swelling properties.23 HA facilitates cell phenotype preservation, angiogenesis, and stem cell differentiation. MC can be modified to allow bio-orthogonal coupling reactions.25 Biotinylated BDNF was conjugated to MC by the strong and selective interaction between biotin and streptavidin. This high-affinity binding did not alter the spatial structure of BDNF, preserving the biologic function. As one of the best-characterized neurotrophic factors, it has been well established that BDNF plays an important role in increasing synaptic plasticity, and promoting neuroprotection and axonal regeneration following SCI.40 Song et al demonstrated that BDNF therapy in SCI rats significantly reduced histopathologic lesions, neuronal loss, and neuronal apoptosis in the injured spinal cord.41 Specifically, RNA sequencing revealed that BDNF expression was absent in SCI lesions.42 In our experiment, rats injected with HAMC-KAFAK/BDNF showed significant improvement in stride width, base of support, and rotation angle at the 8-week time point compared to rats in the untreated SCI group, according to footprint analysis. Moreover, better BBB scores and improved performance in the inclined plane test were observed in the HAMC-KAFAK/BDNF group at 8 weeks after SCI. Traumatic spinal cord injuries are frequently complicated by formation of cystic cavities. As they lack an extracellular matrix and vascularization, cystic cavities do not promote regeneration of axons Hydrogels are biocompatible implants that have been used for creating a permissive environment, and bridging lesion cavities, by releasing neurotrophic substances. Yao et al43 implanted an AFG hydrogel into a rat hemisected SCI model to bridge lesion cavities, which promoted axonal regeneration and locomotor function recovery of rats. Xu et al44 demonstrated that FGF2-loaded dscECM-HP hydrogel can achieve sustained release of FGF2 in vitro and recover both nerve tissue morphology and neuron functions in vivo. Our data showed that injection of HAMC-KAFAK/BDNF hydrogel significantly reduced cavity volume and promoted axonal regeneration and tissue preservation compared to the HAMC or SCI groups. These results demonstrated the functional and histologic benefits of HAMC-KAFAK/BDNF hydrogel in SCI, and its potential to deliver sustained release of BDNF to the SCI lesions.
The limited capacity of axon to regenerate following SCI is mainly attributed to insufficient growth of adult neurons in the spinal cord, coupled with a lack of proper extracellular matrix and stimulating growth factors and an inhibitory microenvironment.45,46 With the goal of attenuating inflammatory response and neuronal apoptosis, we introduced a biofunctionalized delivery platform that combined sustained release of KAFAK and BDNF. The recovery of neural behavior and the histologic outcomes observed in our study has shown the applicability of our delivery platform in SCI. Although the specific mechanism of action of this methodology has not been completely determined, we hypothesize that downregulated secretion of proinflammatory cytokines, as well as exogenous delivery of the protetive, BDNF protein, synergistically enhanced the neuronal regenerative micro-environment through anti-inflammatory and antiapoptotic effects. Due to the numerous, complex pathophysiologic mechanisms that occur after SCI, treatments against a single factor or target often failed to achieve the desired outcome. Remarkably, exogenous drug administration can reach the spinal cord through intravenous, intraperitoneal, intramuscular, and subcutaneous injections. However, due to enzymatic degradation of blood, the concentration of drug in the plasma will decrease. In addition, the blood–brain barrier and/or blood–spinal cord barrier have obstructive effects.47 After dilution by bodily fluids and the blood–spinal cord barrier, little drug remains to reach the injury site, which makes it difficult for these drugs to function biologically. Through thiol–maleimide and biotin–streptavidin bio-orthogonal coupling reactions, HAMC hydrogels have circumvented these limitations and enhanced the release mode of KAFAK and BDNF at the lesion site. Our work here shows that HAMC is a promising drug delivery platform for SCI therapy. As inflammation and apoptosis are major pathologic outcomes following SCI, our findings strongly indicated that HAMC-KAFAK/BDNF decreased neuronal apoptosis and improved neuron survival, which, in turn, promoted functional recovery. Future studies are needed to further evaluate this HAMC controlled-releasing platform for sustained release of multiple growth factors, and assess the validity of cell transplantation in this HAMC hydrogel system.
Conclusion
In summary, using chemical conjugation of thiol–maleimide and biotin–streptavidin, we designed a HAMC injectable hydrogel modified with peptides and proteins for use as a drug delivery system. We demonstrated that injection of the anti-inflammatory peptide, KAFAK, and the neurotrophic factor, BDNF, via a HAMC hydrogel delivery system suppresses local inflammation and promotes neuronal survival, as well as axonal regeneration. Furthermore, the HAMC platform described above can be applied as a vehicle for localized, sustained, and controlled release of other therapeutic molecules, from stem cells to neurotrophic factors, for management of SCI lesion sites, circumventing the complication of systemic delivery. This strategy offers insight for further exploration of the potential therapeutic effects of long-term release of multiple neurotrophic components, their interaction with endogenous axonal growth, and their potential to improve microenvironments.
Acknowledgments
This study was supported by the National Natural Science Foundation Major Program of China (81891003), the Science & Technology Program of Tianjin, China (15ZXLCSY00040, 16ZXHLSY00120), the Technology Research Projects (AWS15J001), and the Tianjin Municipal Natural Science Foundation (17JCYBJC25700).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pertici V, Trimaille T, Laurin J, et al. Repair of the injured spinal cord by implantation of a synthetic degradable block copolymer in rat. Biomaterials. 2014;35(24):6248–6258. doi: 10.1016/j.biomaterials.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. 2014;7(3):a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59(5):957–982. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
- 4.Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34(15):3775–3783. doi: 10.1016/j.biomaterials.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Yong CS, Choi JS, Quan QZ, et al. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm. 2001;226(1–2):195–205. doi: 10.1016/s0378-5173(01)00809-2. [DOI] [PubMed] [Google Scholar]
- 6.Moon YJ, Lee JY, Oh MS, et al. Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J Neurosci Res. 2012;90(1):243–256. doi: 10.1002/jnr.22734. [DOI] [PubMed] [Google Scholar]
- 7.An Y, Tsang KK, Zhang H. Potential of stem cell based therapy and tissue engineering in the regeneration of the central nervous system. Biomed Mater. 2006;1(2):R38–R44. doi: 10.1088/1748-6041/1/2/R02. [DOI] [PubMed] [Google Scholar]
- 8.Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15(3):415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15(3):541–553. doi: 10.1007/s13311-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widenfalk J, Lundströmer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21(10):3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartus K, James ND, Didangelos A, et al. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34(14):4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh JS, An SS, Gwak SJ, et al. Hypoxia-specific VEGF-expressing neural stem cells in spinal cord injury model. Neuroreport. 2012;23(3):174–178. doi: 10.1097/WNR.0b013e32834f4f3a. [DOI] [PubMed] [Google Scholar]
- 13.Fouad K, Ghosh M, Vavrek R, Tse AD, Pearse DD. Dose and chemical modification considerations for continuous cyclic AMP analog delivery to the injured CNS. J Neurotrauma. 2009;26(5):733–740. doi: 10.1089/neu.2008.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer AS, Harvey AR, Plant GW, Hodgetts SI. Systematic review of induced pluripotent stem cell technology as a potential clinical therapy for spinal cord injury. Cell Transplant. 2013;22(4):571–617. doi: 10.3727/096368912X655208. [DOI] [PubMed] [Google Scholar]
- 15.Tsintou M, Dalamagkas K, Seifalian AM. Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res. 2015;10(5):726–742. doi: 10.4103/1673-5374.156966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haggerty AE, Oudega M. Biomaterials for spinal cord repair. Neurosci Bull. 2013;29(4):445–459. doi: 10.1007/s12264-013-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brugnano JL, Chan BK, Seal BL, Panitch A. Cell-penetrating peptides can confer biological function: regulation of inflammatory cytokines in human monocytes by MK2 inhibitor peptides. J Control Release. 2011;155(2):128–133. doi: 10.1016/j.jconrel.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett RL, Sharma S, Panitch A. Cell-penetrating peptides released from thermosensitive nanoparticles suppress pro-inflammatory cytokine response by specifically targeting inflamed cartilage explants. Nanomedicine. 2013;9(3):419–427. doi: 10.1016/j.nano.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ropper AE, Ropper AH. Acute spinal cord compression. N Engl J Med. 2017;376(14):1358–1369. doi: 10.1056/NEJMra1516539. [DOI] [PubMed] [Google Scholar]
- 20.Ritfeld GJ, Patel A, Chou A, et al. The role of brain-derived neurotrophic factor in bone marrow stromal cell-mediated spinal cord repair. Cell Transplant. 2015;24(11):2209–2220. doi: 10.3727/096368915X686201. [DOI] [PubMed] [Google Scholar]
- 21.Brock JH, Rosenzweig ES, Blesch A, et al. Local and remote growth factor effects after primate spinal cord injury. J Neurosci. 2010;30(29):9728–9737. doi: 10.1523/JNEUROSCI.1924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191(2):344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27(11):2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Ziemba AM, Gilbert RJ. Biomaterials for local, controlled drug delivery to the injured spinal cord. Front Pharmacol. 2017;8:245. doi: 10.3389/fphar.2017.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam RY, Cooke MJ, Shoichet MS. A covalently modified hydrogel blend of hyaluronan–methyl cellulose with peptides and growth factors influences neural stem/progenitor cell fate. J Mater Chem. 2012;22(37):19402. [Google Scholar]
- 26.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 27.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47(4):577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 28.Hamers FP, Koopmans GC, Joosten EA. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma. 2006;23(3–4):537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- 29.Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Sandner B, Schackel T, et al. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 2017;60:167–180. doi: 10.1016/j.actbio.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Hong LTA, Kim YM, Park HH, et al. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat Commun. 2017;8(1):533. doi: 10.1038/s41467-017-00583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coll-Miró M, Francos-Quijorna I, Santos-Nogueira E, et al. Beneficial effects of IL-37 after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2016;113(5):1411–1416. doi: 10.1073/pnas.1523212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bracken MB, Shepard MJ, Collins WF, Jr, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76(1):23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998;59(2):135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- 35.Lin JB, Poh S, Panitch A. Controlled release of anti-inflammatory peptides from reducible thermosensitive nanoparticles suppresses cartilage inflammation. Nanomedicine. 2016;12(7):2095–2100. doi: 10.1016/j.nano.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CX, Olschowka JA, Wrathall JR. Increase of interleukin-1beta mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res. 1997;759(2):190–196. doi: 10.1016/s0006-8993(97)00254-0. [DOI] [PubMed] [Google Scholar]
- 37.Lacroix S, Chang L, Rose-John S, Tuszynski MH. Delivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growth. J Comp Neurol. 2002;454(3):213–228. doi: 10.1002/cne.10407. [DOI] [PubMed] [Google Scholar]
- 38.Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng. 2011;8(4):046033. doi: 10.1088/1741-2560/8/4/046033. [DOI] [PubMed] [Google Scholar]
- 39.Wakao N, Imagama S, Zhang H, et al. Hyaluronan oligosaccharides promote functional recovery after spinal cord injury in rats. Neurosci Lett. 2011;488(3):299–304. doi: 10.1016/j.neulet.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki M, Radtke C, Tan AM, et al. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29(47):14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Z, Ye Y, Zhang Z, et al. Noninvasive, targeted gene therapy for acute spinal cord injury using LIFU-mediated BDNF-loaded cationic nanobubble destruction. Biochem Biophys Res Commun. 2018;496(3):911–920. doi: 10.1016/j.bbrc.2018.01.123. [DOI] [PubMed] [Google Scholar]
- 42.Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao S, Yu S, Cao Z, et al. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int J Nanomedicine. 2018;13:2883–2895. doi: 10.2147/IJN.S159356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu HL, Tian FR, Xiao J, et al. Sustained-release of FGF-2 from a hybrid hydrogel of heparin-poloxamer and decellular matrix promotes the neuroprotective effects of proteins after spinal injury. Int J Nanomedicine. 2018;13:681–694. doi: 10.2147/IJN.S152246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32(1):41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Cregg JM, Depaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinhold AK, Rittner HL. Barrier function in the peripheral and central nervous system – a review. Pflugers Arch. 2017;469(1):123–134. doi: 10.1007/s00424-016-1920-8. [DOI] [PubMed] [Google Scholar]